Abstract
The development of reduced intensity conditioning regimens has increased the number of patients diagnosed with chronic lymphocytic leukemia that are referred for allogeneic hematopoietic cell transplantation (allo-HCT). However, given the toxicity of allo-HCT, it should only be offered to eligible patients whose life expectancy is significantly reduced by the disease. Accordingly, the European Group of Blood and Marrow Transplantation has recently identified those patients in whom allo-HCT could be a reasonable therapeutic approach. In this review, we have evaluated the outcome of chronic lymphocytic leukemia patients undergoing allo-HCT, either after conventional or reduced intensity conditioning regimens, in the context of current nontransplantation strategies. We have also analyzed the most important predisposing factors that might interfere with the procedure as well as posttransplantation complications that are particularly common in these patients. Finally, we have addressed the most relevant factors when deciding what patients should be considered for allo-HCT and the timing of the procedure.
Introduction
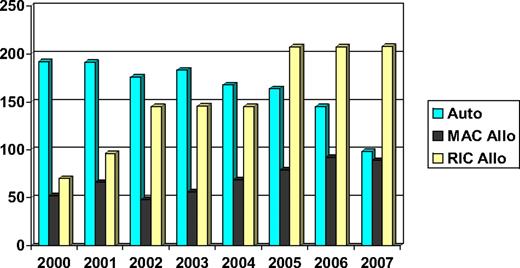
Chronic lymphocytic leukemia (CLL) is a common lymphoid malignancy that has in the past been considered a disease of the elderly and, therefore, not suitable for hematopoietic cell transplantation (HCT). Indeed, the median age at diagnosis is approximately 72 years,1 and a good proportion of patients can be managed effectively with palliative chemotherapy. However, there is a group of younger patients with poor-risk disease whose life expectancy is significantly reduced. As a result, both autologous and allogeneic HCTs have been investigated as potentially curative procedures. Moreover, the development of reduced intensity conditioning (RIC) regimens and the greater availability of volunteer unrelated donors have increased the proportion of patients who might benefit from allogeneic HCT (allo-HCT). Consequently, the number of patients allografted has increased consistently over the past 10 years (Figure 1).
Number of HCTs performed in CLL patients reported to the EBMT (data on file). Auto indicates autologous HCT; MAC Allo, myeloablative conditioning allogeneic HCT; and RIC Allo, reduced intensity conditioning allogeneic HCT.
Number of HCTs performed in CLL patients reported to the EBMT (data on file). Auto indicates autologous HCT; MAC Allo, myeloablative conditioning allogeneic HCT; and RIC Allo, reduced intensity conditioning allogeneic HCT.
Ideally, the place of transplantation in the management of CLL would be established in prospective randomized trials. These have been proven difficult to do in CLL. Not a single published study has prospectively assessed the efficacy of autologous or allogeneic HCT compared with conventional chemotherapy. For this reason, the European Group for Blood and Marrow Transplantation (EBMT) recently published a set of guidelines suggesting situations where allo-HCT might be considered a therapeutic option for CLL patients. Their conclusions were that allo-HCT was reasonable for younger CLL patients refractory to fludarabine, relapsing within 2 years of intensive treatment or with p53 abnormalities requiring treatment.2
Although the use of autologous HCT, which solely relies on dose intensity and does not appear to be curative in CLL, is rapidly declining with the advent of modern immunochemotherapy, allo-HCT remains a unique modality because of its graft-versus-CLL effect and can be effective even in otherwise refractory disease. In this review, we therefore focus on allo-HCT, trying to answer the following key questions: (1) What defines poor-risk CLL? (2) What are the results of myeloablative and reduced intensity transplantation strategies? (3) How do these results compare with the available alternative therapies? (4) What are the specific problems of CLL patients undergoing allogeneic HCT? (5) How can we improve these results?
What defines poor-risk CLL?
