Abstract
Keratinocyte growth factor (KGF), which is given exogenously to allogeneic bone marrow transplantation (allo-BMT) recipients, supports thymic epithelial cells and increases thymic output of naive T cells. Here, we demonstrate that this improved T-cell reconstitution leads to enhanced responses to DNA plasmid tumor vaccination. Tumor-bearing mice treated with KGF and DNA vaccination have improved long-term survival and decreased tumor burden after allo-BMT. When assayed before vaccination, KGF-treated allo-BMT recipients have increased numbers of peripheral T cells, including CD8+ T cells with vaccine-recognition potential. In response to vaccination, KGF-treated allo-BMT recipients, compared with control subjects, generate increased numbers of tumor-specific CD8+ cells, as well as increased numbers of CD8+ cells producing interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α). We also found unanticipated benefits to antitumor immunity with the administration of KGF. KGF-treated allo-BMT recipients have an improved ratio of T effector cells to regulatory T cells, a larger fraction of effector cells that display a central memory phenotype, and effector cells that are derived from a broader T-cell–receptor repertoire. In conclusion, our data suggest that KGF can function as a potent vaccine adjuvant after allo-BMT through its effects on posttransplantation T-cell reconstitution.
Introduction
Keratinocyte growth factor (KGF), also called fibroblast growth factor 7, is indicated and widely used for preventing mucositis associated with high-dose cytotoxic therapy.1,2 Interestingly, its receptor, FgfR2-IIIb, is expressed by thymic epithelium as well as gastrointestinal mucosa. A role for KGF in supporting thymic epithelial cells was first suggested by the observation that FgfR2-IIIb–deficient mice show severely hypoplastic thymi at birth.3 We have previously shown that KGF-deficient mice develop normal thymi but display increased sensitivity to thymic damage and impaired thymic reconstitution, suggesting a role for KGF in supporting thymic epithelium during regeneration.4
The endogenous sources of KGF in the thymus include mesenchymal cells5 as well as αβ-lineage thymocytes.6 When given exogenously, KGF restores the hypoplastic medullary thymic epithelial compartment in Rag-deficient mice.6 In mice with thymic injury secondary to radiation, cyclophosphamide or dexamethasone, or thymic involution attributable to age, KGF restores thymic architecture and improves thymocyte generation,4 translating into improved humoral immune responses.7 KGF directly increases proliferation of both mature and immature thymic epithelial cells, activating the p53, nuclear factor-κB (NF-κB), bone morphogenetic proteins (BMPs), and Wnt pathways, resulting in enhanced T-cell development.8 KGF has been shown to increase intrathymic levels of interleukin-7 (IL-7) and appears to act at least partially via IL-7 signaling.9,10
When given to mice undergoing allogeneic bone marrow transplantation (allo-BMT), KGF preserves thymic architecture and improves thymocyte counts.10 This benefit also has been demonstrated in middle-aged mice4 and mice with active graft-versus-host disease (GVHD).11 KGF also increases numbers of naive T cells in the periphery post–allo-BMT,4,10 with further enhanced T-cell reconstitution when combined with androgen blockade.12 Similar benefits of KGF recently have been reported in rhesus macaques that had received autologous BMT.13 Clinical studies on the effects of KGF on immune function in allo-BMT patients are ongoing at our center.
One potential clinical benefit of accelerated T-cell reconstitution after allo-BMT is improved immunity against pathogens and residual malignancy. In this study, we asked whether KGF leads to improved antitumor immunity in response to vaccination. We and others have reported on the utility of tumor vaccines as a means of enhancing the graft-versus-tumor effect in allo-BMT recipients,14-22 and this strategy also was reported in a clinical trial.23 We previously showed in murine models that DNA tumor vaccination after allo-BMT is quite effective, capable of producing antitumor T-cell responses and rejection of subsequent tumor challenges and markedly improving the long-term survival of tumor-bearing mice in treatment models.14
Strategies to enhance CD8+ T-cell responses to vaccination have not focused on agents with a predominant effect on thymopoiesis. Therefore, we assessed the effects of administrating KGF to allo-BMT recipients receiving DNA tumor vaccination. In this study, we used the murine melanoma model B16, as well as a DNA plasmid vaccine targeting the melanoma antigen tyrosinase-related protein 1 (TRP1), to examine the effects of KGF on responses to tumor vaccination after allo-BMT.
