Abstract
Invariant natural killer T (iNKT) cells are unique T cells that regulate the immune response to microbes, cancers, and autoimmunity. We assessed the characteristics of iNKT cells from persons infected with human T-lymphotropic virus type 1 (HTLV-1). Whereas most infected persons remain asymptomatic carriers (ACs) throughout their lives, a small proportion, usually with high equilibrium proviral loads,develop 2 diseases: HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP) and adult T-cell leukemia (ATL). We demonstrated that the frequency of iNKT, NK, and dendritic cells in the peripheral blood of HAM/TSP and ATL patients is decreased. We also observed an inverse correlation between the iNKT cell frequency and the HTLV-1 proviral load in the peripheral blood of infected persons. Notably, in vitro stimulation of peripheral blood cells with α-galactosylceramide led to an increase in the iNKT cell number and a subsequent decrease in the HTLV-1–infected T-cell number in samples from ACs but not HAM/TSP or ATL patients. Our results suggest that iNKT cells contribute to the immune defense against HTLV-1, and iNKT-cell depletion plays an important role in the pathogenesis of HAM/TSP and ATL. Therefore, iNKT cell–based immunotherapy may be an effective strategy for preventing these HTLV-1–associated disorders.
Introduction
Human T-cell lymphotropic virus type 1 (HTLV-1) causes persistent infection. Whereas most infected persons remain asymptomatic carriers (ACs), 3% to 5% develop a T-cell malignancy termed adult T-cell leukemia (ATL), and another 0.25% to 3% develop a chronic progressive inflammatory neurologic disease known as HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP).1-3 One of the most important pathogenic factors in HAM/TSP is an increased HTLV-1 load in the peripheral blood mononuclear cells (PBMCs) and cerebrospinal fluid,4-6 which suggests that in affected persons, virus control is inadequate. A higher HTLV-1 load increases the risk of HAM/TSP and ATL.4,7 Therefore, the precise mechanisms controlling HTLV-1–infected cells must be better understood. With regard to the host defense mechanisms involved in HTLV-1 infection, the role of HTLV-1–specific CD8+ cytotoxic T lymphocytes (CTLs) has been studied.8-10 The HTLV-1–specific CTL response is critical for a low viral load to be maintained.9-11 Despite the high frequency of HTLV-1–specific CTLs, the number of HTLV-1–infected T cells is surprisingly high in HAM/TSP patients.5,6 We and others have reported that the maturation and functioning of HTLV-1–specific CTLs are inadequate in HAM/TSP patients, although in vitro studies have shown that these CTLs exert cytolytic activity against HTLV-1–expressing target cells.12,13 Therefore, we hypothesize that there may be another cell population without CTLs that contributes to the control of HTLV-1–infected T cells.
A unique T-cell subpopulation, natural killer T (NKT) cells, constitute a subset of lymphocytes that share the features of both innate and adaptive immune cells. Unlike conventional T cells, NKT cells express a T-cell receptor (TCR) that recognizes glycolipids instead of protein antigens. Moreover, these cells share properties and receptors with NK cells. They rapidly produce granzymes and perforins on stimulation. Among the CD3+ T cells in human blood, 10% to 25% express NK cell–surface molecules, such as CD161, and these can be classified as NKT cells.14,15 A small population of T cells within this NKT cell subset expresses a highly conserved Vα24Jα18 TCR chain that associates preferentially with Vβ11.16-18 These T cells are referred to as invariant NKT (iNKT) cells. Very recently, a novel clonotypic monoclonal antibody 6B11 specific for the Vα24Jα18 TCR chain has been shown to selectively stain human iNKT cells.18,19 Activation of human iNKT cells requires the presentation of glycolipids, such as α-galactosylceramide (α-GalCer) on the major histocompatibility complex class I–like molecule CD1d.20 α-GalCer stimulation induces rapid cytokine production and potent antitumor and antipathogen responses by iNKT cells.21-25 Human α-GalCer–stimulated iNKT cells also induce the rapid maturation of dendritic cells (DCs) and activation of NK cells, thereby enhancing antitumor and antipathogen cytotoxicity.24,26 Furthermore, one subset of DCs, myeloid DCs (mDCs), induces the activation of iNKT cells by increasing CD1d expression on their surface.27 Another subset of DCs, plasmacytoid DCs (pDCs), secretes type1 interferon and supports mDCs in promoting iNKT cell activation.28 Furthermore, activated iNKT cells also enhance acquired immunity. Thus, human iNKT cells bridge the innate and adaptive immune responses by interacting with both DCs and NK cells, and they play crucial roles in controlling viral infection and tumor cells.29 Therefore, we investigated the frequency and activity of iNKT cells, NK cells, and DCs in HTLV-1–infected persons to determine whether iNKT cells have anti-HTLV-1 activity and play a pivotal role in the pathogenesis of HTLV-1–associated disorders.
We demonstrated that the frequency of iNKT cells, NK cells, and DCs is significantly decreased among PBMCs from HAM/TSP and ATL patients. We have also reported an inverse correlation between the frequency of iNKT cells and the proviral load in the PBMCs of HTLV-1–infected persons. We showed that in vitro α-GalCer stimulation of cells from ACs leads to an increase in the number of iNKT cells and that, after stimulation, the number of infected cells decreases. On the other hand, the number of iNKT cells among PBMCs from HAM/TSP and ATL patients did not increase on α-GalCer stimulation and, after stimulation, the number of infected cells did not decrease. Our results suggest that iNKT cells play a critical role in controlling HTLV-1 infection and that their activity is selectively depleted in both HAM/TSP and ATL patients.
Methods
Subjects and cell preparation
Written informed consent was obtained from all subjects before the study. The study complied with the tenets of the Declaration of Helsinki and was part of a clinical protocol reviewed and approved by the institutional ethics committee of each participating institution.
The study included 12 healthy donors (HDs; 5 men and 7 women; mean age, 55.64 years), 12 ACs (4 men and 8 women; mean age, 55.75 years), 13 HAM/TSP patients (3 men and 10 women; mean age, 58.15 years), and 11 ATL patients (3 with smoldering ATL and 8 with chronic ATL; 2 men and 9 women; mean age, 55.73 years). None of the subjects had comorbidities or had received prior immunosuppressive or cytotoxic chemotherapy. HAM/TSP was diagnosed according to the World Health Organization guidelines; ATL diagnosis was based on the criteria of Shimoyama.30 HTLV-1 seropositivity was determined by the particle agglutination method (Serodia-HTLV-1; Fujirebio) and confirmed by Western blot (SRL Inc). Peripheral blood samples were collected; PBMCs were prepared by centrifugation over Ficoll-Hypaque gradients and viably cryopreserved in liquid nitrogen. Samples were collected at 3 different hospitals, numbered, and sent to our institute, after which they were processed equally. The investigators knew only the sample number and were unaware of the patient allocation. The identity of the allocated patient was shared only after the analysis.
