Abstract
Adequate recovery of hematopoietic stem cell (HSC) niches after cytotoxic conditioning regimens is essential to successful bone marrow transplantation. Yet, very little is known about the mechanisms that drive the restoration of these niches after bone marrow injury. Here we describe a profound disruption of the marrow microenvironment after lethal total body irradiation of mice that leads to the generation of osteoblasts restoring the HSC niche, followed by a transient, reversible expansion of this niche. Within 48 hours after irradiation, surviving host megakaryocytes were observed close to the endosteal surface of trabecular bone rather than in their normal parasinusoidal site concomitant with an increased stromal-derived factor-1 level. A subsequent increase in 2 megakaryocyte-derived growth factors, platelet-derived growth factor-β and basic fibroblast growth factor, induces a 2-fold expansion of the population of N-cadherin-/osteopontin-positive osteoblasts, relative to the homeostatic osteoblast population, and hence, increases the number of potential niches for HSC engraftment. After donor cell engraftment, this expanded microenvironment reverts to its homeostatic state. Our results demonstrate the rapid recovery of osteoblastic stem cell niches after marrow radioablation, provide critical insights into the associated mechanisms, and suggest novel means to manipulate the bone marrow microenvironment to promote HSC engraftment.
Introduction
Transplantation of whole bone marrow (BM) into recipients that have undergone cytotoxic conditioning regimen leads to engraftment of hematopoietic stem cells (HSCs) and renewed blood production. One factor in this engraftment may be the number of specialized BM niches available to the donor HSCs.1 Generally defined as a cellular microenvironment that nurtures and protects stem cells, HSC niches can be generated by several different cell types, such as the osteopontin+/N-cadherin+ osteoblasts lining the endosteal surface of trabecular bone,2-5 the vascular marrow sinusoids,6 stromal-derived factor-1 (SDF-1)–secreting stromal cells,7 as well as adipocytes and macrophages.8 Investigators focusing on the endosteal surface niche have concluded that direct contact with osteoblast-synthesized protein, osteopontin, is fundamental to the biology of HSCs in situ.2,3 Hence, sufficient numbers of osteoblasts and other niche components must be available in the BM after cytoreductive treatment to ensure the integrity of HSC niches and thus high levels of donor HSC engraftment; however, irradiation of the BM compartment before transplantation produces a cytotoxic effect on osteoblasts as well as hematopoietic cells.9
Recent research has focused almost entirely on how HSCs are regulated by their microenvironmental niches,2,3,8,10 with little attention paid to the recovery of osteoblasts and other cellular constituents of these niches after use of cytotoxic preparative regimens. Moreover, although several models have been proposed, the anatomic location of HSC niches within the marrow microenvironment is not well understood.11 We therefore undertook a detailed study in mice treated with lethal total body irradiation (TBI) to assess the effect of this extreme stress on osteoblastic HSC niches and to identify potential mechanisms of nonhematopoietic cell reconstitution that might be exploited to enhance donor HSC engraftment.
Methods
Irradiation and BM transplantation procedures
Six- to 8-week-old FVB/N mice (n = 6; The Jackson Laboratory) were lethally irradiated with 1125 cGy with a 137Cs source (Mark II Irradiator, J. L. Sheppard and Associates) on a rotating platform (TBI group). Nonirradiated age-matched FVB/N mice (n = 6) were used as controls. To test the uptake of bromodeoxyuridine (BrdU) by bone and marrow cells, we injected mice twice intraperitoneally (after 24 and 42 hours after irradiation) with this reagent (75 mg/kg; Sigma-Aldrich). Forty-eight hours after irradiation, the bones were collected and processed for hematoxylin and eosin staining and immunohistochemical analyses. For the BM transplantation studies, 6- to 8-week-old FVB/N mice (The Jackson Laboratory) were lethally irradiated (1125 cGy) and injected intravenously with 2 × 106 enhanced green fluorescence protein transgenic (FVB/N background) BM cells as previously reported.12 After 48 hours and 10 days after transplantation, the bones were harvested and processed. Experiments were performed at least in triplicate. All animal protocols were approved by the authors' respective Institutional Animal Use and Care Committees.
Histology
Formalin-fixed, decalcified, paraffin-embedded sections were stained with standard hematoxylin-and-eosin stain (Sigma-Aldrich). Single and double immunohistochemical staining was performed with rabbit anti–green fluorescent protein (GFP) antibody (Ab; 1:300; Invitrogen), rabbit anti–collagen I Ab (1:100; Chemicon International), rabbit anti-osteocalcin Ab (1:100; Cosmo Bio), rabbit anti–SDF-1 Ab (1:15; Abcam), rabbit anti–CXCR4 Ab (4 μg/mL; a kind gift from Dr Stefan Schulz Laboratory, Otto-von-Guericke University, Magdeburg, Germany), rabbit anti–N-cadherin Ab (1:80; Abcam), rabbit anti-osteopontin Ab (1:100; Cosmo Bio), rabbit anti–cathepsin K (1:50; Abcam), rabbit anti–VE cadherin, rabbit anti-CD31 (both 1:50; Abcam), and rat anti–mouse CD9 Ab (1:80; BD Biosciences PharMingen), using a goat anti–rat and anti–rabbit biotinylated secondary Ab (1:200; Vector Laboratories) as previously described.13 After incubation with Vectastain ABC (Vector Laboratories), horseradish peroxidase–labeled Abs were visualized with NovaRED (Vector Laboratories). BrdU labeling was detected with an anti–BrdU-POD Ab (1:15; Roche Diagnostic) according to the manufacturer's instructions. Color development was performed with diaminobenzidine-nickel (black), followed by a second step with anti–collagen I, anti-CD9, and anti–N-cadherin and anti–GFP Ab developed with NovaRED (red), as described.12 All slides were counterstained with Harris hematoxylin (Bio Optica). Negative control specimens were bone sections from the experimental mice stained with a rabbit isotypic IgG primary Ab (Vector Laboratories) and rat IgG2a isotypic Ab (BD Biosciences PharMingen) and visualized as reported. In all cases, background staining was not apparent. To evaluate the presence of the tartrate-resistant acid phosphatase (TRAP)–positive osteoclasts, we treated either TBI or control bone sections from decalcified bones with TRAP-kit (Sigma-Aldrich) according to the manufacturer's instructions.
Stained slides were examined by at least 2 investigators on a Zeiss Axiovert 200M (Carl Zeiss) with a either a 10×/0.25 NA or a 40×/0.6 NA dry objective. Photomicrographs were acquired with the attached Axiocam HR color camera and Axiovision 4.5 SP1 software (Carl Zeiss). Images were cropped and labeled with Photoshop 7.0 and Illustrator 10.0 (Adobe Systems).
Platelet count
The platelet count was determined in peripheral blood obtained by retro-orbital puncture using a hematology analyzer (Hemavet, Model HV950FS; Drew Scientific).
RT-PCR
Total RNA was isolated from flushed marrow cells using TRIzol Reagent (Invitrogen). First-strand cDNA was synthesized from 2 μg total RNA using SuperScript First-Strand (Invitrogen) according to the manufacturer's protocol. Semiquantitative polymerase chain reaction (PCR) was performed with MasterAmp Taq DNA Polymerase (Epicentre Biotechnologies). Preliminary experiments to verify exponential amplifications at different cycle numbers (from 20 to 40) were performed to validate the semiquantitative measurements of amplified products (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Conditions for amplification were as follows: 25 cycles at 94°C for 1 minute, 50°C for 1 minute, and 72°C for 1 minute for GAPDH, collagen I, osteocalcin, Ki-67, SDF-1, CXCR4, transforming growth factor-β (TGF-β), and osteopontin, 25 cycles at 94°C for 1 minute, 52°C for 1 minute, and 72°C for 1 minute for platelet-derived growth factor-β (PDGF-β); and 36 cycles at 94°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute for basic fibroblast growth factor (bFGF). VE-cadherin and factor VIII amplifications were as follows: 25 cycles at 94°C for 1 minute, 56°C for 1 minute, and 72°C for 1 minute. N-cadherin amplifications were as follows: 25 cycles at 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute. Amplified products were then identified by 1.5% and 2.5% (exclusively for SDF-1) agarose gel electrophoresis and visualized with ethidium bromide and a UV transilluminator (Gel Doc 2000; Bio-Rad). To quantify relative differences in mRNA expression, we measured relative band intensity (Quantity One software 4.0; Bio-Rad) in relation to GAPDH. Table 1 contains details on the primers used (from MWG Biotech).
Quantitative real-time PCR (ABI 7500 Fast Real-Time PCR System; Applied Biosystems) was performed in a volume of 15 μL containing 1/200 of cDNA (corresponding to 5 ng original RNA), 1× Power SYBR green master mix (Applied Biosystems), and 300 nM reverse and forward primers. Murine GAPDH was amplified as an endogenous control to normalize for input RNA. Conditions for amplification were as follows: 95°C for 10 minutes followed by 50 cycles of 95°C for 15 seconds, and 55°C for 60 seconds using the relative standard curve program. The melting curve protocol was performed for each primer set to confirm specificity. All samples were measured in duplicate PCRs.
Osteoblast isolation and culture
Osteoblast cultures were established as previously described.13 Briefly, 6- to 8-week-old FVB/N mice (n = 6; The Jackson Laboratory) were irradiated and their bones harvested. Metaphyses and epiphyses were carefully dissected under a dissection scope (Olympus SZ60; Olympus). After extended mincing into conical glass vials (REACTI VIALS; Thermo Fisher Scientific), bone fragments were digested for 180 minutes at 37°C with collagenase P (0.2 mg/mL; Roche Diagnostic) into Dulbecco modified Eagle medium–Ham F12 mixture (Invitrogen) supplemented with 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Euroclone). Enzyme was then inactivated with 10% fetal bovine serum (HyClone Laboratories) and the digested bone chips (20 mg/mL) were placed in Dulbecco modified Eagle medium–Ham F12 mixture, 10% fetal bovine serum, L-ascorbic acid-2-phosphate (0.1 mM; Sigma-Aldrich), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. In some experiments, bone samples were placed in the same media but supplemented (5%, vol/vol) with platelet lysate (PL)/human serum (108 platelets/mL) as previously described.14 After 2 weeks, osteoblast colonies originating from bone fragments were washed twice with phosphate-buffered saline (Euroclone), stained with a methanol-crystal violet solution (0.4%; wt/vol; Sigma-Aldrich), and scored by microscopic examinations (Zeiss Axiovert 200M). Photomicrographs were acquired with the attached Axiocam HR color camera and Axiovsion 4.5 SP1 software (Carl Zeiss). Bones from nonirradiated mice (n = 6) were similarly processed as a control for osteoblast growth with and without PL.
