Abstract
Tumor-induced immune suppression can permit tumor cells to escape host immune resistance. To elucidate host factors contributing to the poor response of adoptively transferred tumor-reactive cytotoxic T lymphocytes (CTLs), we used a systemic model of murine acute myeloid leukemia (AML). AML progression resulted in a progressive regulatory T-cell (Treg) accumulation in disease sites. The adoptive transfer of in vitro–generated, potently lytic anti–AML-reactive CTLs failed to reduce disease burden or extend survival. Compared with non–AML-bearing hosts, transferred CTLs had reduced proliferation in AML sites of metastases. Treg depletion by a brief course of interleukin-2 diphtheria toxin (IL-2DT) transiently reduced AML disease burden but did not permit long-term survival. In contrast, IL-2DT prevented anti-AML CTL hypoproliferation, increased the number of transferred CTLs at AML disease sites, reduced AML tumor burden, and resulted in long-term survivors that sustained an anti-AML memory response. These data demonstrated that Tregs present at AML disease sites suppress adoptively transferred CTL proliferation, limiting their in vivo expansion, and Treg depletion before CTL transfer can result in therapeutic efficacy in settings of substantial pre-existing tumor burden in which antitumor reactive CTL infusion alone has proven ineffective.
Introduction
Acute myeloid leukemia (AML) with unfavorable cytogenetics has a poor outcome, even when treated with aggressive chemotherapy.1,2 With chemoradiotherapy and hematopoietic stem cell transplantation, a graft-versus-leukemia effect can be observed even in patients with unfavorable cytogenetics.3 Although donor lymphocyte infusion (DLI) given as adoptive immunotherapy after hematopoietic stem cell transplantation has improved the outcomes of certain types of leukemia,4-6 for patients with AML, DLI has been less effective likely due at least in part to its rapid tumor progression.7,8 As a result of the anti-AML effects of DLI and with the observation that antitumor-specific cytotoxic T cells (CTLs) can be generated in vitro from cancer patients, adoptive CTL therapy has been proposed for decades as cancer treatment.9-14 However, the adoptive transfer of anti–AML-reactive CTLs alone has not solved the problem of AML disease recurrence.15 In patients with chronic myelogenous leukemia, a complete remission was achieved in a patient with accelerated-phase disease after adoptive treatment with leukemia-reactive CTLs.16 In rodents with minimal disease, CTL adoptive transfer also has not been uniformly curative despite the early transfer of large numbers of anti–AML-reactive CTLs.17,18 Enhancing CTL function in vivo via the administration of supportive cytokine therapy such as interleukin-2 (IL-2),19 IL-12,20 and interferons21 can improve antitumor efficacy but has been associated with substantial side effects. Therefore, recent studies have focused on eliminating the suppressive factors in the tumor environment to circumvent CTLs from inhibition.22,23
T-regulatory cells (Tregs) are important regulators of immune responses in transplantation,24,25 allergy,26,27 and autoimmune disease.28,29 In AML patients, the frequency of Tregs was noted to be significantly higher compared with healthy persons, likely due to increased proliferation.30 Human AML cells have been noted to favor the conversion of CD4+25− T cells into Tregs via modulation of tryptophan catabolism.31 Tregs that have been recruited to, or converted either before migration into or within the tumor environment, can have a profound inhibition on T cell–mediated immune response.32 Multiple mechanisms have been defined to be responsible for the suppression, including secretion of transforming growth factor-β33,34 and IL-10,33,34 as well as inhibition of dendritic cell (DC) maturation.33,35 Despite the great potential for Treg depletion in cancer therapies, the efficacy has been limited to prophylactic settings where depletion of Tregs is given before the establishment of tumor.36,37
The present studies were undertaken to determine whether endogenous Tregs present at the site of AML dissemination constrained the antileukemia efficacy of anti-AML CTL adoptive transfer in a rodent model as a prelude to future clinical trials. We observed that AML progression correlated with increased Tregs at the sites of AML disease. Increased Tregs in these sites resulted in decreased proliferation and interferon γ (IFN-γ) secretion of adoptively transferred CTLs. The efficacy of adoptive CTL therapy given to mice with advanced AML disease was markedly improved by prior depletion of Tregs using IL-2 diphtheria toxin (IL-2DT). Treg depletion by IL-2DT restored the proliferation of adoptively transferred CTLs and reduced leukemia burden in the liver and spleen of mice, resulting in a significant increase in the survival of leukemia-bearing mice. Together, these results indicate that the increase in Tregs induced by AML reduces the antileukemia efficacy of adoptively transferred anti–AML-reactive CTLs and suggest that IL-2DT may prove to be promising in improving adoptive CTL therapy for advanced AML disease.
Methods
Mice
C57BL/6 (termed B6, H2b, CD45.2) mice and congenic B6-Ly5.2 mice (H2b, CD45.1), 7 to 12 weeks old at study, were obtained from the National Institutes of Health (NIH). Rag−/− mice were obtained from The Jackson Laboratory. Mice were housed in microisolator cages under specific pathogen-free conditions. All experiments were conducted under approved protocols of the Division of Pediatrics at the University of Minnesota Masonic Cancer Center.
AML cells
C1498, obtained from the ATCC, is an AML cell line that developed spontaneously in B6 mice. C1498FFDsR, stable transfectants of C1498 that express the fluorescent Discoma coral–derived protein DsRed218 and firefly luciferase,38 were prepared using a nonviral vector delivery system as described and used to document tumor burden.
Bone marrow–derived DC isolation and AML lysate pulsing
Bone marrow (BM) was harvested from the femurs and tibiae of mice as described previously.39 Briefly, single-cell suspension was prepared and incubated in Dulbecco modified Eagle complete media with murine granulocyte macrophage colony-stimulating factor (150 units/mL; R&D Systems) for 10 or 7 days at 37°C and 10% CO2. DCs were cultured for 2 additional days with murine interleukin-4 (100 units/mL; R&D Systems) and CpG oligodeoxynucleotides (3 μg/mL) for activation. Day-12 and day-9 DCs were incubated with freeze-thawed tumor lysate as previously described.18 After 4 hours of incubation, DCs were harvested, washed 3 times in RPMI to remove the lysate, and used for T-cell priming.
