Abstract
We previously reported that a dominant-positive activating mutation (Asn505) in the transmembrane domain (TMD) of c-MPL, which encodes the thrombopoietin receptor, caused familial essential thrombocythemia. Here, we show that the Asn505 mutation induces both autonomous dimerization of c-Mpl and signal activation in the absence of its ligand. Signal activation was preserved in a truncated mutant of Asn505 that lacked the extracellular domain of c-MPL. We also found that the substitution of the amino acid (AA) residue at position 505 with others of strong polarity (Glu, Asp, or Gln) also resulted in activated dimerization without ligand stimulation. Overall, these data show that the Asn505 mutation transduced the signal through the autonomous dimerization of the c-MPL protein due to strong AA polarity. This finding provides a new insight into the mechanism of disease causation by mutations in the TMD of cytokine/hematopoietic receptors.
Introduction
Cytokine/hematopoietic receptors possess a single transmembrane domain (TMD). Upon stimulation by ligand binding, some receptors transduce signals into the cytoplasm through dimerization and conformational changes in preformed dimers, and thereby activate a cascade of transphosphorylation signals, such as Jak2, Mek1/2, and Stat5b.1-8 The chemical properties of amino acid (AA) residues in the TMD are thought to play important roles in receptor dimerization and signal activation.8,9 Activating mutations in the TMD of cytokine/hematopoietic receptors have been identified because of oncogenic activity or as a cause of human diseases. For example, Val664Glu in neu/ErbB2-R10 acts as an oncogene, while Gly380Arg and Ala391Glu in fibroblast growth factor receptor 3 (FGFR3) cause achondroplasia and Crouzon syndrome with acanthosis nigricans.11,12
Onishi et al identified an activating mutation in the TMD of c-MPL using polymerase chain reaction (PCR)–driven random mutagenesis13 ; this mutation (Asn505) was demonstrated to be a cause of familial essential thrombocythemia (FET).14 However, it was unclear how Asn505 constitutively activated intracellular signaling. Here, we investigated autonomous dimerization in Asn505 and in a truncated mutant of the entire extracellular domain (ECD) of c-MPL. We also tested whether the substitution of AA residues at the mutated position 505 of c-MPL influenced dimerization and intracellular signaling.
Methods
Wild-type and mutant c-MPL were inserted into pCI-Neo vectors14 and used as templates for PCR amplification. The primers 5′-tactacgcgttccaccatggcctggatctccttggtga-3′ and 3′-tattggcagcagccttgagcggccgctact-5′ were designed to amplify the truncated mutants ΔSer505 and ΔAsn505 that lack the entire ECD, corresponding to AA 490-636 of c-Mpl. The products were inserted into pCI-Neo with MluI and NotI sites.
We also generated a range of c-MPL mutants at position 505 instead of wild-type Ser using the Quick-Change Multi Site-Directed Mutagenesis kit (Stratagene). The constructs were stably transfected into Ba/F3 cells, and their sequences and protein expressions were examined by direct sequencing and immunoblotting, respectively.14 The membrane localization of their c-MPL proteins were confirmed (Figure 1A-B).
c-MPL–expressing Ba/F3 cells were suspended in RPMI medium with 10% fetal bovine serum (FBS), starved for 6 hours to deplete them of IL-3, and stimulated with or without 100 ng/mL human thrombopoietin (TPO; Diaclone Research). The cells were washed with phosphate-buffered saline (PBS) and incubated with or without BS3 (Bis [sulfosuccinimidyl] suberate) or DSG (disuccinimidyl glutarate; Pierce) as a cross-linker for 2 hours at 4°C. Cell lysates were resolved in 2× lysis buffer (Sigma-Aldrich).15 After SDS-PAGE, the membranes were incubated with anti–c-MPL antibody (Upstate Biotechnology), and subsequently with anti–rabbit horseradish peroxidase (HRP; Santa Cruz Biotechnology).
An MTT assay was conducted to estimate the numbers of viable cells.14 The phosphorylation status of MEK1/2 and STAT5 in the c-MPL mutants was determined by immunoblotting without or with IL-3 or TPO.14 Phospho-Mek1/2, Mek1/2, phospho-Stat5, and Stat5 antibodies (Cell Signaling Technology) were used to detect the respective phosphorylation signals.