Flow cytometric analysis
PBMCs were immunostained with various combinations of the following fluorescence-conjugated antibodies to cell surface markers: lineage cocktail (Lin 1) of monoclonal antibodies against CD3, CD14, CD16, CD19, CD20, and CD56 (BD Biosciences), anti–human leukocyte antigen (HLA)-DR (LN3; eBioscience), anti-CD123 (9F5; BD Biosciences), anti-CD11c (3.9; eBioscience), anti-CD3 (UCTH1; eBioscience), anti-CD16 (CB16; eBioscience), anti-CD56 (B159; BD Biosciences), anti-Vα24Jα18 (6B11; BD Biosciences), anti-CD4 (L3T4; eBioscience), anti-CD161 (DX12; BD Biosciences), anti-CD1d (51.1; eBioscience), anti-CD40 (5C3; eBioscience), anti-CD80 (2D10.4; eBioscience), anti-CD86 (IT2.2; eBioscience), anti-CD19 (HB19; eBioscience), anti-CD14 (61D3; eBioscience), and anti-HLA-ABC (W6/32; eBioscience). Each cell phenotype was defined as follows: mDC (Lin−HLA-DR+CD11c+), pDC (Lin−HLA-DR+CD123+), NK cells (CD3−CD16+CD56+), CD56brightCD16− NK cells (CD3−CD16−CD56bright), and iNKT cells (CD3+Vα24Jα18+). Cells were stained with saturating concentrations of antibody (4°C, 30 minutes) in the dark and washed twice before analysis on a FACSCalibur (BD Biosciences). For perforin staining, cells were fixed and permeabilized with a staining buffer set (eBioscience) and intracellularly stained using a perforin reagent set (BD Biosciences). Data were processed using FlowJo software (TreeStar). For cell sorting, we used JSAN (BayBioscience), and the purity exceeded 95%.
Real-time PCR
The HTLV-1 proviral DNA load was measured using ABI Prism 7500 SDS (Applied Biosystems) as described previously.6 Briefly, DNA was extracted, and 100-ng samples were analyzed per well. The proviral DNA load was calculated by the following formula: copy number of HTLV-1 (pX) per 100 cells = (copy number of pX)/(copy number of β actin/2) × 100.
α-GalCer stimulation of iNKT cells
PBMCs from HDs, ACs, and HAM/TSP and ATL patients were plated in 96-well round-bottom plates (105 cells per well) with or without 100 ng/mL α-GalCer (KRN7000; Funakoshi Co Ltd) in the presence or absence of 200 U/mL recombinant human interleukin-2 (IL-2; PeproTech) and cultured for 21 days. Cells were harvested for analysis on days 7, 14, and 21. RPMI-1640 with L-glutamine (Wako) supplemented with 10% fetal bovine serum (Invitrogen), 40 U/mL penicillin, and 40 μg/mL streptomycin (Wako) was used as the culture medium.
Statistical analysis
Nonparametric Scheffé F test was used for multiple comparisons of the frequency of iNKT and NK cells among the subject groups. Multiple comparisons of the mDC and pDC frequency among the subject groups were performed with the parametric Tukey-Kramer test. Scheffé F test was used for multiple comparisons of the expression levels of CD1d, CD86, and HLA-ABC among the subject groups. To assess the correlation between the HTLV-1 proviral load in PBMCs and DCs, Spearman rank correlation test was used to investigate the correlation between the frequency of iNKT cells, NK cells, and DCs and the proviral load in PBMCs. This test was also used to determine the correlation between the expression levels of the surface molecules (CD1d, CD86, and HLA-ABC) on and the proviral load in DCs. The proviral load in DCs of ACs and HAM/TSP patients and that after culture with or without α-GalCer stimulation was compared with the Wilcoxon signed-rank test. Statistical analyses were performed using the Statcel software, version 2 (OMS Publishing Inc).
Results
Decreased frequency of iNKT cells, NK cells, and DCs in PBMCs from HAM/TSP and ATL patients
To investigate the iNKT cell frequency in HTLV-1–infected persons, including HAM/TSP and ATL patients, we assayed the frequency of CD3+Vα24Jα18+ T cells in PBMCs from HDs (n = 12), ACs (n = 12), HAM/TSP patients (n = 13), and ATL patients (n = 11) using flow cytometry (Figure 1A-B). The percentage of iNKT cells among the total PBMCs was significantly decreased in HAM/TSP (0.00%-0.05%, mean = 0.0159%) and ATL patients (0.00%-0.04%, mean = 0.0150%) compared with that in HDs (0.01%-0.14%, mean = 0.0638%). The iNKT cell frequency in ACs (0.02%-0.14%, mean = 0.0525%) was lower than that in HDs and higher than that in HAM/TSP and ATL patients (Figure 1B). Analysis of the frequency of NK cells (CD3−CD56+CD16+) in the total PBMCs showed that the number of NK cells was lower in HAM/TSP and ATL patients than in HDs (Figure 1C). As in the case of iNKT cells, the frequency of NK cells in the ACs was lower than that in HDs but higher than that in HAM/TSP and ATL patients (Figure 1B). We also analyzed the frequency of CD56brightCD16− NK cells, which were recently identified as cells with low cytotoxicity and inhibitory function for T-cell proliferation31 (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The number of CD56brightCD16− NK cells was lower in ATL patients than in HDs with no statistical significance; further, the number of these cells in the HAM/TSP patients and ACs was equivalent to that in HDs. Because DCs are crucial for the in vivo activation of iNKT cells,27,32 we analyzed the frequency of 2 major peripheral blood subtypes of DCs (mDCs and pDCs) in the subjects (Figure 1D-E). The mDC frequency in HAM/TSP patients was lower than that in HDs (P < .01) and similar to that in ACs (Figure 1D). The mDC frequency was significantly lower in ATL patients than in HDs (P < .01), ACs (P < .01), and HAM/TSP patients (P < .01). The mDC frequency was lower in ACs than in HDs, although the difference was not statistically significant (Figure 1D). Furthermore, the pDC frequency was lower in both HAM/TSP and ATL patients than in HDs (Figure 1E). The pDC frequency was also lower in ACs than in HDs, but the difference was not statistically significant. To summarize, the frequency of cell subsets that play a role in innate immunity was lower in the HTLV-1–infected persons, especially HAM/TSP and ATL patients.
Decreased frequency of iNKT and NK cells, mDCs, and pDCs among PBMCs from HAM/TSP and ATL patients. (A) Representative FACS plot of iNKT cells from HDs, ACs, HAM/TSP patients (HAM), and ATL patients. PBMCs were stained for CD3 and Vα24Jα18, and the expression of CD3 and Vα24Jα18 is plotted. Numbers adjacent to the outlined area indicate the percentage of iNKT cells in total PBMCs. Plots of the frequency of iNKT cells (B), CD56+CD16+ NK cells (C), mDCs (D), and pDCs (E) among PBMCs from 12 HDs, 12 ACs, 13 HAM/TSP patients, and 11 ATL patients. The horizontal bar represents the mean value for each group. Scheffé F test was used for statistical analysis. Statistically significant differences: *P < .05, **P < .01.
Decreased frequency of iNKT and NK cells, mDCs, and pDCs among PBMCs from HAM/TSP and ATL patients. (A) Representative FACS plot of iNKT cells from HDs, ACs, HAM/TSP patients (HAM), and ATL patients. PBMCs were stained for CD3 and Vα24Jα18, and the expression of CD3 and Vα24Jα18 is plotted. Numbers adjacent to the outlined area indicate the percentage of iNKT cells in total PBMCs. Plots of the frequency of iNKT cells (B), CD56+CD16+ NK cells (C), mDCs (D), and pDCs (E) among PBMCs from 12 HDs, 12 ACs, 13 HAM/TSP patients, and 11 ATL patients. The horizontal bar represents the mean value for each group. Scheffé F test was used for statistical analysis. Statistically significant differences: *P < .05, **P < .01.