In vitro generation, activation, and expansion of AML-reactive CTLs
B6 mouse bone marrow–derived DCs pulsed with C1498 AML lysate were added to naive splenocytes in the presence of murine IL-15 (50 ng/mL; AMGEN). After 5 days, cells were harvested and replated with RPMI complete media (RPMI-c) containing refreshed IL-15 and cultured for 2 days before second coculture with AML lysate–pulsed DCs for another 5 days. After a total of 12 days in culture, cells were further activated in the presence of anti-CD3ϵ (hybridoma 145-2C11; kindly provided by Dr Jeffrey Bluestone, Memorial Sloan-Kettering Cancer Center, NY)/anti-CD28 (hybridoma 37.51; provided by Dr Jim Allison, University of California, Berkeley) monoclonal antibody (mAb)–coated beads at a 1:1 bead-to-cell ratio produced as previously reported.18 IL-2 (20 IU/mL; AMGEN) and IL-7 (5 ng/mL; R&D Systems) were added. The anti-CD3/CD28 beads were removed after 2 days of culture. Cells were then expanded using IL-15, IL-2, and IL-7 at the given concentrations for 2 more days and harvested 16 days after initial culture for analysis and injection.
Flow cytometric analysis
Cells (106) were washed and incubated with α-FC receptor (CD16/CD32; BD PharMingen) at 4°C for 10 minutes to block nonspecific binding of fluorochromes. One or more of the following directly conjugated antibodies (BD PharMingen) were incubated with CTLs at 4°C for 30 minutes: CD8α–fluorescein isothiocyanate (FITC); CD11b-FITC, CD44–phycoerythrin (PE); CD69-PE; NK1.1-PE; TRAIL-PE; Fas-PE; CD4–peridinin-chlorophyll-protein complex–cyanin 5.5 (Cy5.5); and CD62L–allophycocyanin (APC); CD25-APC; CD19-APC; CD11c-APC. For detection of intracellular IFN-γ in CTLs, monensin solution (eBiosciences) was added and cultured for 5 hours without additional stimulation. Cells were then labeled with CD8α-FITC, fixed and permeabilized with FACSlyse (Caltag), and labeled with IFN-γ–PE. Cells were washed in phosphate-buffered saline (PBS) containing 2% fetal bovine serum and analyzed using the FACSCalibur (Becton Dickinson) and were gated on 10 000 live events. For Tregs, cells were further gated on CD4+ cells. For cell counts, 50 μL counting beads was added to each tube before reading on a flow cytometer. A ratio of beads to cells was measured for each sample, and cell number was calculated with given counting bead concentration.
In vitro CTL killing assay
The cytolytic activity of the generated CTL product was measured with JAM assay, well established previously40 by measuring DNA fragmentation of target cells. Briefly, C1498FFDsR and EL-4 cells were pulsed with 3H-thymidine (5 μCi/mL [0.185 MBq]) overnight. On the day of assay, 3H-labeled target cells were harvested, washed with RPMI-c, counted, and adjusted to 105 cells/mL in RPMI-c. CTLs (106) in 100 μL RPMI-c were plated in a 96-well tissue culture plate and made in 8 2-fold serial dilutions. C1498FFDsR or EL-4 cells (104) in 100 μL RPMI-c were then added to each well. RPMI-c (200 μL) was used as blank controls and 100 μL RPMI-c with 100 μL C1498FFDsR or EL-4 cells was used as tumor only controls. The cells were incubated at 37°C, 5% CO2 for 5 hours before they were harvested and counted with a β-plate reader. The percentage of killing of C1498FFDsR or EL-4 target cells was calculated and presented as the mean percentage of specific killing of triplicate samples (± SD) from a representative experiment.
Treg suppression assay
Tregs were isolated from spleens and lymph nodes (LNs) of either naive or AML-bearing mice 25 days after tumor injection by magnetic-activated cell sorting column selection (Miltenyi) and enriched by sorting the CD4+CD25+ fraction (FACSDiva; BD Biosciences). For in vitro suppression assay, responder CD44−CD8+ T cells from OT I mice (kindly provided by Dr Kris Hogquist, University of Minnesota) were isolated and enriched by magnetic-activated cell sorting column selection. Splenocytes from naive mice were irradiated (3000 cGy) and used as antigen-presenting cells. OT I responder cells (106) were stimulated with 106 irradiated splenocytes in RPMI-c and 100 nM SIINFEKL peptide. Tregs (106) from naive or AML-bearing mice were added to the culture, and RPMI-c was added to the controls. Six days after coculture at 37°C, 5% CO2, cell supernatant was harvested and IFN-γ level in the cell supernatant was determined by using Luminex technology (R&D Systems).
For in vivo suppression assay, 106 CTLs with or without 106 Tregs isolated either from naive or AML-bearing mice were adoptive transferred into Rag−/− mice injected with 2 × 104 C1498 cells 7 days before the adoptive transfer. Thirteen days after the CTL injection, spleens and livers were harvested, and single-cell suspension was prepared. Leukocytes from livers were isolated with discontinuous Percoll gradient centrifugation (Sigma-Aldrich). Cells were stimulated in vitro with anti-CD3 and IL-2 in the presence of monensin (eBiosciences) for 5 hours at 37°C, 5% CO2. Cells were then labeled with CD8α–peridinin-chlorophyll-protein complex and IFN-γ–APC before measured using the FACSCalibur. Cells were gated on 10 000 live events and percentage of IFN-γ–producing CD8+ CTLs was measured.
Diphtheria toxin reagents
Human IL-2DT, containing the first 390 amino acids of diphtheria toxin, and its control, human anti-CD3ϵ diphtheria toxin (ctrl DT), were generated, purified, and tested by D.A.V. as previously reported.41
In vivo CTL adoptive transfer and assessment of proliferation
C1498FFDsR cells were injected into B6 mice via an intravenous route at a lethal dose (106 cells/mouse) on day 0. IL-2DT or ctrl DT was given 4 and 13 days at 1 μg/dose in 0.2 mL PBS via the intraperitoneal route. CTLs were infused intravenously into the leukemia-bearing animals on day 14 at a dose of 30 × 106/mouse in 0.5 mL RPMI. Mice were monitored for survival or killed days 18, 20, and 25 for immune parameter analysis.
In vivo CTL proliferation was measured by 5-bromo-2-deoxyuridine (BrdU) incorporation. Briefly, IL-2DT– or ctrl DT–treated C1498FFDsR-bearing B6 mice were injected intravenously with congenic CD45.1 CTLs (30 × 106/dose) 14 days after tumor injection. BrdU (Sigma-Aldrich) was provided in the drinking water throughout the duration of the study. Four mice per group were killed and spleens, bone marrow, and livers were harvested 18 and 25 days after C1498FFDsR injection. Single-cell suspensions were made. Liver leukocytes were isolated with discontinuous Percoll gradient centrifugation. BrdU incorporation was measured according to the manufacturer's manual (BD Pharmingen).
