Abstract
During the past decade, progress in basic immunology has been impressive. In parallel, whereas our understanding of the pathophysiology of acute graft-versus-host disease (GVHD) has greatly improved, so has our knowledge of the complexities of the immune system. Much of the immunobiology of acute GVHD has been gleaned from preclinical models and far less from correlations with clinical observations or therapeutic interventions. In this review, we summarize some of the major advances in GVHD pathophysiology, including the translation of these from the bench to the bedside, and discuss preclinical approaches that warrant further exploration in the clinic.
Introduction
Much of our understanding of the biology of graft-versus-host disease (GVHD) has developed from 2 preclinical animal models, the mouse and the dog (reviewed in Welniak et al,1 Ferrara et al,2 Shlomchik,3 van den Brink and Burakoff,4 Feinstein et al,5 and Schleuning6 ). Because the inbred mouse model has been used as the basis for much of our knowledge of the immunologic mechanisms of GVHD, this review will focus on murine models but also will highlight several aspects of the canine model. Because there are significant species differences between humans and mice, 5 points are important to consider when drawing conclusions from studies with animal models and before correlation to the clinical allogeneic hematopoietic stem cell transplantation (HSCT) scenario (Table 1).
GVHD is a complex disease resulting from donor T-cell recognition of a genetically disparate recipient that is unable to reject donor cells after allogeneic HSCT. The classical scheme of GVHD1,2,7 development includes 5 basic steps.
Step 1: priming of the immune response. Cytoreductive conditioning induces tissue damage and the release of a storm of proinflammatory cytokines that promote the activation and maturation of antigen-presenting cells (APCs) and the rapid amplification of donor T cells.8-10
Step 2: T-cell activation and costimulation. Activation occurs as the result of the recognition and interaction of the T-cell receptor (TCR) and costimulatory molecules with their cognate ligands expressed on the surface of the APC.
Step 3: alloreactive T-cell expansion and differentiation.
Step 4: activated T-cell trafficking. Activated T-cell migration to GVHD target tissues (gut, liver, skin, and lung) is followed by the recruitment of other effector leukocytes.11
Step 5: destruction of the target tissues by effector T cells. Destruction occurs via exposure to cell surface and release of soluble immune effector molecules. Tissue damage then leads to increased inflammatory signals, perpetuating and augmenting the disease process by contributing to the cytokine storm that fuels GVHD.
Previous reviews1,2,7,8,11,12 have detailed these phases of GVHD initiation and tissue destruction. We will focus on recent advances in GVHD pathophysiology and their translation into clinical knowledge or therapies. In each section, we briefly summarize key experimental data and then provide a perspective as to how these data succeeded or failed to be translated to the bedside. We also discuss potential preclinical leads that warrant consideration for future clinical translation.
Priming of the immune response
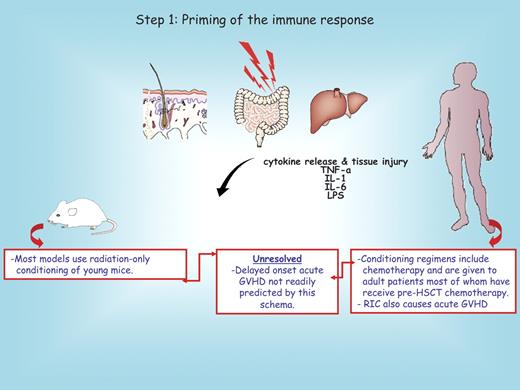
The earliest phase of acute GVHD is set into motion by the damage caused by the underlying disease and exacerbated by conditioning regimens (Figure 1). Damaged host tissues secrete proinflammatory cytokines, such as tumor necrosis factor α (TNF-α) and interleukin-1 (IL-1), which contribute to the “cytokine storm” increasing the expression of adhesion molecules, costimulatory molecules, major histocompatibility complex (MHC) antigens, and chemokine gradients that alert the residual host and the infused donor immune cells. These “danger signals” activate host tissue cells including APCs. Damage to the gastrointestinal tract from the conditioning is particularly important in this process, because it allows for systemic translocation of lipopolysaccharide (LPS) that further enhances host APC activation.7,13 This scenario is in accord with the increased GVHD risk associated with intensive conditioning regimens in some human randomized trials.14-16 Preclinical studies in dogs5,6,17-19 and clinical studies have indicated that reduced-intensity conditioning is associated with less morbidity and less early acute GVHD.20
Step 1: priming of the immune response. Conditioning regimens used to prepare recipients for allogeneic hematopoietic stem cell transplantation (HSCT) cause graft-versus-host disease (GVHD) parenchymal organ injury and the release of proinflammatory cytokines that initiates allogeneic priming. The red boxes below the mouse and the human recipient serve to highlight distinct features between these species. A major unresolved issue not explained by this schema is shown in the middle red box. RIC denotes reduced-intensity conditioning.