Enzyme-linked immunosorbent assay
SDF-1 protein expression was assessed as reported by Slayton et al.15 Briefly, irradiated and control femurs were flushed with 2 mL of phosphate-buffered saline containing a protease inhibitor cocktail (Roche Diagnostics), followed by the addition of Nonidet P40 (1% vol/vol) to the cell suspension. Cells were lysed by freeze/thaw, and the cell debris was removed by centrifugation and discarded. The concentration of SDF-1 was measured using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) according to the manufacturer's instructions.
Statistical methods
Data are presented as mean (± SD). At least 3 biologic replicates were analyzed, and differences were considered statistically significant by a 2-tailed Student t test if they attained a P value of .05 or less. All statistical analyses were performed with the Excel 2003 program (Microsoft) or Prism, Version 4 (GraphPad Software).
Results
Osteoblasts proliferate after marrow radioablation
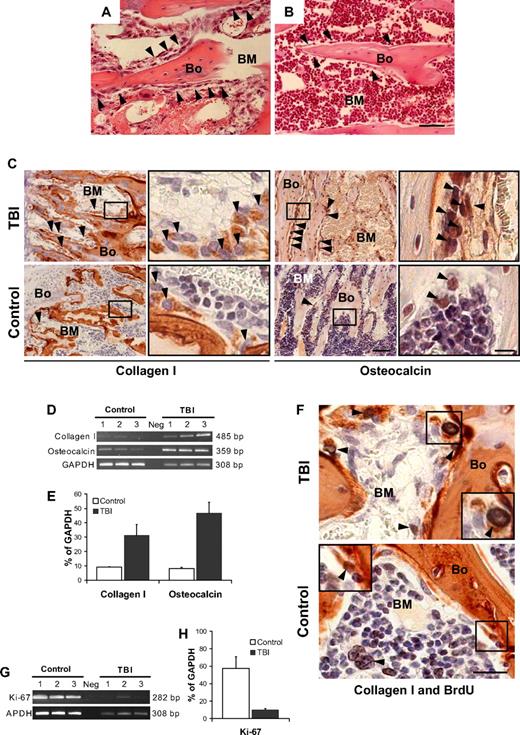
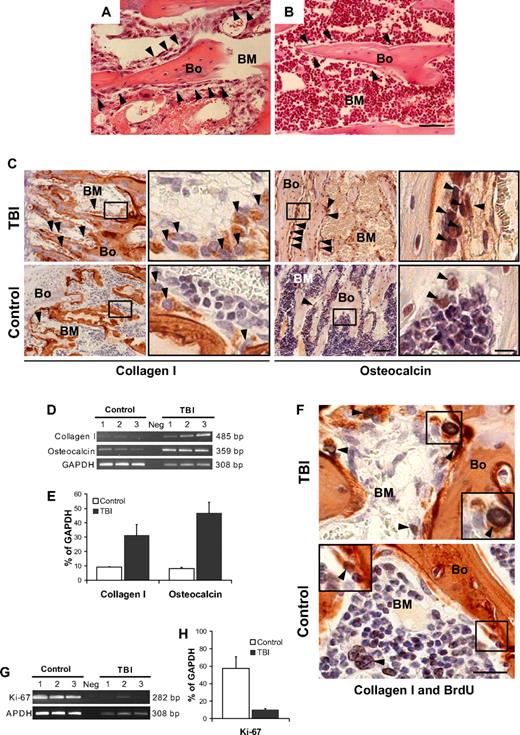
We first examined the histology of the BM microenvironment along the endosteal surface where marrow stem cells interface with blood and bone. Femora taken from mice (n = 6) at 48 hours after TBI without donor BM infusion showed a significant decrease in overall BM cellularity compared with nonirradiated controls (18% ± 11% vs 92% ± 6%, P < .001; Figure 1A-B). Surprisingly, bone-lining cells along the endosteal surface, which usually are arranged as a single tier (Figure 1B), were markedly expanded in the irradiated animals, resulting in multiple layers of flattened cells (Figure 1A). Visual inspection of bone sections immunohistochemically stained for collagen I and osteocalcin, 2 reliable markers of osteopoietic cells, indicated an increase in expression of these proteins among cells lining the bones of irradiated compared with control animals (Figure 1C). The most striking increases of collagen I– and osteocalcin-expressing cells were observed in the trabecular bone of the metaphyses (Figure 1C), whereas the diaphyses consistently lacked these changes (supplemental Figure 2A), suggesting that the microenvironment formed by endosteal osteoblasts of the metaphysis is distinct from that produced by cells of the diaphysis. To confirm this apparent expansion of endosteal osteoblasts cells, we assessed the expression of collagen I and osteocalcin by semiquantitative reverse-transcriptase (RT) PCR and then verified the outcome by quantitative real-time PCR (supplemental Figure 3). The results (Figure 1D-E), based on RNA isolated from the cellular contents of the marrow space, demonstrate 3- and 5-fold increases (P = .01 and P = .001, respectively), in the expression of collagen I and osteocalcin in irradiated versus nonirradiated mice.
Osteoblast proliferation after marrow radioablation. (A) Bone (Bo) and BM section taken from a metaphysis at 48 hours after TBI shows an increased number of cells lining the bone ( ) compared with (B) the nonirradiated control (hematoxylin-and-eosin stain). (A,B) Scale bar represents 50 μm. (C) Immunohistochemical staining (in red) to identify the bone-lining cells in panels A and B revealed an increase of collagen I (left) and osteocalcin (right) producing osteoblasts (
) compared with (B) the nonirradiated control (hematoxylin-and-eosin stain). (A,B) Scale bar represents 50 μm. (C) Immunohistochemical staining (in red) to identify the bone-lining cells in panels A and B revealed an increase of collagen I (left) and osteocalcin (right) producing osteoblasts ( ). Scale bar (third column) represents 50 μm; scale bar (fourth column) represents 10 μm. (D) Semiquantitative RT-PCR analysis confirming an increased expression of collagen I and osteocalcin in TBI mice. The negative control (Neg, without template) is shown in the middle lane. (E) Comparison of transcript band intensities, for genes encoding collagen I and osteocalcin, expressed in irradiated and nonirradiated mice. The values were calculated relative to GAPDH levels and reported as mean percentages (± SD) of 6 mice per group. Both collagen I (P = .01) and osteocalcin (P = .001) levels are significantly increased over control values (Student t test). (F) Double staining of sections from TBI and control mice with anti-BrdU (black) and anticollagen I (red) antibodies. The typical nuclear pattern of the BrdU labeling and the simultaneous collagen I expression (
). Scale bar (third column) represents 50 μm; scale bar (fourth column) represents 10 μm. (D) Semiquantitative RT-PCR analysis confirming an increased expression of collagen I and osteocalcin in TBI mice. The negative control (Neg, without template) is shown in the middle lane. (E) Comparison of transcript band intensities, for genes encoding collagen I and osteocalcin, expressed in irradiated and nonirradiated mice. The values were calculated relative to GAPDH levels and reported as mean percentages (± SD) of 6 mice per group. Both collagen I (P = .01) and osteocalcin (P = .001) levels are significantly increased over control values (Student t test). (F) Double staining of sections from TBI and control mice with anti-BrdU (black) and anticollagen I (red) antibodies. The typical nuclear pattern of the BrdU labeling and the simultaneous collagen I expression ( ) demonstrates the presence of proliferating osteoblasts after TBI. The BrdU-positive cells in the control mice are predominantly present in the BM space (
) demonstrates the presence of proliferating osteoblasts after TBI. The BrdU-positive cells in the control mice are predominantly present in the BM space ( ), with rare double-positive osteoblasts (inset). Scale bar represents 20 μm. (G) Semiquantitative RT-PCR analysis of Ki-67 expression by marrow cells from TBI and control mice demonstrates the persistence of proliferating BM cells after irradiation. (H) Comparison of transcript band intensities for Ki-67 expressed in irradiated versus nonirradiated mice. Values were calculated relative to GAPDH levels and reported as mean percentages (± SD) of 6 mice per group. Ki-67 expression was significantly decreased (P < .01) but persisted in irradiated mice.
), with rare double-positive osteoblasts (inset). Scale bar represents 20 μm. (G) Semiquantitative RT-PCR analysis of Ki-67 expression by marrow cells from TBI and control mice demonstrates the persistence of proliferating BM cells after irradiation. (H) Comparison of transcript band intensities for Ki-67 expressed in irradiated versus nonirradiated mice. Values were calculated relative to GAPDH levels and reported as mean percentages (± SD) of 6 mice per group. Ki-67 expression was significantly decreased (P < .01) but persisted in irradiated mice.