Bioluminescent imaging studies
A Xenogen IVIS imaging system (Caliper Life Sciences) was used for live animal imaging. AML-bearing mice were anesthetized with 0.25 mL pentobarbital (1:10 diluted in PBS). Firefly luciferin substrate (0.1 mL; 5 mg/mL in PBS; Caliper Life Sciences) was injected intraperitoneally and the IVIS imaging was performed immediately after substrate injection. Data were analyzed and presented as photon counts per area.
Immunofluorescence imaging
Spleens and livers from mice were harvested and cryopreserved in optimal cutting temperature medium (Sakura Finetek USA Inc) at −80°C. Six-micrometer thick frozen sections were mounted on positively charged glass slides and fixed in acetone for 5 minutes at room temperature. Slides were blocked with undiluted normal horse serum then incubated with an AB blocking kit (Vector Labs). These tissues were stained with primary antibodies biotin rat anti–mouse-FoxP3 and CD4-FITC, (eBiosciences), rabbit anti–mouse DsRed (Clontech), CD45.1-FITC and intercellular adhesion molecule-1 (ICAM-1)–FITC (Abcam), and CD31-PE (BD PharMingen). Secondary antibody streptavidin Cy3 was used with the FoxP3, and donkey anti–rabbit Cy3 was used in combination with DsRed (Jackson Immunoresearch). Slides were examined by confocal microscopy using Olympus BX51 FluoView 500 confocal microscope and FluoView software. All images were taken at 60×/1.45 oil objective.
Statistics
The Kaplan-Meier product-limit method was used to calculate survival curve. Differences between groups in survival studies were determined using log-rank statistics. One-way analysis of variance with posthoc Tukey test was used to determine significant differences between each group in bar graph. P values of .05 or less were considered significant.
Results
Phenotype of CTLs generated and activated in vitro
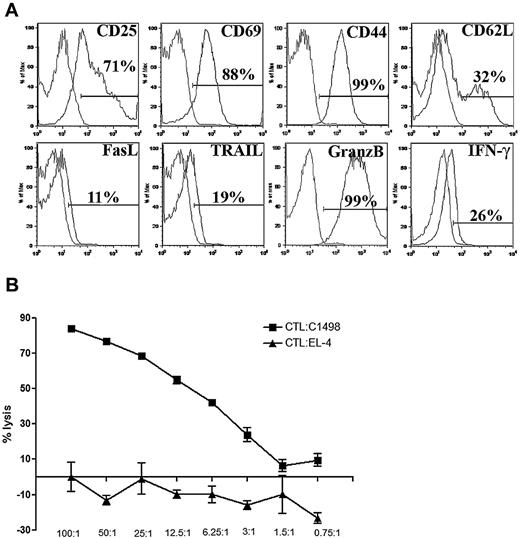
AML-reactive CTLs were generated in vitro from B6 splenocytes by twice stimulating with syngeneic AML lysate–pulsed bone marrow–derived DCs and then expanding with anti-CD3/CD28 mAb-coated magnetic beads in the presence of exogenous cytokines as described. After 16 days of culture, more than 90% of cells are CD8+ T cells (data not shown). Approximately 5% of cells were natural killer cells. B cells, CD4+ T cells, CD11b+ cells, and CD11c+ cells each comprised less than 1% of final CTL culture and no Tregs were detected (data not shown). CTLs were activated as evidenced by high CD25 and CD69 expression and had an effector memory T-cell phenotype denoted by high CD44 and low CD62L expression (Figure 1A). All CTLs expressed granzyme B and approximately 20% expressed IFN-γ without in vitro restimulation. A minority of CTLs expressed low levels of FasL and TRAIL (Figure 1A). To determine whether CTLs were capable of eliminating AML targets, a CTL lytic assay was performed in vitro. Approximately 50% lysis at a ratio of 12.5:1 was seen after 5 hours of incubation (Figure 1B). Specificity of lysis was determined by comparing with EL-4, a different B6 hematopoietic malignancy (T-cell leukemia/lymphoma) cell line (Figure 1B).
Phenotype of in vitro cultured and activated CTLs. (A) CTLs were harvested on day 16 of in vitro cell culture. Flow cytometric analysis of CTLs was performed. Cells were gated on CD8 expression. CTLs displayed an activated and effector memory phenotype. (B) Lysis of C1498FFDsR target or irrelevant tumor target, EL-4, was performed with CTLs 16 days after in vitro culture for 5 hours. CTLs were capable of killing the AML target but not the EL-4 cells in vitro. Error bars represent SD.
Phenotype of in vitro cultured and activated CTLs. (A) CTLs were harvested on day 16 of in vitro cell culture. Flow cytometric analysis of CTLs was performed. Cells were gated on CD8 expression. CTLs displayed an activated and effector memory phenotype. (B) Lysis of C1498FFDsR target or irrelevant tumor target, EL-4, was performed with CTLs 16 days after in vitro culture for 5 hours. CTLs were capable of killing the AML target but not the EL-4 cells in vitro. Error bars represent SD.
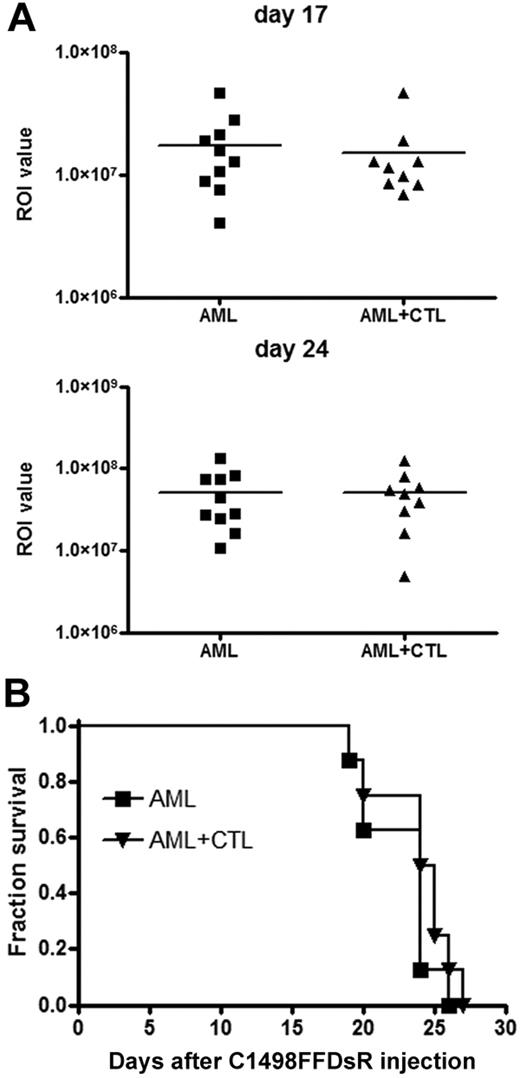
To assess the antitumor effect of CTLs in vivo, 30 × 106 CTLs were adoptively transferred into mice challenged with live AML cells 14 days earlier. Despite the high efficiency observed in vitro, anti–AML-reactive CTLs failed to reduce leukemia burden as measured by bioluminescent imaging (BLI) (Figure 2A) or provide a survival advantage against AML-induced lethality in this model (Figure 2B). Metastases were present in multiple organs including the liver, spleen, ovaries, lymph nodes (LNs), blood, and brain (data not shown). Thus, adoptive CTL transfer had no substantial impact on tumor burden or survival in mice with advanced AML disease.