Step 1: priming of the immune response. Conditioning regimens used to prepare recipients for allogeneic hematopoietic stem cell transplantation (HSCT) cause graft-versus-host disease (GVHD) parenchymal organ injury and the release of proinflammatory cytokines that initiates allogeneic priming. The red boxes below the mouse and the human recipient serve to highlight distinct features between these species. A major unresolved issue not explained by this schema is shown in the middle red box. RIC denotes reduced-intensity conditioning.
It is noticeable that IL-1 blockade21 or protection of epithelial tissue damage by infusion of keratinocyte growth factor, although partially efficacious in some experimental GVHD models,22,23 thus far has proved ineffective in preventing acute GVHD in a randomized human trial performed in matched sibling donors.24 Because the mechanisms associated with acute (late-onset) GVHD after reduced (eventually minimal) conditioning have not been well elucidated, additional studies are warranted that go back to the bench to develop so-called mini transplant in the mouse setting that may complement the aforementioned canine investigations.
T-cell activation and costimulation
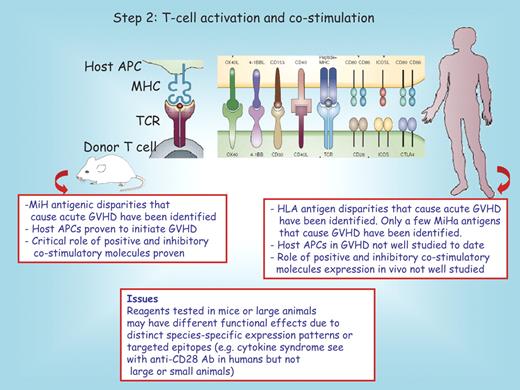
The core of the graft-versus-host immune reaction lies within the second step, in which donor T cells proliferate and differentiate in response to host APCs25,26 (Figure 2). Recent advances have indicated the presence of a subset of postmitotic, self-renewing CD44lo/CD62Lhi/CD8+ T cells that can generate and sustain all allogeneic T-cell subsets in GVHD reactions, including central memory, effector memory, and effector CD8+ T cells.27 The danger signals generated in the first phase augment this activation, at least in part, by increasing expression of costimulatory molecules. In mouse models, in which genetic differences between donor and recipient strains can be tightly controlled, CD4+ T cells induce acute GVHD to MHC class II differences and CD8+ T cells induce acute disease to MHC class I differences.25,28-35 Murine studies with minor histocompatibility antigen (MiHA)–disparate models have demonstrated that GVHD initiation requires donor T-cell recognition of host antigen in the context of host APCs.25,28-35 Donor-derived APCs then are able to augment CD8+ T cell–mediated GVHD by acquiring and presenting host antigens.35 In dogs, large-scale ex vivo expansion of MiHA-specific T cells has been achieved.36 However, even though decades of experimentation in the preclinical canine HSCT model have substantially improved our understanding of the biology of GVHD, the in vivo phenotype of potent APCs in dogs has not been defined to date. Although CD11c+/HLA-DR+/CD14−/DM5− cells obtained from canine peripheral blood have functional and morphologic characteristics similar to those of human myeloid dendritic cells,37 the role of host dendritic cells in the early development of acute GVHD in dogs remains to be fully elucidated.
Step 2: T-cell activation and costimulation. Donor T cells that express positive or inhibitory costimulatory pathway receptors encounter host antigen-presenting cells (APCs) that express major histocompatibility complex (MHC) antigens and ligands for these receptors. A host peptide is shown in the context of MHC/T-cell receptor (TCR) interactions. The red boxes below the mouse and the human recipient serve to highlight distinct features between these species. MiH indicates minor histocompatibility; MiHa, minor histocompatibility antigen; and Ab, antibody.
Step 2: T-cell activation and costimulation. Donor T cells that express positive or inhibitory costimulatory pathway receptors encounter host antigen-presenting cells (APCs) that express major histocompatibility complex (MHC) antigens and ligands for these receptors. A host peptide is shown in the context of MHC/T-cell receptor (TCR) interactions. The red boxes below the mouse and the human recipient serve to highlight distinct features between these species. MiH indicates minor histocompatibility; MiHa, minor histocompatibility antigen; and Ab, antibody.