Inverse correlation between frequency of iNKT cells and proviral load in peripheral blood of HTLV-1–infected persons
To examine whether iNKT and NK cells, mDCs, and pDCs in HTLV-1–infected persons (Figure 1) contribute to the defense against HTLV-1, we assayed the correlation between the frequency of each cell population and the HTLV-1 proviral load as measured by quantitative real-time PCR of PBMCs from ACs (n = 12) and HAM/TSP patients (n = 13; Figure 2). As shown in Figure 2A, the frequency of iNKT cells was inversely and significantly correlated with the proviral load (P = .008 by Spearman rank correlation test); the frequency of NK cells (P = .449), mDCs (P = .712), and pDCs (P = .894) was not (Figure 2B-D). These results suggest that iNKT cells play an important role in the defense against HTLV-1.
Inverse correlation between the frequency of iNKT cells and the proviral load in peripheral blood from HTLV-1–infected persons. The correlation between the HTLV-1 proviral load in PBMCs and the frequency of iNKT cells (A), CD56+CD16+ NK cells (B), mDCs (C), and pDCs (D) from HTLV-1–infected persons (ACs, n = 12; HAM/TSP patients, n = 13) was statistically analyzed using the Spearman rank correlation test.
Inverse correlation between the frequency of iNKT cells and the proviral load in peripheral blood from HTLV-1–infected persons. The correlation between the HTLV-1 proviral load in PBMCs and the frequency of iNKT cells (A), CD56+CD16+ NK cells (B), mDCs (C), and pDCs (D) from HTLV-1–infected persons (ACs, n = 12; HAM/TSP patients, n = 13) was statistically analyzed using the Spearman rank correlation test.
Equivalent expression level of CD1d in all study groups
iNKT cells recognize their cognate antigen expressed on the major histocompatibility complex class I–like molecule CD1d, and mDCs induce the activation of iNKT cells by increasing surface CD1d expression.27 Therefore, we examined whether the frequency of iNKT cells in HAM/TSP and ATL patients decreases because of the decreased expression of CD1d on mDCs. We analyzed CD1d expression on mDCs from 9 HDs, 7 ACs, 9 HAM/TSP patients, and 3 ATL patients using flow cytometry (Figure 3A). The CD1d expression level per mDC was similar among the study subjects. Because CD1d is also expressed on monocytes and B cells, we analyzed the CD1d expression level on the Lin+ population (which includes monocytes and B cells) from the subjects. As shown in Figure 3B, the CD1d expression level per Lin+ cell was also similar among the study subjects. We further analyzed the CD1d expression level on monocytes (CD14+) and B cells (CD19+) in PBMCs from 9 HDs, 4 ACs, 9 HAM/TSP patients, and 3 ATL patients using flow cytometry (supplemental Figure 2); the CD1d expression level per monocyte and B cell was equivalent among the subject groups.
Equivalent expression levels of CD1d on mDCs and lineage-positive (Lin+) cells from HDs, ACs, and HAM/TSP patients, and ATL patients. Plots of the expression level (mean fluorescence intensity [MFI]) of CD1d molecules on mDCs (A) and Lin+ cells (B) among PBMCs from HDs (n = 9), ACs (n = 7), HAM/TSP patients (n = 9), and ATL patients (n = 3). The horizontal bar represents the mean value for each group. Statistical analysis of the expression level for each study subject revealed no statistically significant differences by 1-way analysis of variance and Scheffé F test.
Equivalent expression levels of CD1d on mDCs and lineage-positive (Lin+) cells from HDs, ACs, and HAM/TSP patients, and ATL patients. Plots of the expression level (mean fluorescence intensity [MFI]) of CD1d molecules on mDCs (A) and Lin+ cells (B) among PBMCs from HDs (n = 9), ACs (n = 7), HAM/TSP patients (n = 9), and ATL patients (n = 3). The horizontal bar represents the mean value for each group. Statistical analysis of the expression level for each study subject revealed no statistically significant differences by 1-way analysis of variance and Scheffé F test.
pDCs cooperate with mDCs to promote antiviral activity by activating iNKT cells.28,33 Therefore, we subsequently analyzed the surface expression level of DC-activation markers, such as CD40, CD80, CD86, and HLA-ABC on the mDCs and pDCs of 9 HDs, 7 ACs, 9 HAM/TSP patients, and 3 ATL patients (supplemental Figure 3). CD40 and CD80 expression on both circulating mDCs and pDCs (data not shown) and CD86 expression on pDCs (supplemental Figure 3A) were negligible in all subjects. CD86 expression on mDCs and HLA-ABC expression on mDCs and pDCs (supplemental Figure 3) were detectable in all subjects, but there was no statistically significant difference in the expression of either among the study subjects.
No correlation between the high HTLV-1 proviral load in DCs of HAM/TSP patients and CD1d expression level
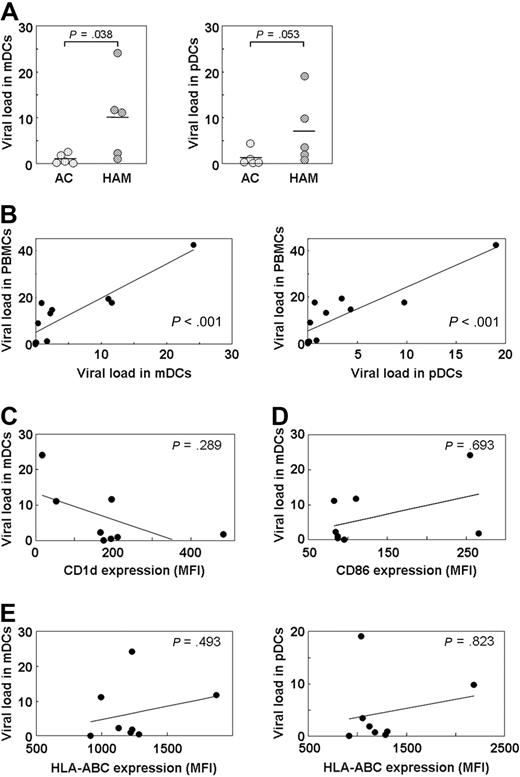
HTLV-1 infects DCs,34 and the extracellular HTLV-1 Tax protein has been reported to induce the overexpression of CD86 and decreased expression of HLA-ABC on DCs.35 Moreover, human immunodeficiency virus type 1 (HIV-1) reportedly down-regulates CD1d expression.36,37 We hypothesized that HTLV-1 infection of DCs may alter their function by affecting the expression level of CD1d, CD86, and HLA-ABC on the cell surface. Therefore, we first separated mDCs and pDCs from 5 ACs and 5 HAM/TSP patients by fluorescence-activated cell sorter (FACS) and quantified the HTLV-1 proviral load. As shown in Figure 4A, the proviral load was significantly higher in the mDCs of HAM/TSP patients than in those of ACs (P = .038). Moreover, it was also higher in the pDCs of HAM/TSP patients than in those of ACs (Figure 4A), although the difference was not statistically significant (P = .053). We analyzed the correlation between the proviral load in PBMCs and that in mDCs or pDCs and found a positive correlation (Figure 4B). Thus, in infected persons, in vivo HTLV-1 infection of mDCs and pDCs is apparent, and the proviral load in DCs is associated with the total proviral load in PBMCs.
