Abstract
Epstein-Barr virus (EBV)–driven posttransplantation lymphoproliferative disease (PTLD) is a serious complication of immunosuppression after either stem cell transplantation (SCT) or solid organ transplantation (SOT). Adoptive transfer of EBV-specific cytotoxic T lymphocytes (EBV-CTLs) is an effective prophylaxis and treatment for PTLD after SCT, but not for PTLD after SOT when pharmacologic immunosuppression cannot be discontinued. We report the generation of calcineurin (CN) mutants that render EBV-CTL resistant to the immunosuppressants tacrolimus (FK506) and cyclosporin A (CsA): mutant CNa12 confers resistance to CsA but not FK506, and mutant CNa22 confers resistance to FK506 but not CsA, whereas mutant CNb30 renders CTLs resistant to both calcineurin inhibitors. Untransduced EBV-CTLs do not proliferate in the presence of FK506/CsA. However, EBV-CTLs transduced with a retroviral vector coding for these mutants retain the ability to both proliferate and secrete normal levels of interferon-γ in the presence therapeutic levels of FK506 (CNa12), CsA (CNa22), or both (CNb30). The cytotoxicity and phenotype of EBV-CTL lines were unaffected by expression of these mutant CNs. This approach should allow effective immunotherapy with EBV-CTLs in the SOT setting without risking the graft by reduction in immunosuppression, and represents a generic approach to improving immunotherapy in the face of immunosuppression.
Introduction
Posttransplantation lymphoproliferative disease (PTLD) is a serious complication of immunosuppression after either stem cell transplantation (SCT) or solid organ transplantation (SOT) and is associated with significant mortality.1-5 B-cell proliferation leading to the development of PTLD is driven by Epstein-Barr virus (EBV), a ubiquitous γ herpesvirus which establishes latency in a pool of memory B cells after primary infection.6,7 In immunocompetent individuals, outgrowth of EBV-infected B cells is prevented by virus-specific cytotoxic T lymphocytes (EBV-CTLs).8 The lack of such an immune response early after T cell–depleted SCT, or as a result of the lifelong immune suppression necessary to prevent graft rejection after SOT, can result in uncontrolled EBV-driven B-cell proliferation and the development of PTLD.7,9
Because loss of cellular immunity to EBV plays a critical role in the pathogenesis of PTLD, adoptive immunotherapy to restore viral-specific immunity represents a logical approach to preventing this complication. In SCT, EBV-CTL lines generated ex vivo from the stem cell donor and infused prophylactically can prevent the development of PTLD in high-risk patients.10-12 Furthermore, these donor EBV-CTLs have also been shown to induce durable responses in patients with established PTLD.10,13 In these studies, immunosuppression was withdrawn or tapered before adoptive transfer of CTLs.
In contrast, in the SOT setting where immunosuppression could not be stopped, the use of EBV-CTLs has met with more limited success. Autologous EBV-CTLs generated from the organ recipient induce a transient increase in circulating EBV-CTL frequency, but variable reduction in the viral load and limited long-term clinical efficacy.14,15 A clinical response was observed in one patient despite therapeutic doses of cyclosporin A (CsA); however, the patient relapsed 10 weeks later.16 In addition, partly HLA-matched third-party EBV-CTLs induced clinical responses in 17 of 28 SOT patients with established life-threatening PTLD. However, it was notable that all patients in this study had a reduction of immunosuppression; 26 patients' suppressions stopped completely before EBV-CTL infusion.17 These results demonstrate the potential clinical utility of EBV-CTLs infused after SOT; however, the success of immunotherapy seen in PTLD after SCT has not been matched thus far.
It is likely that the function of the infused EBV-CTLs is impaired by ongoing immunosuppression after SOT. Indeed, the major immunosuppressants used in this setting, tacrolimus (FK506) and CsA, have been shown to inhibit both the proliferation and function of EBV-CTLs in vitro.18-20 Although reduction in immunosuppression is frequently used to restore some EBV-specific immunity in SOT patients with PTLD,21,22 this must be balanced with the concurrent increased risk of graft rejection. For instance, in one major series, death from graft loss was as common as death from PTLD.4 To address this, we investigated the possibility of engineering ex vivo–generated EBV-CTLs to be resistant to calcineurin inhibitors. We anticipate that these resistant CTLs could function effectively to treat PTLD in SOT patients without requiring a reduction in immunosuppression.