Methods
Mice and stem cell transplantation
Female C57BL/6 (B6, H-2b) and LP (H-2b) mice 8-10 weeks of age were obtained from The Jackson Laboratory (Bar Harbor, ME). On day 0, bone marrow cells were removed aseptically from femurs and tibias of LP mice. Donor bone marrow was T-cell depleted by incubation with anti–Thy-1.2 for 40 minutes at 4°C followed by incubation with Low-TOX-M rabbit complement (Cedarlane Laboratories, Burlington, NC) for 40 minutes at 37°C. A total of 5 million cells were transplanted by tail vein injection into lethally irradiated B6 recipients (11 Gy of total body irradiation from a 137Cs source as a split dose with a 2.5- to 3-hour interval between doses). Mice were housed in sterilized microisolator cages and received normal chow and autoclaved hyperchlorinated drinking water (pH 3.0). BMT protocols were approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee.
KGF administration
Recombinant human KGF was administered daily at 5 mg/kg per day subcutaneously on days −6, −5, and −4 before allo-BMT as previously described.4 Recombinant human KGF was kindly provided by Amgen (Thousand Oaks, CA). Control mice received phosphate-buffered saline (PBS) instead of KGF.
DNA plasmid vaccine construct
VP22-opt-TRP1 is a DNA plasmid vaccine based on a tyrosinase-related protein 1 (TRP1) vaccine described previously,24 designed with the use of software based on the concept of heteroclitic peptides.25 The coding sequence of mouse TRP1 was modified to optimize major histocompatibility complex (MHC) class I binding to both Kb and Db, and subsequently fused to VP22, an HSV1 protein that has been shown to enhance vaccine potency.26 We have previously shown that immunization with the plasmid vector alone did not induce tumor rejection or antigen-specific responses.27
DNA vaccine administration
Mice were immunized by helium-driven particle bombardment, as previously reported.28 In brief, plasmid DNA was purified, coated onto 1-μm diameter gold particles (Alfa Aesar, Ward Hill, MA), and allowed to settle on bullets of Teflon tubing. Gold particles containing 1 μg DNA were delivered to each abdominal quadrant by the use of a helium-driven gene gun (Accell; PowderMed, Oxford, United Kingdom), for a total of 4 μg DNA per mouse. Mice were immunized every 5 days starting at day 14 for a total of either 3 or 5 immunizations and harvested 6 days after the last immunization for cell counts and flow cytometry studies. For survival studies, tumor-bearing mice were vaccinated every 5 days, starting at day 14.
Mouse tumor studies and in vivo bioluminescence
Tumor challenge experiments were conducted with melanoma B16 cells. In brief, 5 × 104 B16F10 melanoma cells (gift from I. Fidler, M. D. Anderson Cancer Center, Houston, TX) were injected intravenously via the tail vein. Mice were monitored daily and were euthanized when experiencing respiratory distress or found to have progressive large bulky tumors greater than 1 cm in diameter. Survival was assessed from the day of tumor challenge. Kaplan-Meier survival curves were generated and compared with the use of the log-rank test. For quantitating tumor burden, B16-TGL cells (B16 retrovirally transduced to express a triple fusion protein consisting of herpes simplex virus thymidine kinase, enhanced green fluorescent protein, and firefly luciferase)29 were administered intravenously at a dose of 10 × 105. Bioluminescent signal intensity of tumor-bearing mice was determined weekly starting 2 weeks after BMT. At 10 minutes after the intraperitoneal injection of 3 mg/mouse D-luciferin (Caliper Life Sciences, Hopkinton, MA), mice were anesthetized and placed into the light tight chamber of an IVIS 200 bioluminescence imaging system (Xenogen, Alameda, CA). Grayscale photographic images of the mice were acquired first, and then a low-level bioluminescent signal was recorded. Pseudocolor images showing the whole-body distribution of bioluminescent signal intensity were superimposed on the grayscale photographs, and total flux (photons/second) was determined for individual mice.
Cells
Single-cell suspensions were prepared from spleen, thymus, and lymph nodes (LNs) according to standard protocols and analyzed by flow cytometry.
Antibodies and flow cytometry
Anti–murine CD16/CD32 Fc receptor block (2.4G2) and all of the following fluorochrome-labeled antibodies against antigens (all of which are murine unless otherwise indicated) were obtained from BD Pharmingen: CD3ϵ (500A2), CD4 (RM4-5), CD45 (30-F11), CD8α (53-6.7), cleaved caspase-3 (C92-605), human KI-67 (B56), Ly 9.1 (30C7), interferon-γ (IFN-γ; XMG1.2), tumor necrosis factor-α (TNF-α; MP6-XT22), and Vβ T-cell receptor (TCR) screening panel. Antibodies against CD44 (IM7), CD62L (MEL-14), and Foxp3 (FJK-16s) were obtained from BioLegend (San Diego, CA), Caltag (Burlingame, CA), and eBioscience (San Diego, CA), respectively. iTAg MHC tetramer with peptide TAPDNLGYM (amino acids 455-463 from murine TRP1, optimized for H-2Db binding) was custom ordered from Beckman Coulter (Fullerton, CA). Intracellular cytokine production was assayed with overnight stimulation of whole splenocytes with peptide TAPDNLGYA (amino acids 455-463 from murine TRP1, wild-type sequence) at a concentration of 1 μg/mL in the presence of Golgiplug from BD Pharmingen (Franklin Lakes, NJ) per the manufacturer's directions. Cells were acquired on a LSRII cytometer (BD Biosciences, San Jose, CA) with FACSDiva software. Data were analyzed with FlowJo software (TreeStar, Ashland, OR).