In humans, the incidence of acute GVHD is directly related to the degree of mismatch between HLA determinants,38 mapped by high-resolution DNA typing of HLA genes with polymerase chain reaction–based techniques, largely replacing earlier cellular methods. However, recipients of HLA-identical grafts still can develop systemic acute GVHD due to genetic differences that lie outside the MHC loci and that encode proteins referred to as minor histocompatibility antigens (MiHAs). Thus, there is strong evidence for MiHA-mismatch–mediated GVHD in humans.39-41 Although individual human MiHA antigens associated with GVHD have been identified, the relative contribution of diverse MiHAs and the existence (if any) of single, dominant MiHAs in humans (such as B6dom and H60 that have been well characterized in rodents42,43 ) is unknown. With respect to the donor versus host origin of APCs initiating GVHD in humans, few data are available. However, recent studies on the fate of human Langerhans cells, dermal dendritic cells, and macrophages in patients suggest that host-derived APCs participate at least to the early stage of the disease.44-46 Donor and recipient polymorphisms of cytokine genes ascribed to the cytokine storm in rodents and humans also have been implicated as risk factors for the disorder. For example, TNF-α, interleukin 10, and interferon-γ variants have correlated with GVHD in some, but not all, studies (reviewed in Dickinson and Holler47 ). Genetic polymorphisms of proteins connected with innate immunity, such as NOD2, have been associated with acute GVHD in patients.48 Lastly, in some experimental models, polymorphisms in members of the Toll-like receptor family have been linked to GVHD risk.49
Costimulatory molecules play pivotal roles in experimental GVHD
A major role for GVHD initiation has been ascribed to CD28/cytotoxic T-lymphocyte antigen 4 (CTLA-4) (CD152):B7 interactions, which consist of both a positive (CD28/B7) and an inhibitory (CTLA-4:B7) pathway. Reduction in GVHD lethality has been seen in mice treated with B7 antagonists, most prominently using anti-B7 antibody but also in some studies using CTLA-4–Ig that binds to B7 ligands (CD80, CD86) expressed on host APCs and competitively inhibits CD28 binding to its natural ligands.50-52 However, CTLA4-Ig that has been in clinical use for autoimmune disorders failed to induce tolerance beyond that achieved with methotrexate/cyclosporine alone in a canine model.53 Another B7 supergene family member, inducible costimulator (ICOS; CD278), binds the ligand B7h (CD275) expressed on host APCs and thereby promotes T-effector responses. Blockade or absence of ICOS on donor T cells diminishes gut and liver GVHD.54,55 Members of the TNF receptor (TNFR) family also function as costimulatory molecules and modulate GVHD. OX40 (CD134) is present on both activated CD4+ and CD8+ T cells, and its cognate ligand, OX40L, is present on activated APCs. Despite the presence of the receptor on both T-cell subsets, the absence of either the receptor or ligand or the use of blocking antibody demonstrates that activation of OX40 promotes CD4+ but not CD8+ T cell–mediated GVHD.56,57 In contrast, CD40L (CD154) is expressed only on activated CD4+ T cells. Endogenous CD40:CD40L interaction increases acute GVHD lethality by promoting both direct CD4+ T cell–mediated tissue destruction and CD8+ T-cell expansion.58 Two other members of the TNFR family are 4-1BB (CD137) and glucocorticoid-induced tumor necrosis factor receptor (GITR). Blockade of 4-1BB reduces CD8+ T cell–mediated acute GVHD lethality and CD4+ T-helper type 1 (proinflammatory) generation.50 In GVHD, stimulation of GITR on CD4+CD25− T cells reduced GVHD in MHCII-disparate recipients, whereas stimulation of GITR of CD8+CD25− T cells increased proliferation and acute GVHD in an MHCI-disparate murine model.59
Inhibitory pathways that down-regulate GVHD
In response to tissue injury and activated T cells, inhibitory pathways are up-regulated in an attempt to protect the host against injury. CTLA-4 and programmed death-1 (CD279) are up-regulated on donor T cells during acute GVHD and serve to dampen the immune response. Both also are primarily expressed in the cytoplasm of activated T cells and CD4+CD25+ regulatory T cells (Tregs; reviewed in Welniak et al1 ). In rodents, selective blockade of CTLA-4:B7 interactions accelerated acute GVHD lethality. Thus, an ideal reagent for inhibiting GVHD would be one that selectively blocks CD28/B7. Blockade or absence of programmed death-1 on donor cells accelerates GVHD and is associated with increased interferon-γ (IFN-γ) production.60 Other coinhibitory pathways such as BTLA and B7H3 have not been extensively studied in classical acute GVHD models. The roles of other inhibitory pathways such as B7H4 have not yet been elucidated in GVHD. In addition to surface molecules, the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase, induced by IFN-γ in GVHD target organs especially in the gastrointestinal tract, diminishes T-effector cell destruction via local mechanisms that result in both an increased donor T-cell apoptosis and decreased proliferation.61-63
Translating data on costimulatory molecules for GVHD prevention into the clinic turns out to be much more difficult. Data on a limited number of patients suggest that costimulatory blockade accomplished by adding CTLA4-Ig to an in vitro mixed lymphocyte reaction culture resulted in donor antihost hyporesponsive T cells that supported relatively rapid T-cell immune recovery and a seemingly low propensity to cause acute GVHD when added to a haploidentical stem cell graft.64,65 More broadly directed in vitro methodologies have been recently devised to depleted alloreactive T cells and such methodologies have been applied to studies in a limited number of patients.66 The new CTLA4-Ig derivative abatacept, which preferentially blocks CD28/B7 interactions, is highly efficient in the treatment of rheumatoid arthritis and psoriasis and in preventing acute solid organ graft rejection, but has not been tested to date for acute GVHD prophylaxis.67
Acute GVHD and T-cell subpopulations
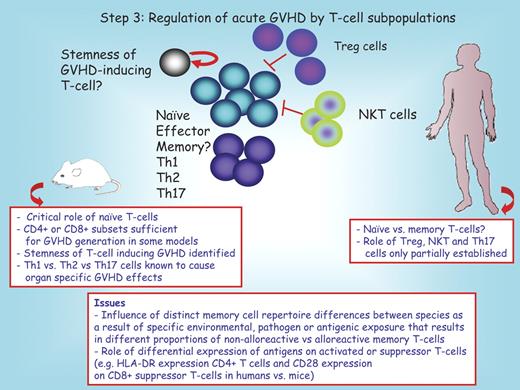
Diverse subpopulations are implicated in GVHD (Figure 3).