There is no doubt that there has been a significant change in the way we approach the diagnosis and management of CLL patients.3 Historically, only half of the patients had early-stage disease at diagnosis. These patients were typically placed under a “watchful waiting” strategy and told they would probably die of CLL-unrelated causes. At the other end of the spectrum, patients diagnosed at advanced stage were considered to have poor-risk disease, with a median overall survival (OS) of approximately 2 years.4-6 Nowadays, the clinical picture has changed considerably. Thanks to automated blood counters, 70% of CLL patients are diagnosed at early stage and have asymptomatic disease.7 However, 50% of CLL patients with early-stage disease eventually die of disease progression or disease-related complications,3 and almost all patients younger than 55 years at the time of diagnosis invariably die of their disease regardless of clinical stage.8
The development of new prognostic markers in the last 10 years has had a great influence on this change of scenery. We are now able to identify those patients with early-stage disease who have biologically aggressive disease and are therefore at high risk of clinical progression.9-11 In addition, patients whose tumor cells have specific biologic characteristics, such as the presence of a deletion in the short arm of chromosome 17, not only have a poor prognosis but also are more frequently refractory to conventional chemotherapy12 and might benefit from alternative treatment strategies.13
Although we are now in a better position to predict the clinical outcome of a given patient, CLL remains incurable with conventional therapy. Even modern chemoimmunotherapy combinations, for example, fludarabine, cyclophosphamide, and rituximab, able to achieve objective responses in 95% of patients when given in initial treatment, have a long-term progression-free survival (PFS) approximately 50% at 6 years with no plateau in the curve. Furthermore, these impressive responses are costly in terms of early and late toxicity.14
The prognosis of CLL patients who are refractory to fludarabine is dismal. Before the availability of monoclonal antibodies, the overall response rate (ORR) for the first salvage therapy was 22%, and the median OS ranged from 10 to 12 months.15,16 These poor results obviously encouraged the search for more effective agents. Alemtuzumab, a monoclonal antibody targeting CD52, was subsequently licensed for fludarabine-refractory patients, but the ORR was only 33% and the median OS was 16 months (Table 1).17 Almost identical results were obtained by the German CLL Study Group (GCLLSG),18 whereas a third study revealed a somewhat higher ORR approximately 50% in fludarabine-refractory patients.28 Furthermore, Tam et al29 have recently evaluated the natural history of CLL patients who are refractory to both fludarabine and alemtuzumab (double-refractory) or that, being fludarabine-refractory, are also unlikely to respond to alemtuzumab because of bulky lymphadenopathy (bulky-refractory). In this study, they observed a 23% ORR for the first salvage therapy and a median OS of 9 months for the whole group of patients.
Other groups have evaluated chemoimmunotherapy combinations in this situation. A single-center study analyzing the fludarabine, cyclophosphamide, and rituximab combination as salvage therapy reported a 58% response rate in fludarabine-refractory patients,19 but a more aggressive combination of rituximab, cytarabine, oxaliplatin, and fludarabine only obtained a 33% ORR with a median OS barely approaching 12 months.20 Table 1 summarizes the results obtained with these and other promising alternatives, such as lenalidomide,21 flavopiridol,22 and rituximab plus high-dose methylprednisolone.23 Unfortunately, none of these strategies appears curative or provides acceptable long-term disease control.
The case of patients with 17p deletion, found in 5% to 8% of newly diagnosed CLL, is not better. Even though there is still some controversy regarding the most significant cutoff for this test, the outcome of these patients is uniformly poor.12,30-32 Results using fludarabine combinations at first line, either with or without rituximab, remain disappointing with a median PFS no longer than 12 months and a median OS approximately 24 months (Table 1).24 The use of alemtuzumab as initial therapy does not improve matters. A recent international randomized study reported a promising 64% ORR but a median PFS of only 11 months.25 When given at relapse, alemtuzumab only achieved a 40% ORR (all partial responses) and a median time to progression of 8 months in patients with 17p deletion or TP53 mutations.26
Recently, minimal residual disease (MRD) has been identified as a prognostic marker in patients receiving cytotoxic therapy. One study revealed that patients not achieving an MRD− status after the administration of alemtuzumab as salvage therapy had a significantly worse OS, suggesting that MRD might be helpful for characterizing poor-risk CLL.28 In contrast, the OS of patients achieving an MRD+ complete remission (CR) after first-line fludarabine combinations was not significantly inferior to that of patients achieving an MRD− CR.33 Therefore, further studies are needed to confirm whether MRD results can be used for defining the indication of allo-HCT.
In conclusion, CLL patients with poor-risk features, as identified by the EBMT group, have a dismal outcome with nontransplantation strategies. Can these results be improved by allo-HCT?
What are the results of myeloablative and reduced intensity transplantation strategies?