Statistics
Statistical comparisons of experimental data were performed with the nonparametric unpaired Mann-Whitney t test. A P value less than .05 was considered statistically significant. For Vβ experiments, background usage for each Vβ was determined by the 20% trimmed-mean percentage of Vβ+ tetramer-negative cells from the same experiments.
Results
KGF administration increases thymic cellularity and peripheral T-cell numbers in allo-BMT recipients
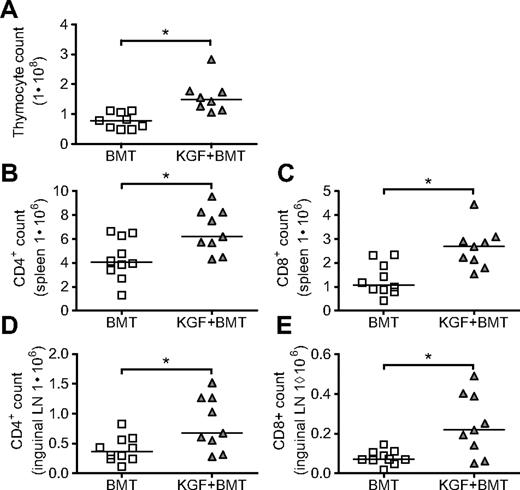
The authors of a previous study of KGF in mice undergoing T cell–depleted MHC-disparate allo-BMT used the BALB/c→B6 (H2d→H2b) model and found enhanced thymopoiesis and increased T-cell numbers in the periphery as early as day 28 after BMT.10 In the current study, we examined the effects of KGF in a model with minor histocompatibility antigen disparities, LP→B6 (H2b→H2b), which we used previously in our studies of DNA tumor vaccination.14 On day 0, B6 mice were lethally irradiated and intravenously injected with T cell–depleted LP bone marrow. KGF was given subcutaneously before BMT on days −6, −5, and −4. We found in this model that KGF produces a 2-fold increase in thymic cellularity as early as day 19 (Figure 1A), similar to the results in the MHC-disparate model. Splenic and inguinal LN cellularities were unchanged in KGF-treated recipients (data not shown), but the composition of cells in these peripheral lymphoid organs were markedly changed, with increased total numbers of CD4+ and CD8+ cells in both spleens (Figure 1B,C) and inguinal LNs (Figure 1D,E), as well as increased percentages of CD4+ and CD8+ cells (data not shown).
KGF increases thymic cellularity and numbers of peripheral T cells on day 19 after allogeneic BMT. Eight- to 10-week-old B6 mice were lethally irradiated (11 Gy) and transplanted with T cell–depleted LP BM (5 × 106). Some recipients received KGF (5 mg/kg/day) or PBS subcutaneously pretransplant on days −6 to −4. On day 19, thymic cellularity (A, *P = .002), CD4+ and CD8+ populations from spleens (B, *P = .02, and C, *P = .003), and inguinal LN (D, *P = .02, and E, *P = .02) were determined by flow cytometry. Values from individual mice are shown with medians. Data are pooled from 2 experiments with similar results.
KGF increases thymic cellularity and numbers of peripheral T cells on day 19 after allogeneic BMT. Eight- to 10-week-old B6 mice were lethally irradiated (11 Gy) and transplanted with T cell–depleted LP BM (5 × 106). Some recipients received KGF (5 mg/kg/day) or PBS subcutaneously pretransplant on days −6 to −4. On day 19, thymic cellularity (A, *P = .002), CD4+ and CD8+ populations from spleens (B, *P = .02, and C, *P = .003), and inguinal LN (D, *P = .02, and E, *P = .02) were determined by flow cytometry. Values from individual mice are shown with medians. Data are pooled from 2 experiments with similar results.
KGF increases the overall survival of tumor-bearing mice treated with allo-BMT with and without DNA tumor vaccination
We hypothesized that the accelerated T-cell reconstitution observed with KGF administration would lead to more robust T-cell responses to tumor vaccination. To test this hypothesis, we used a DNA plasmid vaccine targeting the melanoma differentiation antigen TRP1. Mice were inoculated intravenously with B16 melanoma 7 days before allo-BMT, received DNA immunizations starting on day 14 after allo-BMT delivered by gene gun every 5 days, and were monitored for survival. We found that BMT alone had a significant impact on survival (Figure 2A). Although the addition of DNA vaccination to BMT did not produce an appreciable benefit, mice treated with both KGF and DNA vaccination showed marked improvement in median survival and 37% 90-day survival (Figure 2B). The combination of KGF and vaccination trended toward a benefit compared with KGF alone (P = .08). We found a similar benefit when we replaced the DNA vaccine with a granulocyte-macrophage colony-stimulating factor (GM-CSF)–secreting B16-cell vaccine (data not shown).