Increased HTLV-1 proviral load in DCs of HAM/TSP patients not associated with CD1d expression level. (A) The HTLV-1 proviral load was higher in mDCs (P = .038) and pDCs (P = .053) of HAM/TSP patients (n = 5) than in those of ACs (n = 5). The Mann-Whitney U test was used for statistical analysis. (B) Correlation analysis of the proviral load in PBMCs of HTLV-1–infected persons (5 ACs and 5 HAM/TSP patients) and that in mDCs (P < .001) and pDCs (P < .001) using Spearman rank correlation test. (C-E) No correlation was found between the proviral load in mDCs or pDCs of HTLV-1–infected persons (3 ACs and 5 HAM/TSP patients) and the expression level of CD1d on mDCs (P = .289; C), CD86 on mDCs (P = .693; D), and HLA-ABC on mDCs (P = .493) and pDCs (P = .823; E) by Spearman rank correlation test.
Increased HTLV-1 proviral load in DCs of HAM/TSP patients not associated with CD1d expression level. (A) The HTLV-1 proviral load was higher in mDCs (P = .038) and pDCs (P = .053) of HAM/TSP patients (n = 5) than in those of ACs (n = 5). The Mann-Whitney U test was used for statistical analysis. (B) Correlation analysis of the proviral load in PBMCs of HTLV-1–infected persons (5 ACs and 5 HAM/TSP patients) and that in mDCs (P < .001) and pDCs (P < .001) using Spearman rank correlation test. (C-E) No correlation was found between the proviral load in mDCs or pDCs of HTLV-1–infected persons (3 ACs and 5 HAM/TSP patients) and the expression level of CD1d on mDCs (P = .289; C), CD86 on mDCs (P = .693; D), and HLA-ABC on mDCs (P = .493) and pDCs (P = .823; E) by Spearman rank correlation test.
To determine whether HTLV-1 infection of DCs affects CD1d expression on the DC surface, we analyzed the correlation between the proviral load in the mDCs and the expression level of CD1d on the mDCs of 8 HTLV-1–infected persons (3 ACs and 5 HAM/TSP patients). We observed no correlation (Figure 4C). Furthermore, we found no correlation between the proviral load in DCs and the expression levels of CD86 (Figure 4D) and HLA-ABC (Figure 4E). These results suggest that HTLV-1 infection of DCs has less effect on the expression levels of surface molecules important for the activation of T and iNKT cells.
Expansion of iNKT cells by in vitro α-GalCer stimulation can decrease the number of HTLV-1–infected T cells in ACs but not HAM/TSP and ATL patients
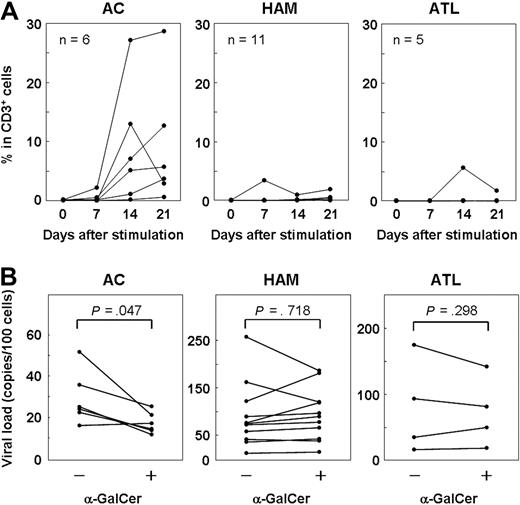
The inverse correlation between the frequency of iNKT cells and the proviral load in HTLV-1–infected persons (Figure 2A) suggests that iNKT cells play a role in the immune defense against HTLV-1. To explore whether iNKT cells have an anti–HTLV-1 effect, we cultured PBMCs from 6 ACs, 11 HAM/TSP patients, and 5 ATL patients with or without α-GalCer (100 ng/mL), and we analyzed the frequency of iNKT cells on days 0, 7, 14, and 21 using flow cytometry. As shown in Figure 5A, the number of iNKT cells in the PBMCs of most of the ACs increased after α-GalCer stimulation. On the other hand, the iNKT cells in the PBMCs from most of the HAM/TSP and ATL patients showed little or no response to α-GalCer stimulation.
Expansion of iNKT cells by α-GalCer stimulation decreased the number of HTLV-1–infected T cells in ACs but not in HAM/TSP and ATL patients. (A) PBMCs from ACs (n = 6) and HAM/TSP (n = 11) and ATL patients (n = 5) were cultured with α-GalCer (100 ng/mL), and the percentage of iNKT cells on days 0, 7, 14, and 21 was plotted. (B) PBMCs from ACs (n = 6) and HAM/TSP (n = 11) and ATL patients (n = 4) were cultured for 14 days with or without α-GalCer, and the proviral load in each sample was quantified by real-time PCR. In ACs, the proviral load in PBMCs cultured with α-GalCer was significantly lower than that of cultured PBMCs grown without α-GalCer (P = .047). No significant difference was observed between HAM/TSP (P = .718) and ATL (P = .298) patients. Wilcoxon signed-rank test was used for statistical analysis.
Expansion of iNKT cells by α-GalCer stimulation decreased the number of HTLV-1–infected T cells in ACs but not in HAM/TSP and ATL patients. (A) PBMCs from ACs (n = 6) and HAM/TSP (n = 11) and ATL patients (n = 5) were cultured with α-GalCer (100 ng/mL), and the percentage of iNKT cells on days 0, 7, 14, and 21 was plotted. (B) PBMCs from ACs (n = 6) and HAM/TSP (n = 11) and ATL patients (n = 4) were cultured for 14 days with or without α-GalCer, and the proviral load in each sample was quantified by real-time PCR. In ACs, the proviral load in PBMCs cultured with α-GalCer was significantly lower than that of cultured PBMCs grown without α-GalCer (P = .047). No significant difference was observed between HAM/TSP (P = .718) and ATL (P = .298) patients. Wilcoxon signed-rank test was used for statistical analysis.
Using real-time PCR, we also quantified the HTLV-1 proviral load in PBMCs from ACs (n = 6), HAM/TSP patients (n = 11), and ATL patients (n = 4) on day 14 of culture (Figure 5B). Notably, the proviral load in cultured PBMCs decreased (P = .047) only in samples from ACs, in which the number of iNKT cells increased on α-GalCer stimulation. However, in the samples from HAM/TSP and ATL patients, in which α-GalCer stimulation did not result in an increase in the number of iNKT cells, there was no difference in the proviral load of PBMCs grown in the presence or absence of α-GalCer.
