Abstract
Abstract 48
We recently reported on the use of the proteasome-inhibitor bortezomib as a novel strategy to control acute graft-versus-host-disease (GVHD). We evaluated bortezomib, tacrolimus and mini-methotrexate for GVHD prophylaxis after reduced-intensity conditioning (RIC) hematopoietic stem cell transplantation (HSCT) with unrelated donors mismatched at 1-2 HLA loci (-A, -B, -C, -DRB1). The regimen was safe, with zero non-relapse mortality. There was a 13% incidence of grade II-IV acute GVHD.
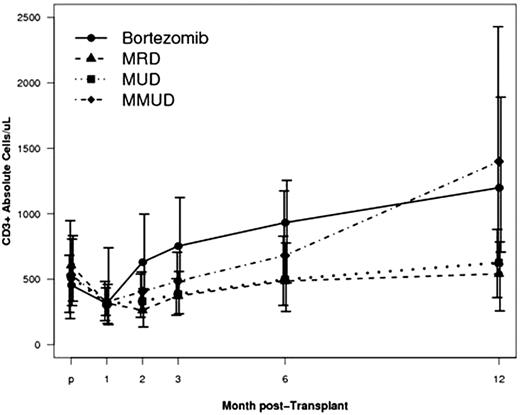
We analyzed immune reconstitution in the bortezomib cohort (n=24) [Bort] vs. i) 8/8 matched-related donor (-A, -B, -C, -DRB1) RIC HSCT cohort (n=96) [MRD]; ii) 8/8 matched-unrelated donor RIC HSCT cohort (n=139) [MUD]; and iii) 1-2 HLA mismatched-unrelated donor RIC HSCT cohort (n=24) [MMUD]. Comparator groups received sirolimus, tacrolimus and mini-methotrexate GVHD prophylaxis. All received fludarabine/busulfan conditioning and G-CSF mobilized adult-donor peripheral blood stem cells.
All groups had rapid hematopoietic engraftment and >90% median donor-chimerism by day +45 post-HSCT. They were not different with regards to age (p=0.53), sex (p=0.39) and acute grade II-IV GVHD (p=0.63). There was a difference in the proportion of good-risk disease: [Bort] (46%), [MRD] (21%), [MUD] (14%) and [MMUD] (21%) (p=0.005). Chronic GVHD was also different: [Bort] (41%), [MRD] (39%), [MUD] (53%) and [MMUD] (67%) (p=0.03).
Peripheral blood cells incubated with directly conjugated monoclonal antibodies defining distinct lymphocyte populations were analyzed by flow cytometry. Lymphocyte subsets included T (CD3+, CD4+, CD8+) B (CD19+, CD20+) NK (CD56+CD16+) NK-T (CD56+CD3+) and regulatory-T (Treg; CD4+CD25+) cells.
Unadjusted analyses revealed no significant differences in B, NK, NK-T or Treg recovery. In contrast, CD3+, CD4+ and CD8+ T-cell reconstitution was significantly higher in the bortezomib group, especially at 3 months post-HSCT.