KGF improves overall survival and tumor burden of tumor-bearing mice undergoing allogeneic BMT and tumor vaccination. B6 mice were inoculated intravenously with syngeneic B16 melanoma (5 × 104 cells) 7 days before T cell–depleted BMT. BMT alone had a significant effect on overall survival (A, *P < .001). Mice were treated with KGF as described in Figure 1, and some mice were vaccinated every 5 days starting on day 14 with VP22-opt-TRP1 plasmid DNA. Mice were followed daily for survival and were euthanized when experiencing respiratory distress or found to have progressive large bulky tumors greater than 1 cm in diameter. Data shown are pooled from 4 experiments with similar results (B, *P = .003, **P = .01, KGF + BMT vs KGF + BMT + DNA not significant, P = .08). In a similar experiment, B16-TGL (105 cells) expressing firefly luciferase was substituted for B16. Weekly, starting day 21 after tumor challenge, mice were evaluated with in vivo bioluminescence imaging, with median values of luminescence shown in panel C, pooled from 2 experiments with similar results. Individual data from day 35 are shown in panel D (*P = .047, **P = .008).
KGF improves overall survival and tumor burden of tumor-bearing mice undergoing allogeneic BMT and tumor vaccination. B6 mice were inoculated intravenously with syngeneic B16 melanoma (5 × 104 cells) 7 days before T cell–depleted BMT. BMT alone had a significant effect on overall survival (A, *P < .001). Mice were treated with KGF as described in Figure 1, and some mice were vaccinated every 5 days starting on day 14 with VP22-opt-TRP1 plasmid DNA. Mice were followed daily for survival and were euthanized when experiencing respiratory distress or found to have progressive large bulky tumors greater than 1 cm in diameter. Data shown are pooled from 4 experiments with similar results (B, *P = .003, **P = .01, KGF + BMT vs KGF + BMT + DNA not significant, P = .08). In a similar experiment, B16-TGL (105 cells) expressing firefly luciferase was substituted for B16. Weekly, starting day 21 after tumor challenge, mice were evaluated with in vivo bioluminescence imaging, with median values of luminescence shown in panel C, pooled from 2 experiments with similar results. Individual data from day 35 are shown in panel D (*P = .047, **P = .008).
To evaluate the effect of our treatments on tumor burden, we used an in vivo bioluminescence technique that included the use of luciferase-positive B16-TGL cells. We found that, again, DNA vaccination did not add significantly to BMT alone, but treatment with KGF plus DNA resulted in improved control of tumor burden (Figure 2C,D). With the more immunogenic B16-TGL cell line, which expresses a triple fusion protein consisting of herpes simplex virus thymidine kinase, enhanced green fluorescent protein, and firefly luciferase, we found that KGF plus DNA was no longer trending toward a benefit compared with KGF alone. This observation raised the possibility that KGF may act independently of vaccination, and we thus performed a series of experiments to assess whether KGF was improving the efficacy of DNA vaccination by augmenting immune responses.