Step 3: regulation of acute GVHD by T-cell subpopulations. T-cell subsets that have been implicated in GVHD generation include naive and effector T cells, and Th1, Th2, and Th17 cells. More uncertain is the role of memory T cells. Inhibitory T-cell populations include CD4+CD25+ regulator T cells and natural killer T cells. In rodents, a T-cell population with stem cell properties has been implicated in acute GVHD generation. The red boxes below the mouse and the human recipient serve to highlight distinct features between these species. Question marks denote uncertain conclusions. Treg indicates T regulatory cell; and NKT, natural killer T cell.
Step 3: regulation of acute GVHD by T-cell subpopulations. T-cell subsets that have been implicated in GVHD generation include naive and effector T cells, and Th1, Th2, and Th17 cells. More uncertain is the role of memory T cells. Inhibitory T-cell populations include CD4+CD25+ regulator T cells and natural killer T cells. In rodents, a T-cell population with stem cell properties has been implicated in acute GVHD generation. The red boxes below the mouse and the human recipient serve to highlight distinct features between these species. Question marks denote uncertain conclusions. Treg indicates T regulatory cell; and NKT, natural killer T cell.
Naive T cells
Using new methods in rodents including green fluorescent protein marking or bioluminescence technology, it has been reported that T cells can undergo a massive and much earlier than previously thought expansion in lymph nodes and Peyer patches.68 In mice, naive CD44loCD62Lhi CD8+ T cells generate and sustain allogeneic CD8+ T cells in GVHD reactions.69,70 Murine memory T cells isolated from nonallosensitized donors fail to induce GVHD in experimental models.69 In contrast, alloantigen-sensitized effector memory CD44hiCD62Llo as well as naive phenotype CD44loCD62Lhi T cells, but not central memory CD44hiCD62Lhi CD8+ T cells, cause GVHD after adoptive transfer into secondary recipients.27 Both alloantigen-sensitized memory CD4+ and CD8+ T cells are involved in the transfer of GVHD under these conditions.
In the clinic, quantification of the degree and location of early T-cell expansion is not readily possible given the limitations of current technology that can be applied to persons undergoing HSCT. Nonetheless, clinical studies are planned for this year to extrapolate in vivo rodent data by transferring enriched memory T cells rather than naive T cells to the recipient at the time of HSCT. Such studies will provide important proof-of-concept as to whether the removal of naive T cells from the donor graft is sufficient to reduce or prevent acute GVHD.