| Phenotype . | Month . | N . | [Bort] median cells/uL (range) . | N . | [MRD] median cells/uL (range) . | N . | [MUD] median cells/uL (range) . | N . | [MMUD] median cells/uL (range) . | p-value . |
|---|---|---|---|---|---|---|---|---|---|---|
| CD3+ | Pre | 21 | 455 (84, 4363) | 36 | 607 (1, 1551) | 67 | 519 (10, 1698) | 10 | 533 (64, 2622) | 0.872 |
| 1 | 22 | 315 (3, 5888) | 84 | 317 (5, 1692) | 123 | 311 (20, 3742) | 18 | 328 (52, 551) | 0.848 | |
| 3 | 21 | 753 (31, 1777) | 74 | 373 (8, 3114) | 107 | 386 (0, 2338) | 15 | 485 (22, 2274) | <0.001 | |
| 6 | 18 | 932 (47, 1682) | 63 | 486 (37, 3701) | 86 | 498 (59, 3785) | 15 | 681 (60, 1572) | 0.036 | |
| CD4+ | Pre | 21 | 249 (79, 4320) | 36 | 273 (1, 1043) | 67 | 283 (2, 1265) | 10 | 271 (47, 705) | 0.930 |
| 1 | 22 | 187 (2, 4313) | 83 | 210 (2, 851) | 123 | 193 (7, 1682) | 18 | 179 (15, 471) | 0.939 | |
| 3 | 21 | 350 (20, 856) | 73 | 237 (6, 1763) | 107 | 215 (0, 654) | 15 | 323 (5, 896) | 0.033 | |
| 6 | 18 | 340 (21, 682) | 62 | 306 (10, 1302) | 85 | 261 (21, 1082) | 15 | 346 (43, 648) | 0.274 | |
| CD8+ | Pre | 21 | 173 (6, 392) | 36 | 207 (1, 970) | 67 | 165 (2, 807) | 10 | 232 (9, 2151) | 0.531 |
| 1 | 22 | 111 (1, 1744) | 84 | 70 (3, 681) | 123 | 81 (3, 1266) | 18 | 94 (14, 293) | 0.292 | |
| 3 | 21 | 379 (7, 1208) | 74 | 96 (1, 1281) | 107 | 101 (0, 1661) | 15 | 146 (2, 1762) | <0.001 | |
| 6 | 18 | 421 (9, 834) | 62 | 142 (18, 3343) | 85 | 197 (25, 2940) | 15 | 189 (15, 1123) | 0.030 |
| Phenotype . | Month . | N . | [Bort] median cells/uL (range) . | N . | [MRD] median cells/uL (range) . | N . | [MUD] median cells/uL (range) . | N . | [MMUD] median cells/uL (range) . | p-value . |
|---|---|---|---|---|---|---|---|---|---|---|
| CD3+ | Pre | 21 | 455 (84, 4363) | 36 | 607 (1, 1551) | 67 | 519 (10, 1698) | 10 | 533 (64, 2622) | 0.872 |
| 1 | 22 | 315 (3, 5888) | 84 | 317 (5, 1692) | 123 | 311 (20, 3742) | 18 | 328 (52, 551) | 0.848 | |
| 3 | 21 | 753 (31, 1777) | 74 | 373 (8, 3114) | 107 | 386 (0, 2338) | 15 | 485 (22, 2274) | <0.001 | |
| 6 | 18 | 932 (47, 1682) | 63 | 486 (37, 3701) | 86 | 498 (59, 3785) | 15 | 681 (60, 1572) | 0.036 | |
| CD4+ | Pre | 21 | 249 (79, 4320) | 36 | 273 (1, 1043) | 67 | 283 (2, 1265) | 10 | 271 (47, 705) | 0.930 |
| 1 | 22 | 187 (2, 4313) | 83 | 210 (2, 851) | 123 | 193 (7, 1682) | 18 | 179 (15, 471) | 0.939 | |
| 3 | 21 | 350 (20, 856) | 73 | 237 (6, 1763) | 107 | 215 (0, 654) | 15 | 323 (5, 896) | 0.033 | |
| 6 | 18 | 340 (21, 682) | 62 | 306 (10, 1302) | 85 | 261 (21, 1082) | 15 | 346 (43, 648) | 0.274 | |
| CD8+ | Pre | 21 | 173 (6, 392) | 36 | 207 (1, 970) | 67 | 165 (2, 807) | 10 | 232 (9, 2151) | 0.531 |
| 1 | 22 | 111 (1, 1744) | 84 | 70 (3, 681) | 123 | 81 (3, 1266) | 18 | 94 (14, 293) | 0.292 | |
| 3 | 21 | 379 (7, 1208) | 74 | 96 (1, 1281) | 107 | 101 (0, 1661) | 15 | 146 (2, 1762) | <0.001 | |
| 6 | 18 | 421 (9, 834) | 62 | 142 (18, 3343) | 85 | 197 (25, 2940) | 15 | 189 (15, 1123) | 0.030 |
T-cell immune reconstitution parameters are improved after HLA-mismatched RIC HSCT and bortezomib-based GVHD prophylaxis. Importantly this can be accomplished with rapid hematopoietic engraftment and without increased GVHD incidence in patients without HLA-matched donors.
Off Label Use: bortezomib for GVHD control.
Author notes
Asterisk with author names denotes non-ASH members.