Results and discussion
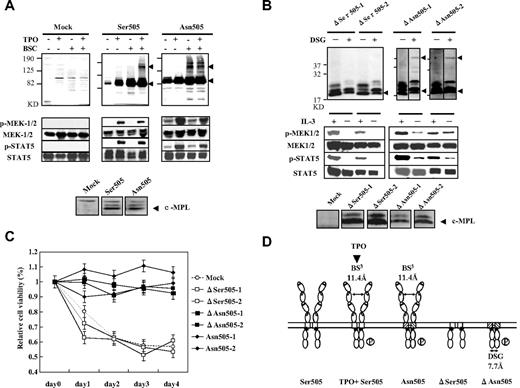
Homodimerization and conformational changes in receptors have been shown to be essential for signal activation in some cytokine/hematopoietic receptors.1-8 Thus, we evaluated homodimerization in the various c-MPL–expressing Ba/F3 cells under nonreducing conditions with the chemical cross-linker BS3. In Tpo-free conditions, Asn505-expressing cells, but not Ser505-expressing cells, contained a band double the size of the c-MPL monomer (Figure 1A). The detection of dimerized bands depended on the presence of BS3, suggesting that dimerization was not due to a disulfide bond. TPO-independent phosphorylation of MEK1/2 and STAT5 was detected by the occurrence of the dimerized bands.
The relationship of autonomous homodimerization and signal activation to cell-survival capacity in Asn505 and ΔAsn505 mutants of c-Mpl. (A) Detection of homodimers and phosphorylation of intracellular signals in the Asn505 mutant of c-MPL. The “mock” group comprised Ba/F3 cells with the PCI-Neo vector, and acted as the negative control. Ser505 and Asn505 indicate Ba/F3 cells expressing wild-type (Ser505) or Asn505, respectively. The cross-linking assay was conducted under nonreducing conditions to detect dimers resulting from disulfide or hydrogen bonds. Noncleavable, water-soluble BS3 was used as a cross-linker to detect the homodimers. (The reaction product is an amine-containing compound with a spacer length of 11.4 Å.) The c-MPL monomeric (∼ 85 kDa) and dimerized bands (∼ 170 kDa) are indicated by ◀. The dimerized band was detected in Asn505-expressing Ba/F3 cells in the absence of TPO (top panel). The experiments were repeated using reducing conditions (with 5% 2-mercaptoethanol in the sample buffer) and gave the same results as under nonreducing conditions (data not shown). The phosphorylation status of MEK1/2 and STAT5 is shown in the middle panel. Phosphorylation of MEK1/2 and STAT5 was observed independently of TPO stimulation in Asn505-expressing cells, although the phosphorylated signals in TPO-free conditions were weaker than those in the presence of TPO. The bottom panel shows the expressions of the c-MPL in the membrane fractions, which were separated using Mem-PER Eukaryotic membrane protein extraction reagent kit (Thermo Scientific) in immunoblotting. The c-MPL expressions in the whole cell lysates, the membrane fractions, and the cytoplasmic fractions were examined, respectively, and only the gel images of the membrane fractions were picked up and aligned here. (B) Detection of homodimers and phosphorylation of intracellular signals in the truncated c-MPL. The ΔSer505-1 and ΔSer505-2 mutants have truncation of the entire ECD of the wild-type (Ser505) of c-MPL, while the ΔAsn505-1 and ΔAsn505-2 mutants have truncation of the entire ECD of the Asn505 mutants of c-MPL. The anti–c-MPL antibody (Upstate Biotechnology), used here for detection of dimerization, recognizes the C-terminal of c-MPL. Noncleavable membrane-permeable DSG was used as a cross-linker to detect homodimers of the ECD-truncated c-MPL mutants. (The reaction product is an amine-containing compound with a spacer length of 7.7 Å.) The cross-linking assay was conducted in the absence of factor (IL-3 or TPO) stimulation. The truncated c-MPL monomers (∼ 20 kDa) and dimers (∼ 40 kDa) are indicated by ◀. Dimerized bands were detected in both ΔAsn505-1 and ΔAsn505-2, but not in either of the ΔSer505 mutants under nonreducing conditions (top panel). Vertical lines have been inserted to indicate a repositioned gel lane. MEK1/2 and STAT5 showed phosphorylation in both ΔAsn505 clones even in the absence of IL-3 stimulation (middle panel). The bottom panel shows the expressions of the truncated c-MPL in the membrane fractions in immunoblotting (see legend for panel A). The truncated c-MPL expressions in the whole cell lysates, the membrane fractions, and the cytoplasmic fractions were examined, respectively, and only the gel images of the membrane fractions were picked up and aligned here. (C) MTT assay of the c-MPL mutants without factor stimulation. Briefly, transfected Ba/F3 cells were washed in PBS and resuspended in RPMI medium containing 10% FBS. A total of 5 × 104 cells per well in 100 μL medium were plated in 96-well plates. A total of 10 μL thiazolyl blue tetrazolium bromide (5 mg/mL; Sigma-Aldrich) was used for daily estimation of the relative cell viability (%) over a period of 4 days. The results are shown as means ± SEM of 3 separate experiments. Asn505-1 and Asn505-2 are Ba/F3 transfectants expressing Asn505. (D) Schematic diagram of Ser505, Asn505, ΔSer505, and ΔAsn505 mutants of c-MPL, and the results of phosphorylation of intracellular signals. Two different cross-linkers, BS3 and DSG, were used here to detect homodimer formation; the lengths of the spacer are indicated (↔). A circled “P” indicates phosphorylation of intracellular signals (MEK1/2 and STAT5) with homodimer formation.
