Abstract
Acute lymphoblastic leukemia (ALL) diagnosed in the first month of life (congenital ALL) is very rare. Although congenital ALL is often assumed to be fatal, no studies have been published on outcome except for case reports. The present study reports the outcome of 30 patients with congenital ALL treated with the uniform Interfant-99 protocol, a hybrid regimen combining ALL treatment with elements designed for treatment of acute myeloid leukemia. Congenital ALL was characterized by a higher white blood cell count and a strong trend for higher incidence of MLL rearrangements and CD10-negative B-lineage ALL compared with older infants. Induction failure rate was 13% and not significantly different from that in older infants (7%, P = .14), but relapse rate was significantly higher in congenital ALL patients (2-year cumulative incidence [SE] was 60.0 [9.3] vs 34.2 [2.3], P < .001). Two-year event-free survival and survival of congenital ALL patients treated with this protocol was 20% (SE 9.1%). Early death in complete remission and treatment delays resulting from toxicity were not different. The survival of 17% after last follow-up, combined with a toxicity profile comparable with that in older infants, justifies treating congenital ALL with curative intent. This trial was registered at www.clinicaltrials.gov as no. NCT 00015873, and at www.controlled-trials.com as no. ISRCTN24251487.
Introduction
Acute lymphoblastic leukemia (ALL) in infants (up to 1 year of age) is known to be biologically different from ALL in older children diagnosed with ALL. ALL in infants is more often associated with a higher tumor load at diagnosis,1,2 a rearrangement in the mixed lineage leukemia (MLL) gene, and very immature B-cell phenotype (pro-B ALL) without CD10 expression.1-3 Infant ALL cells are more resistant to several standard chemotherapeutic agents,3,4 and the disease is also characterized by a poorer prognosis compared with older children.5-14
Congenital ALL is diagnosed at birth or within the first month of life and is very rare. Although it is assumed to be inevitably fatal and the toxicity of the chemotherapeutic agents in these very young infants is unclear, to the best of our knowledge, no series have been published on congenital ALL except for case reports. Bresters et al15 reviewed 24 patients with congenital ALL diagnosed over 25 years who were described in case reports: all patients died.
We recently reported the results of a large international collaborative trial, Interfant-99, in infants younger than 1 year with ALL.14 Here, we detail the outcome and characteristics of 30 patients with congenital ALL who received uniform therapy with curative intent.
Methods
Patients
The Interfant-99 trial design, the inclusion criteria, and recruitment methods have been published earlier.14 Individual study groups obtained ethics approval from all participating institutions, and patient informed consent was obtained in accordance with the Declaration of Helsinki. Of the 518 infants diagnosed with ALL, which account for approximately 3% of the ALL population, 35 patients were younger than 1 month, confirming the rarity of congenital ALL (∼ 2 children per every 1000 with ALL). The present study reports on 30 cases that were treated with Interfant-99 (Figure 1).
Procedures
Enrolled patients were stratified into standard-risk and high-risk groups on the basis of their response to 1 week of daily systemic prednisone (at a dose of 60 mg/m2) and 1 intrathecal dose of methotrexate. Patients were classified as standard risk if their peripheral blood blast count was less than 1000 cells per microliter at day 8 and high risk if the blast count was equal to or greater than 1000 per microliter.4,7 Patients were tested for MLL gene rearrangement with split-signal fluorescent in situ hybridization, polymerase chain reaction, or both. Absence of MLL rearrangement was defined as a negative split signal for fluorescent in situ hybridization.
The Interfant-99 treatment was a hybrid regimen combining standard ALL treatment with elements designed for treatment of acute myeloid leukemia. The protocol consisted of multiagent phases of induction and consolidation chemotherapy, followed by maintenance treatment with antimetabolites.16 Doses were adjusted according to patients' ages at the start of each treatment phase: children younger than 6 months were given two-thirds of the full dose, and children 6 to 12 months of age received three-fourths of the full dose. Total treatment duration was 104 weeks.