CTLs fail to control advanced AML disease in vivo. B6 mice (10 mice/group) were injected with 106 C1498FFDsR cells through an intravenous route 14 days before being infused with 30 × 106 CTLs. Mice were then monitored for survival. Infusion of CTLs 14 days after tumor injection showed no reduction of AML burden (A, ■ vs ▴) or survival advantage (B, ■ vs ▴). Results from 1 of 5 representative experiments are shown.
CTLs fail to control advanced AML disease in vivo. B6 mice (10 mice/group) were injected with 106 C1498FFDsR cells through an intravenous route 14 days before being infused with 30 × 106 CTLs. Mice were then monitored for survival. Infusion of CTLs 14 days after tumor injection showed no reduction of AML burden (A, ■ vs ▴) or survival advantage (B, ■ vs ▴). Results from 1 of 5 representative experiments are shown.
The frequency of tumor-localized Tregs was inversely correlated with the proliferation and absolute number of adoptively transferred CTLs present in sites of AML metastases
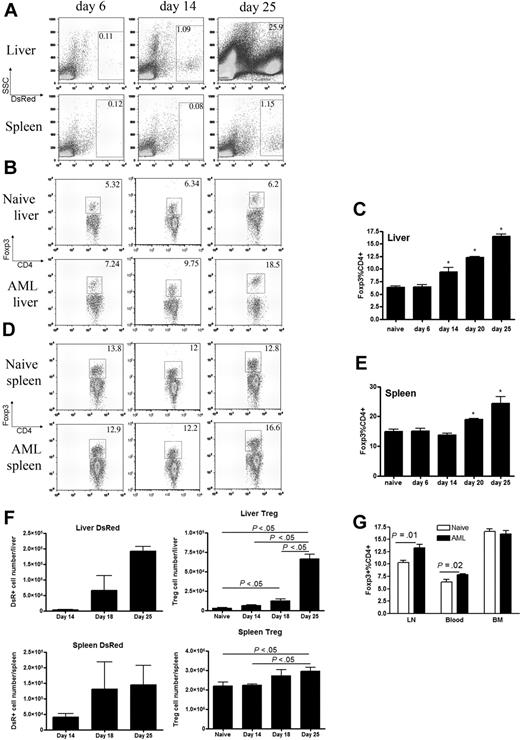
Tregs are known contributors to immune suppression in the tumor environment,32 and the presence of Tregs is often correlated with aggressive disease in cancer patients.42,43 Therefore, studies were performed to determine whether a higher Treg frequency existed at the sites of AML dissemination. Live animal whole-body BLI, coupled with necropsy studies, indicated that the liver and spleen were the primary initial sites of AML seeding in this model. To physically track AML progression in mice by flow cytometry, we took the advantage of the incorporated DsRed gene expression in the C1498 cell line used. Leukemia cells were readily detected at 14 days after injection in the liver and at 25 days after injection in the spleens (Figure 3A). The frequency of Foxp3+ Tregs increased in the liver and spleen in parallel to that observed for AML cells. Thus, by 14 days after injection, the frequency of Tregs in the livers was significantly higher than noninjected controls (Figure 3B-C). Similarly, by day 20 days after AML injection, the frequency of Tregs was significantly higher in the spleen compared with noninjected controls (Figure 3D-E). The absolute numbers of Tregs in the liver and spleen also increased as AML disease burden progressed in those organs over time (Figure 3F). Furthermore, an increased percentage of Tregs was also seen in other sites of AML, including lymph nodes and peripheral blood 25 days after AML injection (Figure 3G). This phenomenon was not shown in the bone marrow (Figure 3G) by day 25, possibly due to limited AML presence in the bone marrow at this time point (data not shown).
AML progression results in increased Tregs present at the primary sites of AML metastases. B6 mice were injected intravenously with 106 C1498FFDsR cells and killed 6, 14, or 25 days after tumor injection. Flow cytometric analysis was performed on liver leukocytes and splenocytes. (A) Increased percentage of DsR+ tumor cells was found in the livers and spleens of mice. (B-C) An increased percentage of Foxp3+ Tregs in the CD4+ T-cell population was found in the livers of mice, shown in flow dot plot (B) and bar graph (C, P < .05). (D-E) An increased percentage of Foxp3+ Tregs in the CD4+ T-cell population was found in the spleens of mice, shown in flow dot plot (D) and bar graph (E, P < .05). (F) An increased number of tumor cells was correlated with an increased number of Treg cells in the livers and spleens of mice (P < .05). (G) An increased percentage of Tregs was found in the LNs (P < .05) and blood (P < .05) but not in the BM. Results from 1 of 3 representative experiments are shown. Error bars represent SD.
AML progression results in increased Tregs present at the primary sites of AML metastases. B6 mice were injected intravenously with 106 C1498FFDsR cells and killed 6, 14, or 25 days after tumor injection. Flow cytometric analysis was performed on liver leukocytes and splenocytes. (A) Increased percentage of DsR+ tumor cells was found in the livers and spleens of mice. (B-C) An increased percentage of Foxp3+ Tregs in the CD4+ T-cell population was found in the livers of mice, shown in flow dot plot (B) and bar graph (C, P < .05). (D-E) An increased percentage of Foxp3+ Tregs in the CD4+ T-cell population was found in the spleens of mice, shown in flow dot plot (D) and bar graph (E, P < .05). (F) An increased number of tumor cells was correlated with an increased number of Treg cells in the livers and spleens of mice (P < .05). (G) An increased percentage of Tregs was found in the LNs (P < .05) and blood (P < .05) but not in the BM. Results from 1 of 3 representative experiments are shown. Error bars represent SD.
