Abstract
Abstract 1189
Poster Board I-211
In the last decade, several groups have demonstrated that non-HLA gene polymorphisms can impact on outcome after HSCT. So far, their role appears as a matter of debate; non HLA genotyping is not yet routinely used. In addition, the main clinical factors influencing outcome after HSCT have also been described. Most experience has been gained through analyses of patients undergoing HSCT for chronic myeloid leukemia (CML). Patient age, stage of the disease, time interval from diagnosis to transplant, histocompatibility and donor and patient gender combination were initially identified as key pre-transplant risk factors for CML in the European Group for Blood and Marrow Transplantation (EBMT) clinical risk score, recently confirmed as risk factors for all patients with haematological indications for HSCT.
In this study, we assessed the additional impact of polymorphisms within the tumour necrosis factor receptor II (TNFRSF1B), estrogen receptor (ESR1), vitamin D receptor (VDR), interleukin 6 (IL6), interleukin 1 receptor antagonist (ILRN), interferon gamma (IFNG) and interleukin 4 (IL4) genes on overall survival in a EUROBANK cohort of 915 ( median patient age 41 years, range 16 years to 67 years; 59 % male patients) HLA identical sibling (n=501; 55%) and matched unrelated donor (MUD) (n=414; 45%) transplants consisting of patients having either acute leukaemia (AL) (n=463; 51%), CML (N=187; 20%), plasma cell neoplasia (n=120; 13%) or lymphoma (n=145; 16%) from 8 transplant centres (Barcelona, Paris, Munich, Newcastle-upon-Tyne, Prague, Regensburg, Vienna and Rostock). The statistical analysis was performed for the full cohort and for the AL subgroup. Potential influential genetic factors were assessed using the log rank test (p value < 0.2). These candidate factors were further included in addition to the EBMT clinical risk score in a stepwise Cox regression procedure to select final genes. The power of a model to predict overall survival was assessed through prediction error curves.
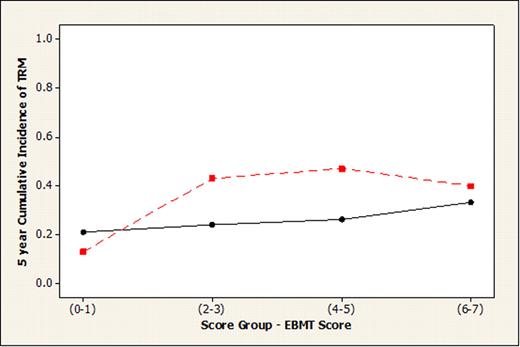
For the full cohort, the SNP IL6−174 within the IL6 gene in donors improved the prediction of the outcome by EBMT clinical risk score. Specifically, the presence of allele C in the donor IL-6 genotype was associated with lower survival time. This SNP assessment only held true for the (combined) CML, lymphoma and plasma cell neoplasia subgroups (log rank, p=0.016), not for AL (log rank, p= 0.638). The AL subgroup, the largest, was further assessed separately. Absence of patient IL4 (any T) and presence of patient IL1RN (any C) were associated with lower survival time as shown by Kaplan Meier survival plots. When viewed together with the EBMT score, these two genotypes improved the predictive value. Patients with absence of patient IL4 (any T) and presence of patient IL1RN (any C) were assigned to the ‘high risk’ group; remaining patients were assigned to the low risk group - Figure 1A and B illustrate the lower probability of survival and higher incidence of transplant related mortality (TRM) in the high risk polymorphism AL group compared with the low risk group.
Figure 1 (A) Survival probability at 5 years for Acute Leukemia by EBMT risk score, with high risk (red line) and low risk (black line) SNP genetic profile. (B) Cumulative incidence of TRM at 5 years for Acute Leukemia by EBMT risk score, with high risk (red line) and low risk (black line) SNP genetic profile.
Figure 1 (A) Survival probability at 5 years for Acute Leukemia by EBMT risk score, with high risk (red line) and low risk (black line) SNP genetic profile. (B) Cumulative incidence of TRM at 5 years for Acute Leukemia by EBMT risk score, with high risk (red line) and low risk (black line) SNP genetic profile.
This study confirms the important role of non-HLA genotyping for risk assessment in allogeneic HSCT. Improvement of fit of the EBMT risk score presents a powerful novel tool to assess this impact of cytokine gene polymorphisms in a complex heterogeneous patient population such as HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.