The relationship of autonomous homodimerization and signal activation to cell-survival capacity in Asn505 and ΔAsn505 mutants of c-Mpl. (A) Detection of homodimers and phosphorylation of intracellular signals in the Asn505 mutant of c-MPL. The “mock” group comprised Ba/F3 cells with the PCI-Neo vector, and acted as the negative control. Ser505 and Asn505 indicate Ba/F3 cells expressing wild-type (Ser505) or Asn505, respectively. The cross-linking assay was conducted under nonreducing conditions to detect dimers resulting from disulfide or hydrogen bonds. Noncleavable, water-soluble BS3 was used as a cross-linker to detect the homodimers. (The reaction product is an amine-containing compound with a spacer length of 11.4 Å.) The c-MPL monomeric (∼ 85 kDa) and dimerized bands (∼ 170 kDa) are indicated by ◀. The dimerized band was detected in Asn505-expressing Ba/F3 cells in the absence of TPO (top panel). The experiments were repeated using reducing conditions (with 5% 2-mercaptoethanol in the sample buffer) and gave the same results as under nonreducing conditions (data not shown). The phosphorylation status of MEK1/2 and STAT5 is shown in the middle panel. Phosphorylation of MEK1/2 and STAT5 was observed independently of TPO stimulation in Asn505-expressing cells, although the phosphorylated signals in TPO-free conditions were weaker than those in the presence of TPO. The bottom panel shows the expressions of the c-MPL in the membrane fractions, which were separated using Mem-PER Eukaryotic membrane protein extraction reagent kit (Thermo Scientific) in immunoblotting. The c-MPL expressions in the whole cell lysates, the membrane fractions, and the cytoplasmic fractions were examined, respectively, and only the gel images of the membrane fractions were picked up and aligned here. (B) Detection of homodimers and phosphorylation of intracellular signals in the truncated c-MPL. The ΔSer505-1 and ΔSer505-2 mutants have truncation of the entire ECD of the wild-type (Ser505) of c-MPL, while the ΔAsn505-1 and ΔAsn505-2 mutants have truncation of the entire ECD of the Asn505 mutants of c-MPL. The anti–c-MPL antibody (Upstate Biotechnology), used here for detection of dimerization, recognizes the C-terminal of c-MPL. Noncleavable membrane-permeable DSG was used as a cross-linker to detect homodimers of the ECD-truncated c-MPL mutants. (The reaction product is an amine-containing compound with a spacer length of 7.7 Å.) The cross-linking assay was conducted in the absence of factor (IL-3 or TPO) stimulation. The truncated c-MPL monomers (∼ 20 kDa) and dimers (∼ 40 kDa) are indicated by ◀. Dimerized bands were detected in both ΔAsn505-1 and ΔAsn505-2, but not in either of the ΔSer505 mutants under nonreducing conditions (top panel). Vertical lines have been inserted to indicate a repositioned gel lane. MEK1/2 and STAT5 showed phosphorylation in both ΔAsn505 clones even in the absence of IL-3 stimulation (middle panel). The bottom panel shows the expressions of the truncated c-MPL in the membrane fractions in immunoblotting (see legend for panel A). The truncated c-MPL expressions in the whole cell lysates, the membrane fractions, and the cytoplasmic fractions were examined, respectively, and only the gel images of the membrane fractions were picked up and aligned here. (C) MTT assay of the c-MPL mutants without factor stimulation. Briefly, transfected Ba/F3 cells were washed in PBS and resuspended in RPMI medium containing 10% FBS. A total of 5 × 104 cells per well in 100 μL medium were plated in 96-well plates. A total of 10 μL thiazolyl blue tetrazolium bromide (5 mg/mL; Sigma-Aldrich) was used for daily estimation of the relative cell viability (%) over a period of 4 days. The results are shown as means ± SEM of 3 separate experiments. Asn505-1 and Asn505-2 are Ba/F3 transfectants expressing Asn505. (D) Schematic diagram of Ser505, Asn505, ΔSer505, and ΔAsn505 mutants of c-MPL, and the results of phosphorylation of intracellular signals. Two different cross-linkers, BS3 and DSG, were used here to detect homodimer formation; the lengths of the spacer are indicated (↔). A circled “P” indicates phosphorylation of intracellular signals (MEK1/2 and STAT5) with homodimer formation.