Conventional (myeloablative) allogeneic HCT
Traditionally, conventional allo-HCT has been associated with unacceptable toxicity and mortality in CLL patients (Table 2). Results published by the EBMT together with the International Bone Marrow Transplantation Registry revealed a long-term nonrelapse mortality (NRM) and OS of 46% each.34 More recent registry data, considering recipients of unrelated donors only, showed a 38% NRM and 33% OS at 5 years.35 However, compared with autologous HCT, late relapses are uncommon in most published series, stressing the rationale for allo-HCT.
Single-center studies have shown slightly better results. The Dana-Farber Cancer Institute enrolled 162 high-risk CLL patients in a study where patients with HLA-matched siblings underwent T cell–depleted allo-HCT, whereas patients without sibling donors underwent autologous HCT. At 6 years, there were no significant differences in OS between the groups (55% vs 58% for patients allografted and autografted, respectively), but PFS was superior in the autologous group (24% vs 30%, P = .04).36 In Canada, 30 CLL patients were conditioned with myeloablative chemo-irradiation and received grafts from 20 and 10 HLA-matched siblings and unrelated donors, respectively. Actuarial OS and PFS were 39% at 5 years, significantly better for sibling compared with unrelated-donor recipients (48% vs 20%, P = .02).37 At Hospital Clinic, Barcelona, 12 young patients underwent conventional allo-HCT from HLA-matched sibling donors, and both OS and PFS were 65% at a median follow-up of 43 months.38 At the M. D. Anderson Cancer Center (MDACC), 28 young patients were allografted after myeloablative conditioning, 20 of them from HLA-identical siblings. The 5-year OS was 45%, significantly worse for chemo-refractory patients compared with those with chemo-sensitive disease at transplantation (78% vs 31%, P = .05).39
However, taking into account that median age at CLL diagnosis is 72 years and that conventional allo-HCT is rarely performed above 50 years of age, very few patients are ever considered candidates for this procedure. Indeed, the median age at transplantation ranged from 39 to 48 years in the studies previously mentioned (Table 2). Furthermore, at least 2 studies have retrospectively compared myeloablative with RIC regimens for CLL patients. The first study by the EBMT group found a significantly lower NRM in patients receiving RIC regimens but also a trend toward a higher relapse rate. However, these differences did not translate into a prolonged OS or PFS.40 Second, a recent retrospective analysis performed at the Fred Hutchinson Cancer Research Center (FHCRC) revealed that CLL patients with comorbidities, whether young or old, had better long-term survival after RIC compared with conventional allo-HCT. In contrast, patients without comorbidities had comparable NRM, PFS, and OS. The same study also showed that the conditioning regimen only played a minor role in terms of disease control, with very similar relapse rates for both the myeloablative and RIC groups.41 Moreover, the lack of curative activity of autologous HCT emphasizes that dose intensity may not be a crucial factor for long-term disease con-trol in CLL.
In conclusion, evidence that myeloablative conditioning is superior to RIC, even in younger or patients with fewer comorbidities, is lacking, although a potential role of conditioning intensity in patients with refractory/bulky disease or HLA-mismatched donor-recipient pairs cannot be excluded.42,43
RIC allogeneic HCT
RIC regimens were developed in the 1990s to allow allo-HCT in older patients or younger patients with comorbidities. Understandably, CLL was one of the diseases that benefited most from the implementation of RIC transplantation programs around the globe. In Europe, the total number of allogeneic transplantations for CLL patients has almost tripled in the last 7 years (Figure 1).