Osteoblast proliferation after marrow radioablation. (A) Bone (Bo) and BM section taken from a metaphysis at 48 hours after TBI shows an increased number of cells lining the bone ( ) compared with (B) the nonirradiated control (hematoxylin-and-eosin stain). (A,B) Scale bar represents 50 μm. (C) Immunohistochemical staining (in red) to identify the bone-lining cells in panels A and B revealed an increase of collagen I (left) and osteocalcin (right) producing osteoblasts (
) compared with (B) the nonirradiated control (hematoxylin-and-eosin stain). (A,B) Scale bar represents 50 μm. (C) Immunohistochemical staining (in red) to identify the bone-lining cells in panels A and B revealed an increase of collagen I (left) and osteocalcin (right) producing osteoblasts ( ). Scale bar (third column) represents 50 μm; scale bar (fourth column) represents 10 μm. (D) Semiquantitative RT-PCR analysis confirming an increased expression of collagen I and osteocalcin in TBI mice. The negative control (Neg, without template) is shown in the middle lane. (E) Comparison of transcript band intensities, for genes encoding collagen I and osteocalcin, expressed in irradiated and nonirradiated mice. The values were calculated relative to GAPDH levels and reported as mean percentages (± SD) of 6 mice per group. Both collagen I (P = .01) and osteocalcin (P = .001) levels are significantly increased over control values (Student t test). (F) Double staining of sections from TBI and control mice with anti-BrdU (black) and anticollagen I (red) antibodies. The typical nuclear pattern of the BrdU labeling and the simultaneous collagen I expression (
). Scale bar (third column) represents 50 μm; scale bar (fourth column) represents 10 μm. (D) Semiquantitative RT-PCR analysis confirming an increased expression of collagen I and osteocalcin in TBI mice. The negative control (Neg, without template) is shown in the middle lane. (E) Comparison of transcript band intensities, for genes encoding collagen I and osteocalcin, expressed in irradiated and nonirradiated mice. The values were calculated relative to GAPDH levels and reported as mean percentages (± SD) of 6 mice per group. Both collagen I (P = .01) and osteocalcin (P = .001) levels are significantly increased over control values (Student t test). (F) Double staining of sections from TBI and control mice with anti-BrdU (black) and anticollagen I (red) antibodies. The typical nuclear pattern of the BrdU labeling and the simultaneous collagen I expression ( ) demonstrates the presence of proliferating osteoblasts after TBI. The BrdU-positive cells in the control mice are predominantly present in the BM space (
) demonstrates the presence of proliferating osteoblasts after TBI. The BrdU-positive cells in the control mice are predominantly present in the BM space ( ), with rare double-positive osteoblasts (inset). Scale bar represents 20 μm. (G) Semiquantitative RT-PCR analysis of Ki-67 expression by marrow cells from TBI and control mice demonstrates the persistence of proliferating BM cells after irradiation. (H) Comparison of transcript band intensities for Ki-67 expressed in irradiated versus nonirradiated mice. Values were calculated relative to GAPDH levels and reported as mean percentages (± SD) of 6 mice per group. Ki-67 expression was significantly decreased (P < .01) but persisted in irradiated mice.
), with rare double-positive osteoblasts (inset). Scale bar represents 20 μm. (G) Semiquantitative RT-PCR analysis of Ki-67 expression by marrow cells from TBI and control mice demonstrates the persistence of proliferating BM cells after irradiation. (H) Comparison of transcript band intensities for Ki-67 expressed in irradiated versus nonirradiated mice. Values were calculated relative to GAPDH levels and reported as mean percentages (± SD) of 6 mice per group. Ki-67 expression was significantly decreased (P < .01) but persisted in irradiated mice.
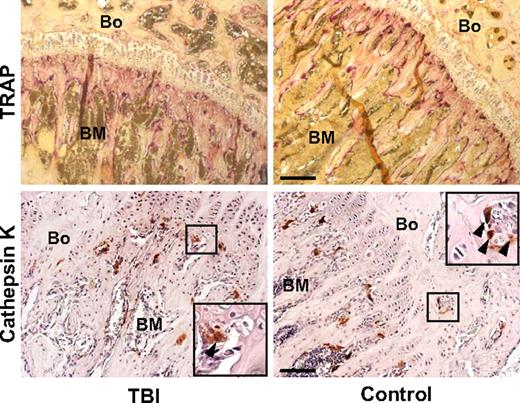
To determine whether the increased mesenchymal cellularity of trabecular bone was the result of proliferation, we injected lethally irradiated mice (n = 10) with BrdU at 24 and 42 hours after irradiation and killed the animals at 48 hours. Immunohistochemical staining (Figure 1F, supplemental Figure 2B-C) revealed BrdU uptake in 10.0% (± 3.6%) of the collagen I–expressing cells in the irradiated mice compared with 1.9% (± 0.9%) of cells in control animals (P = .005), indicating that the amplified cellularity along the endosteal surface of trabecular bone resulted from expansion of the resident cell population, presumably from a relatively radioresistant endogenous osteoprogenitor. Further molecular analyses, based on Ki-67 expression, a cell-cycle marker with a short half-life,16 confirmed that a population of proliferating marrow cells persists after irradiation (Figure 1G-H). Osteoclasts, which are normal constituents of the bone-lining cell compartment,10 did not contribute to this expansion, as indicated by the results of staining for TRAP and cathepsin K, 2 specific osteoclast markers (Figure 2). Thus, an expanding population of osteoblasts can be found proximal to the endosteal surface of trabecular bone after irradiation of the marrow space and would be expected to contribute to the restoration of depleted niches.
Osteoclast compartment staining in irradiated (TBI) and control mice. Photomicrographs of a bone (Bo) and BM section from the metaphysis stained with tartrate-resistant acid phosphatase (top; purple) and with anti–cathepsin K antibody (bottom; red), as 2 reliable osteoclast markers. Staining intensities did not differ appreciably between the 2 groups. Scale bar (top) represents 200 μm; scale bar (bottom) represents 100 μm.
Osteoclast compartment staining in irradiated (TBI) and control mice. Photomicrographs of a bone (Bo) and BM section from the metaphysis stained with tartrate-resistant acid phosphatase (top; purple) and with anti–cathepsin K antibody (bottom; red), as 2 reliable osteoclast markers. Staining intensities did not differ appreciably between the 2 groups. Scale bar (top) represents 200 μm; scale bar (bottom) represents 100 μm.
Megakaryocyte-osteoblast interactions after radioablation
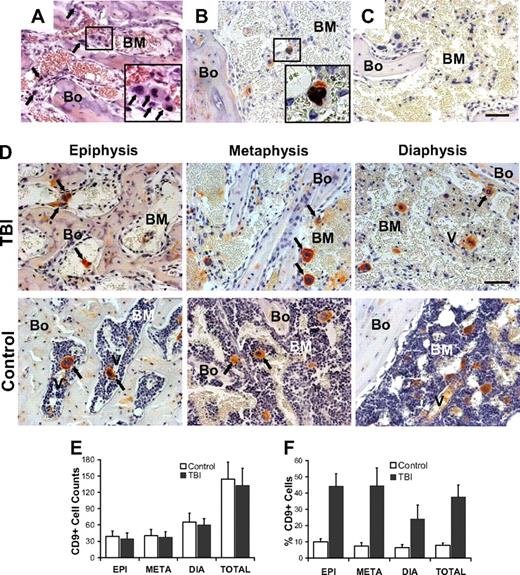
What stimuli might account for these observations? Because HSC niches are highly interactive units, organized to facilitate self-renewal and differentiation decisions in a timely manner,11 we hypothesized that one or more types of cells remain functional in the face of lethal irradiation and provide the regulatory signals needed to induce and sustain osteoblast proliferation. Megakaryocytes were considered the most probable candidates because they remain functional in rodents for at least 7 to 10 days after irradiation and BM transplantation17,18 and interact closely with bone.19,20 Investigation of the marrow space at 48 hours after lethal irradiation revealed a substantial number of large, multinucleated cells (Figure 3A inset) whose morphologic appearance and expression of CD9 (Figure 3B) were indicative of megakaryocytes.21 To assess the postirradiation viability of these cells, we analyzed the colocalization of BrdU and CD9 in bone sections from lethally irradiated mice (Figure 3B inset). BrdU uptake was detected in the nucleus of 25.0% (± 5.9%) of the megakaryocytes (defined throughout as large, multinucleated, CD9+ cells) compared with 33.9% (± 5.2%) of those in nonirradiated control animals (P = .04), indicating that approximately one-fourth of the megakaryocytes remained viable and were undergoing endomitosis for at least 24 hours after irradiation. Consistent with this observation, the peripheral blood platelet count did not significantly change within 48 hours after irradiation (P > .2, supplemental Figure 4).
Irradiation and marrow distribution of host surviving megakaryocytes. (A) Bone (Bo) and BM section of a metaphysis taken at 48 hours after irradiation. Among the residual stromal cells and osteoblasts are large cells ( ) with large multilobulated nuclei that appear as megakaryocytes (inset). (B) Immunohistochemical staining with anti-CD9 (red) and anti-BrdU (black) antibodies confirms the presence of physiologically active megakaryocytes after TBI. The inset represents an enlarged image of the results of CD9 and BrdU double staining. (C) Bone section stained with isotype control primary antibody. (D) Specific pattern of megakaryocyte distribution in response to TBI as revealed by staining for CD9 (red). After radioablation, megakaryocytes (
) with large multilobulated nuclei that appear as megakaryocytes (inset). (B) Immunohistochemical staining with anti-CD9 (red) and anti-BrdU (black) antibodies confirms the presence of physiologically active megakaryocytes after TBI. The inset represents an enlarged image of the results of CD9 and BrdU double staining. (C) Bone section stained with isotype control primary antibody. (D) Specific pattern of megakaryocyte distribution in response to TBI as revealed by staining for CD9 (red). After radioablation, megakaryocytes ( ) can be found in close contact with the bone-lining cells, whereas in the control they are located primarily in the central areas of the BM, often in close proximity to the vessels (V). (A-D) Scale bar represents 50 μm. (E) Total CD9+ multilobulated megakaryocyte counts in the epiphysis, metaphysis, diaphysis, and total marrow space of control versus irradiated mice (n = 4). Mean (± SD) values for randomly selected 200× fields (288 sections scored for each group) are shown. Differences between the control and irradiated groups are not significant (P > .05). (F) Mean percentages (± SD) of CD9+ multilobulated megakaryocytes in contact with the cells lining the bone in control versus irradiated mice (n = 4). Percentages of bone-associated megakaryocytes were significantly higher in irradiated mice in all 3 major bone regions (P ≤ .01), with the epiphysis and metaphysis both showing greater accumulations of megakaryocytes at the bone surface than was apparent in the diaphysis (P < .001).
) can be found in close contact with the bone-lining cells, whereas in the control they are located primarily in the central areas of the BM, often in close proximity to the vessels (V). (A-D) Scale bar represents 50 μm. (E) Total CD9+ multilobulated megakaryocyte counts in the epiphysis, metaphysis, diaphysis, and total marrow space of control versus irradiated mice (n = 4). Mean (± SD) values for randomly selected 200× fields (288 sections scored for each group) are shown. Differences between the control and irradiated groups are not significant (P > .05). (F) Mean percentages (± SD) of CD9+ multilobulated megakaryocytes in contact with the cells lining the bone in control versus irradiated mice (n = 4). Percentages of bone-associated megakaryocytes were significantly higher in irradiated mice in all 3 major bone regions (P ≤ .01), with the epiphysis and metaphysis both showing greater accumulations of megakaryocytes at the bone surface than was apparent in the diaphysis (P < .001).