Regulatory T cells
CD4+CD25+Foxp3+ regulatory T cells (Tregs) have potent suppressor activity both in vitro and in vivo. Donor Treg infusion blocks acute GVHD. Murine L-selectin (CD62L)–expressing Tregs preferentially home to secondary lymphoid organs, and in particular lymph nodes, resulting in GVHD prevention.71 Conversely, depletion of CD25+ T cells from the donor graft or in the recipient immediately after allogeneic HSCT promotes acute and chronic GVHD in various mouse models while still maintaining a graft–versus–hematopoietic cell malignancy response in most but not all studies.59,72-75 Because of the relatively low frequency of Tregs in lymphoid organs, ex vivo expansion of Tregs often has been used to increase the number available for in vivo infusion. Immunosuppressive drugs given to prevent or control GVHD also affect Treg expansion and function. Calcineurin inhibitors such as cyclosporine decrease IL-2 production, leading to a reduction in Treg proliferation and function. In contrast, rapamycin preferentially spares, induces, or may functionally increase murine and human Tregs in ex vivo culture systems, albeit at the expense of overall cell yield.76
Some challenges have arisen in the manipulation of human Tregs during allogeneic HSCT when isolation procedures are based solely on CD4 and CD25 coexpression. Although the expression of the intracellular protein, Foxp3, typically is used in conjunction with CD4 and CD25 to define Treg populations, permeabilization of cells to assess Foxp3 expression precludes the use of Foxp3 as a marker suitable for cell isolation procedures without impairing cell viability and function. More recently, a combination of CD4, CD25, and CD127 (IL-7Rα) has permitted the isolation of a highly purified Treg population that included both CD4+CD25+ and CD4+CD25− T-cell subsets, both of which were as suppressive as the classic CD4+CD25hi Treg subset.77,78 However, it is unknown whether the expansion of this Treg subpopulation will permit retention of as high a level of suppressor function as the CD4+CD25+ population.79 Besides these important technical problems and despite the encouraging rodent data, future studies will be needed to determine whether Treg infusion in persons undergoing HSCT adversely affects an allogeneic graft-versus-tumor response. Furthermore experimental data both in mice and humans have demonstrated the extraordinary potential of T-helper cell subsets (Th1, Th2, and Th17) and of Tregs to exhibit plasticity, shifting from one phenotype to another (reviewed in Annunziato and Romagnani80 ; and Rowell and Wilson81 ). This aspect of “plasticity” may also be of concern when Tregs will move from the bench to the bedside.
NKT cells
A second inhibitory population shown to inhibit acute GVHD lethality is the natural killer T (NKT) subset that coexpresses NK- and T-cell surface determinants.82 In rodents, total lymphoid irradiation combined with antithymocyte globulin has been shown to induce host NKT cells that also promote the generation of Tregs and the production and release of anti-inflammatory cytokines.83 In humans undergoing HSCT, recent studies indicate that the reduced acute GVHD lethality seen despite the infusion of high numbers of T cells contained in a granulocyte colony-stimulating factor–mobilized peripheral blood stem cell graft is associated with increased donor NKT cells.84,85
Th17 cells
Finally, Th17 cells86 have recently emerged as a new player in GVHD. Although the role of this new T-cell subset has been dissected in certain experimental models including inflammatory bowel disease, and lung and skin GVHD, the study of its role in experimental GVHD has led to seemingly discordant GVHD lethality results that may be ascribed to different experimental conditions.87-89 As yet, the potent role of Th17 cells in humans is unknown. Preliminary data from one of us (G.S., unpublished observations, September 2009) indicate that Th17 cells in human intestinal GVHD Th17 cells are not as numerous as in Crohn disease.
T-cell trafficking
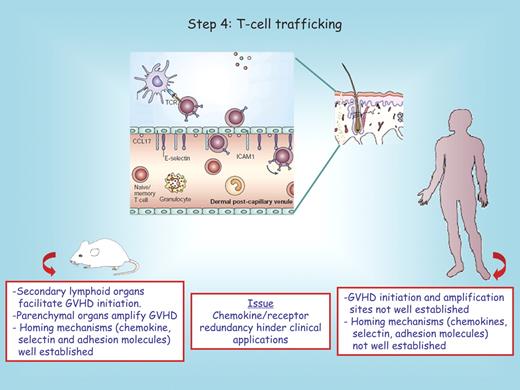
How T cells are recruited into tissues could be pivotal for understanding the stereotypical involvement of skin, liver, and bowel in GVHD (Figure 4). Although the migration of T cells into secondary lymphoid organs during GVHD and other inflammatory responses has been well characterized, the migration of leukocytes into parenchymal organs is less well understood. This process may involve changes in vascular permeability and, in certain systems, has been shown to require specific selectin-ligand, chemokine-receptor, and integrin-ligand interactions (reviewed in Wysocki et al11 ). During a GVHD reaction, donor T cells initially migrate to spleen and peripheral lymphoid tissues within hours.90 Naive donor T cells traffic to lymphoid tissues, where the subset of alloreactive T cells receives activation signals by APCs, and then subsequently migrate to specific GVHD target organ sites, essential for the induction and pathogenesis of acute GVHD.91 Almost all tissues express transplantation antigens; however, acute GVHD pathology is primarily limited to only a few locations—gut, skin, liver, lung, secondary lymphoid organs, and thymus. With respect to GVHD target organ-specific migration, the combination of CD62L and beta7 integrin expression is required to induce acute colitis and facilitate entry of CD4+ donor T cells in the mesenteric nodes associated with lethal GVHD in allogeneic hosts.92,93 β7−/− donor T cells caused less GVHD morbidity and mortality than WT donor T cells because of selectively decreased T-cell infiltration of the liver and intestines.94 The ability of alloreactive donor T cells to home to specific organs is regulated by a unique combination of signals that bind to corresponding receptors on host tissues and counterreceptors expressed on donor T