Previous studies showed that the ECD of cytokine receptors mediated dimerization16,17 and controlled receptor activity.18 Thus, we generated 2 c-MPL mutants, ΔSer505 and ΔAsn505, in which the entire ECD was deleted. The ΔAsn505 mutant displayed homodimerization with the membrane-permeable cross-linker DSG and phosphorylation of MEK1/2 and STAT5 independently of IL-3 stimulation (Figure 1B). Cells carrying ΔAsn505 survived (but did not proliferate) in factor-free conditions, similarly to the Asn505 mutant (Figure 1C). These data indicate that the Asn505 mutation induced autonomous homodimerization and intracellular activation independently of growth factors and the ECD of c-MPL (Figure 1D).
The functions of the cytoplasmic domain and the ECD of c-MPL have been described in various reports.15,19,20 Our observation that ΔSer505 did not induce constitutive activation (Figure 1B) is of interest because Sabath et al previously showed that deletion of the distal ECD (CRM-1) induced constitutive activation independently of TPO stimulation.18 We also found that ΔAsn505 had weaker phosphorylation of intracellular signals (Figure 1B), suggesting that the ECD of c-MPL may contribute to the stabilization of dimerization and signaling. The precise function of the ECD of c-MPL will be confirmed with further analysis using such mutants.
In the Asn505 mutant, MEK1/2 and STAT5 were phosphorylated more weakly without TPO stimulation than with TPO (Figure 1A), and cell survival rather than proliferation was observed without TPO. Under TPO stimulation, the proliferation capacity of the Asn505 mutant was not significantly different from the wild-type (Ser505; data not shown); this is consistent with another study.13 These findings suggest that Asn505 is not a fully constitutive activating mutation and does not have hypersensitivity to TPO; these characteristics might explain the relatively mild phenotype of patients with FET.14 Asn505 may not only promote dimerization of c-MPL but also change the conformation/rotation of dimer to an active form, as is the case with EPOR,1,2,21 EGFR,3 and GHR.22 Further study is required to clarify whether preformed dimers of c-MPL exist on the membrane.
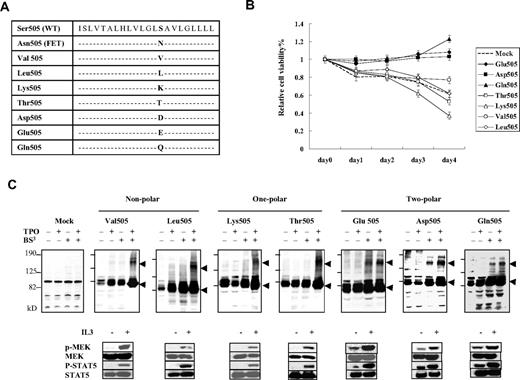
The Asn residue in the TMD of receptors was previously shown to induce interhelical hydrogen bonds with strong polarity.8,9 Thus, we hypothesized that autonomous dimerization in Asn505 was induced by hydrogen bonding due to its strong polarity. To test this, we examined dimerization in various Ba/F3/c-Mpl mutants in which Ser505 was substituted with residues with different polarities: 2-polar Glu, Asp, or Gln; 1-polar Lys or Thr; and nonpolar Val or Leu (Figure 2A). The mutants with 2-polar residues presented factor-independent cell survival and constitutive phosphorylation of MEK1/2 and STAT5 with dimer formation, but the mutants with a 1-polar or nonpolar residue did not show such effects (Figure 2B-C). These results strongly suggest that autonomous dimerization of c-MPL in Asn505 was induced by hydrogen bonding due to strong AA polarity.
