In this issue of Blood, Miao and colleagues demonstrate that Tregs from a FoxP3 transgenic mouse injected with a normally immunogenic FVIII plasmid not only suppress the response to FVIII but also may “turn on” host Tregs upon adoptive transfer.
The tantalizing promise of replacement gene therapy for the treatment of diseases like hemophilia has been around for many years. Indeed, significant advances have been made toward the therapeutic expression of human proteins, such as factor VIII (FVIII). However, one of the daunting challenges remains the immunogenicity not only of the vector, but also of the expressed replacement protein, due to lack of tolerance to this protein in many potential recipients. Fortunately, immunologists and gene therapy mavens have now joined forces to approach this problem. Many different and successful approaches toward the induction of tolerance to FVIII and other gene therapy products have been reported recently.1-6 In most of these cases, tolerance requires regulatory T cells (Tregs) for either the induction or the maintenance of tolerance. These cells, defined as CD25+ and FoxP3+ in the mouse, have the ability to suppress both T-cell and B-cell responses in vivo and in vitro.
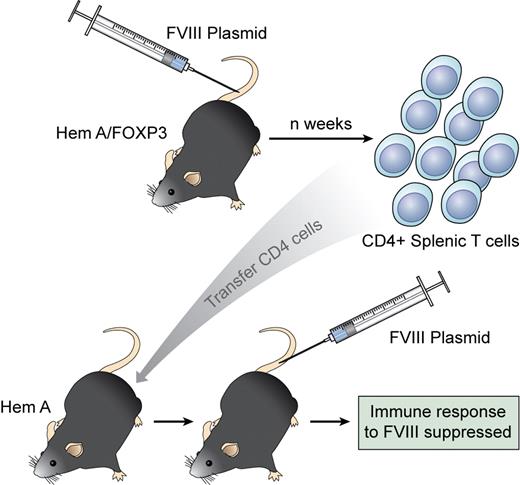
Regulatory CD4 T cells are generated in a FoxP3 transgenic mouse injected with an immunogenic FVIII vector. When transferred to a naive recipient, these CD4 cells suppress the response to FVIII and generate Tregs from the host. Professional illustration by Kenneth X. Probst.
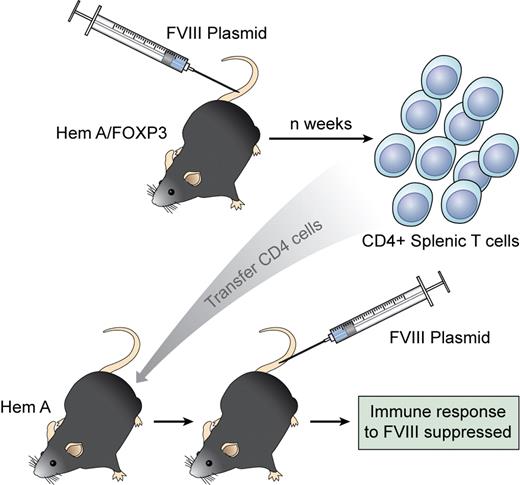
Regulatory CD4 T cells are generated in a FoxP3 transgenic mouse injected with an immunogenic FVIII vector. When transferred to a naive recipient, these CD4 cells suppress the response to FVIII and generate Tregs from the host. Professional illustration by Kenneth X. Probst.
In this issue of Blood, Miao et al have taken advantage of a transgenic mouse (generated by the Ziegler laboratory) that overexpresses the transcription factor FoxP3.7 They crossed this mouse with a hemophilia A line (FoxP3HemA) to demonstrate that CD4 Tregs can be engendered during FVIII gene therapy. Under normal circumstances, injection of a liver-specific plasmid for FVIII into wild-type hemophilia A mice leads to only transient expression of FVIII and the generation of high-titered inhibitors. However, in FoxP3HemA mice, no antibodies to FVIII developed and FVIII expression was as high as 30% of normal. This is an important finding both for gene therapists and immunologists! CD4 T cells expressing typical Treg markers (CD25, GITR, and CTLA-4) increased in the spleen and peripheral blood of FoxP3HemA transgenic mice injected with the FVIII plasmid. Importantly, regulatory T cells from plasmid-treated FoxP3HemA mice could then suppress inhibitor formation in secondary HEMA recipients and appeared to “turn on” host Tregs to modulate subsequent responsiveness to the highly immunogenic plasmid. The donor Tregs did not persist in the host, but led to long-lasting inhibition of inhibitor formation. Hence, the FoxP3+ CD4 Tregs seem to initiate a regulatory circuit in the recipient (see figure).
These are important observations because they suggest that it might be possible to generate Tregs ex vivo that are able to recruit host Tregs to maintain suppression when antigen or plasmid gene therapy is applied. Several hurdles need to be overcome for this to become a reality. First, FoxP3 is not as definitive a Treg marker in humans as it is in mice. The surface phenotype of these cells in humans needs to be better defined so that the cells can be purified and isolated. Clearly, “transgenics” overexpressing FoxP3 (or any acceptable Treg marker) cannot be constructed in humans, but transduced donor cell lines may be able to be created. Nevertheless, methods do exist to induce and expand natural Tregs in vitro, for example, with anti-CD3 + TGFβ,8 albeit the plasticity of these cells is a concern.9 The specificity of these Tregs needs to be tightly determined and controlled. Otherwise, we will be creating an expensive and nonspecific immunosuppressive cellular therapy.
Despite these caveats, this model offers promise that future cellular and gene therapies can be combined to initiate specific immunoregulatory controls for the success of replacement therapy in the future.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■