cells, including members of the chemokine family. Macrophage inflammatory protein 1a and other chemokines (such as CCL2-CCL5, CXCL2, CXCL9, CXCL10, CXCL11, CCL17, and CCL27) are overexpressed during GVHD generation and enhance the homing of cellular effectors to GVHD target organs.11,95,96 Blockade of the chemokine receptor CCR5 or of MadCAM, the gut-associated ligand for lymphocyte Peyer patch adhesion molecule (α4β7 integrin), or precluding the formation of Peyer patches has been reported by some authors, but not by others, to be sufficient to reduce acute GVHD dependent on the intensity of conditioning.30,92,97 The perceived requirement for alloreactive T-cell priming within secondary lymphoid organs as a critical step in the development of GVHD led to experiments using FTY720 (a pharmacologic agent that can trap T cells in secondary lymphoid tissues). In experimental models, administration of FTY720 inhibited GVHD, although the responsible mechanism may be due to inhibition of APC migration and not donor T-cell trapping in lymphoid organs.98 However, FTY720 administration into dogs with active GVHD did not reduce lethality of the disease,99 and human development for GVHD prevention has not proceeded due to toxicity concerns in solid organ transplant recipients. CCR6+ donor CD4+ T cells target cells to the gut and skin in murine GVHD models.100 Expression of CXCR3 or CCR2 on donor CD8+ T cells targets these cells to the gut and liver but not lung.101-104
Step 4: T-cell trafficking. In rodents, secondary lymphoid organs are known to facilitate GVHD initiation. In both rodents and humans, GVHD tissue injury requires migration of such activated donor T cells into GVHD target organs that is orchestrated by chemokines, selectin, and adhesion molecules. An example of the homing process into the skin is depicted. The red boxes below the mouse and the human recipient serve to highlight distinct features between these species. Question marks denote uncertain conclusions. In the center red box, we note that clinical translational approaches to prevent GVHD by blocking individual chemokine/receptor interactions may be difficult due to known redundancies that exist for many pathways.
Step 4: T-cell trafficking. In rodents, secondary lymphoid organs are known to facilitate GVHD initiation. In both rodents and humans, GVHD tissue injury requires migration of such activated donor T cells into GVHD target organs that is orchestrated by chemokines, selectin, and adhesion molecules. An example of the homing process into the skin is depicted. The red boxes below the mouse and the human recipient serve to highlight distinct features between these species. Question marks denote uncertain conclusions. In the center red box, we note that clinical translational approaches to prevent GVHD by blocking individual chemokine/receptor interactions may be difficult due to known redundancies that exist for many pathways.
These results suggest that strategies that influence T-cell migration, particularly to GVHD target organs, may offer promise for reducing GVHD target organ–specific injury, although the redundancy of chemokines and their receptors may hinder clinical efficacy in the context of GVHD prevention or therapy. As such, targeting lymphocyte/integrin interaction may be a more promising way to explore. Indeed the research of targeting lymphocyte trafficking has been taken into the clinic in diseases related to GVHD, such as rheumatoid arthritis and colitis. For example, natalizumab, a humanized monoclonal antibody against alpha4 integrin, has been tested in phase 3 clinical trials in Crohn disease and beneficial results have been reported.105,106
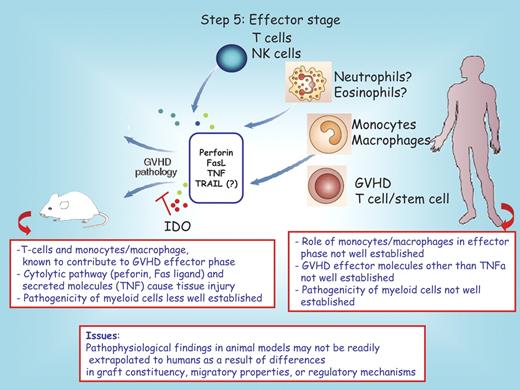
Effector stage: T cells and others
After migration of alloreactive effector T cells to the target tissues of GVHD, these cells can mediate tissue destruction through both direct cytotoxic activity and the recruitment of other leukocytes (Figure 5). Targeting these effector pathways has been studied as a strategy to prevent or reduce GVHD severity. Researchers have considered acute GVHD to be a Th1/Tcytotoxic-type (IL-12, IL-2, and IFN-γ) disease on the basis of the predominance of cytotoxic T cell–mediated pathology and of increased production of Th1-type cytokines, including IFN-γ,107 that is detectable in the serum or donor T cells during acute GVHD. However, several recent studies have suggested that the influence of Th1 and Th2 cytokines in acute and chronic GVHD is not so simply explained (reviewed in Welniak et al1 ). The production of high levels of IFN-γ by both CD4+ and CD8+ donor T cells early after bone marrow transplantation can limit the severity of acute GVHD in recipient mice after myeloablative conditioning due, in part, to the induction of T-cell apoptosis.108 Likewise, the proinflammatory cytokine IL-18, which promotes IFN-γ production, can inhibit CD4+-dependent GVHD. The use of donor T cells from mice lacking signal transducer and activator of transcription 4 or signal transducer and activator of transcription 6, transcription factors required for development to Th1 or Th2 phenotypes, respectively, or the ablation of either IL-2–producing (Th1-type) or IL-4–producing (Th2-type) donor T cells after the onset of clinical symptoms of GVHD demonstrates that both Th1- and Th2-type donor T cells can induce acute GVHD.107,109-114
Step 5: effector phase. Cells implicated in the GVHD effector process are illustrated. In the gray box are the known mediators of tissue injury. IDO inhibits GVHD pathology by reducing the frequency of T-effector cells present in the colon. The red boxes below the mouse and the human recipient serve to highlight distinct features between these species. Question marks denote uncertain conclusions. NK indicates natural killer; TNF, tumor necrosis factor; and TNFa, tumor necrosis factor alpha.