Irradiation and marrow distribution of host surviving megakaryocytes. (A) Bone (Bo) and BM section of a metaphysis taken at 48 hours after irradiation. Among the residual stromal cells and osteoblasts are large cells ( ) with large multilobulated nuclei that appear as megakaryocytes (inset). (B) Immunohistochemical staining with anti-CD9 (red) and anti-BrdU (black) antibodies confirms the presence of physiologically active megakaryocytes after TBI. The inset represents an enlarged image of the results of CD9 and BrdU double staining. (C) Bone section stained with isotype control primary antibody. (D) Specific pattern of megakaryocyte distribution in response to TBI as revealed by staining for CD9 (red). After radioablation, megakaryocytes (
) with large multilobulated nuclei that appear as megakaryocytes (inset). (B) Immunohistochemical staining with anti-CD9 (red) and anti-BrdU (black) antibodies confirms the presence of physiologically active megakaryocytes after TBI. The inset represents an enlarged image of the results of CD9 and BrdU double staining. (C) Bone section stained with isotype control primary antibody. (D) Specific pattern of megakaryocyte distribution in response to TBI as revealed by staining for CD9 (red). After radioablation, megakaryocytes ( ) can be found in close contact with the bone-lining cells, whereas in the control they are located primarily in the central areas of the BM, often in close proximity to the vessels (V). (A-D) Scale bar represents 50 μm. (E) Total CD9+ multilobulated megakaryocyte counts in the epiphysis, metaphysis, diaphysis, and total marrow space of control versus irradiated mice (n = 4). Mean (± SD) values for randomly selected 200× fields (288 sections scored for each group) are shown. Differences between the control and irradiated groups are not significant (P > .05). (F) Mean percentages (± SD) of CD9+ multilobulated megakaryocytes in contact with the cells lining the bone in control versus irradiated mice (n = 4). Percentages of bone-associated megakaryocytes were significantly higher in irradiated mice in all 3 major bone regions (P ≤ .01), with the epiphysis and metaphysis both showing greater accumulations of megakaryocytes at the bone surface than was apparent in the diaphysis (P < .001).
) can be found in close contact with the bone-lining cells, whereas in the control they are located primarily in the central areas of the BM, often in close proximity to the vessels (V). (A-D) Scale bar represents 50 μm. (E) Total CD9+ multilobulated megakaryocyte counts in the epiphysis, metaphysis, diaphysis, and total marrow space of control versus irradiated mice (n = 4). Mean (± SD) values for randomly selected 200× fields (288 sections scored for each group) are shown. Differences between the control and irradiated groups are not significant (P > .05). (F) Mean percentages (± SD) of CD9+ multilobulated megakaryocytes in contact with the cells lining the bone in control versus irradiated mice (n = 4). Percentages of bone-associated megakaryocytes were significantly higher in irradiated mice in all 3 major bone regions (P ≤ .01), with the epiphysis and metaphysis both showing greater accumulations of megakaryocytes at the bone surface than was apparent in the diaphysis (P < .001).
The number of megakaryocytes after irradiation did not differ in irradiated versus control animals (P > .5). However, the distribution of megakaryocytes differed significantly among the major bone regions, with more CD9+ megakaryocytes found in the diaphyseal marrow than in the marrow of either the epiphysis or the metaphysis (P < .05; Figure 3D-E). Moreover, the distribution pattern of megakaryocytes within the marrow space, in each of the bone regions, strikingly differed after irradiation compared with the normal marrow, where murine and human megakaryocytes preferentially localize near the sinusoidal endothelial cells in the central area of the marrow,22,23 as can been seen in our control specimens (Figure 3D-F). Throughout the entire BM after irradiation, more than a third of megakaryocytes (37.5% ± 7.4%) were observed adjacent to the endosteal surface rather than in their normal parasinusoidal location. In the metaphysis, 44.4% (± 10.9%) of the CD9+ megakaryocytes appeared to be in physical contact with the cells lining the bone, compared with only 7.4% (± 2.06%) in the control animals (Figure 3F, P < .001). Similarly, in the epiphysis, 44.1% (± 7.6%) of the CD9+ megakaryocytes in irradiated animals, compared with 9.9% (± 2.0%) in control animals (P < .001), were in contact with bone. A less pronounced but still significant difference was found in the diaphysis of irradiated versus control animals (24.0% ± 8.6% vs 6.4% ± 2.0%; Figure 3F, P = .007). Thus, irradiation of the marrow space is followed by a marked repositioning of megakaryocytes to trabecular bone in all bone regions but is associated with osteoblast proliferation only in the metaphyses and epiphyses, areas of early hematopoietic engraftment after transplantation.24
Growth factor–mediated survival and proliferation
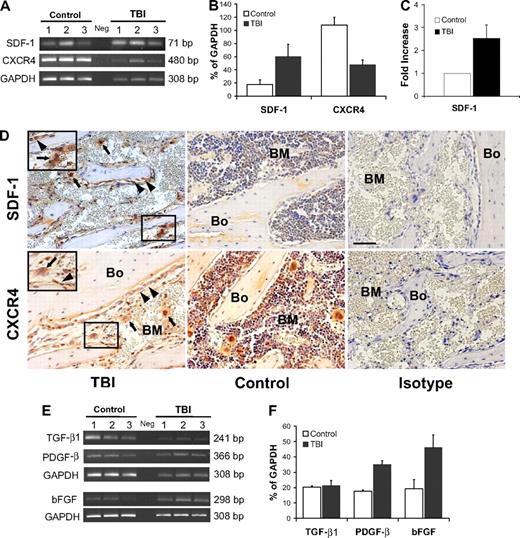
To begin to address the mechanisms by which megakaryocytes and osteoblasts survive and proliferate after irradiation, we focused on SDF-1, a factor that acts as a strong promoter of megakaryocyte homing and survival25-27 and is secreted in increased amounts by osteoblasts after irradiation.28 Using RT-PCR, we found increased levels of SDF-1 transcripts after irradiation (3.3-fold, P = .03; Figure 4A-B). Significantly higher levels of SDF-1 protein were similarly found by ELISA after irradiation (2.5-fold, P = .008; Figure 4C). By immunohistochemical staining, we further confirmed an increase of SDF-1 protein expression in sections of metaphysis (Figure 4D), where osteoblast proliferation was most pronounced. In addition, low-level SDF-1 expression was observed in megakaryocytes along the endosteal surface, where it may be functioning as an autocrine survival factor.25,28,29
SDF-1/CXCR4 axis and megakaryocyte-derived growth factors after irradiation. (A) Semiquantitative RT-PCR analyses of SDF-1 and CXCR4 expression in BM cells flushed from mice in the TBI and nonirradiated control groups (n = 3 per group). The negative control (Neg, without template) is shown in the middle lane. (B) Comparison of SDF-1 and CXCR4 expression levels (calculated relative to GAPDH levels and reported as mean percentages ± SD) in irradiated versus nonirradiated control mice. Increase of SDF-1 expression and the decrease of CXCR4 expression in the irradiated groups are both significant (P = .03 and P = .003, respectively). (C) Comparison of the SDF-1 protein level measured by ELISA in irradiated versus nonirradiated control mice (n = 3 per group; P = .008). (D top) Immunohistochemical staining (red) confirming an increase of SDF-1 levels in osteoblasts ( , left panel) and megakaryocytes (
, left panel) and megakaryocytes ( ) after 48 hours after TBI vs nonirradiated control mice (middle panel). Bone (Bo) from a TBI mouse stained using an isotype primary antibody was used as control (right panel). (Bottom) In contrast to SDF-1, the CXCR4 levels decreased at 48 hours after TBI and remained localized into the osteoblasts (
) after 48 hours after TBI vs nonirradiated control mice (middle panel). Bone (Bo) from a TBI mouse stained using an isotype primary antibody was used as control (right panel). (Bottom) In contrast to SDF-1, the CXCR4 levels decreased at 48 hours after TBI and remained localized into the osteoblasts ( ) and in the surviving megakaryocytes (
) and in the surviving megakaryocytes ( ). Controls and isotype-stained sections as described for SDF-1. Scale bar represents 50 μm. (E) Semiquantitative RT-PCR analyses of TGF-β1, PGDF-β, and bFGF in BM cells flushed from mice in the TBI and nonirradiated control groups (n = 3). The negative control (Neg, without template) is shown in the middle lane. (F) Levels of PGDF-β and bFGF, but not TGF-β1, calculated relative to GAPDH levels and reported as mean percentages (± SD), were significantly higher in the TBI group (P = .006, P = .02, P > .05, respectively).
). Controls and isotype-stained sections as described for SDF-1. Scale bar represents 50 μm. (E) Semiquantitative RT-PCR analyses of TGF-β1, PGDF-β, and bFGF in BM cells flushed from mice in the TBI and nonirradiated control groups (n = 3). The negative control (Neg, without template) is shown in the middle lane. (F) Levels of PGDF-β and bFGF, but not TGF-β1, calculated relative to GAPDH levels and reported as mean percentages (± SD), were significantly higher in the TBI group (P = .006, P = .02, P > .05, respectively).
SDF-1/CXCR4 axis and megakaryocyte-derived growth factors after irradiation. (A) Semiquantitative RT-PCR analyses of SDF-1 and CXCR4 expression in BM cells flushed from mice in the TBI and nonirradiated control groups (n = 3 per group). The negative control (Neg, without template) is shown in the middle lane. (B) Comparison of SDF-1 and CXCR4 expression levels (calculated relative to GAPDH levels and reported as mean percentages ± SD) in irradiated versus nonirradiated control mice. Increase of SDF-1 expression and the decrease of CXCR4 expression in the irradiated groups are both significant (P = .03 and P = .003, respectively). (C) Comparison of the SDF-1 protein level measured by ELISA in irradiated versus nonirradiated control mice (n = 3 per group; P = .008). (D top) Immunohistochemical staining (red) confirming an increase of SDF-1 levels in osteoblasts ( , left panel) and megakaryocytes (
, left panel) and megakaryocytes ( ) after 48 hours after TBI vs nonirradiated control mice (middle panel). Bone (Bo) from a TBI mouse stained using an isotype primary antibody was used as control (right panel). (Bottom) In contrast to SDF-1, the CXCR4 levels decreased at 48 hours after TBI and remained localized into the osteoblasts (
) after 48 hours after TBI vs nonirradiated control mice (middle panel). Bone (Bo) from a TBI mouse stained using an isotype primary antibody was used as control (right panel). (Bottom) In contrast to SDF-1, the CXCR4 levels decreased at 48 hours after TBI and remained localized into the osteoblasts ( ) and in the surviving megakaryocytes (
) and in the surviving megakaryocytes ( ). Controls and isotype-stained sections as described for SDF-1. Scale bar represents 50 μm. (E) Semiquantitative RT-PCR analyses of TGF-β1, PGDF-β, and bFGF in BM cells flushed from mice in the TBI and nonirradiated control groups (n = 3). The negative control (Neg, without template) is shown in the middle lane. (F) Levels of PGDF-β and bFGF, but not TGF-β1, calculated relative to GAPDH levels and reported as mean percentages (± SD), were significantly higher in the TBI group (P = .006, P = .02, P > .05, respectively).