KGF administration increases the number of T cells with vaccine-recognizing potential in unvaccinated allo-BMT recipients, as well as the number of CD4+Foxp3+ Tregs
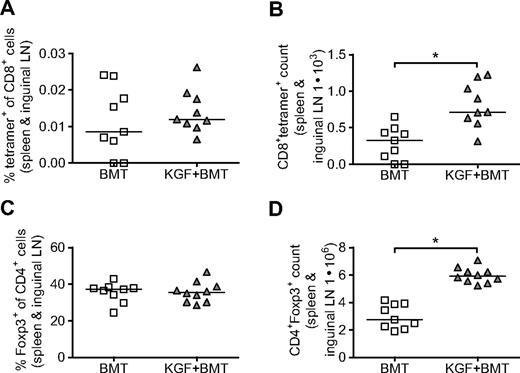
We first evaluated the frequency of CD8+ T cells that could potentially respond to vaccination. CD8+ T cells from spleens and inguinal LNs were purified with magnetic beads on day 19 after BMT and stained with 455-opt-TRP1 tetramer to identify CD8+ cells that, in the absence of vaccination, recognize the immunodominant heteroclitic epitope of our DNA vaccine. We found that although the tetramer+ percentage of CD8+ cells does not change with KGF, the total number of tetramer+ cells in each individual mouse was increased in the KGF-treated group (Figure 3A,B). Thus, the overall increase in CD8+ cells that we observed in KGF-treated mice resulted in a concurrent increase in the number of CD8+ T cells that recognized our DNA vaccine. In a similar manner, we found that although KGF-treated mice had percentages of CD4+ cells that expressed Foxp3 similar to mice not treated with KGF, an overall increase in CD4+ T cells led to an increased total number of regulatory T cells (Tregs; Figure 3C,D). In nontransplanted mice, KGF was reported previously to increase both the frequency and total numbers of Tregs.30
On day 19 after BMT without vaccination, KGF does not change the percentage of CD8+tetramer+ cells that could respond to vaccination or the percentage of CD4+ cells that express Foxp3 but does increase the overall number CD8+tetramer+ and CD4+Foxp3+ cells in each mouse. B6 mice underwent T cell-depleted BMT and were treated with KGF as described in Figure 1. On day 19, frequency and total number of CD8+tetramer+ cells were determined by purifying peripheral CD8+ cells (combined spleens and inguinal LNs) with magnetic beads, and staining with opt-TRP1-455 tetramer. Purified CD8+ cells staining for tetramer (A and B, *P = .004) and splenocytes staining for Foxp3 (C,D, *P < .001) were analyzed with flow cytometry. Data are pooled from 2 experiments with similar results.
On day 19 after BMT without vaccination, KGF does not change the percentage of CD8+tetramer+ cells that could respond to vaccination or the percentage of CD4+ cells that express Foxp3 but does increase the overall number CD8+tetramer+ and CD4+Foxp3+ cells in each mouse. B6 mice underwent T cell-depleted BMT and were treated with KGF as described in Figure 1. On day 19, frequency and total number of CD8+tetramer+ cells were determined by purifying peripheral CD8+ cells (combined spleens and inguinal LNs) with magnetic beads, and staining with opt-TRP1-455 tetramer. Purified CD8+ cells staining for tetramer (A and B, *P = .004) and splenocytes staining for Foxp3 (C,D, *P < .001) were analyzed with flow cytometry. Data are pooled from 2 experiments with similar results.
After vaccination, KGF-treated allo-BMT recipients have increased numbers of tumor-specific T cells without increased Tregs
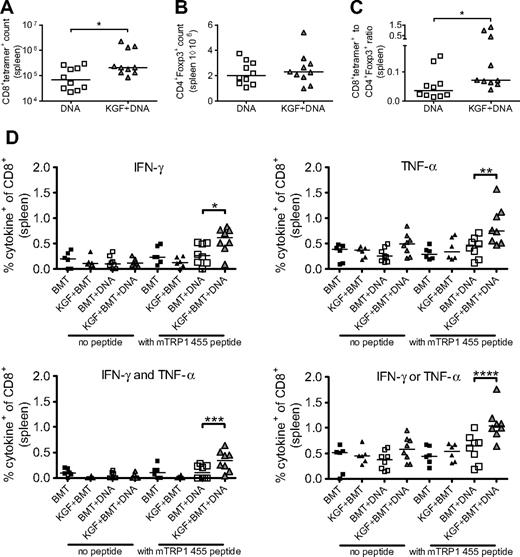
We then performed vaccination experiments, administering DNA immunizations every 5 days starting on day 14 after BMT. After the third vaccination, we found a marked increase in tetramer+ cells in KGF-treated vaccinated mice compared with untreated, vaccinated mice (Figure 4A). We also again assessed the numbers of Tregs in our KGF-treated versus untreated allo-BMT recipients, now both vaccinated, and found no significant difference (Figure 4B). The ratio of T effector cells to Tregs has been suggested to predict effective immune responses.31 In our model, we found that KGF-treated allo-BMT recipients indeed displayed an increased tumor-specific T cells to Tregs ratio compared with control allo-BMT recipients (Figure 4C). Our data demonstrate that posttransplantation vaccination of KGF-treated allo-BMT recipients produces improved T-cell tumor immunity through an increase in tumor-specific T cells and the ratio of T effector cells to Tregs.
KGF produces increased CD8+ T-cell responses to DNA tumor vaccine without increased CD4+Foxp3+ cells in day 30 recipients of allogeneic BMT. B6 mice underwent T cell–depleted BMT and were treated with KGF as described in Figure 1. On days 14, 19, and 24, mice were vaccinated with VP22-opt-TRP1 plasmid DNA. On day 30, the numbers of CD8+tetramer+ cells (A, *P = .04), CD4+Foxp3+ cells (B), and ratio of CD8+tetramer+ to CD4+Foxp3+ cells (C, *P = .01) from spleens were determined by flow cytometry. Whole splenocytes from transplanted groups also were stimulated overnight in the absence or presence of wild-type murine TRP1 455 peptide, and intracellular production of IFN-γ and TNFα by CD8+ cells was determined by flow cytometry (D, *P = .04, **P = .009, ***P = .03, ****P = .01). Data are pooled from 2 experiments with similar results.