iNKT cells functionally impaired in proliferation and perforin production are partially infected with HTLV-1 and CD4− iNKT cells are depleted in HTLV-1–infected persons
The poor capacity of iNKT cells from HAM/TSP and ATL patients (Figure 5A) to expand in response to α-GalCer stimulation suggested 2 possibilities: (1) the frequency of iNKT cells before α-GalCer stimulation is low in these patients, as demonstrated in Figure 1, or (2) the proliferative response of iNKT cells in these patients is impaired. Although these possibilities are not mutually exclusive, to address whether iNKT cells of these patients have impaired proliferative response, we cultured PBMCs from 3 HDs, 3 ACs, 3 HAM/TSP patients, and 3 ATL patients, each with or without α-GalCer, and counted the absolute number of iNKT cells on days 0 and 14. We calculated the proliferative response ratio (absolute number of iNKT cells on day 14/absolute number of iNKT cells on day 0). As shown in Figure 6A, the proliferative response ratio of the iNKT cells was lower in HAM/TSP and ATL patients than in HDs and ACs. It was also lower in ACs than in HDs. Further, we examined whether this low response in HAM/TSP and ATL patients could be restored by IL-2, as has previously been reported in the case of cancer patients.38 However, this was not possible (Figure 6A). Therefore, we conclude that the iNKT cells of patients with HTLV-1–associated disorders have impaired proliferation.
iNKT cells impaired in proliferation and perforin production are partially infected with HTLV-1 and CD4– iNKT cells are depleted in HTLV-1–infected persons. (A) Proliferative response ratio of iNKT cells. PBMCs from HDs (n = 3), ACs (n = 3), and HAM/TSP (n = 3) and ATL patients (n = 3) were cultured without any treatment, with 100 ng/mL α-GalCer, or with α-GalCer (100 ng/mL) + IL-2 (100 U/mL). The fold change in the number of iNKT cells was calculated as the ratio of the absolute number of iNKT cells on day 14 divided by the absolute number of iNKT cells on day 0. Data are represented as mean ± SEM. (B) Representative FACS histogram of perforin expression by iNKT cells from HDs, ACs, and HAM/TSP patients. PBMCs were stained for perforin, Vα24Jα18, and CD3, and the expression levels of perforin in CD3+Vα24Jα18+ cells are plotted. (C) Levels of perforin expression (mean fluorescence intensity [MFI]) by CD3+Vα24Jα18+ iNKT cells of HDs, ACs, and HAM/TSP patients. PBMCs from 7 HDs, 4 ACs, and 6 HAM/TSP and 2 ATL patients were stained for perforin, Vα24Jα18, and CD3, and the expression level of perforin in each CD3+Vα24Jα18+ cell was plotted. In 2 of the 6 HAM/TSP patients and 2 ATL patients, perforin expression could not be analyzed because the frequency of iNKT cells was low. The perforin expression levels were lower in iNKT cells of ACs (P = .038), and HAM/TSP patients (P = .038) were lower than in those of HDs. The Mann-Whitney U test was used for statistical analysis. The horizontal bar represents the mean value of each group. (D) The HTLV-1 proviral load in CD3+Vα24Jα18+ (iNKT) cells and CD3+Vα24Jα18− (CD3+ cells lacking iNKT cells) cells of 4 ACs and 1 HAM/TSP patient. Paired t test was used for statistical analysis; n.s. indicates not significant. (E) The percentage of CD4− iNKT cells among total iNKT cells. PBMCs from 6 HDs and 7 HTLV-1–infected persons (1 AC, 5 HAM/TSP patients, and 1 ATL patient with detectable iNKT cells) were stained for CD3, CD4, and Vα24Jα18, the percentage of CD4+/CD4− cells among CD3+Vα24Jα18+ iNKT cells was analyzed by FACS, and the percentage of CD4− iNKT cells among total iNKT cells of each person was calculated. CD4− iNKT cells were found to be dominantly depleted in HTLV-1–infected persons compared with HDs (P = .001 by Student t test).
iNKT cells impaired in proliferation and perforin production are partially infected with HTLV-1 and CD4– iNKT cells are depleted in HTLV-1–infected persons. (A) Proliferative response ratio of iNKT cells. PBMCs from HDs (n = 3), ACs (n = 3), and HAM/TSP (n = 3) and ATL patients (n = 3) were cultured without any treatment, with 100 ng/mL α-GalCer, or with α-GalCer (100 ng/mL) + IL-2 (100 U/mL). The fold change in the number of iNKT cells was calculated as the ratio of the absolute number of iNKT cells on day 14 divided by the absolute number of iNKT cells on day 0. Data are represented as mean ± SEM. (B) Representative FACS histogram of perforin expression by iNKT cells from HDs, ACs, and HAM/TSP patients. PBMCs were stained for perforin, Vα24Jα18, and CD3, and the expression levels of perforin in CD3+Vα24Jα18+ cells are plotted. (C) Levels of perforin expression (mean fluorescence intensity [MFI]) by CD3+Vα24Jα18+ iNKT cells of HDs, ACs, and HAM/TSP patients. PBMCs from 7 HDs, 4 ACs, and 6 HAM/TSP and 2 ATL patients were stained for perforin, Vα24Jα18, and CD3, and the expression level of perforin in each CD3+Vα24Jα18+ cell was plotted. In 2 of the 6 HAM/TSP patients and 2 ATL patients, perforin expression could not be analyzed because the frequency of iNKT cells was low. The perforin expression levels were lower in iNKT cells of ACs (P = .038), and HAM/TSP patients (P = .038) were lower than in those of HDs. The Mann-Whitney U test was used for statistical analysis. The horizontal bar represents the mean value of each group. (D) The HTLV-1 proviral load in CD3+Vα24Jα18+ (iNKT) cells and CD3+Vα24Jα18− (CD3+ cells lacking iNKT cells) cells of 4 ACs and 1 HAM/TSP patient. Paired t test was used for statistical analysis; n.s. indicates not significant. (E) The percentage of CD4− iNKT cells among total iNKT cells. PBMCs from 6 HDs and 7 HTLV-1–infected persons (1 AC, 5 HAM/TSP patients, and 1 ATL patient with detectable iNKT cells) were stained for CD3, CD4, and Vα24Jα18, the percentage of CD4+/CD4− cells among CD3+Vα24Jα18+ iNKT cells was analyzed by FACS, and the percentage of CD4− iNKT cells among total iNKT cells of each person was calculated. CD4− iNKT cells were found to be dominantly depleted in HTLV-1–infected persons compared with HDs (P = .001 by Student t test).
In assessing the characteristics of the iNKT cells of HTLV-1–infected persons, we were interested in not only their proliferative capacity but also the expression levels of perforin, which is important for their cytotoxicity. We analyzed the expression levels of perforin in iNKT cells from 7 HDs, 4 ACs, and 4 HAM/TSP patients (none of the ATL patients and 4 of the 6 HAM/TSP patients could be analyzed because the frequency of iNKT cells was low). As shown in Figure 6B and C, the expression levels of perforin were lower in iNKT cells from ACs and HAM/TSP patients than in those from HDs, suggesting that the iNKT cells of HTLV-1–infected persons have lower cytotoxicity.
It has been reported that iNKT cells are highly susceptible to HIV-1 infection, and direct infection may be the primary cause of their low number and poor function.39,40 Therefore, we analyzed HTLV-1 infection of iNKT cells from infected persons. Using PBMCs from 4 ACs and 1 HAM/TSP patient whose iNKT cell number was adequate for the assay, we separated CD3+Vα24Jα18+ (iNKT) and CD3+Vα24Jα18− (CD3+ cells without iNKT cells) cells by FACS and quantified the HTLV-1 proviral DNA load of each population by real-time PCR. As shown in Figure 6D, the proviral load in the iNKT cells (mean, 20.6 copies per 100 cells) was equivalent to that in the CD3+ lacking iNKT cells (mean, 21.2 copies per 100 cells). Thus, HTLV-1 infection of iNKT cells was demonstrated. However, iNKT cells were not found to be more favorable targets of HTLV-1 infection than conventional T cells.