The induction phase consisted of a standard 4-drug induction: dexamethasone, vincristine, daunorubicin, and L-asparaginase (Escherichia coli) with the addition of low-dose cytarabine.16 The MARAM (6-mercaptopurine, methotrexate, leucovorin rescue, prednisone, cytarabine, L-asparaginase) phase was a consolidation course17 that included high-dose cytarabine and high-dose methotrexate. OCTADD (dexamethasone, 6-thioguanine, vincristine, daunorubicin, cytarabine, prednisone, cyclophosphamide) was a reinduction block derived from the consolidation phase of the Berlin-Frankfurt-Münster trials for treatment of acute myeloid leukemia,18 except that prednisone was replaced by dexamethasone. Standard-risk patients were given a maintenance phase of oral 6-mercaptopurine and methotrexate, combined with pulses of dexamethasone and vincristine and intrathecal methotrexate with steroid in the first 3 cycles. High-risk patients were given standard maintenance therapy intensified with pulses of cytarabine and etoposide in the first 3 cycles. Patients were randomly assigned to receiving an extra late intensification (VIMARAM) phase (similar to the MARAM maintenance block but with the addition of vincristine).17 In case of availability of a suitable donor, high-risk patients were eligible for allogenic bone marrow transplantation. Only one congenital ALL patient underwent a bone marrow transplantation instead of maintenance therapy. For more details on the treatment protocol, see Pieters et al.14
Statistical analysis
The Interfant-99 trial database was used for analysis. The Fisher exact test was applied to investigate the association between age group (congenital ALL patients vs older infants) and both patients' characteristics and rate of induction failures. The Wilcoxon test was used to compare durations of treatment phases between age groups. Endpoints were early death (during induction); resistance to induction (ie, no complete remission [CR] at the end of the induction phase); relapse; death in CR; and second malignancy. Outcome measures were event-free survival (EFS), defined as the time from diagnosis to any one of the endpoints and survival, defined as time to death from any cause. Time was censored at the latest follow-up available if no events were recorded. Follow-up was updated in December 2007. EFS and survival curves were computed with the Kaplan-Meier estimator and their SE with the Greenwood formula. The log-rank test was used for univariate comparisons. A one-step Cox model was applied to estimate the hazard of relapse for congenital ALL compared with older infants, adjusting for relevant factors. The probabilities of relapse and death in CR were estimated by applying the cumulative incidence estimator, which accounts for competing risks, and were compared according to Gray.18 All tests were 2-sided. SPSS 16.0 statistical software (SPSS Inc), SAS 8.2 package (SAS Institute), and R statistical software (http://www.R-project.org) were used for data analysis.
Role of the funding source
The many foundations that collaborated in and supported this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Patient characteristics
Thirty-five congenital ALL patients were eligible to enter the Interfant-99 protocol. Five patients did not enter for various reasons (Figure 1), all of whom died, respectively, at the day of diagnosis, 2 days after diagnosis, 1 month after diagnosis (2 patients), and 1 patient who was treated died 5 months after diagnosis (2 weeks after relapse). Table 1 shows the distribution of relevant characteristics in 30 patients with congenital ALL and in the remaining 452 infants treated with Interfant-99. The percentage of patients with a poor response to prednisone was not significantly different between the congenital ALL group (39%) and the older infants 1 to 12 months of age (30%) (P = .29). In addition, sex and central nervous system involvement did not differ between these 2 groups (P = .71 and P = .48, respectively). Congenital ALL cases more often presented with a high white blood cell (WBC) count (P = .01): only 23% had a WBC count at diagnosis less than 100 × 109/L compared with 46% in the group 1 to 12 months of age. There was a trend toward a higher incidence of a CD10−, B-lineage immunophenotype in the congenital ALL group (76%) compared with the older patients (62%) (P = .09) and for a higher incidence (P = .09) of MLL rearrangement in congenital ALL (93%) versus older patients (78%). The distribution of the different MLL fusion partners did not differ (P = .53). In both age groups, t(4;11) was the most common type of translocation (congenital ALL 48% vs 53% in older patients). A t(11;19) was found in 32% of congenital ALL patients, whereas this was seen in 19% of older infants.
Outcome
Outcomes are described for 482 enrolled patients overall (30 with congenital ALL; Table 2), with a median follow-up time from diagnosis of 58 months (range, 1-102 months). Nine patients were diagnosed at the age of 0 to 7 days and 21 patients at the age of 8 to 30 days. One congenital ALL patient (diagnosed at the age of 24 days) and 2 older patients died during the first week of prednisone treatment (and could therefore not be further stratified in the standard- or high-risk group). Two of 30 congenital ALL patients (7%, diagnosed both at the age of 0-7 days) died during the induction phase, whereas 16 of 452 (4%) older patients died during induction. One congenital ALL patient did not achieve CR at the end of induction and died thereafter (age at diagnosis, 0 days), whereas 11 patients were resistant among the older infants (8 died). The induction failure rate in congenital cases (13%) was not significantly different from that in older patients (7%, P = .14).