Tregs have been shown to decrease in vitro T-cell proliferation in response to antigenic stimulation.24 To determine whether the proliferation of exogenous transferred CTLs was reduced in the presence of Tregs induced by AML, congenic anti–AML-reactive CTLs (CD45.1+) were generated and infused into mice 14 days after leukemia challenge. Mice were supplied with BrdU in their drinking water, which is incorporated into the DNA of proliferating cells. CD45.1 and CD8 expression was used to distinguish exogenous versus endogenous CTLs by FACs. Compared with naive mice without AML, the proliferation of CTLs was significantly decreased in the spleens and livers of AML-bearing mice when measured early (day 4) after infusion (Figure 4A). Thus, despite the anticipated increase in CTL proliferation that might have occurred due to encounter of anti–AML-reactive CTLs with their target antigen, CTL proliferation was decreased and appeared to correlate with increased Treg numbers in leukemia-bearing mice. To verify the suppressive function of AML-induced Tregs on CTLs, Tregs were isolated from naive or AML-bearing mice and adoptively transferred to AML-bearing Rag−/− mice with CTLs. Thirteen days after transfer, the percentage of IFN-γ–producing CTLs was significantly decreased in the spleen and liver of AML-Treg transferred mice. Moreover, the suppression capacity of Tregs isolated from AML-bearing mice was superior to those from naive mice, indicating AML-induced Tregs were more activated (Figure 4B-C). To determine whether AML-associated Tregs were specific in suppressing T-cell functions after activation by leukemia cells, an in vitro suppression assay was performed with OT I CD8+ T cells stimulated with SIINFEKL peptide. Consistent with another study on virus-induced Tregs,44 Tregs isolated from leukemia-bearing mice could suppress OT I CD8+ T cells evidenced by decreased IFN-γ levels found in the cocultured cell supernatant. However, naive Tregs were not capable of suppressing OT I CD8+ T cells in the absence of in vitro activation stimuli (Figure 4D). This indicates that leukemia-associated Tregs have the capacity to inhibit CD8+ T-cell response nonspecifically after activation by AML.
AML-associated Tregs reduced proliferation and IFN-γ secretion of adoptive transferred CTLs. (A) B6 mice were injected with 106 C1498FFDsR cells. Congenic B6-ly5.2 (CD45.1+) CTLs (30 × 106) were infused through an intravenous route 14 days after tumor injection or to naive mice. BrdU was added to the drinking water to track proliferation. Eighteen days after tumor injection, 4 mice per group were killed. Flow Cytometric Analysis (FACs) was done with liver leukocytes and splenocytes. Tumor-bearing mice had significantly reduced proliferation of CTLs compared with naive mice in the livers and spleens (P < .05). (B-C) CTLs (106) and Tregs (106) isolated from AML-bearing or naive mice were adoptively transferred to AML-bearing Rag−/− mice. Thirteen days after transfer, FACs was performed on splenocytes (B) and liver leukocytes (C). Cells were gated on CD8 expression for CTLs. Intracellular IFN-γ expression was measured on gated CTLs. Tregs from AML-bearing mice significantly reduced the percentage of IFN-γ–producing CTLs in the spleen and liver. (D) Tregs isolated from AML-bearing or naive mice were cocultured with OT I CD8+ T cells stimulated with SIINFEKL peptide (Ovap) for 6 days. Cell supernatant was harvested and the IFN-γ level was determined. Tregs from AML-bearing mice inhibited IFN-γ production by OT I CD8+ T cells (P < .01), whereas naive Tregs were not suppressive. Results from 1 of 2 representative experiments are shown. Error bars represent SD.
AML-associated Tregs reduced proliferation and IFN-γ secretion of adoptive transferred CTLs. (A) B6 mice were injected with 106 C1498FFDsR cells. Congenic B6-ly5.2 (CD45.1+) CTLs (30 × 106) were infused through an intravenous route 14 days after tumor injection or to naive mice. BrdU was added to the drinking water to track proliferation. Eighteen days after tumor injection, 4 mice per group were killed. Flow Cytometric Analysis (FACs) was done with liver leukocytes and splenocytes. Tumor-bearing mice had significantly reduced proliferation of CTLs compared with naive mice in the livers and spleens (P < .05). (B-C) CTLs (106) and Tregs (106) isolated from AML-bearing or naive mice were adoptively transferred to AML-bearing Rag−/− mice. Thirteen days after transfer, FACs was performed on splenocytes (B) and liver leukocytes (C). Cells were gated on CD8 expression for CTLs. Intracellular IFN-γ expression was measured on gated CTLs. Tregs from AML-bearing mice significantly reduced the percentage of IFN-γ–producing CTLs in the spleen and liver. (D) Tregs isolated from AML-bearing or naive mice were cocultured with OT I CD8+ T cells stimulated with SIINFEKL peptide (Ovap) for 6 days. Cell supernatant was harvested and the IFN-γ level was determined. Tregs from AML-bearing mice inhibited IFN-γ production by OT I CD8+ T cells (P < .01), whereas naive Tregs were not suppressive. Results from 1 of 2 representative experiments are shown. Error bars represent SD.