We were also interested in determining the ratio of CD4+ and CD4− iNKT cells because it has been reported that the former may be more susceptible than the latter to HIV-1 infection and subsequent depletion39,40 Therefore, we measured CD4 expression on CD3+Vα24Jα18+ iNKT cells from 6 HDs and 7 HTLV-1–infected persons (1 AC, 5 HAM/TSP patients, and 1 ATL patient with detectable iNKT cells). In the HTLV-1–infected persons, CD4− iNKT cells were notably more dominantly depleted than CD4+ iNKT cells (Figure 6E).
Discussion
HTLV-1 causes 2 different diseases: HAM/TSP, a neuroimmunologic disorder, and ATL, a T-cell malignancy. Their common characteristic is the increased number of HTLV-1–infected cells (HTLV-1–infected leukemia cells in ATL).4,7 Furthermore, the high viral load in ACs increases the risk of developing HAM/TSP or ATL.4,7 These findings suggest that common mechanisms underlie the insufficient control of HTLV-1 infection in both diseases. iNKT cells have been implicated in infection control.41-43 To test the hypothesis that iNKT cells may contribute to the immune defense against HTLV-1, we first measured the frequency of iNKT cells among PBMCs from the study groups. We demonstrated, for the first time, that the frequency of iNKT cells was significantly decreased in HTLV-1–infected persons, especially HAM/TSP or ATL patients (Figure 1).
To investigate the mechanisms underlying the decrease in iNKT cells in HTLV-1–infected persons, we measured the frequency of DCs and analyzed the expression levels of CD1d and activation markers (CD40, CD80, CD86, and HLA-ABC) on DCs from the study subjects because DCs are essential for the in vivo activation of iNKT cells27,32 and mDCs induce activation by increasing the expression of CD1d on their cell surface.27 As shown in Figure 1D and E, the DC frequency was decreased in patients with HTLV-1–associated disorders, especially ATL patients. The decreased frequency of DCs in ATL patients is consistent with a previous report.44 We are the first to demonstrate the decreased DC frequency in HAM/TSP patients compared with HDs. Whereas the DC frequency in ATL patients was significantly lower, the expression level of CD1d on mDCs, monocytes, and B cells and that of activation markers (CD86 and HLA-ABC) on circulating DCs was similar among the study groups (Figure 3, supplemental Figure 3). These results suggest that the total number of CD1d-expressing mDCs and activated DCs is significantly decreased in ATL patients; this may be one of the mechanisms underlying the low frequency of iNKT cells in ATL patients. However, the total number of mDCs, and the expression level of CD1d and that of activation markers on mDCs, was equivalent between ACs and HAM/TSP patients (Figure 3), suggesting that the low number of iNKT cells in HAM/TSP patients is attributable to another mechanism.
Therefore, we next tested the hypothesis that HTLV-1 infection of DCs may result in the decreased frequency of iNKT cells, as has been reported for another human retrovirus, namely, HIV-1.36,37 The HTLV-1 proviral load was significantly higher in DCs from HAM/TSP patients than from ACs (Figure 4). To determine the effect of HTLV-1 on CD1d expression and DC activation, we statistically analyzed the correlation between the proviral load in DCs and the expression level of CD1d and DC-activation markers. We found no correlation among the subjects (Figure 4). Although no statistical significance could be demonstrated, we observed a tendency toward an inverse correlation between the HTLV-1 proviral load in and the expression level of CD1d on mDCs (Figure 4C). Further in vitro studies are necessary to investigate the effect of HTLV-1 infection on CD1d expression on mDCs. Moreover, the absence of a correlation between the proviral load in DCs and the expression level of CD1d and activation markers on circulating DCs suggests other mechanisms by which HTLV-1 infection affects the functioning, maturation, and/or viability of DCs. HTLV-1–induced alterations in the DC function have been reported: Monocyte-derived DCs infected in vitro are poor stimulators of autologous T-cell differentiation.45 Moreover, the ability of pDCs from ACs to produce type 1 interferon, an important antiviral mechanism of the innate immune system, is impaired.44 Further studies must address the effect of HTLV-1 infection on DC functions, especially the function to activate iNKT cells.
Recently, it has been reported that iNKT cells are highly susceptible to HIV-1 infection, and direct infection may be a primary cause of their depletion and functional impairment.39,40 Therefore, we quantified the rate of infection in iNKT cells in HTLV-1–infected persons to determine whether direct HTLV-1 infection is also an important mechanism underlying the decreased frequency and functional impairment of iNKT cells in such persons. As shown in Figure 6D, HTLV-1 was shown to infect iNKT cells to the same extent as conventional CD3+ T cells, suggesting that iNKT cells are partially but not selectively infected with HTLV-1. However, we cannot exclude the possibility that HTLV-1 can induce iNKT cell depletion through direct infection. Therefore, we next compared the frequency of CD4+ and CD4− iNKT cells between HDs and HTLV-1–infected persons (Figure 6E). Because HTLV-1 is known to preferentially infect CD4+ cells over CD4− cells,46 we hypothesized that the number of CD4+ iNKT cells might be preferentially decreased if direct HTLV-1 infection of iNKT cells causes their depletion, as has been reported in the case of HIV-1.39,40 However, contrary to our expectations, we clearly found that CD4− iNKT cells were selectively depleted in HTLV-1–infected persons, especially HAM/TSP and ATL patients (Figure 6E). These results suggest that direct HTLV-1 infection of iNKT cells is not the main cause of their depletion in infected persons. Recently, it has been reported that CD4− iNKT cells preferentially induce the Th1 response and are more important than CD4+ iNKT cells in controlling viral infection and cancers.47,48 The predominant depletion of CD4− iNKT cells in HAM/TSP and ATL patients (Figure 6E) may contribute to the common underlying mechanisms of insufficient control of HTLV-1 infection in both diseases.
iNKT cells are known to exhibit antiviral activity.49 We demonstrated an inverse correlation between the iNKT cell frequency and proviral load in the peripheral blood of HTLV-1–infected persons (Figure 2). In vitro stimulation of PBMCs by α-GalCer resulted in a decrease in the number of HTLV-1–infected T cells only in ACs, in whom the number of iNKT cells increased on α-GalCer stimulation (Figure 5). These results suggest that iNKT cells possess anti–HTLV-1 activity. There was no decrease in the proviral load in α-GalCer–stimulated PBMCs from HAM/TSP and ATL patients, in whom the number of iNKT cells did not increase on stimulation (Figure 5). This result further confirms the anti–HTLV-1 effect of iNKT cells. Moreover, iNKT cells in these patients may be functionally defective, as indicated by their low proliferative response on α-GalCer stimulation and low perforin production (Figure 6A). Because iNKT cells play a crucial role in DC- and NK-cell activation,29 the decreased number and the functional impairment of iNKT cells in HAM/TSP and ATL patients may be one cause of the decreased frequency of DCs and NK cells (Figure 1) in these patients. Thus, iNKT depletion in HAM/TSP and ATL patients directly and/or indirectly diminishes the immune defense against HTLV-1, resulting in the insufficient control of HTLV-1–infected cells, including leukemia cells, in patients with HTLV-1–associated disorders.