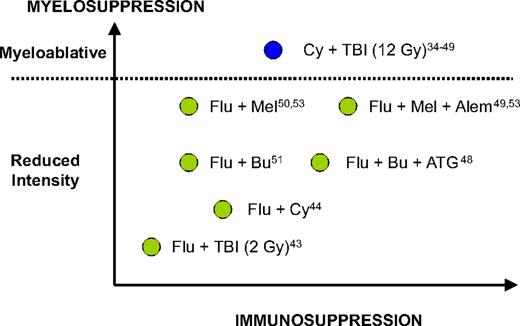
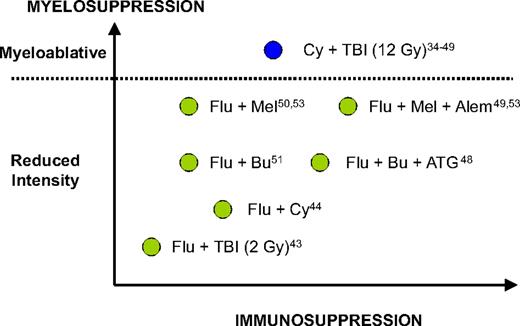
In the past decade, multiple reports from both sides of the Atlantic have suggested that RIC allo-HCT may be a potentially curative strategy for CLL patients. This suggestion was based on circumstantial evidence of a clinically effective graft-versus-leukemia effect in CLL patients undergoing RIC allo-HCT, such as achievement of long-term molecular responses,44 reduced relapse rates in patients with chronic graft-versus-host disease (GVHD),40,45 increased relapse rates associated with T cell–depleted grafts,46,47 and some efficacy of donor lymphocyte infusions (DLIs).46,48,49 Multiple single-center phase 2 studies have evaluated several different RIC regimens with different myeloablative and immunoablative potential (Figure 2; Table 3).43-46,48-54
The different myelosuppressive and immunosuppressive potential of most conditioning regimens mentioned in this report. Adapted from Storb et al54 with permission. Flu indicates, fludarabine; Bu, busulfan; Mel, melphalan; Cy, cyclophosphamide; ATG, thymoglobulin; Alem; alemtuzumab; TBI, total body irradiation.
The different myelosuppressive and immunosuppressive potential of most conditioning regimens mentioned in this report. Adapted from Storb et al54 with permission. Flu indicates, fludarabine; Bu, busulfan; Mel, melphalan; Cy, cyclophosphamide; ATG, thymoglobulin; Alem; alemtuzumab; TBI, total body irradiation.
The outcome of 82 patients treated at the FHCRC consortium using a nonablative conditioning regimen (fludarabine and low-dose total body irradiation) was recently updated.43 Eighty-seven percent of patients had fludarabine refractory disease and 9% had del(17p). At 5 years, the NRM and relapse rate were 23% and 38%, respectively, which translated into a 50% OS and 39% PFS. Patients with bulky lymphadenopathy (> 5 cm) at the time of transplantation had a particularly poor outcome, with a 5-year relapse rate and PFS of 71% and 8%, respectively. Extensive chronic GVHD was diagnosed in 50% of patients, but it resolved in most of them eventually.
At the MDACC, 39 patients were conditioned with a nonablative fludarabine-cyclophosphamide-rituximab regimen. NRM was 28% at 4 years. Current PFS (ie, not counting patients who relapsed after transplantation but achieved a remission with DLIs + rituximab) was 44%, and OS was 48% at 4 years. Patients with mixed chimerism at 3 months or with chemorefractory disease at the time of transplantation had a significantly increased re-lapse rate.52
Preliminary results from the GCLLSG CLL3X trial have been recently published. Thirty patients underwent fludarabine plus cyclophosphamide-conditioned allo-HCT from 13 HLA-matched siblings and 17 HLA-matched unrelated donors. The cumulative incidence of extensive chronic GVHD was 59% at 1 year, and the NRM was 11% at 3 years. OS and PFS were 71% and 58% at 3 years, respectively. MRD eradication was achieved in more than 50% of patients and was a very strong predictor of PFS. Of note, patients with significant comorbidities were excluded from the study, which probably contributed to the significant reduction in NRM and improved outcomes.44
The British and Spanish Cooperative groups used a fairly similar, and slightly more intensive, RIC regimen. The Spanish group used fludarabine plus melphalan,50 whereas the United Kingdom groups added alemtuzumab to the combination. In this context, alemtuzumab is used to deplete recipient and incoming donor T cells, providing sustained engraftment while reducing the incidence of GVHD.49 This reduced GVHD rate is counterbalanced by delayed immune reconstitution, increased risk of severe infections, and increased relapse rate.55 We have recently compared and updated the results from 4 institutions selected from these collaborative groups.53 NRM, relapse rate, PFS, and OS, with and without alemtuzumab, were 28% versus 34% (P = .735), 32% versus 20% (P = .112), 39% versus 47% (P = .361), and 65% versus 57% (P = .629) at 3 years. Patients receiving alemtuzumab had a higher risk of mixed chimerism at 6 months, and there was a trend toward a higher viral infection rate in the same group. In contrast, the incidence of extensive chronic GVHD was significantly higher in patients not receiving alemtuzumab (48% vs 10%, P = .03).