Transient Treg depletion improves the antitumor efficacy of adoptive CTL transfer in advanced AML
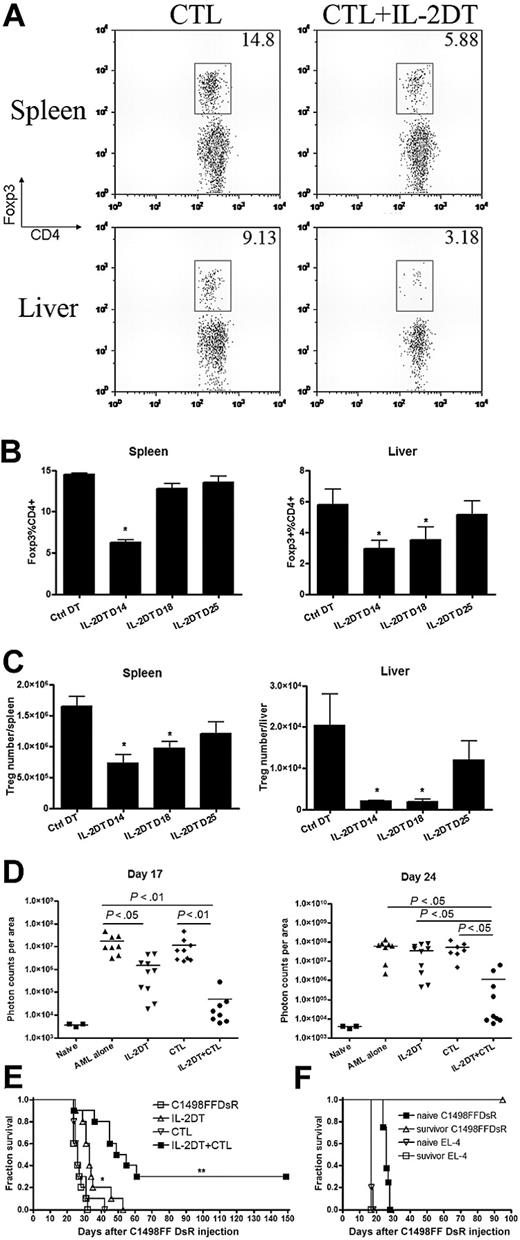
To determine whether the efficacy of CTL therapy could be enhanced by eliminating the Tregs, mice were injected with AML and treated with IL-2DT to deplete Tregs on day 4 and 13 after leukemia injection. The latter time period is 1 day before the day-14 adoptive transfer of anti–AML-reactive CTLs and corresponds to a time when Treg numbers are increased in AML-bearing recipients compared with naive, tumor-free controls (Figure 3). To quantify the level and duration of Treg depletion, AML-bearing mice were given IL-2DT on day 13 and killed 1, 5, and 12 days later for FACs (Figure 5A-C). Treg frequency in the spleen was reduced by 60% and in the liver by 70% on day 14, 1 day after the second injection of IL-2DT. Four days later (day 18), the frequency and absolute number of splenic Tregs recovered to control levels present in naive mice, whereas an additional 7 days (day 25) was required for normalization of liver Tregs. To investigate the potential impact of IL-2DT treatment on other CD25+ T cells, we assessed the percentage of CD25+CD8+ T-cell population in the spleen, LNs, and blood. We found that the CD25+CD8+ T-cell population was not affected by IL-2DT administration at the time when maximum depletion of Tregs was achieved (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). This finding is consistent with the results shown in similar studies performed by others.45
IL-2DT treatment followed by anti–AML-reactive CTLs significantly prolonged the survival of mice with advanced AML. B6 mice (10 mice/group) were injected with 106 C1498FFDsR cells followed by IL-2DT and CTL treatment as described. (A) Dot plots showing depletion of Tregs 1 day after IL-2DT treatment in the liver and spleen of mice. (B-C) Kinetics of Treg depletion by IL-2DT. (D) Combination of IL-2DT and CTL treatment significantly decreased tumor burden 17 and 24 days after tumor injection (■ vs □, P < .01). (E) IL-2DT treatment alone significantly prolonged the survival mice compared with either control mice or mice treated with CTLs alone (▵ vs ■ or ▿, P < .01). Combined IL-2DT and CTLs had superior effect (by log-rank test, P < .005) compared with either control mice or mice receiving IL-2DT or CTLs alone. Representative data from 1 of 3 similar experiments are shown. (F) Combed IL-2DT and CTL treatment promotes anti-AML memory response. Naive C57BL/6 or surviving mice (7-8 mice/group) were challenged with either EL-4 (105/dose) or C1498FFDsR (106/dose) cells. Both naive and surviving mice succumbed to EL-4 challenge. Significant increases in survival were observed only in rechallenged recipients of C1498FFDsR (■ vs ▵, P < .001). Error bars represent SD.
IL-2DT treatment followed by anti–AML-reactive CTLs significantly prolonged the survival of mice with advanced AML. B6 mice (10 mice/group) were injected with 106 C1498FFDsR cells followed by IL-2DT and CTL treatment as described. (A) Dot plots showing depletion of Tregs 1 day after IL-2DT treatment in the liver and spleen of mice. (B-C) Kinetics of Treg depletion by IL-2DT. (D) Combination of IL-2DT and CTL treatment significantly decreased tumor burden 17 and 24 days after tumor injection (■ vs □, P < .01). (E) IL-2DT treatment alone significantly prolonged the survival mice compared with either control mice or mice treated with CTLs alone (▵ vs ■ or ▿, P < .01). Combined IL-2DT and CTLs had superior effect (by log-rank test, P < .005) compared with either control mice or mice receiving IL-2DT or CTLs alone. Representative data from 1 of 3 similar experiments are shown. (F) Combed IL-2DT and CTL treatment promotes anti-AML memory response. Naive C57BL/6 or surviving mice (7-8 mice/group) were challenged with either EL-4 (105/dose) or C1498FFDsR (106/dose) cells. Both naive and surviving mice succumbed to EL-4 challenge. Significant increases in survival were observed only in rechallenged recipients of C1498FFDsR (■ vs ▵, P < .001). Error bars represent SD.
In the absence of Treg depletion, the adoptive transfer of CTLs failed to reduce tumor burden as measured by BLI on days 17 or 24 after AML challenge (Figure 5D). As also shown in Figure 2B, anti–AML-reactive CTL adoptive immunotherapy did not prolong the survival of AML-bearing mice (Figure 5E). A brief course (2 injections) of IL-2DT reduced leukemia burden in mice on day 18, although the reduction was transient because, by day 25, tumor burden did not differ from mice receiving AML cells alone. The transient reduction in AML burden resulted in a slightly, albeit significantly, prolonged survival rate compared with nontreated controls, although no mice survived beyond day 55 (Figure 5E). In contrast to individual therapy, IL-2DT treatment followed by anti-AML CTL adoptive transfer was able to reduce the AML burden on days 17 and 25 after injection (Figure 5D) and this reduction permitted the long-term survival of 30% of mice (Figure 5E). Combined IL-2DT and anti-AML CTL therapy was significantly better than either therapy alone in extending survival in mice with advanced AML.
To determine whether AML-bearing mice that received IL-2DT and CTL and could mount a memory response to AML rechallenge, mice surviving more than 80 days after initial AML challenge were rechallenged with the same dose of leukemia cells and compared with naive mice. Surviving mice were resistant to AML rechallenge (> 120 days), whereas naive mice succumbed to AML within 35 days (Figure 5F). In contrast, no survival advantage was seen when given a different syngeneic lymphohematopoietic malignant cell line, EL-4 (Figure 5F). Thus, the resistance to a rechallenge with leukemia in AML-bearing mice was seen only when rechallenge was performed with the original leukemia line, consistent with an AML-reactive memory cell response.