Nineteen of 26 (73%) congenital ALL patients had a relapse after achieving CR, whereas relapses in the infants of 1 to 12 months were 176 of 423 (42%) (P < .001). All relapses in congenital ALL patients presented in the bone marrow, and none was detected in the central nervous system. Ten relapses occurred within 6 months from CR, 6 between 7 and 12 months, and the remaining 3 after 1 year (of whom only 1 after the end of therapy, at 2.6 years). All relapsed patients died. The cumulative incidence (SE) of relapse in the congenital ALL patients was 60.0 (9.3) at 2 years, whereas the corresponding value for older infants with ALL was 34.2 (2.3; P < .001). Results of the multivariable Cox model indicate that congenital ALL cases had a significantly higher risk of relapse than older infants (hazard ratio = 2.4; 95% confidence interval [CI], 1.5-3.9, P < .001), even after adjusting for known prognostic factors (WBC count at diagnosis, MLL gene rearrangement, and response to prednisone) and other characteristics (sex, immunophenotype).
Two of 26 (8%) congenital ALL patients in CR died from toxicity: 1 from a brain abscess and 1 from septic complications (2 and 5 months from diagnosis, respectively). A total of 23 deaths (9%) occurred in older ALL infants. The cumulative incidence (SE) of death in CR at 2 years was 6.7 (4.7) and 5.1 (1.0) in congenital ALL and older ALL infants, respectively (P = .70).
Five patients (17%) with congenital ALL were still in CR at last follow-up, after 29 up to 103 months from diagnosis. No second malignancies were diagnosed among the congenital ALL patients.
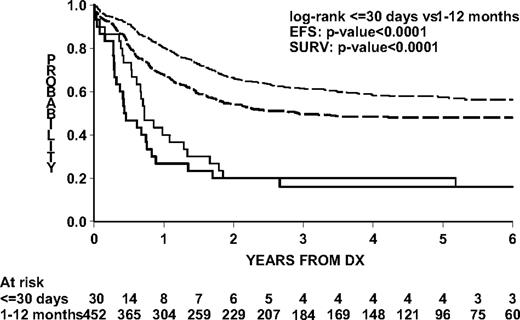
EFS and survival for congenital ALL were significantly lower than for older infants (Figure 2). Two-year EFS was 20.0% (SE 9.1; 95% CI, 2.2-37.8) and 54.2% (SE 2.4; 95% CI, 49.5-58.9) and overall 2-year survival was 20.0% (SE 9.1; CI, 2.2-37.8) and 66.4% (SE 2.4; 95% CI, 62.1-70.7) for congenital ALL and older infants with ALL, respectively.
Outcome by age group in the Interfant-99 protocol. Log-rank test for difference in EFS and survival between congenital ALL patients and ALL patients 1 to 12 months of age. Continuous lines represent congenital ALL patients; dashed lines, ALL patients 1 to 12 months of age; thick lines (both groups), EFS; thin lines (both groups), survival.
Outcome by age group in the Interfant-99 protocol. Log-rank test for difference in EFS and survival between congenital ALL patients and ALL patients 1 to 12 months of age. Continuous lines represent congenital ALL patients; dashed lines, ALL patients 1 to 12 months of age; thick lines (both groups), EFS; thin lines (both groups), survival.
As expected, a treatment delay, as an indirect measure for toxicity, was observed in all phases of chemotherapy in congenital ALL patients as well as in older infants (Table 3). Nineteen congenital ALL patients and 335 infants 1 to 12 months of age of whom data were available had a median induction phase delay of 1 versus 0.3 weeks, respectively. The consecutive phases, consolidation and reinduction, were started with a median delay in congenital ALL patients of 2.1 weeks (1.8 weeks in older infants), 1.6 weeks (2.1 weeks in older infants), and 2.8 weeks (2.7 weeks in older infants). None of these differences in treatment delay between the 2 groups was statistically significant.
Discussion
The limited, single case report based, literature to date suggested that congenital ALL was invariably fatal. The present study is the first that reports on a series of 30 patients with congenital ALL treated uniformly with curative intent. The Interfant database includes all diagnosed infants with ALL, even if they were not treated or not treated according to protocol. The present analysis demonstrates that the outcome, although worse than in older infants, is not inevitably fatal. A total of 17% of the congenital ALL patients were still alive at last follow-up.