The Cooperative German Transplant Study Group analyzed the clinical outcome of 30 CLL patients conditioned with fludarabine, busulfan, and thymoglobulin. At 2 years, OS and PFS were 72% and 67%, respectively. NRM was 16% at 2 years, being significantly higher in unrelated donor compared with sibling donor recipients (28% vs 0%, P = .04). GVHD was considered the underlying cause of death in all these patients.48
Finally, the Dana-Farber Cancer Institute evaluated the use of fludarabine plus busulfan as an RIC regimen in 43 patients. The patients received unmanipulated grafts from 15 (33%) siblings and 31 (67%) unrelated donors. At 2 years, NRM was 17%, but the relapse rate was 48%. Median PFS was 10 months (34% at 2 years) and the 2-year OS was 54%. The most important factor associated with disease relapse and NRM was chemorefractory disease at the time of transplantation. Increased number of prior therapy lines and CLL marrow involvement were also associated with OS and PFS.51
In summary, RIC allo-HCT was associated with a 11% to 34% NRM, a 34% to 67% PFS, and a 48% to 72% OS depending on the conditioning regimen and follow-up. Long-term disease control was obtained in a substantial proportion of patients, particularly in those with chemosensitive and nonbulky disease at the time of transplantation. Extensive chronic GVHD rates can be as low as 10% when in vivo T-cell depletion is used or as high as 59% when unmanipulated grafts are infused. However, the low incidence of primary chronic GVHD after T-cell depletion may be offset by higher rates of secondary chronic GVHD induced by preemptive or therapeutic DLIs as suggested by a recent EBMT analysis.56
How do these results compare with the available alternative therapies?
As previously described, patients with fludarabine-refractory disease or 17p deletion have a poor outcome without transplantation, with a median PFS of 11 to 15 months and a median OS of 12 to 40+ months. Comparatively, the results obtained with RIC allo-HCT are apparently better. In the FHCRC study, where most patients were refractory to fludarabine, the median OS was 60 months.43 In the GCLLSG study, both OS and PFS were more than 60% with a median follow-up of 34 months when fludarabine-refractory patients were evaluated separately.44 Finally, in the Spanish-British comparative study, median OS and PFS were only 17 and 16 months, respectively, for fludarabine-refractory patients. Indeed, fludarabine refractoriness was one of the most important predictors of OS in this study.53
For patients with del(17p), the situation is complicated by the low incidence of this cytogenetic aberration. The Spanish Collaborative Group as well as the FHCRC Consortium reported individual patients with long-term PFS after RIC allo-HCT.43,50 A recent EBMT retrospective study evaluated the outcome of 44 CLL patients with del(17p) who underwent allo-HCT. Five patients were conditioned with myeloablative chemo-irradiation, whereas 39 were included in RIC protocols. At 3 years, OS and PFS were 44% and 37%, respectively, with a median OS and PFS approximately 32 and 18 months, respectively. Of note, 16 (36%) patients were alive and disease-free after a median follow-up of 39 months.47
A recent study compared the outcomes of poor-risk CLL patients undergoing RIC allo-HCT with a group of matched controls who did not have a suitable donor or refused the procedure. Of note, all controls fulfilled EBMT criteria for allo-HCT. Taking into account all the limitations of risk-matched comparisons, this study suggested a survival advantage for patients undergoing RIC allo-HCT, but only when calculated from time of first therapy.57
By looking at these results, it could be argued that allogeneic HCT seems very reasonable for poor-risk CLL because it is the only modality capable of providing long-term disease control in a significant proportion of otherwise incurable patients. However, it could also be argued that these results seem to be worse than those obtained in other indolent lymphoproliferative disorders, particularly follicular lymphoma.58-61 The reasons for these differences are unclear, but there are some possible explanations.
What are the specific problems of CLL patients undergoing allogeneic HCT?
High graft rejection rates, either with conventional or RIC
CLL patients undergoing allogeneic HCT have remarkably high graft rejection rates. Some initial reports using myeloablative conditioning regimens showed rejection rates approximately 18% to 20%.34,62 Indeed, a study performed in Nebraska acknowledged the fact that only 65% of all CLL allograft patients achieved sustained trilineage engraftment and transfusion independence.63 In the case of RIC regimens, the incidence of graft rejection varies from 12% to 25%, when grafts are T cell–depleted in vivo or in vitro,46,49 to 5% to 6% in the case of unmanipulated grafts.44,52,64 The recent British-Spanish comparative study found a secondary rejection rate of 12% and 9% for patients conditioned with or without alemtuzumab, respectively.53 In comparison, patients with follicular lymphoma undergoing transplantation with the same conditioning regimens are reported to have a 3% to 6% rejection rate.58,60,61
A possible explanation for this phenomenon could be the significant marrow infiltration allowed in CLL patients at the time of transplantation, which would probably preclude transplantation in patients with other malignancies. As such, at least 2 studies have shown an inverse correlation between the degree of marrow involvement and outcome.51,64 Other hypotheses, such as the role played by host dendritic cells, which are seriously defective in CLL patients,53 or the effect of prior alemtuzumab therapy are still speculative at this time.