IL-2DT pretreatment restored the proliferation and increased the number of adoptively transferred CTLs in AML-bearing mice
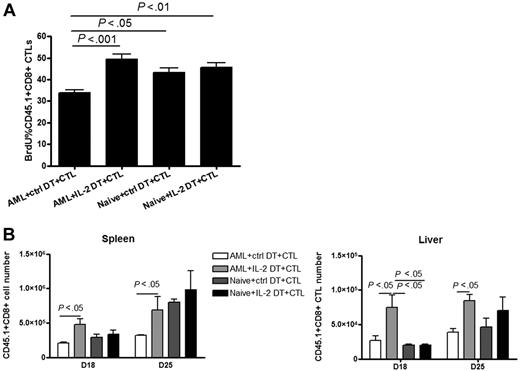
The reduction in CTL proliferation in tumor-bearing mice (Figure 4) paralleled the increase in Treg frequency (Figure 3), both of which appeared to correlate with the lack of efficacy of CTL adoptive immunotherapy in reducing tumor burden or prolonging survival (Figures 2,5). Thus, we reasoned that Treg depletion would enhance the proliferation and potential efficacy of adoptively transferred CTLs. Naive or AML-bearing mice were given IL-2DT or ctrl DT on days 4 and 13. On day 14, all mice were infused with CD45.1+ CTLs and proliferation was assessed by BrdU incorporation on day 18. IL-2DT treatment given before CTL infusion significantly enhanced CTL proliferation, restoring proliferation to that seen in naive nonleukemia-bearing mice (Figure 6A). This increased proliferation of CTLs by IL-2DT treatment was not evident in the bone marrow (supplemental Figure 2) mainly due to no change of the Treg population in the bone marrow observed at this time point (Figure 3G). Interestingly, no difference was found in naive mice with or without IL-2DT treatment, suggesting that the suppression of exogenous CTLs by Tregs on day 18 was associated with a state of high AML burden (Figure 6A). Augmented CTL proliferation resulted in an increase in the absolute number of adoptively transferred CTLs found in the livers and spleens of leukemia-bearing mice on days 18 and 25 (Figure 6B).
Combined IL-2DT treatment restored the proliferation and number of CTLs at sites of AML metastases. Naive or tumor-bearing mice were treated with IL-2DT or control DT (1 μg/dose) 4 and 13 days after tumor injection. Congenic B6-Ly5.2 (CD45.1+) CTLs (30 × 106/dose) were then injected intravenously 14 days after tumor injection and mice were fed with BrdU water to track proliferation. Eighteen days after tumor injection, 4 mice per group were killed. Flow cytometric analysis was done with liver leukocytes and splenocytes. IL-2DT treatment significantly augmented the percentage of BrdU+ CTLs in the spleens compared with control DT treatment. IL-2DT treatment significantly increased the percentage (A) and number (B) of CTLs found in the livers and spleens compared with control DT treatment. Representative data from 1 of 2 similar experiments are shown. Error bars represent SD.
Combined IL-2DT treatment restored the proliferation and number of CTLs at sites of AML metastases. Naive or tumor-bearing mice were treated with IL-2DT or control DT (1 μg/dose) 4 and 13 days after tumor injection. Congenic B6-Ly5.2 (CD45.1+) CTLs (30 × 106/dose) were then injected intravenously 14 days after tumor injection and mice were fed with BrdU water to track proliferation. Eighteen days after tumor injection, 4 mice per group were killed. Flow cytometric analysis was done with liver leukocytes and splenocytes. IL-2DT treatment significantly augmented the percentage of BrdU+ CTLs in the spleens compared with control DT treatment. IL-2DT treatment significantly increased the percentage (A) and number (B) of CTLs found in the livers and spleens compared with control DT treatment. Representative data from 1 of 2 similar experiments are shown. Error bars represent SD.
To determine the spatial location of CTLs, Tregs, and AML cells as influenced by IL-2 DT treatment, confocal microscopy of the spleen and liver from control DT– versus IL-2DT– and CTL-treated mice was analyzed 20 days after AML challenge. The frequency of Tregs (CD4+Foxp3+) found in the spleen and liver of IL-2DT–treated mice was comparable with naive mice, both of which were lower than in control DT–treated AML-bearing mice (Figure 7A). AML cells (DsR+, Figure 7B) in the liver appeared to inversely correlate with the frequency of adoptively transferred CTLs within sites of tumor metastases, best observed when comparing with AML-bearing mice treated with CTLs and control versus IL-2DT. Too few tumor cells were seen in the spleen at this time to be analyzable for colocalization of AML cells and CTLs (data not shown). In both the liver and the spleen, colocalization studies revealed an inverse correlation between the frequency of Tregs and adoptively transferred CTLs (Foxp3+, Figure 7C), evident when comparing findings in IL-2DT with ctrl DT treatment.
Treg depletion by IL-2DT treatment augments CTL infiltration into sites of AML metastases. AML-bearing mice were treated with IL-2DT or control DT (1 μg/dose) 4 and 13 days after tumor injection. Congenic B6-Ly5.2 (CD45.1+) CTLs (30 × 106/dose) were then injected intravenously 14 days after tumor injection and organs were harvested 6 days after CTL infusion. Confocal microscopy was performed. Naive B6 mice were used as normal tissue controls. (A) CD4 (green) and Foxp3 (red); (B) CD45.1 (green) and DsR (red); (C) CD45.1 (green) and Foxp3 (red); and (D) CD45.1 (green) and CD31 (red). Representative data from 1 of 2 similar experiments are shown.
Treg depletion by IL-2DT treatment augments CTL infiltration into sites of AML metastases. AML-bearing mice were treated with IL-2DT or control DT (1 μg/dose) 4 and 13 days after tumor injection. Congenic B6-Ly5.2 (CD45.1+) CTLs (30 × 106/dose) were then injected intravenously 14 days after tumor injection and organs were harvested 6 days after CTL infusion. Confocal microscopy was performed. Naive B6 mice were used as normal tissue controls. (A) CD4 (green) and Foxp3 (red); (B) CD45.1 (green) and DsR (red); (C) CD45.1 (green) and Foxp3 (red); and (D) CD45.1 (green) and CD31 (red). Representative data from 1 of 2 similar experiments are shown.
A recent study has shown correlation between down-regulation of adhesion molecules in the tumor microenvironment and diminished T-cell infiltration.36 CD31, an adhesion molecule involved in the trans-endothelial emigration of cells, was used to determine CTL migration. IL-2DT treatment significantly increased CTL infiltration in sites adjacent to CD31 expression in the spleens and livers of AML-bearing mice (Figure 7D). Augmented access of CTLs to C1498FFDsR cells may subsequently control leukemia progression and therefore enhance the efficacy of adoptive CTL therapy. In contrast to this recent report using a subcutaneous murine melanoma model, we could not observe a consistent effect of IL-2DT on augmenting ICAM-1 expression on CD31+ cells in our systemic AML disease model, which could have provided a direct facilitating mechanism for leukocyte function-associated antigen 1/ICAM-1–assisted CTL entry into the tumor site from blood vessels (data not shown).