CD1d-restricted iNKT cells play an important role to protect infection as well as to control tumor immunity and to regulate the deleterious immune responses in autoimmune diseases.50 Therefore, the decreased frequency of iNKT cells in patients with HAM/TSP and ATL may underlie not only insufficient infected cell control but also the pathogenesis of ATL and HAM/TSP. Currently, evidence is accumulating about the potential use of iNKT-mediated immunotherapy as a clinical modulator in cancer and autoimmune disorders. iNKT cell–based immunotherapy using α-GalCer–loaded DCs can induce more efficient iNKT–cell activation; it has been well tolerated in clinical trials and effectively produces antitumor cytotoxic effects, leading to prolonged stable disease in some patients.51 Furthermore, α-GalCer–loaded tumor cells have recently been shown to induce the activation of iNKT cells and NK cells more efficiently.27 Moreover, IL-10–treated tolerogenic DCs can induce the regulatory function of iNKT cells, which enables them to control autoimmune diseases by suppressing deleterious immune responses.52 These findings indicate the potential usefulness of immunotherapy targeting iNKT cells as one of the clinical therapeutic strategies for treating ATL and HAM/TSP. However, iNKT cells in HAM/TSP and ATL patients demonstrated poor proliferation on in vitro α-GalCer stimulation (Figures 5–6). This impaired proliferation could not be restored by IL-2, as has been successful in some cancers38 (Figure 6A). These results suggest severe functional impairment of such iNKT cells. Therefore, α-GalCer–based therapy as a clinical modulator in HAM/TSP and ATL patients will be challenging. On the other hand, although the iNKT cells of ACs produced less perforin than those of HDs (Figure 6), the decreased proviral load after expansion of the iNKT cells by in vitro α-GalCer stimulation in ACs suggests that immunotherapy targeting iNKT cells will be potentially useful as a clinical therapeutic strategy to prevent HTLV-1–associated disorders.
In conclusion, this report highlights the ability of iNKT cells to reduce the HTLV-1 viral load in infected persons and suggests the importance of these cells in the pathogenesis of HAM/TSP and ATL. Our findings provide a platform to explore the potential use of α-GalCer as an immune modulator to prevent and/or treat HTLV-1–associated disorders. Studies on the implications of the loss of iNKT-cell subsets in the pathogenesis of HTLV-1 infection and associated diseases are underway in our laboratory.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. Takahashi, Y. Ishikura, Y. Kunitomo, and A. Une for technical assistance and Dr K. Arimura and members of the Department of Neurology and Geriatrics, Kagoshima University Graduate School of Medical and Dental Sciences, for useful support.
This work was supported in part by the Japan Society for the Promotion of Science (Grant-in Aid for Scientific Research); the Ministry of Education, Culture, Sports, Science and Technology, Japanese Ministry of Health, Labour, and Welfare (H21-NANCHI-IPPAN-008); National Institute of Biomedical Innovation; Uehara Memorial Foundation; Nagao Takeshi Nanbyo Foundation; Kanagawa Nanbyo Foundation; Mishima Kaiun Memorial Foundation; Takeda Science Foundation; ITSUU Laboratory Research Foundation; Kanae Foundation for Life and Socio-Medical Science; Japan Research Foundation for Clinical Pharmacology; Kanagawa Academy of Science and Technology (research grants); Japan College of Rheumatology; Nakajima Foundation; Osaka Foundation for Cancer Research; New Energy and Industrial Technology Development Organization; Mochida Pharmaceutical Company Ltd; Kanagawa High-Technology Foundation; Kanto Bureau of Economy, Trade and Industry; Mitsui Life Insurance Company Ltd; Heiwa Nakajima Foundation; Sagawa Foundation for Promotion of Cancer Research; and Tokyo Biochemical Research Foundation.
National Institutes of Health
Authorship
Contribution: K.A. and T.S. performed the experiments and data analysis and wrote the manuscript; Y.Y. developed the project, performed the experiments and data analysis, and wrote the manuscript; N.A., A.U., R.K., K.S., D.H., T.I., H.F., S.A., R.F., N.Y., and H.K. contributed to sample collection and preparation; and A.U., R.K., T.K., K.S., K.N., and T.N. reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshihisa Yamano, Department of Molecular Medical Science, Institute of Medical Science, St Marianna University School of Medicine, 2-16-1 Sugao, Miyamae-ku, Kawasaki, Kanagawa 216-8512, Japan; e-mail: yyamano@marianna-u.ac.jp.
References
Author notes
*K.A. and T.S. contributed equally to this work.



![Figure 3. Equivalent expression levels of CD1d on mDCs and lineage-positive (Lin+) cells from HDs, ACs, and HAM/TSP patients, and ATL patients. Plots of the expression level (mean fluorescence intensity [MFI]) of CD1d molecules on mDCs (A) and Lin+ cells (B) among PBMCs from HDs (n = 9), ACs (n = 7), HAM/TSP patients (n = 9), and ATL patients (n = 3). The horizontal bar represents the mean value for each group. Statistical analysis of the expression level for each study subject revealed no statistically significant differences by 1-way analysis of variance and Scheffé F test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/15/10.1182_blood-2009-02-203042/4/m_zh89990942790003.jpeg?Expires=1767890889&Signature=KhWe01eFtEc8K0sDNKW3KwR609IjYP8I0tZBW-ktlesE2eJNplCx-uDox1Fhqlmg07~I~1rOB5NVo2IT0ttkTDCFuabioTQbf5WJZkPBJTXn74ak1eqNALo62LqGnsy55vf41vL8OsK5gxDVyW-UddZSzimzTN~8MuDiMdzcJFr1FF~ikkqn95NVQRT80L2UHK2EHz4yiSHsJ4T6-Pm7ulcOFbId-sxiJDofqWEAavqvcncTBs7Xq7tlmpRsV4Beo6SCUF~o~if51yG5kgjWVsIBq7GBSPTmQyrczePuiYsJ8kHerD2Txq9ApOHodU6icQf5wNuEnrxbNGmYIp8zFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 6. iNKT cells impaired in proliferation and perforin production are partially infected with HTLV-1 and CD4– iNKT cells are depleted in HTLV-1–infected persons. (A) Proliferative response ratio of iNKT cells. PBMCs from HDs (n = 3), ACs (n = 3), and HAM/TSP (n = 3) and ATL patients (n = 3) were cultured without any treatment, with 100 ng/mL α-GalCer, or with α-GalCer (100 ng/mL) + IL-2 (100 U/mL). The fold change in the number of iNKT cells was calculated as the ratio of the