High infection rates resulting from preexisting immunosuppression
Infections account for up to 50% of all CLL-related deaths.65,66 These percentages are probably higher in patients who are refractory to fludarabine and/or alemtuzumab,15,29 precisely those patients who are referred for allo-HCT. This susceptibility is both disease and therapy-related and is secondary to multiple factors, including hypogammaglobulinemia, defective T and natural killer cell function, neutropenia, and deficient complement activity.67 It is not surprising, then, that CLL patients subjected to allo-HCT had a high incidence of infections, particularly, but not exclusively, when alemtuzumab was part of the conditioning regimen. Indeed, more than 60% of all nonrelapse deaths were the result of infections in the Spanish-British comparative study, most of them related to GVHD.53 Other similar studies have shown similar rates (30%-66% of all nonrelapse deaths) with a clear correlation between the risk of infections and the presence of GVHD.43,50,52 Comparatively, this degree of immunosuppression before HCT is rarely encountered in patients with other lymphoproliferative disorders, such as follicular lymphoma, whose main cause of death is disease progression.68
The role of DLIs: is there any evidence of graft-versus-CLL effect?
There is strong circumstantial evidence of the existence of graft-versus-CLL (GVCLL) effect in patients undergoing RIC allo-HCT. Examples are the achievement of long-term molecular responses on withdrawal of immunosuppression,44 reduced relapse rates in patients with chronic GVHD,40,45 reduced relapse rates in recipients of unrelated donor grafts compared with HLA-matched siblings,64 increased relapse rates associated with T cell–depleted grafts,36,47 and the efficacy of DLIs.46,48,49
The efficacy of DLI has been specifically studied in 12 patients conditioned with fludarabine, busulfan, and thymoglobulin and allografted with T cell–depleted HLA-matched stem cell products. No GVHD prophylaxis was given, and DLIs were anticipated in all patients. Eleven patients finally received DLI: 1 for mixed chimerism, 7 for both mixed chimerism and persistent disease, and 3 for progressive disease. In patients with progressive disease, DLI was not effective, but in the remaining 8 patients DLI achieved a CR in 5 of them, translating into a 2-year OS and PFS of 67% and 33%, respectively. Of note, the authors could detect CLL-reactive T-cell clones in patients who responded to DLI, but not in those with unresponsive disease.46 Similar results were obtained after an alemtuzumab containing a RIC regimen. DLIs were given to 18 patients for various reasons. They were effective for reverting mixed chimerism in more than 50% of patients but only achieved sustained responses in less than one-third of patients with progressive disease.49 Other studies using T cell–replete grafts have used less DLIs because of higher GVHD rates, but the results have been broadly similar, with response rates approximately 15% or less in patients with relapsed disease after HCT.48,51,64 Better results might be achieved with DLI given preemptively for persistent MRD.44 At the MDACC, 50% of patients with relapsed disease after HCT responded to DLI, but the interpretation of these results is complicated by the concomitant administration of rituximab. Interestingly, the presence of mixed chimerism was a negative predictor of response to DLI in this study.52 One explanation for the relatively poor efficacy of DLI in T-replete patients despite strong evidence for GVCLL might be the “secondary GVCLL resistance” phenomenon described by Ritgen et al.44
The occurrence of early and late relapses
Disease relapse remains the major cause of failure after RIC allo-HCT in CLL patients (Table 3). Early relapses are thought to occur in patients with chemorefractory disease at the time of transplantation, for which RIC regimens are ineffective in controlling the disease before the GVCLL effect can take place. This phenomenon also occurs in other lymphoproliferative disorders.