). Controls and isotype-stained sections as described for SDF-1. Scale bar represents 50 μm. (E) Semiquantitative RT-PCR analyses of TGF-β1, PGDF-β, and bFGF in BM cells flushed from mice in the TBI and nonirradiated control groups (n = 3). The negative control (Neg, without template) is shown in the middle lane. (F) Levels of PGDF-β and bFGF, but not TGF-β1, calculated relative to GAPDH levels and reported as mean percentages (± SD), were significantly higher in the TBI group (P = .006, P = .02, P > .05, respectively).
Because SDF-1 acts through its receptor, CXCR4, which is widely expressed on steady-state BM cells,30 we next sought to evaluate the effects of irradiation on CXCR4 expression. As expected, given the radiation-induced reduction in overall BM cellularity, there was at least a 2-fold decrease of CXCR4 levels at 48 hours after irradiation (P = .003, Figure 4A-B). Nonetheless, immunohistochemical staining revealed that the surviving megakaryocytes continued to express CXCR4 (Figure 4D) and therefore could respond to the striking increase in expression levels of SDF-1, which may act in either a paracrine or autocrine manner. This stimulus may account for the prolonged survival of megakaryocytes and their translocation to the endosteal surface, where they could interact with osteoblasts to promote their proliferation.
If this model of megakaryocyte translocation followed by megakaryocyte-associated osteoblast stimulation is correct, we would predict an increased expression of megakaryocyte-derived mesenchymal growth factors, such as PDGF-β, TGF-β1, and bFGF, which are known to induce preosteoblast and osteoblast proliferation.19,20 Semiquantitative RT-PCR of RNA isolated from the flushed cellular contents of the marrow space 48 hours after lethal irradiation demonstrated a 2-fold increase of PDGF-β (P = .006) and bFGF (P = .02) transcripts compared with controls, whereas the expression of TGF-β1 was not significantly higher than the control value (Figure 4E-F).
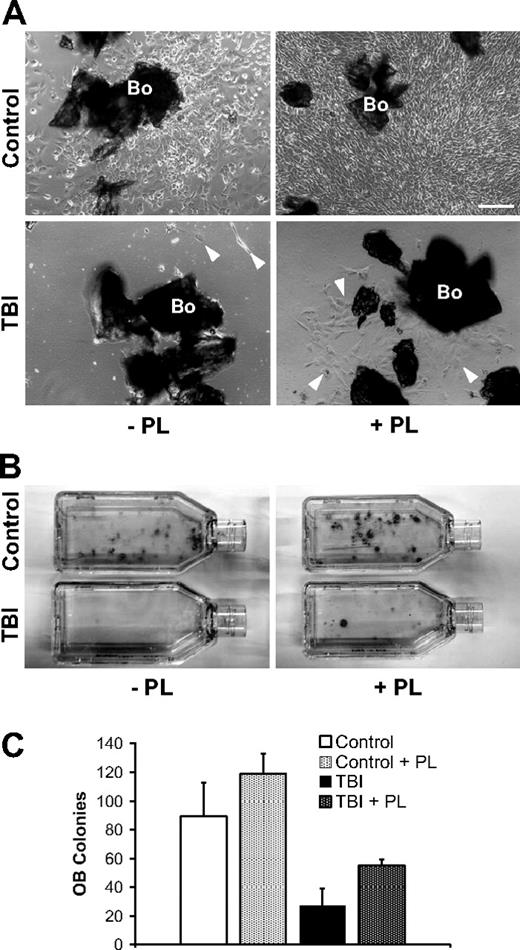
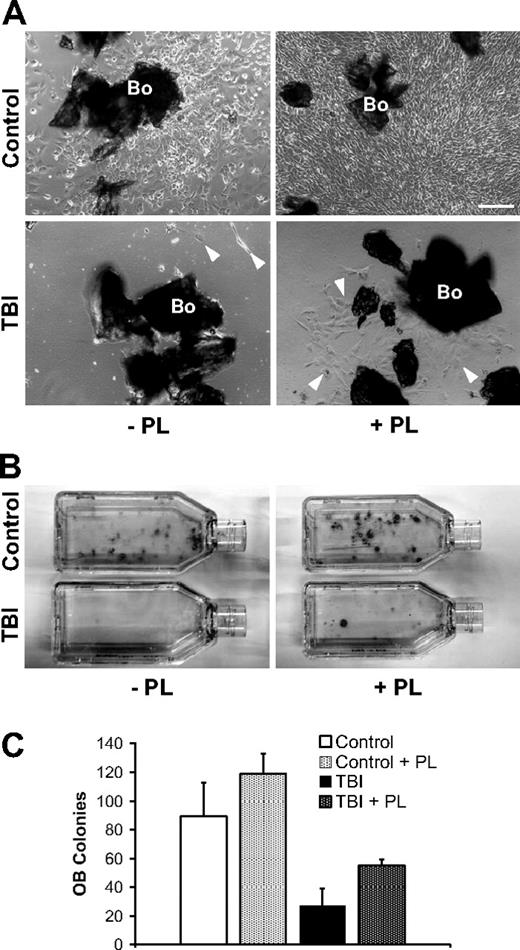
To examine the role of megakaryocyte-derived factors for osteoblast survival and proliferation after marrow radioablation, selectively isolated osteoblasts from bone explants taken after irradiation were maintained in vitro with or without serum/PL supplementation of the otherwise same culture media, as a surrogate for the proposed megakaryocyte function in vivo. In cultures lacking PL, osteoblasts from the irradiated mice show a striking reduction of both proliferative and clonogenic potentials compared with controls (Figure 5). Remarkably, osteoblasts from the irradiated mice expanded with PL-supplemented media show greater proliferation and clonogenic colony formation compared with cultures without PL, suggesting that PL partially rescues the radiation-induced osteoblast injury. A similar effect is also observed in cultures from the nonirradiated controls, indicating a broad osteoblast growth-promoting effect of PL (Figure 5). Within the limits of an in vitro model, these data support the concept that the repopulating osteoblasts in situ are derived from a rare endogenous osteoprogenitor and that the megakaryocyte-derived mitogenic stimuli for proliferation of the resident osteoprogenitors after irradiation are critically important.
Isolation of irradiated osteoblasts from megakaryocyte-conditioned marrow microenvironment. (A) Representative photomicrographs of in vitro–cultured osteoblasts selectively obtained from bones (Bo) in control (n = 6) and irradiated (TBI; n = 6) mice. The bones are harvested and completely depleted of hematopoietic marrow fraction. Left panels represent nonirradiated control (top) and irradiated (bottom) samples after 7 days of culture without PL (−PL). Osteoblasts in control samples begin to proliferate generating an adherent cell layer; conversely, after TBI the separation of irradiated osteoblasts ( ) from marrow microenvironment is followed by a defective in vitro behavior. The addition of PL (+PL, right) increased the proliferation of osteoblasts from controls (top right) as well as from the irradiated animals (bottom right). Osteoblasts are indicated (
) from marrow microenvironment is followed by a defective in vitro behavior. The addition of PL (+PL, right) increased the proliferation of osteoblasts from controls (top right) as well as from the irradiated animals (bottom right). Osteoblasts are indicated ( ). Scale bar represents 200 μm. (B) Representative pictures of osteoblast colonies from irradiated or control mice, after 2 weeks in culture with or without PL. (C) In vitro osteoblast colony count (per 200 mg processed bone chips) from irradiated and control mice, with or without PL. A reduction of the average colony number is observed after irradiation (89.2 ± 23.7 vs 27 ± 12, P = .006), indicating a damage that is not restored because of the absence of megakaryocyte-derived growth factors in the cultures. PL was able to significantly increase the colony number both in control samples (89.2 ± 23.7 vs 118.7 ± 14.4, P = .04) and after TBI (27 ± 12 vs 55 ± 4.3, P = .03). Experiments were performed at least in triplicate.
). Scale bar represents 200 μm. (B) Representative pictures of osteoblast colonies from irradiated or control mice, after 2 weeks in culture with or without PL. (C) In vitro osteoblast colony count (per 200 mg processed bone chips) from irradiated and control mice, with or without PL. A reduction of the average colony number is observed after irradiation (89.2 ± 23.7 vs 27 ± 12, P = .006), indicating a damage that is not restored because of the absence of megakaryocyte-derived growth factors in the cultures. PL was able to significantly increase the colony number both in control samples (89.2 ± 23.7 vs 118.7 ± 14.4, P = .04) and after TBI (27 ± 12 vs 55 ± 4.3, P = .03). Experiments were performed at least in triplicate.
Isolation of irradiated osteoblasts from megakaryocyte-conditioned marrow microenvironment. (A) Representative photomicrographs of in vitro–cultured osteoblasts selectively obtained from bones (Bo) in control (n = 6) and irradiated (TBI; n = 6) mice. The bones are harvested and completely depleted of hematopoietic marrow fraction. Left panels represent nonirradiated control (top) and irradiated (bottom) samples after 7 days of culture without PL (−PL). Osteoblasts in control samples begin to proliferate generating an adherent cell layer; conversely, after TBI the separation of irradiated osteoblasts ( ) from marrow microenvironment is followed by a defective in vitro behavior. The addition of PL (+PL, right) increased the proliferation of osteoblasts from controls (top right) as well as from the irradiated animals (bottom right). Osteoblasts are indicated (
) from marrow microenvironment is followed by a defective in vitro behavior. The addition of PL (+PL, right) increased the proliferation of osteoblasts from controls (top right) as well as from the irradiated animals (bottom right). Osteoblasts are indicated ( ). Scale bar represents 200 μm. (B) Representative pictures of osteoblast colonies from irradiated or control mice, after 2 weeks in culture with or without PL. (C) In vitro osteoblast colony count (per 200 mg processed bone chips) from irradiated and control mice, with or without PL. A reduction of the average colony number is observed after irradiation (89.2 ± 23.7 vs 27 ± 12, P = .006), indicating a damage that is not restored because of the absence of megakaryocyte-derived growth factors in the cultures. PL was able to significantly increase the colony number both in control samples (89.2 ± 23.7 vs 118.7 ± 14.4, P = .04) and after TBI (27 ± 12 vs 55 ± 4.3, P = .03). Experiments were performed at least in triplicate.