absolute number of iNKT cells on day 14 divided by the absolute number of iNKT cells on day 0. Data are represented as mean ± SEM. (B) Representative FACS histogram of perforin expression by iNKT cells from HDs, ACs, and HAM/TSP patients. PBMCs were stained for perforin, Vα24Jα18, and CD3, and the expression levels of perforin in CD3+Vα24Jα18+ cells are plotted. (C) Levels of perforin expression (mean fluorescence intensity [MFI]) by CD3+Vα24Jα18+ iNKT cells of HDs, ACs, and HAM/TSP patients. PBMCs from 7 HDs, 4 ACs, and 6 HAM/TSP and 2 ATL patients were stained for perforin, Vα24Jα18, and CD3, and the expression level of perforin in each CD3+Vα24Jα18+ cell was plotted. In 2 of the 6 HAM/TSP patients and 2 ATL patients, perforin expression could not be analyzed because the frequency of iNKT cells was low. The perforin expression levels were lower in iNKT cells of ACs (P = .038), and HAM/TSP patients (P = .038) were lower than in those of HDs. The Mann-Whitney U test was used for statistical analysis. The horizontal bar represents the mean value of each group. (D) The HTLV-1 proviral load in CD3+Vα24Jα18+ (iNKT) cells and CD3+Vα24Jα18− (CD3+ cells lacking iNKT cells) cells of 4 ACs and 1 HAM/TSP patient. Paired t test was used for statistical analysis; n.s. indicates not significant. (E) The percentage of CD4− iNKT cells among total iNKT cells. PBMCs from 6 HDs and 7 HTLV-1–infected persons (1 AC, 5 HAM/TSP patients, and 1 ATL patient with detectable iNKT cells) were stained for CD3, CD4, and Vα24Jα18, the percentage of CD4+/CD4− cells among CD3+Vα24Jα18+ iNKT cells was analyzed by FACS, and the percentage of CD4− iNKT cells among total iNKT cells of each person was calculated. CD4− iNKT cells were found to be dominantly depleted in HTLV-1–infected persons compared with HDs (P = .001 by Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/15/10.1182_blood-2009-02-203042/4/m_zh89990942790006.jpeg?Expires=1767890889&Signature=qg8YWRg-vFj-b8TrGNDYBj0nmXhsEnXHjF9fU6aiAZkutXMD0dYbW1ASOdliI8afhnjvickUK2cIp4-qjvtmVpBanlHEE2gjuE0LwrVzfWaiwOG3SZGkMhABZlNVPt~4ZgiIUgnFAN-fNHenUsY3F-hNgSXNQ4h2RbTdcqqezhh0PnA1grcPhZgC8jMvh5WWZulGwmBuCsjBCaAyOQ2o1u8f~uEhXfS0BU1rv4gFKUOsrl8CKxXvTqkwOLjZTeLBAfrp2vh4X7Jc0~O1cwxhd6P4bf6aRa6Sfm5OK4fgx5aE0UooXBr7bKGAZVqBtOgynZRQhz6u2WZALM9ZJf9Jjw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 3. Equivalent expression levels of CD1d on mDCs and lineage-positive (Lin+) cells from HDs, ACs, and HAM/TSP patients, and ATL patients. Plots of the expression level (mean fluorescence intensity [MFI]) of CD1d molecules on mDCs (A) and Lin+ cells (B) among PBMCs from HDs (n = 9), ACs (n = 7), HAM/TSP patients (n = 9), and ATL patients (n = 3). The horizontal bar represents the mean value for each group. Statistical analysis of the expression level for each study subject revealed no statistically significant differences by 1-way analysis of variance and Scheffé F test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/15/10.1182_blood-2009-02-203042/4/m_zh89990942790003.jpeg?Expires=1767890890&Signature=MwJ7KT4KZQ15vsezQfdz37D7eaMvu~OulwK8la8n5~o5hvp-EskP11Q-xObpHhebZLEMsVcj34C7xSqHeoJ9S4n4h31zycP81FF9dcQALhvur6hwgHv2Ii4waWka7YdpqGKhf4qvLASx-jqKULOLlGi0ea2h6v6lnD4q0HIDkwokNx~GCsDpKCJLibZ18KdVlS176QOD~NzRFJ14vrnhoQhDnzWAHlTp78WCISThSvOAvvoYQSdlL0wiYUU3NdTSsauH-vvCTzkmyof1V9NoyXfUJbciEpENLGAYQnBEHRY0lO3GB-4pGWRElsvdlv-8ikV6d5jcOleR2By8rdvRNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. iNKT cells impaired in proliferation and perforin production are partially infected with HTLV-1 and CD4– iNKT cells are depleted in HTLV-1–infected persons. (A) Proliferative response ratio of iNKT cells. PBMCs from HDs (n = 3), ACs (n = 3), and HAM/TSP (n = 3) and ATL patients (n = 3) were cultured without any treatment, with 100 ng/mL α-GalCer, or with α-GalCer (100 ng/mL) + IL-2 (100 U/mL). The fold change in the number of iNKT cells was calculated as the ratio of the absolute number of iNKT cells on day 14 divided by the absolute number of iNKT cells on day 0. Data are represented as mean ± SEM. (B) Representative FACS histogram of perforin expression by iNKT cells from HDs, ACs, and HAM/TSP patients. PBMCs were stained for perforin, Vα24Jα18, and CD3, and the expression levels of perforin in CD3+Vα24Jα18+ cells are plotted. (C) Levels of perforin expression (mean fluorescence intensity [MFI]) by CD3+Vα24Jα18+ iNKT cells of HDs, ACs, and HAM/TSP patients. PBMCs from 7 HDs, 4 ACs, and 6 HAM/TSP and 2 ATL patients were stained for perforin, Vα24Jα18, and CD3, and the expression level of perforin in each CD3+Vα24Jα18+ cell was plotted. In 2 of the 6 HAM/TSP patients and 2 ATL patients, perforin expression could not be analyzed because the frequency of iNKT cells was low. The perforin expression levels were lower in iNKT cells of ACs (P = .038), and HAM/TSP patients (P = .038) were lower than in those of HDs. The Mann-Whitney U test was used for statistical analysis. The horizontal bar represents the mean value of each group. (D) The HTLV-1 proviral load in CD3+Vα24Jα18+ (iNKT) cells and CD3+Vα24Jα18− (CD3+ cells lacking iNKT cells) cells of 4 ACs and 1 HAM/TSP patient. Paired t test was used for statistical analysis; n.s. indicates not significant. (E) The percentage of CD4− iNKT cells among total iNKT cells. PBMCs from 6 HDs and 7 HTLV-1–infected persons (1 AC, 5 HAM/TSP patients, and 1 ATL patient with detectable iNKT cells) were stained for CD3, CD4, and Vα24Jα18, the percentage of CD4+/CD4− cells among CD3+Vα24Jα18+ iNKT cells was analyzed by FACS, and the percentage of CD4− iNKT cells among total iNKT cells of each person was calculated. CD4− iNKT cells were found to be dominantly depleted in HTLV-1–infected persons compared with HDs (P = .001 by Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/15/10.1182_blood-2009-02-203042/4/m_zh89990942790006.jpeg?Expires=1767890890&Signature=GzAQ5JeUPmuYoAgHKjpU9OHIl80ReWFPSfpuggN~-ODnslQDFV2iUqPXvFKmnRfHPLuVDzxzHZlLFmMs6Wo1FqW~GwmlADhyZmdOeep1EV0ZXWiw0qrOxMvsIfKB1yXbTjlHkXgXLXFOYyJe9vSfP56X87s5GhkQ5q5zvevTYE8Bxt0N5oD6S7vYfE0l8rEDvQ4TLEuYOSy9LpQ~ixeJrwj~B6EUX~vvl0Z9WqFZMDVDSVVuFtPXN93hR9CP8HTHPogxtE7-Inwc9-~xeRxZ2gOFimEBkq8yBmFUqSOjcrzCiU~h2KdlZxLs0B3YvXpff-jffDcXNEKr6UsG4q4KJw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)