Resistance to prednisone prephase did not differ between the neonates and older infants. Neither was induction failure or mortality in CR significantly higher in the congenital ALL cases compared with older infants. Because of the small sample size, lack of significance in this study does not prove equivalence; therefore, results should be interpreted with caution. However, patients with congenital ALL did have a significantly higher relapse rate. Congenital ALL was characterized by a significantly higher WBC count, a trend toward a higher incidence of MLL gene rearrangements, and a CD10− B-lineage immunophenotype than ALL in older infants. These biologic characteristics have all been associated with a dismal outcome, as also a very young age per se has been established as a risk factor for an inferior outcome.14 Nevertheless, a multivariate analysis showed that congenital ALL cases had a higher relapse rate also after adjustment of risk factors other than age. Possibly, because of fear for severe side effects in these young patients, the reduced doses administered to these patients contribute to the higher relapse rate.
As there is evidence for a prenatal initiation of acute leukemia in young patients,19 we realize the definition of congenital ALL defined as diagnosed within the first month of life is arbitrary. The definition is based on previous literature (eg, Bresters et al15 ), although the type of MLL rearrangement shifts as infants are diagnosed later. A total of 91% of infants younger than 6 months had MLL rearrangements compared with 66% of infants 6 to 12 months of age. Two-thirds of patients with t(4;11) or t(11;19) were younger than 6 months at diagnosis compared with one-third of patients with t(9;11).14 A longer latency from initiation to diagnosis, and by this possibly a less aggressive form of leukemia, could be in part an explanation for the better outcome of the older infants with MLL-rearranged ALL.
Improvement of outcome for the congenital ALL patients is still urgently needed. New treatment strategies are under current investigation to improve outcome of infants with ALL. Unpublished observations of the Interfant-99 trial suggest that infants with MLL rearrangements, younger age, and a very high WBC might benefit from stem cell transplantation. In the current Interfant-06 study, different early intensification strategies are studied to prevent early bone marrow relapses. New therapeutic targets have to be identified by unraveling the biology of MLL-rearranged ALL. Phase 1/2 trials with FLT3 inhibitors20 are currently being initiated in infant MLL-rearranged ALL.
In conclusion, the current study shows a survival of 17% for congenital ALL and a toxicity profile comparable with that in older infants. This proves that congenital ALL is not invariably fatal and justifies treatment with curative intent. For now, the treatment to be used should be the same as for older infants, but their poor outcome also necessitates the testing of newer approaches, such as new strategies to prevent early relapses as tested in the ongoing Interfant-06 and new targeted treatments as FLT3 inhibitors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
M.H.v.d.L. is supported by KiKa. The many participating institutes of all study groups are acknowledged for their support. This study in Associazione Italiana Ematologia Oncologia Pediatrica is partially supported by Associazione Italiana Ricerca sul Cancro Regional Grant Cod 1105 (to A.B. and M.G.V.). The international study operating center was partially supported by Fondazione Tettamanti and Comitato MM Verga (to M.G.V. and P.D.L.). T.S. was supported by the Polish Ministry of Science and Higher Education (grant 2 P054 095 30). Czech Paediatric Haematology was supported by the Czech Ministry of Education (grant MSM0021620813). Berlin-Frankfurt-Münster Germany was supported by Deutsche Krebshilfe.
M.H.v.d.L. is a PhD student at the research laboratory of Pediatric Oncology of the Erasmus University, and this work is submitted in partial fulfillment of the requirement for the PhD.
Authorship
Contribution: R.P., P.D.L., and M.G.V. designed and planned the study; M.H.v.d.L., P.D.L., M.G.V., and R.P. wrote the report; M.H.v.d.L. and P.D.L. were in charge of data pooling, data checking, reporting, and analyses; M.G.V. was the study statistician; A.M., G.J., T.M.L., M.F., A.B., M.C., I.H., J.E.R., J.S., T.S., A.V., A.F., L.H., and L.B.S. have commented on and approved the final version of the report; and all authors coordinated the study in their own countries.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete listing of participating study group centers appears in the supplemental Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Rob Pieters, Erasmus MC-Sophia Children's Hospital, Department of Paediatric Oncology and Haematology, Dr Molewaterplein 60, 3015GJ Rotterdam, The Netherlands; e-mail: rob.pieters@erasmusmc.nl.