). Scale bar represents 200 μm. (B) Representative pictures of osteoblast colonies from irradiated or control mice, after 2 weeks in culture with or without PL. (C) In vitro osteoblast colony count (per 200 mg processed bone chips) from irradiated and control mice, with or without PL. A reduction of the average colony number is observed after irradiation (89.2 ± 23.7 vs 27 ± 12, P = .006), indicating a damage that is not restored because of the absence of megakaryocyte-derived growth factors in the cultures. PL was able to significantly increase the colony number both in control samples (89.2 ± 23.7 vs 118.7 ± 14.4, P = .04) and after TBI (27 ± 12 vs 55 ± 4.3, P = .03). Experiments were performed at least in triplicate.
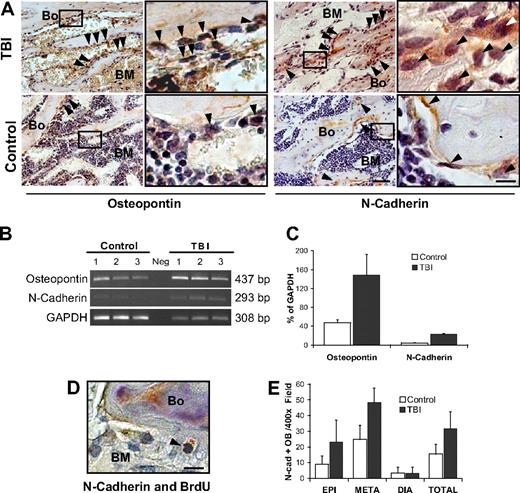
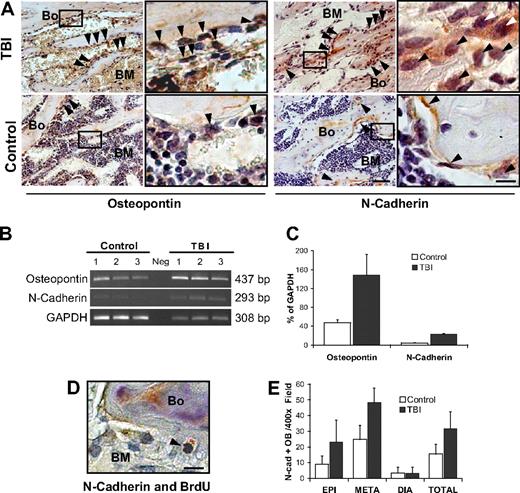
Proliferating osteoblasts constitute the HSC niche
Although providing clear evidence of host osteoblast expansion after myeloablative irradiation, our data do not address whether the proliferating osteoblasts could function as part of a true HSC niche. Hence, we performed immunohistochemical analyses to assess the expression of 2 proteins, N-cadherin and osteopontin, which typify the osteoblast components of this niche.2-4 Increases in both proteins were apparent by immunohistochemical analysis of osteoblast specimens from the metaphysis, but not in those from the diaphysis (Figure 6A and supplemental Figure 5A). Molecular analyses showed a 3-fold increase in osteopontin (P = .03) and 5-fold increase in N-cadherin (P < .001) expression in marrow samples from irradiated mice (Figure 6B-C). Double staining with anti-BrdU (black) and anti–N-cadherin (red) revealed a typical nuclear pattern of BrdU labeling and simultaneous N-cadherin expression, providing evidence for the presence of proliferating N-cadherin–positive osteoblasts in the postirradiation microenvironment (Figure 6D). By scoring the absolute number of N-cadherin–positive osteoblasts in the epiphysis, metaphysic, and diaphysis of radioablated versus control mice (32 928 total cells scored, representing ∼ 420 microscopic fields from 6 mice per group), we could demonstrate a 2-fold increase of N-cadherin-expressing osteoblasts in the metaphysis and epiphysis (P < .001 and P < .001, respectively), but not in the diaphysis (P < .9; Figure 6E). Because RNA expression of N-cadherin in the marrow population increased 5-fold, with only a 2-fold increase of N-cadherin–expressing osteoblasts, irradiation seems to have increased N-cadherin gene expression within the osteoblasts, similar to observations for SDF-1 expression.28 Indeed, the increased expression of N-cadherin, or other niche proteins, may represent a distinct and significant contribution to the functional restoration of the osteoblastic niche.
Stem cell niche expands after marrow radioablation. (A) Immunohistochemical staining of TBI versus control mice reveals an increase in 2 markers of the HSC niche: osteopontin (left) and N-cadherin (right), particularly in the metaphysis ( ). Scale bar (third column) represents 50 μm; scale bar (fourth column) represents 10 μm. (B) Semiquantitative RT-PCR analysis showing increased expression of osteopontin and N-cadherin in the TBI group. The negative control (Neg, without template) is shown in the middle lane. (C) Comparison of osteopontin and N-cadherin expression levels in irradiated versus nonirradiated control mice. Transcript band intensities are calculated in relation with the GAPDH levels and reported as mean percentages (± SD) of 6 measurements per group. Significant increases of osteopontin and N-cadherin are detected after irradiation (P = .03 and P < .001, respectively). (D) Double staining of TBI mice with anti-BrdU (black) and anti–N-cadherin (red) antibodies. Scale bar represents 20 μm. The typical nuclear pattern of the BrdU labeling and simultaneous N-cadherin expression (
). Scale bar (third column) represents 50 μm; scale bar (fourth column) represents 10 μm. (B) Semiquantitative RT-PCR analysis showing increased expression of osteopontin and N-cadherin in the TBI group. The negative control (Neg, without template) is shown in the middle lane. (C) Comparison of osteopontin and N-cadherin expression levels in irradiated versus nonirradiated control mice. Transcript band intensities are calculated in relation with the GAPDH levels and reported as mean percentages (± SD) of 6 measurements per group. Significant increases of osteopontin and N-cadherin are detected after irradiation (P = .03 and P < .001, respectively). (D) Double staining of TBI mice with anti-BrdU (black) and anti–N-cadherin (red) antibodies. Scale bar represents 20 μm. The typical nuclear pattern of the BrdU labeling and simultaneous N-cadherin expression ( ) demonstrates the presence of proliferating N-cadherin–positive osteoblasts after TBI. (E) N-cadherin–positive osteoblasts (OB) in the epiphysis, metaphysis, diaphysis, and total marrow space at 48 hours after irradiation. Mean (± SD) numbers of cells per 400× microscopic field (n = 420 fields/group) are shown.
) demonstrates the presence of proliferating N-cadherin–positive osteoblasts after TBI. (E) N-cadherin–positive osteoblasts (OB) in the epiphysis, metaphysis, diaphysis, and total marrow space at 48 hours after irradiation. Mean (± SD) numbers of cells per 400× microscopic field (n = 420 fields/group) are shown.
Stem cell niche expands after marrow radioablation. (A) Immunohistochemical staining of TBI versus control mice reveals an increase in 2 markers of the HSC niche: osteopontin (left) and N-cadherin (right), particularly in the metaphysis ( ). Scale bar (third column) represents 50 μm; scale bar (fourth column) represents 10 μm. (B) Semiquantitative RT-PCR analysis showing increased expression of osteopontin and N-cadherin in the TBI group. The negative control (Neg, without template) is shown in the middle lane. (C) Comparison of osteopontin and N-cadherin expression levels in irradiated versus nonirradiated control mice. Transcript band intensities are calculated in relation with the GAPDH levels and reported as mean percentages (± SD) of 6 measurements per group. Significant increases of osteopontin and N-cadherin are detected after irradiation (P = .03 and P < .001, respectively). (D) Double staining of TBI mice with anti-BrdU (black) and anti–N-cadherin (red) antibodies. Scale bar represents 20 μm. The typical nuclear pattern of the BrdU labeling and simultaneous N-cadherin expression (
). Scale bar (third column) represents 50 μm; scale bar (fourth column) represents 10 μm. (B) Semiquantitative RT-PCR analysis showing increased expression of osteopontin and N-cadherin in the TBI group. The negative control (Neg, without template) is shown in the middle lane. (C) Comparison of osteopontin and N-cadherin expression levels in irradiated versus nonirradiated control mice. Transcript band intensities are calculated in relation with the GAPDH levels and reported as mean percentages (± SD) of 6 measurements per group. Significant increases of osteopontin and N-cadherin are detected after irradiation (P = .03 and P < .001, respectively). (D) Double staining of TBI mice with anti-BrdU (black) and anti–N-cadherin (red) antibodies. Scale bar represents 20 μm. The typical nuclear pattern of the BrdU labeling and simultaneous N-cadherin expression ( ) demonstrates the presence of proliferating N-cadherin–positive osteoblasts after TBI. (E) N-cadherin–positive osteoblasts (OB) in the epiphysis, metaphysis, diaphysis, and total marrow space at 48 hours after irradiation. Mean (± SD) numbers of cells per 400× microscopic field (n = 420 fields/group) are shown.
) demonstrates the presence of proliferating N-cadherin–positive osteoblasts after TBI. (E) N-cadherin–positive osteoblasts (OB) in the epiphysis, metaphysis, diaphysis, and total marrow space at 48 hours after irradiation. Mean (± SD) numbers of cells per 400× microscopic field (n = 420 fields/group) are shown.
Besides proliferating (N-cadherin+) osteoblasts, perivascular reticular cells might also serve as an important source of SDF-1 in the marrow microenvironment.7 Microscopic examination of bone specimens allowed us to identify reticular cells, flattened cells expressing high levels of SDF-1 and relatively lower levels of N-cadherin compared with the endosteal osteoblasts (Figure 4D and supplemental Figure 5B), as originally identified by Sugiyama et al,7 which appear to be lining the bone. To test the impact of myeloablation on this cellular compartment, we determined the absolute number of these elongated reticular cells per 400× field in the BM space before and after TBI. Although this compartment expanded after irradiation, the increase per 400× field was not significant (4.29 ± 2.87 vs 3.08 ± 1.61, P = 0.1), suggesting a marginal contribution of perivascular reticular cells to restoration of the HSC niche after marrow radioablation.