KGF produces increased CD8+ T-cell responses to DNA tumor vaccine without increased CD4+Foxp3+ cells in day 30 recipients of allogeneic BMT. B6 mice underwent T cell–depleted BMT and were treated with KGF as described in Figure 1. On days 14, 19, and 24, mice were vaccinated with VP22-opt-TRP1 plasmid DNA. On day 30, the numbers of CD8+tetramer+ cells (A, *P = .04), CD4+Foxp3+ cells (B), and ratio of CD8+tetramer+ to CD4+Foxp3+ cells (C, *P = .01) from spleens were determined by flow cytometry. Whole splenocytes from transplanted groups also were stimulated overnight in the absence or presence of wild-type murine TRP1 455 peptide, and intracellular production of IFN-γ and TNFα by CD8+ cells was determined by flow cytometry (D, *P = .04, **P = .009, ***P = .03, ****P = .01). Data are pooled from 2 experiments with similar results.
We also evaluated the functionality of CD8+ T cells in vaccinated allo-BMT recipients by assaying for production of IFN-γ and TNF-α in response to stimulation with wild-type TRP1 peptide. We found that KGF-treated mice had increased numbers of CD8+ T cells producing either IFN-γ, TNF-α, either, or both cytokines. Polyfunctionality has been identified as an indicator of superior CD8+ T-cell responses in terms of control of HIV replication.32
KGF does not affect the proliferation, survival, or chimerism of vaccine-generated tumor-specific T cells but does increase the proportion with a central memory phenotype
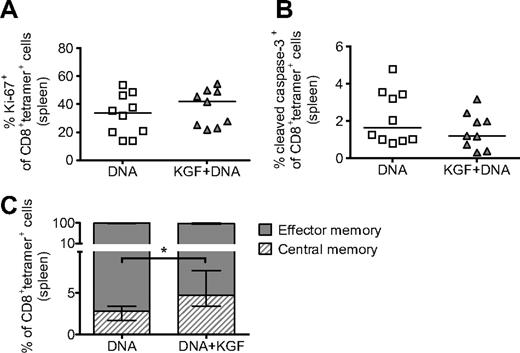
We then asked whether the increased T-cell vaccine responses observed in KGF-treated mice were the result of changes in the proliferation or survival of CD8+ cells responding to the vaccine. For these studies, we found that assaying after 5 immunizations allowed for adequate numbers of tetramer+ cells in both groups for analysis. When using flow cytometry, we found no significant changes in expression of either Ki-67 or cleaved caspase-3 in tetramer+ cells from mice treated with KGF, suggesting that KGF administration has no effect on the proliferation or apoptosis of tetramer+ cells (Figure 5A,B). We also assayed for donor or host origin at this time point and found no changes in chimerism of tetramer+ cells as the result of KGF (data not shown).
KGF does not affect proliferation or apoptosis but does increase the central memory phenotype of tetramer+ cells in vaccinated mice after allogeneic BMT. B6 mice underwent T cell–depleted BMT and were treated with KGF as described in Figure 1. On days 14, 19, 24, 29, and 34, mice were vaccinated with VP22-opt-TRP1 plasmid DNA. On day 40, tetramer+ cells from spleens were evaluated for proliferation (A), apoptosis (B), and central versus effector memory phenotype (C, *P = .009) by staining for Ki-67, cleaved caspase-3, and CD44 and CD62L, respectively, and analyzing with flow cytometry. Central memory and effector memory cells are defined as CD44highCD62Lhigh and CD44highCD62Llow, respectively. Data are pooled from 2 experiments with similar results.
KGF does not affect proliferation or apoptosis but does increase the central memory phenotype of tetramer+ cells in vaccinated mice after allogeneic BMT. B6 mice underwent T cell–depleted BMT and were treated with KGF as described in Figure 1. On days 14, 19, 24, 29, and 34, mice were vaccinated with VP22-opt-TRP1 plasmid DNA. On day 40, tetramer+ cells from spleens were evaluated for proliferation (A), apoptosis (B), and central versus effector memory phenotype (C, *P = .009) by staining for Ki-67, cleaved caspase-3, and CD44 and CD62L, respectively, and analyzing with flow cytometry. Central memory and effector memory cells are defined as CD44highCD62Lhigh and CD44highCD62Llow, respectively. Data are pooled from 2 experiments with similar results.