Step 5: effector phase. Cells implicated in the GVHD effector process are illustrated. In the gray box are the known mediators of tissue injury. IDO inhibits GVHD pathology by reducing the frequency of T-effector cells present in the colon. The red boxes below the mouse and the human recipient serve to highlight distinct features between these species. Question marks denote uncertain conclusions. NK indicates natural killer; TNF, tumor necrosis factor; and TNFa, tumor necrosis factor alpha.
The concentration and timing of cytokine release into the circulation and relevant target organs appear to be critical for GVHD. For example, IL-10 promotes Th2 and type 1 regulatory T-cell responses, which can be important in the induction of tolerance to allografts (reviewed in Welniak et al1 ). Higher production of IL-10, as demonstrated in human recipients with IL-10 production polymorphism, is associated with reduced occurrence and severity of GVHD.115 Paradoxically, high-dose IL-10 administration can accelerate GVHD in a murine model, and high-serum IL-10 levels in patients after HSCT are associated with a fatal outcome. However, conversely, low-dose IL-10 administration can inhibit acute GVHD in mice (reviewed in Welniak et al1 ). These findings highlight the pleiotropic, sometimes opposing, nature of cytokines during the different phases of GVHD pathogenesis and on various effector and regulatory cell populations.
T cells mediate the final effector pathway in GVHD by multiple pathways.27,116,117 The expression of both Fas and Fas ligand (FasL) is increased on CD8+ and CD4+ donor T cells during acute GVHD in patients and mice, and serum levels of soluble FasL and Fas were found to correlate with GVHD severity or the response to GVHD therapy. Several studies in experimental mouse models have analyzed the role of the Fas-FasL and perforin-granzymes pathways in the development of GVHD using mice that are deficient for FasL (gld mice), perforin, or granzyme B as donors, or by the in vivo administration of neutralizing anti-FasL antibodies. Although these differences in experimental design affect the opportunity to draw a uniform conclusion, most studies have shown a role for the Fas-FasL pathway in GVHD mortality. With respect to the perforin-granzyme pathway, approximately two-thirds of studies demonstrated the importance of this pathway in GVHD pathogenesis (reviewed in van den Brink and Burakoff4 ). Although TNF/TNFR interaction can contribute to GVHD mortality and histopathology,118 pegylated TNFα receptor failed to prevent acute GVHD in the dog model.119
In studies of transplant recipients, polymorphisms in the TNFα gene of patients undergoing HSCT are associated with higher levels of production of the cytokine and are correlated with a higher incidence of severe acute GVHD (Cavet et al120 and reviewed in Dickinson and Holler47 ), which suggests that, in humans, induction of TNFα from recipient cells may make an important contribution to disease. Regardless of the source of TNFα, its importance in GVHD is borne out with the demonstration that treatment of steroid-resistant GVHD with a TNFα blocker has shown efficacy, especially against gastrointestinal disease, in some studies121-123 or when administrated for GVHD prophylaxis.124 Although preliminary studies by the University of Michigan also suggested that an anti-TNFα antibody (etanercept) in addition to steroids was superior to steroids alone as initial treatment for acute GVHD,122 a recent multicenter 4-arm Blood and Marrow Transplant Clinical Trials Network (BMT-CTN) randomized trial designed to identify the most promising agent(s) for initial therapy for acute GVHD indicated that etanercept was not the most effective agent to be combined with steroids for GVHD therapy.125
The black box: promise from molecular biology and proteomics?