Finally, we considered the potential role of endothelial cells, which comprise an HSC niche6 within the marrow and have been reported to express N-cadherin.31 In the marrow of irradiated mice, we noted a striking reduction of vascular structures in contrast to normal marrow (supplemental Figure 6A-B). Semiquantitative RT-PCR of RNA isolated from the flushed cellular contents of the marrow revealed a significant decrease in the levels of VE-cadherin (P < .001) and factor VIII (P < .002), 2 specific endothelial cell markers,32,33 after irradiation, compared with controls (supplemental Figure 6C-D). These data suggest that the endothelial niche is extensively damaged by the irradiation and has not recovered within 48 hours, consistent with previous reports.15,34 Moreover, the endothelial niche does not seem to contribute to the early osteoblast niche restoration and expansion, which spans the time frame of initial engraftment in the marrow after hematopoietic cell transplantation.35,36
Reversibility of osteoblastic niche expansion after donor hematopoietic cell engraftment
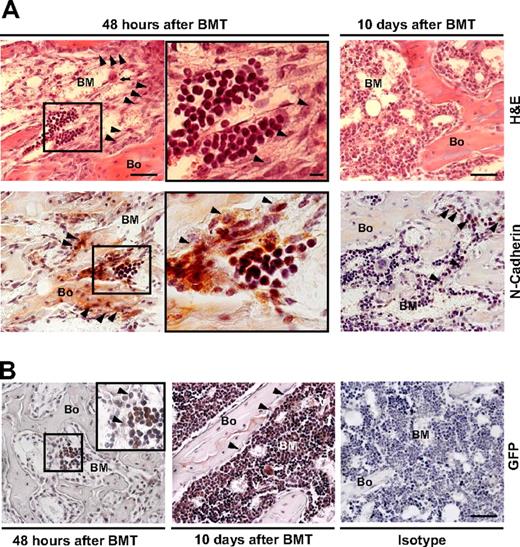
To test the effects of donor hematopoietic cells on the integrity of HSC niches formed exclusively from host cells, we compared the architecture of these niches in the BM microenvironments of irradiated/transplanted versus irradiated/nontransplanted mice. Routine staining showed that the structural features of the marrow microenvironments in transplanted mice (Figure 7A top left) were similar (if not identical) to those in nontransplanted mice at 48 hours after irradiation (compare with Figure 1A). Importantly, after transplantation, we observed small clusters of engrafted donor hematopoietic cells (1-6 cell clusters per femur section) in close proximity to the expanded osteoblasts along the trabeculae of the metaphysis (Figure 7A center). Moreover, the transplanted marrow cells engrafted only at sites of N-cadherin–positive cells (Figure 7A bottom left and central, red), indicating the functionality of host cell–derived niches. Finally, GFP staining (red) showed that the cell clusters were donor-derived (Figure 7B left) and double-staining for GFP and BrdU demonstrated that they were undergoing mitosis at less than 48 hours after transplantation (not shown).
Anatomic localization of stem cell niches and reversibility of the microenvironmental changes after transplantation. (A) Sections of a metaphysis obtained 48 hours (left and center) or 10 days (right) after TBI and donor (GFP+) BM transplantation (BMT), stained with hematoxylin and eosin (top) or immunostained for N-cadherin (red; bottom). An increased number of osteoblasts ( ) and the persistence of megakaryocytes in their proximity (
) and the persistence of megakaryocytes in their proximity ( ; top left panel) are apparent at 48 hours after BMT. The engrafted cells appear as discrete clusters of small round cells close to the endosteum (center panels are the insets indicated in left panels). Scale bar (left and right panels) represents 50 μm; scale bar (center panels) represents 10 μm. (B) Sections obtained 48 hours (left) or 10 days (center) after donor (GFP+) BMT immunostained for GFP. The previously observed cells appear as donor derived clusters (≤ 6/femur) located in the metaphysis and epiphysis of recipients. At 10 days after transplantation, the marrow cellularity increased (center), megakaryocytes (⇑) were frequently found close to vessels (V) and bone lining cells returned to a single tier (
; top left panel) are apparent at 48 hours after BMT. The engrafted cells appear as discrete clusters of small round cells close to the endosteum (center panels are the insets indicated in left panels). Scale bar (left and right panels) represents 50 μm; scale bar (center panels) represents 10 μm. (B) Sections obtained 48 hours (left) or 10 days (center) after donor (GFP+) BMT immunostained for GFP. The previously observed cells appear as donor derived clusters (≤ 6/femur) located in the metaphysis and epiphysis of recipients. At 10 days after transplantation, the marrow cellularity increased (center), megakaryocytes (⇑) were frequently found close to vessels (V) and bone lining cells returned to a single tier ( ). Isotype control is shown for comparison (right). Scale bar: 50 μm.
). Isotype control is shown for comparison (right). Scale bar: 50 μm.
Anatomic localization of stem cell niches and reversibility of the microenvironmental changes after transplantation. (A) Sections of a metaphysis obtained 48 hours (left and center) or 10 days (right) after TBI and donor (GFP+) BM transplantation (BMT), stained with hematoxylin and eosin (top) or immunostained for N-cadherin (red; bottom). An increased number of osteoblasts ( ) and the persistence of megakaryocytes in their proximity (
) and the persistence of megakaryocytes in their proximity ( ; top left panel) are apparent at 48 hours after BMT. The engrafted cells appear as discrete clusters of small round cells close to the endosteum (center panels are the insets indicated in left panels). Scale bar (left and right panels) represents 50 μm; scale bar (center panels) represents 10 μm. (B) Sections obtained 48 hours (left) or 10 days (center) after donor (GFP+) BMT immunostained for GFP. The previously observed cells appear as donor derived clusters (≤ 6/femur) located in the metaphysis and epiphysis of recipients. At 10 days after transplantation, the marrow cellularity increased (center), megakaryocytes (⇑) were frequently found close to vessels (V) and bone lining cells returned to a single tier (
; top left panel) are apparent at 48 hours after BMT. The engrafted cells appear as discrete clusters of small round cells close to the endosteum (center panels are the insets indicated in left panels). Scale bar (left and right panels) represents 50 μm; scale bar (center panels) represents 10 μm. (B) Sections obtained 48 hours (left) or 10 days (center) after donor (GFP+) BMT immunostained for GFP. The previously observed cells appear as donor derived clusters (≤ 6/femur) located in the metaphysis and epiphysis of recipients. At 10 days after transplantation, the marrow cellularity increased (center), megakaryocytes (⇑) were frequently found close to vessels (V) and bone lining cells returned to a single tier ( ). Isotype control is shown for comparison (right). Scale bar: 50 μm.
). Isotype control is shown for comparison (right). Scale bar: 50 μm.
At 10 days after transplantation, the marrow space was replete with donor hematopoietic cells, the endosteal osteoblasts again formed a single layer, and the megakaryocytes had relocated from the endosteum to the center of the marrow space (Figure 7B center). In addition, N-cadherin expression had returned to homeostatic levels (Figure 7A bottom left). Together, these observations demonstrate the reversibility of the osteoblast niche expansion on engraftment and repopulation of hematopoietic marrow cell compartment.
Discussion
Transplantation of whole BM into radioablated hosts leads to engraftment of HSCs and the restoration of hematopoiesis. Our understanding of this homing process has greatly improved with recognition of the role of SDF-1/CXCR4,30 the identification of HSC niches,4,6,7,37 and most recently, the role of calcium gradients.38 Although many cell types in the BM microenvironment seem capable of regulating HSC function, we have focused on the osteoblastic niche because of the recognized role of N-cadherin+/osteopontin+ osteoblasts in HSC maintenance,2-5 and the responsiveness of this niche to signaling through bone-specific growth pathways, such as those mediated by parathyroid hormone and bone morphogenetic protein.4,37 We reasoned that the ability of the osteoblastic niche to recover from specific insults such as TBI might be an important factor in HSC engraftment and successful recovery of normal hematopoiesis. Thus, we sought to identify the exact anatomic site(s) of osteoblastic niches within the marrow space and monitor them during the early postirradiation interval to detect dynamic changes in their distribution, number, and architecture, In addition, we investigated some of the chemotactic and mitogenic stimuli that might underlie the mechanism of these fluctuations.
Here we show that exposure to radiation leads to a specific and reversible expansion of the osteoblastic niche. The surviving pool of radioresistant osteoprogenitors proliferate close to the endosteal bone areas and indeed may account for 40-year-old data indicating the existence of bone-related radioresistant nonhematopoietic cells capable of providing better hematopoietic support than control cells.39
The exclusive proliferation of osteoblasts in the metaphysis and epiphysis also suggests that such osteoblasts may differ from those in the diaphysis and may afford the only niches where engraftment can occur.40 Long-term hematopoietic repopulating cells must reside in the diaphysis in mice during homeostasis because this anatomic location is the primary source of marrow grafts; however, our findings suggest that the transplanted HSCs first engraft in the metaphysis and epiphysis and then populate the diaphysis on in situ expansion.