A growing body of literature has demonstrated that central memory CD8+ cells, described as CD44highCD62Lhigh, compared with effector memory cells (CD44highCD62Llow),33 are longer lasting, have improved proliferative potential, and produce more effective immune responses.34 By using flow cytometry, we found that although the majority of tetramer+ cells in both groups displayed an effector memory phenotype, KGF-treated allo-BMT recipients had a greater than 2-fold increase in the percentage of cells with a central memory phenotype (Figure 5C).
Vaccine responses in KGF-treated allo-BMT recipients are derived from a more varied TCR repertoire
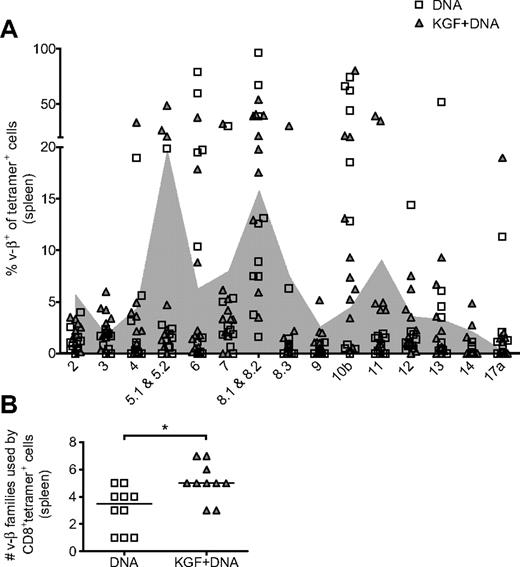
Because the administration of KGF enhances posttransplantation thymopoiesis, we hypothesized that CD8+ cells responding to vaccine should be derived from a broader TCR repertoire. The superiority of a diverse TCR repertoire in controlling viral infections has been suggested by others.35,36 We assayed for TCR repertoire breadth by flow cytometry with Vβ family-specific antibodies. We compared Vβ+ percentages of tetramer+ cells with background Vβ usage, determined by Vβ+ percentages of tetramer− cells from the same experiments (Figure 6A). We defined tetramer+ usage of a particular Vβ as having a Vβ+ percentage greater than background. With this analysis, we found that, in the KGF-treated group, tetramer+ cells in a given individual are derived from a median of 5 Vβ families compared with 3.5 Vβ families in the control group (Figure 6B). This finding suggests that mice that received KGF before allo-BMT have vaccine-responsive cells that are derived from a broader TCR repertoire.
KGF broadens the TCR repertoire of CD8+ cells responding to vaccine in mice after allogeneic BMT. B6 mice underwent T cell–depleted BMT, were treated with KGF, and were vaccinated as described in Figure 5. On day 40, CD8+tetramer+ cells from spleens were evaluated for TCR Vβ family origin with the use of flow cytometry (A). Shaded in gray is the background Vβ family usage, determined by the 20% trimmed mean of tetramer− cells from the same experiments. The number of Vβ families used by tetramer+ cells from individual mice, defined as usage above background, is also shown (B, *P = .01). Data are pooled from 2 experiments with similar results.
KGF broadens the TCR repertoire of CD8+ cells responding to vaccine in mice after allogeneic BMT. B6 mice underwent T cell–depleted BMT, were treated with KGF, and were vaccinated as described in Figure 5. On day 40, CD8+tetramer+ cells from spleens were evaluated for TCR Vβ family origin with the use of flow cytometry (A). Shaded in gray is the background Vβ family usage, determined by the 20% trimmed mean of tetramer− cells from the same experiments. The number of Vβ families used by tetramer+ cells from individual mice, defined as usage above background, is also shown (B, *P = .01). Data are pooled from 2 experiments with similar results.
Discussion
The administration of KGF to patients receiving high-dose cytotoxic therapy can reduce the duration and severity of mucositis.1,2 Preclinical studies also suggest that KGF may ameliorate GVHD37-39 and enhance post-BMT thymopoiesis.4,10-13 In this study, we demonstrate an additional potential benefit of KGF: the enhancement of responses to vaccination after T cell–depleted allo-BMT.
Our data suggest that KGF exerts its effects primarily by accelerating T-cell reconstitution, thus increasing the availability of naive T cells capable of responding to vaccination. With vaccination, this leads to increased numbers of tumor-specific T cells, which then translates into improved survival in tumor-bearing mice. To our knowledge, this report is the first demonstration of a thymopoietic agent acting as a CD8+ T-cell vaccine adjuvant.
We also found unanticipated benefits to antitumor immunity with KGF administration, including an improved T effector to Treg ratio. Several studies have demonstrated that T-cell responses in the lymphopenic setting are markedly enhanced.40 Several mechanisms for this phenomenon have been identified, including the relative absence or dysfunction of immune regulatory cells such as Tregs and the increased availability of prosurvival T-cell cytokines, including IL-7 and IL-15. Our data suggest that administering KGF in the setting of allo-BMT allows for the generation of more vaccine-responsive precursors. Upon vaccination, KGF-treated allo-BMT recipients generate more tumor-specific T cells with an improved T effector to Treg ratio. These T effector cells can then benefit from the increased availability of IL-7 and IL-15 in the lymphopenic setting.