Discussion
Adoptive CTL therapy has been proposed in cancer therapy for decades, however clinical applications can be limited by suppressive factors induced in the tumor microenvironment in vivo.46-48 Little progress has been made in achieving long-term survival in advanced disease settings. Enhancement of cancer immunotherapy by depletion of regulatory T cells has been well studied and characterized as prophylaxis in tumor models. The therapeutic effect of Treg depletion in established tumor models is rather disappointing.36,37 This study provides the first evidence that efficient antileukemia activity of adoptively transferred CTLs when given after the establishment of tumor can be achieved upon the prior removal of Tregs even in an advanced disease setting.
For Treg depletion, we used IL-2DT, a similar form of clinically tested reagent used for depleting Tregs and as an immunotherapeutic reagent in treating melanoma in humans.49,50 IL-2DT has a very short half-life in vivo (< 1 day), in contrast to the extended duration of mAbs,51 resulting in maximum Treg depletion before CTL transfer with minimal effect on infused CTLs. Despite providing IL-2DT only 1 day before adoptive CTL therapy, a prolonged antitumor effect was observed, suggesting that IL-2DT had a more dominant effect on the depletion of Tregs than on adoptively transferred CTLs. This phenomenon may be due to the higher expression levels of CD25 on Tregs, perhaps especially tumor-reactive Tregs, than on activated adoptively transferred CTLs.
The timing of the IL-2DT treatment proved to be important in the combination therapy. Delay of the initiation of IL-2DT treatment from day 4 to day 7, a time of greater tumor burden, reduced the efficacy of combined therapy (data not shown), although giving IL-2DT and CTL therapy earlier (7 days after tumor implantation) would achieve a more robust immune response to AML (supplemental Figure 3). We chose to focus on the more clinically relevant advanced disease setting. Treg depletion alone enhanced the endogenous immune responses and reduced tumor burden by BLI measurements and prolonged survival after initial tumor challenge. Consistent with studies by others that Tregs in tumor sites can limit infiltration by endogenous CTLs,36 we observed increased numbers of adoptively transferred CTLs in the vicinity of tumor vasculature in IL-2DT–treated mice. Treg depletion may augment antigen presentation by DCs,35 activate antitumor T lymphocytes,52 and promote natural killer cell function.53 Early treatment with IL-2DT is necessary to tune the immune system to be optimally receptive to the antileukemia activity of adoptively transferred CTLs. However, reduced leukemia burden at the time of CTL infusion is not the only reason accounting for improved CTL therapy, because the same anti–AML-reactive CTLs given as early as 1 day after AML challenge failed to rescue mice from leukemia (data not shown).
Multiple studies have focused on elucidating the mechanism that limits CTL function in vivo as a first step in potentially improving the efficacy of adoptively transferred CTL therapy.54 Our studies showed that the proliferation of adoptive CTLs in vivo was significantly reduced in the leukemia environment and that this could be corrected by depletion of Tregs. There was no change in the apoptosis or persistence of anti–AML-reactive CTLs observed with IL-2DT treatment, indicating there are other extrinsic and intrinsic factors that contribute to immunosuppression in the tumor environment. Myeloid-derived suppressor cells (MDSCs) are widely studied now for their suppressive properties in multiple disease models including tumor.55 It is possible that AML can induce/recruit MDSCs to assist the escape from the host immune system. Whether the failure to cure all mice with advanced disease was due to MDSCs that would have been unaffected by IL-2DT or other tumor immune suppressive mechanisms such as those associated with the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase, is unknown.
It is not clear in our studies whether the infused CTLs or the host endogenous CD8+ T cells were responsible for increased memory responses to AML. However, because Tregs comprise a minority cell population and were only partially and transiently depleted, we do not favor the explanation that homeostatic proliferation contributed to the enhanced proliferation of adoptively transferred CTLs in Treg-depleted recipients. Rather, we favor an explanation that Treg depletion unleashes the constraints imposed on adoptively transferred CTLs that may work in concert with endogenous CTLs to mediate an antitumor effect. Support for the activation of endogenous CTLs may be derived from data in which mice were given AML followed by IL-2DT treatment alone. These mice had reduced tumor burden, and a prolongation of survival without long-term cures. Thus, neither the endogenous CTLs alone, unrestrained by antitumor reactive Tregs, nor adoptively transferred CTLs alone were sufficient to provide long-term survival of AML-bearing mice. The short life span of the transfused CTLs is another obstacle of adoptive therapy. However, a sufficient immune response does not require a large number of antigen-specific T-cell precursors. A recent study has shown that a single clone of antigen-specific CD8+ T cells could proliferate and develop into various effector subsets in response to antigen stimuli.56 Because adoptive CTL transfer was required for survival and only survivors could be tested for long-term memory response, we favor the explanation that the recall response was dependent on exogenous CTLs, though we cannot exclude an important contribution from endogenous CTLs.
In summary, progressive AML disease led to an increased number of Tregs at the sites of AML metastases. The increased Treg frequency was associated with reduced infiltration and proliferation of adoptively transferred antitumor reactive CTLs. Treg depletion alone had a transient effect on AML tumor burden and slight survival prolongation, likely due to endogenous CTL activation. Treg depletion before adoptive CTL transfer significantly increased the therapeutic benefits of CTL immunotherapy, likely in conjunction with endogenous antitumor reactive CTLs. The resulting immune response permitted long-term memory, which may be needed to prevent tumor recurrence. We conclude that Treg depletion before CTL transfer can result in therapeutic efficacy in settings of substantial pre-existing tumor burden in which antitumor reactive CTL infusion or Treg depletion alone have proven to be ineffective.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Patricia A. Taylor, Dr Jianying Yang, Dr Lisa K. Jasperson, and Dr Mathangi Srinivasan for their technical help.
This work was supported in part by National Institutes of Health grant R01 CA72669 and the Children's Cancer Research Fund.
National Institutes of Health
Authorship
Contribution: Q.Z. designed, organized, and supervised research, performed experiments, analyzed data, designed the figures, and wrote the paper; C.B. and C.H.J. designed experiments; M.E.M. performed experiments; S.L.H. performed experiments and edited the paper; J.T. designed and supervised immunofluorescent imaging; D.H.M. designed research and edited the paper; B.L.L. and B.J.W. designed experiments and edited the paper; M.R. performed immunofluorescent staining and analyzed data; D.A.V. made the IL-2DT reagent; and B.R.B. designed, organized, and supervised research and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, Department of Pediatrics, MMC 109, University of Minnesota, Twin Cities, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.