Osteoblast proliferation after TBI was accompanied by an increase in the relative amount of TGF-β1 and, in particular, of PDGF-β and bFGF, which are factors known to play an important role in bone homeostasis.41,42 In the BM microenvironment, all of these factors are produced by megakaryocytes, so that these cells have been proposed as important contributors to skeletal homeostasis.20 Moreover, we and others have suggested a role for platelet-derived factors in the stimulation of osteoprogenitor proliferation in vitro,14,43 whereas findings in several animal models and human myeloproliferative syndromes indicate a relationship between increased levels of TGF-β1 and PDGF-β, both secreted by megakaryocytes, and the onset of osteosclerosis.20,44 Thus, the relative increases of osteoprogenitor growth factors seen after TBI in the present study can be attributed to the persistence of resident megakaryocytes, in numbers that did not differ significantly from controls. This suggests that exposure to radiation does not initially alter host thrombocytopoiesis, in agreement with in vivo data indicating stable or increased megakaryocyte-stimulating activity early after TBI.45,46 Notably, our immunohistochemical analysis revealed a specific pattern of megakaryocyte repositioning after TBI. In normal steady-state conditions, the majority of megakaryocytes reside in close proximity to the BM sinusoidal vessels.22 As we report here, irradiation induces a profound perturbation of the BM microenvironment, composed of vascular damage and a shift in the location of megakaryocytes to the endosteal surface, where they maintain close contact with osteoblasts, suggesting interactions between these 2 cell types. Indeed, there are compelling in vitro data to support the concept that osteoblasts can maintain the growth and differentiation of megakaryocytes47,48 and that contact between megakaryocytes and osteoblast precursors facilitates proliferation and differentiation of the latter.49
Although the intent of cytotoxic conditioning regimen is to eradicate host BM cellularity, we noted that many resident megakaryocytes, osteoblasts, and reticular bone lining cells survived and even proliferated up to 48 hours after TBI. Our results implicate SDF-1 as a survival factor, up-regulated after TBI, that could account for the persistence of these mesenchymal elements. The ability of SDF-1 to promote the survival of either megakaryocytes or osteoblast progenitors is well recognized and may contribute to their relative radioresistance.25,28,50 The presence of CXCR4 on both osteoblasts and megakaryocytes suggests that SDF-1 could act in an autocrine as well as paracrine manner to exert an antiapoptotic function analogous to its effect on human CD34+ cells.51 Indeed, the increased concentrations of SDF-1 produced by osteoblasts immediately after irradiation could have 2 distinct outcomes. On the one hand, they could drive the homing of infused stem cells to specific niches, whereas on the other, they could attract the megakaryocytes to the endosteal surface, where their production of TGF-β1, PDGF-β, and SDF-1 would allow the maintenance and proliferation of host osteoblasts and of the megakaryocytes themselves. The latter notion suggests that SDF-1 may act as a megakaryocyte chemotactic factor so that the observed repositioning of megakaryocytes may represent a specific translocation across the marrow space.
Recent identification of the perivascular niche and other BM cellular microenvironments as potential sites of HSC homing6-8 raises an important question. Does the osteoblastic niche at the endosteal surface represent the majority site of donor HSC homing after TBI or only one of several possible sites? Although a possible increase (not statistically significant) of spindle-shaped reticular stromal cells was seen after TBI, we did not observe any proliferation of the components of marrow vessels early after TBI, but rather a disruption of marrow sinusoids, which are known to sustain severe damage from radiation resulting in disruption of their regulatory functions.52,53 Although data published by Kiel et al6 favor the perivascular over the osteoblastic niche as the predominant site of HSC homing in BM, this interpretation may only be valid for steady-state conditions and would not extend to a severely damaged BM microenvironment. For example, Slayton et al15 noted that, after exposure to radiation, restoration of vascular homeostasis by host endothelial cells required 7 to 10 days. Hence, the osteoblastic niche appears to offer the best cellular microenvironment for productive HSC homing during the first few days after TBI, and perhaps after any form of cytoreductive treatment.54 Thereafter, endothelial sinusoids may recover sufficiently to fill an important role in HSC maintenance and proliferation, and are probably required for full histologic normalization of the BM microenvironment by 10 days after transplantation.
The model of postirradiation recovery of HSC niches suggested by our data can be summarized as follows. Proliferating osteoblasts in the marrow cavity secrete increased amounts of SDF-1 that diffuse across the marrow space because of the lack of proximal CXCR4-expressing hematopoietic progenitors and bind to surviving megakaryocytes in the parasinusoidal niche, inducing them to migrate to osteoblasts that line the endosteal surface of trabecular bone within the metaphysis and epiphysis. Secretion of bFGF, PDGF-β, and TGF-β1 by the translocated megakaryocytes stimulates further proliferation of the resident osteoblasts, leading to an expansion in the number of HSC niches. If confirmed, this model would have several therapeutic implications. It would predict, first of all, that delaying marrow graft infusions until 24 to 48 hours after radioablation could result in greater occupancy of expanded HSC niches, thus contributing to enhanced engraftment. Indeed, this prediction has proved valid in animal models.55,56 Second, the leading role played by megakaryocytes in the regeneration of HSC niches after marrow radioablation suggests that modulation of the recruitment of these cells to trabecular bone might further improve HSC engraftment. This cellular mechanism may partly underlie the observations of a decade ago that thrombopoietin promotes HSC recovery after cytotoxic chemotherapy.57,58 Third, the relative radioresistance and expansion of osteoblastic niches after TBI suggest that radiation may not offer the optimal means for eradicating tumorigenic niches.59,60 According to this concept, the efficacy of BM transplantation may be decreased in cases where either substantial microenvironmental dysfunction contributes to disease61 or when the endosteal niche maintains a surviving cancer stem cell pool.62
Future efforts will be directed toward identifying pivotal molecular steps in this process, which can be targeted to improve the magnitude and duration of donor HSC engraftment and to ensure optimal differentiation of the engrafted stem cells.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ilaria Castelli for assistance with the immunohistochemistry, Michele Cilli and Federica Piccardi for assistance in animal care, Professor Stefan Schulz for providing the anti-CXCR4 antibody, and John Gilbert for reviewing the manuscript.
This work was supported in part by grants from National Institutes of Health (R01 HL077643), the Ministero Istruzione Università e Ricerca (PRIN 2006), the Regione Emilia Romagna, the Associazione per il Sostegno dell'Ematologia e dell'Oncologia Pediatrica, and Fondazione Cassa di Risparmio di Modena.
National Institutes of Health
Authorship
Contribution: M.D. designed, performed, and analyzed research and assisted with preparation of the manuscript; V.R., R.B., X.C., F.B., P.P., C.S., D.B., and E.V. performed research; T.J.H. designed and analyzed research; P.C. analyzed research; and E.M.H. oversaw the entire project, designed and analyzed research, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Massimo Dominici, Laboratory of Cell Biology and Advanced Cancer Therapies, University of Modena and Reggio Emilia, Via del Pozzo, 71, 41100, Modena, Italy; e-mail: massimo.dominici@unimore.it; or Edwin M. Horwitz, Children's Hospital of Philadelphia, Abramson Research Center, 1116D, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: horwitze@email.chop.edu.



 ) compared with (B) the nonirradiated control (hematoxylin-and-eosin stain). (A,B) Scale bar represents 50 μm. (C) Immunohistochemical staining (in red) to identify the bone-lining cells in panels A and B revealed an increase of collagen I (left) and osteocalcin (right) producing osteoblasts (
) compared with (B) the nonirradiated control (hematoxylin-and-eosin stain). (A,B) Scale bar represents 50 μm. (C) Immunohistochemical staining (in red) to identify the bone-lining cells in panels A and B revealed an increase of collagen I (left) and osteocalcin (right) producing osteoblasts (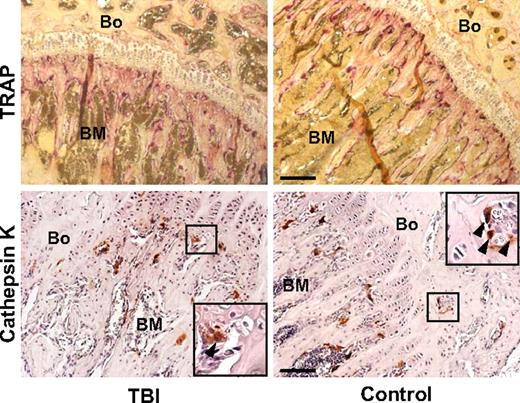

 ) with large multilobulated nuclei that appear as megakaryocytes (inset). (B) Immunohistochemical staining with anti-CD9 (red) and anti-BrdU (black) antibodies confirms the presence of physiologically active megakaryocytes after TBI. The inset represents an enlarged image of the results of CD9 and BrdU double staining. (C) Bone section stained with isotype control primary antibody. (D) Specific pattern of megakaryocyte distribution in response to TBI as revealed by staining for CD9 (red). After radioablation, megakaryocytes (
) with large multilobulated nuclei that appear as megakaryocytes (inset). (B) Immunohistochemical staining with anti-CD9 (red) and anti-BrdU (black) antibodies confirms the presence of physiologically active megakaryocytes after TBI. The inset represents an enlarged image of the results of CD9 and BrdU double staining. (C) Bone section stained with isotype control primary antibody. (D) Specific pattern of megakaryocyte distribution in response to TBI as revealed by staining for CD9 (red). After radioablation, megakaryocytes (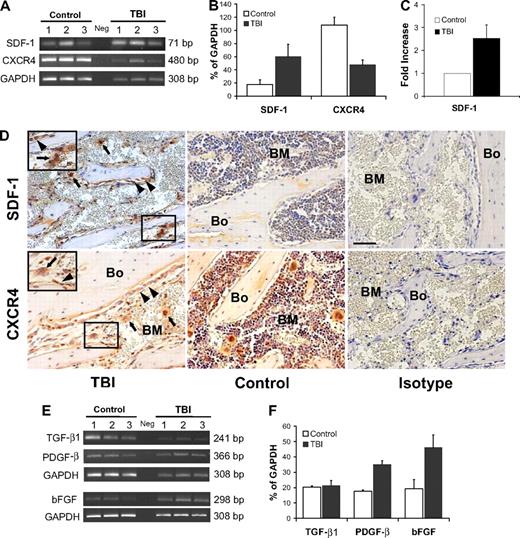
 ; top left panel) are apparent at 48 hours after BMT. The engrafted cells appear as discrete clusters of small round cells close to the endosteum (center panels are the insets indicated in left panels). Scale bar (left and right panels) represents 50 μm; scale bar (center panels) represents 10 μm. (B) Sections obtained 48 hours (left) or 10 days (center) after donor (GFP+) BMT immunostained for GFP. The previously observed cells appear as donor derived clusters (≤ 6/femur) located in the metaphysis and epiphysis of recipients. At 10 days after transplantation, the marrow cellularity increased (center), megakaryocytes (⇑) were frequently found close to vessels (V) and bone lining cells returned to a single tier (
; top left panel) are apparent at 48 hours after BMT. The engrafted cells appear as discrete clusters of small round cells close to the endosteum (center panels are the insets indicated in left panels). Scale bar (left and right panels) represents 50 μm; scale bar (center panels) represents 10 μm. (B) Sections obtained 48 hours (left) or 10 days (center) after donor (GFP+) BMT immunostained for GFP. The previously observed cells appear as donor derived clusters (≤ 6/femur) located in the metaphysis and epiphysis of recipients. At 10 days after transplantation, the marrow cellularity increased (center), megakaryocytes (⇑) were frequently found close to vessels (V) and bone lining cells returned to a single tier (