More intriguing and different from the pattern observed in follicular lymphoma are late relapses. In the GCLLSG CLL3X trial, some patients showed MRD kinetics characterized by a steep decrease coincident with the onset of chronic GVHD followed by a delayed increase of MRD and clinical relapse despite ongoing GVHD (“secondary GVCLL resistance”).44 Several hypotheses were offered by the authors, including CLL clonal evolution, the development of tolerance,46 and the survival of tumor cells in “GVCLL sanctuary sites.” In this respect, there are plenty of reports in the literature of relapses up to 10 years after allo-HCT, and a significant percentage of these late relapses occurred in lymph nodes in the absence of bone marrow or peripheral blood involvement, or even in patients with MRD− status.29,36,37,39,69,70 Furthermore, the presence of bulky lymphadenopathy at the time of allo-HCT had a negative impact on PFS in the FHCRC study, further sustaining the hypothesis of the existence of “GVCLL sanctuary sites.”43 It is thus apparent that, at least in a fraction of patients, the GVCLL effect is inadequate to ensure complete disease eradication. Furthermore, this phenomenon emphasizes the important role of imaging studies, such as CT scans in the early detection of disease relapse after allo-HCT.
How can we improve these results?
Better patient selection
In our opinion, the EBMT guidelines are a good platform to build future trials of RIC allo-HCT for CLL. They identify a group of patients in whom available therapies are unlikely to achieve a prolonged disease-free survival. On the other hand, RIC allo-HCT is able to mitigate the adverse prognosis conferred by purine analog resistance and unfavorable genetics (“How do these results compare with the available alternative therapies?”).
However, one of the most important aims when trying to improve the results obtained with allo-HCT should be to reduce NRM. The FHCRC analysis revealed that comorbidities were more important than CLL-related variables for predicting PFS and OS.43 Another risk factor for NRM, usually correlated with the presence of comorbidities, is refractory disease at transplantation.71 Thus, it would appear prudent, given our current knowledge, to limit allo-HCT to fitter patients without evidence of refractory disease.
Better timing
If a CLL patient is eligible for RIC allo-HCT and has a donor available, he or she should proceed to transplantation as soon as EBMT criteria are met. By definition, these patients have poor-risk disease and one should not wait too long if a reasonable response is obtained with chemoimmunotherapy because the general prognosis and the outcome after allo-HCT are significantly impaired once the disease has reached a state of complete unresponsiveness.
MRD monitoring
Modulation of the GVCLL effect and maintenance therapy
Rituximab given concomitantly with RIC allo-HCT or DLI may facilitate disease control.52 This may be due, not only to direct cytotoxicity, but also to modulation of the GVCLL effect. Interestingly, rituximab has been shown to promote the cross-presentation of tumor-derived peptides by antigen-presenting cells, thus enhancing the formation of cytotoxic T-cell clones and a GVCLL effect.72 A more powerful way of redirecting donor T cells to residual CLL cells could be the posttransplantation administration of bispecific antibody constructs targeting both B- and T-cell antigens, such as blinatumomab.73
In addition, maintenance therapy with monoclonal antibodies or immunomodulatory drugs with proven activity in poor-risk CLL, such as lenalidomide, could be explored in future trials to minimize the risk of relapse.
In conclusion, because of the potent GVCLL effect, allogeneic HCT is a very powerful tool in the management of poor-risk CLL patients, but there is room for improvement. Patients who can expect a significant reduction of life expectancy under alternative therapies should be promptly identified and referred for allo-HCT before the disease becomes unresponsive to salvage treatment, provided there is a related or unrelated donor available. Because of old age, frequent marrow involvement, preexisting immune suppression, and other adverse features, these patients are prone to several transplantation-related complications and should be managed with great care in experienced centers using RIC regimens. The role of more intensive myeloablative conditioning regimens in younger patients and/or those with refractory disease remains to be settled. Novel transplantation strategies, such as MRD-based preemptive immune modulation or posttransplantation maintenance therapy, could improve the results and should be tested in the context of clinical trials. It is important that patients understand the risks and potential benefits of transplantation and are able to make an informed choice. All these facts should be kept in mind if we want to make further progress.
Authorship
Contribution: J.D., D.W.M., and P.D. wrote and proofread all versions of this manuscript.
Conflict-of-interest disclosure: J.D. has received lecturing fees from Bayer Schering Pharma and Roche and consulting fees from Bayer Schering Pharma, Roche, and GlaxoSmithkline. D.W.M. and P.D. declare no competing financial interests.
Correspondence: Julio Delgado, Servei d'Hematologia, Hospital de la Santa Creu i Sant Pau, Sant Antoni Maria Claret 167, 08205, Barcelona, Spain; e-mail: jdelgadog@santpau.cat.