We found that KGF-treated allo-BMT recipients, when vaccinated, generate a larger fraction of T effector cells that display a central memory rather than effector memory phenotype. The superiority of central memory T cells over effector memory T cells in maintaining immune responses has been demonstrated by others.34 This decrease in effector differentiation may be related to the increase in numbers of vaccine-specific T cells that we observed both before and after vaccination. Others have reported that in situations of increased antigen availability and decreased clonal competition, full terminal differentiation to an effector phenotype is accelerated.41 It is possible that, in KGF-treated individuals, the increase in antigen-specific clones leads to less available antigen on a per-cell basis, resulting in increased numbers of tumor-specific T cells with a central memory phenotype.
We also found that T effector cells from KGF-treated allo-BMT recipients are more likely to produce both IFN-γ and TNF-α and are derived from a broader TCR repertoire. Studies in various viral models suggest that T cells exhibiting polyfunctionality and a diverse TCR repertoire may be more effective at controlling viral replication32 and preventing development of escape variants,35,36 respectively.
In our survival experiments, a surprising finding was that the KGF plus BMT group showed a survival benefit compared with BMT alone, the reasons for which are unclear. The effects of KGF on in vivo proliferation and as a radiosensitizer of B16 were studied previously and found to be negligible.42 In our experiments, KGF produced more dramatic benefits with B16-TGL tumors, which are potentially more immunogenic. This finding suggests that KGF in the setting of BMT could be supporting immune-mediated suppression of tumor growth by accelerating T-cell reconstitution even in the absence of tumor vaccine. In retrospective clinical studies, early lymphocyte recovery has been associated with decreased relapse and improved overall survival after autologous BMT for various malignancies.43,44 It is possible that the benefits derived from KGF also may be associated with other strategies to improve T-cell generation, such as sex-steroid blockade,45 growth hormone,46 leptin,47 IL-7,48 and insulin-like growth factor I.48
In summary, KGF administration to allo-BMT recipients produces improved thymopoiesis, resulting in improved T-cell reconstitution. This improved reconstitution leads to enhanced responses to DNA tumor vaccination and improved survival in tumor-bearing allo-BMT recipients. Interestingly, KGF produced additional benefits to antitumor immunity, including an improved T effector to Treg ratio, a larger fraction of effector cells that display a central memory phenotype, more effectors producing both IFN-γ and TNF-α, and effector cells that are derived from a broader TCR repertoire.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by National Institutes of Health (Bethesda, MD) grants RO1-HL069929, RO1-CA107096, RO1-AI080455, and PO1-CA33049 (all M.R.M.v.d.B.). Support also was received from the Ryan Gibson Foundation (Dallas, TX), the Elsa U. Pardee Foundation (Midland, MI), the Byrne Fund (Etna, NH), the Emerald Foundation (New York, NY), and The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by Mr William H. Goodwin and Mrs Alice Goodwin, the Commonwealth Foundation for Cancer Research (Richmond, VA), The Bobby Zucker Memorial Fund (Phoenixville, PA; M.R.M.v.d.B.), and The Lymphoma Foundation (New York, NY). R.R.J. is the recipient of grants from the American Association for Cancer Research (Philadelphia, PA)–MedImmune Fellowship for Research on Biologics-Based Therapies for Cancer and the Leukemia & Lymphoma Society (White Plains, NY) Special Fellowship in Clinical Research.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: R.R.J. designed and performed research, analyzed data, and wrote the paper; C.G.K. performed research and contributed to writing the paper; C.V. performed research and contributed to writing the paper; D.S. performed research and contributed to writing the paper; O.M.S. performed research and contributed to writing the paper; U.K.R. performed research and contributed to writing the paper; N.L.Y. performed research and contributed to writing the paper; A.M.H. contributed to writing the paper; S.X.L. contributed to writing the paper; J.L.Z. contributed to writing the paper; G.L.G. contributed to writing the paper. A.D. designed research and analyzed data; O.A. designed research and analyzed data; O.P. designed research and analyzed data; I.-K.N. designed research and analyzed data; L.W.K. designed research and analyzed data; J.D.W. analyzed data and contributed vital reagents; A.N.H. analyzed data and contributed vital reagents; M.-A.P. designed research and analyzed data; and M.R.M.v.d.B designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcel R. M. van den Brink, Memorial Sloan-Kettering Cancer Center, Department of Medicine, 1275 York Avenue, New York, NY 10065; e-mail: vandenbm@mskcc.org.