The relationship of autonomous homodimerization and signal activation to cell-survival capacity in the artificially generated TMD mutants of c-MPL. (A) A list of the AA substitution mutants generated at position Ser505 of c-MPL. All mutants were transfected into Ba/F3 cells. (B) MTT assay of the artificial TMD mutants of c-MPL in the absence of factor stimulation. Cells transfected with mutants with 2-polar residues (Glu, Asp, or Gln) showed factor-independent cell survival. Mock and Ba/F3 cells were transfected with PCI-Neo vector. The results are shown as means ± SEM of 3 separate experiments. (C) Autonomous homodimerization and signal activation of the artificial TMD mutants of c-MPL. The top panel shows the detection of homodimers in immunoblots under nonreducing conditions. BS3 was used as the cross-linker. ◀ indicate monomers (∼ 85 kDa) and dimers (∼ 170 kDa). The bottom panel depicts the phosphorylation status of MEK1/2 and STAT5 in the mutants of c-MPL with or without IL-3 stimulation. Only mutants carrying 2-polar residues (Glu, Asp, or Gln) showed autonomous homodimerization and phosphorylation of MEK1/2 and STAT5 in the absence of factor stimulation.
The relationship of autonomous homodimerization and signal activation to cell-survival capacity in the artificially generated TMD mutants of c-MPL. (A) A list of the AA substitution mutants generated at position Ser505 of c-MPL. All mutants were transfected into Ba/F3 cells. (B) MTT assay of the artificial TMD mutants of c-MPL in the absence of factor stimulation. Cells transfected with mutants with 2-polar residues (Glu, Asp, or Gln) showed factor-independent cell survival. Mock and Ba/F3 cells were transfected with PCI-Neo vector. The results are shown as means ± SEM of 3 separate experiments. (C) Autonomous homodimerization and signal activation of the artificial TMD mutants of c-MPL. The top panel shows the detection of homodimers in immunoblots under nonreducing conditions. BS3 was used as the cross-linker. ◀ indicate monomers (∼ 85 kDa) and dimers (∼ 170 kDa). The bottom panel depicts the phosphorylation status of MEK1/2 and STAT5 in the mutants of c-MPL with or without IL-3 stimulation. Only mutants carrying 2-polar residues (Glu, Asp, or Gln) showed autonomous homodimerization and phosphorylation of MEK1/2 and STAT5 in the absence of factor stimulation.
We reviewed all the reported activating mutations in the TMD of cytokine receptors (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article) and found that most contain AA substitutions that result in the replacement of nonpolar or 1-polar AA with 2-polar AA. Thus, these mutations appear to have a similar effect to Asn505 in c-MPL. The exceptions were the mutations of a 1-polar Arg residue in FGFR223 and FGFR3.11 Arg380 of FGFR3 predominantly showed ligand-dependent dimerization and overexpression with a selective delay in the down-regulation of the mutant receptor,11 suggesting a different mechanism from Asn505. Our future studies will include 3-D structural analyses,24,25 which should be valuable for understanding the precise mechanism by which Asn505 exerts its effects.
Overall, the Asn505 mutation of c-MPL, a cause of FET, activates intracellular signals through homodimerization in a factor-independent manner. Homodimerization resulted from hydrogen bonding due to the strong polarity of the Asn residue. Our results have provided a new insight into the mechanism of activating mutations in the TMD of cytokine/hematopoietic receptors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Chiori Fukuyama and Chiharu Nakagawa for excellent technical assistance.
This work was supported by the Japan Society for the Promotion of Science (J. D.) and the Grants-in-Aid for Scientific Research (no. 17591004 to H.K.) in Japan.
Authorship
Contribution: J.D. designed and performed the experiment and wrote the paper; H.K., S.I., and M.N. designed research and discussed the data; H.Y., A. Inagaki, F.M., M.R., and A. Ito generated the expression vectors of c-MPL; S.K., T.I., and A.W. contributed to the design of the research and discussed the data; and R.U. guided the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hirokazu Komatsu, Department of Medical Oncology and Immunology, Graduate School of Medical Sciences, Nagoya City University; 1-Kawazumi Mizuho-ku, Nagoya, 467-8601, Japan; e-mail: komatsu@med.nagoya-cu.ac.jp.