New molecular tools including proteomic and gene profiling have already begun to pave the way of such response, allowing for a more precise definition of acute GVHD or the construction of a predictive model for acute GVHD severity both in humans and in the experimental setting. In this regard, a recent paper measured the gene-expression profiles of CD4+ and CD8+ T cells from 50 donors with microarray technology. Pre-HSCT gene-expression profiling segregates donors based on whether their transplant recipient suffered from chronic GVHD. Using quantitative polymerase chain reaction, established statistical tests, and analysis of multiple independent training-test datasets, the authors found that “dangerous donor” trait (occurrence of GVHD in the recipient) is under polygenic control and is shaped by the activity of genes that regulate transforming growth factor-beta signaling and cell proliferation.126 These findings strongly suggest that the donor gene-expression profile can have a dominant influence on the occurrence of chronic GVHD in the recipient. However, it should be stressed that currently no gene-expression profiling data are available on acute GVHD. The application of proteomic tools that allow screening for differentially expressed or excreted proteins in body fluids has generated considerable interest in the field. Using proteomics, authors have screened for plasma proteins specific for GVHD in a mouse model and identified peak that discriminated 2 diagnostic groups (GVHD and normal controls) with increased expression during murine GVHD. Purification and mass analysis identified this molecule as CCL8, a member of a large chemokine family. In human samples, the serum concentration of CCL8 correlated closely with GVHD severity.127 In humans, studies by Weissinger et al used capillary electrophoresis and mass spectrometry to discriminate 31 GVHD-linked polypeptides.128 More recently, Paczesny et al129 aimed to isolate candidate proteins using high-throughput assays on a large number of patient samples, and to determine their significance with respect to patient outcome. They screened plasma with antibody microarrays for 120 proteins in a discovery set of 42 patients who underwent transplantation that revealed 8 potential biomarkers for diagnostic of GVHD. Using enzyme-linked immunosorbent assay they then measured the levels of these biomarkers in samples from more than 400 patients who underwent transplantation divided into training and validation sets. Statistical analysis of these 8 proteins led to the identification of a panel of 4 proteins (IL-2 receptor-α, TNF receptor-1, IL-8, and hepatocyte growth factor) that best discriminated patients with and without acute GVHD.
Conclusions
Substantial progress has been made in our understanding of acute GVHD through experimental models. However, major clinically relevant questions are still unanswered. Among others, one can cite the following questions:
What are the pathophysiologic mechanisms responsible for late-onset acute GVHD seen after reduced intensity conditioning?
What are the mechanisms underlying steroid-resistant disease?
Small-animal models are typically designed to study GVHD in the absence of posttransplantation immunosuppressive medications. In the absence of T-cell depletion approaches, humans develop GVHD despite the administration of posttransplantation immunosuppressive medications. From this point of view, it could be asked why GVHD is resistant to these medications in some humans but not in others. This clinically relevant question has never been fully addressed in small-animal models or even in large-animal models in which immunosuppressive medications used in the clinic are administered. Further complicating the performance of such studies, there are substantial differences in the pharmacokinetics, pharmacodynamics, and toxicities of some GVHD prophylactic agents in rodents and larger animals compared with humans. The corollary question is whether small-animal models of GVHD in the absence of posttransplantation immunosuppressive medications adequately simulate GVHD pathophysiology that occurs in larger animals or in humans who receive posttransplantation immunosuppressive medications. Although the answer to this question is unknown, there are many similarities in GVHD pathology and immune responses between small- and large-animal models and with humans.
Considerable challenges remain as to how to most efficiently integrate the many components of a response and microenvironment that make up a cell, organism, or ecological community in a way that helps us to understand the function of the whole using lessons learned from preclinical models and clinical trials; that is, to quote the title of an article published 10 years ago130 on GVHD pathophysiology, the fundamental problem we face is how to best design prevention and therapy approaches given “Cytokines, T cells, chaos and complexity” that dictate the acute GVHD reaction?
Acknowledgments
The authors thank past and current laboratory members and team members who focus on the care of patients undergoing allogeneic HSCT, especially those who are focused on GVHD prevention and therapy.
This work was supported in part by National Institutes of Health (NIH) grants R01 AI 34495, R01 HL56067, P01 056299, P01 CA142106, and P01 CA067493, and grants from the Leukemia & Lymphoma Society and the Children's Cancer Research Fund.
National Institutes of Health
Authorship
Contribution: G.S. and B.R.B. contributed to the review of literature and writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gérard Socié, Service d'Hématologie Greffe, and Inserm U728, Hôpital Saint-Louis, 1 Av Vellefaux, 75010 Paris, France; e-mail: gerard.socie@sls.aphp.fr.