We focused on the interactions between calcineurin (CN) and FK506/CsA, bound by their carrier proteins FKBP12/cyclopilin A (CyPA). CN is a heterodimeric serine-threonine phosphatase which is central to T-cell activation. After engagement of the T-cell receptor, CN dephosphorylates the transcription factor NFAT, allowing it to translocate to the nucleus and activate key target genes such as IL2.23 FK506 in complex with FKBP12, or CsA in complex with CyPA, block NFAT access to CN′s active site, preventing its dephosphorylation, and thereby inhibiting T-cell activation.24,25 We modified CN at key amino acid residues to disrupt docking of either or both FK506-FKBP12 and CsA-CyPA to produce CN mutants resistant to FK506 and/or CsA.26,27 In a parallel approach, we mutated FKBP12 to disrupt its interaction with CN, thereby sequestering free FK506 in complex with mutant FKBP12. We anticipated that expression of such mutants in EBV-CTLs would render them resistant to immunosuppression.
Here, we demonstrate that EBV-CTLs can indeed be rendered resistant to the CN inhibitors CsA or FK506 by genetic engineering. This approach may allow EBV-CTLs to retain their function in immunosuppressed patients, allowing effective adoptive immunotherapy of PTLD while maintaining therapeutic immunosuppression.
Methods
Design and generation of CN and FKBP12 mutants
Crystallographic data were used to identify critical residues involved in the interaction between CN and the FK506/FKBP12 or CsA/CyPA heterodimers.26-29 These were substituted with alternatives predicted to interrupt binding based on their charge and side-chain characteristics. Mutations were generated using overlap extension polymerase chain reaction (PCR) as published previously.30 In brief, 2 fragments of CN or FKBP12 genes adjacent to the target site were amplified in initial mutagenesis PCRs using one complimentary external primer and one internal primer containing the desired sequence change. A total of 35 cycles of amplification were performed (40 seconds at 98°C, 30 seconds at 64°C, and 90 seconds at 72°C) using Phusion polymerase (NEB). Products were purifed using PCR clean-up columns (QIAGEN) and joined in a fusion PCR using the 2 external primers and amplification conditions described. CN and FKBP12 mutants were cloned as MluI-NotI fragments into the SFG-eGFP retroviral vector. This vector consists of Moloney murine leukemia virus (MMLV) long-terminal repeats LTRs driving transgene expression from the start codon of the deleted viral env gene, followed by an internal ribosomal entry site and an eGFP reporter gene.31 Codon optimization was performed in-house. First, we designing an optimal sequence based on weighted optimization of codon usage for Homo sapiens, raising GC content to 70%, reduction of local hairpins and literal repeats to a minimum, and avoidance of splice signals. Next, the entire sequence was assembled with ligation-by-PCR using Phusion polymerase from overlapping oligonucleotides (IDT DNA). Finally, the synthetic DNA was directly cloned into the retroviral vector and confirmed to be correct by capillary sequencing (ABI 3130xl Genetic Analyzer; Applied Biosystems). The sequences of the CN genes wtCNa, CNa12, CNa22, wtCNb and CNb30 have been published in GenBank, with accession numbers GQ463593-GQ463597 and are available from AddGene with reference numbers 22489-22493.
Dual luciferase assay
293T cells were plated at 7 × 104 per well in 24-well tissue-culture plates (24 wp) in Dulbecco modified Eagle medium (DMEM; Gibco, Invitrogen) containing 10% fetal bovine serum (Sigma-Aldrich) without antibiotics. The following day, cells were triple-transfected with 400 ng of NFAT-RE_FLuc NFAT–responsive Firefly luciferase plasmid (Clontech), 8 ng of phRG-TK constitutively expressed Renilla luciferase control plasmid (Promega), and 600 ng of SFG-CN plasmid using Lipofectamine 2000 (Invitrogen). Two days later, cultures were stimulated with 20 ng/mL PMA and 1 μg/mL ionomycin (both Sigma-Aldrich) with or without 10 ng/mL FK506 (Astellas Pharma) or 200 ng/mL CsA (Novartis Pharmaceuticals). The next day, a dual luciferase assay was performed according to the manufacturer's instructions (Promega) and analyzed using a FLUOstar Optima Luminometer (BMG Labtech). Firefly luciferase activity was normalized to Renilla luciferase activity to control for transfection efficiency. Results were expressed as the percentage of activity of the stimulated sample for each construct without FK506/CsA (percentage of resistance).
Generation of retrovirus
For Jurkat transductions, RD114 pseudotyped transient retroviral supes were generated by triple transfection of 4.69 μg of Peq-Pam plasmid (Moloney GagPol), 3.125 μg of RDF plasmid (RD114 envelope32 ), and 4.69 μg of SFG-CN or SFG-FKBP12 plasmids into 293T cells using GeneJuice (Novagen). Supernatant was harvested after 48 and 72 hours. High-titer producer lines were generated by multiple transduction of FLY-RD18 cells33 with GALV pseudotyped SFG retrovirus containing CN mutants, produced as described. FLY-RD18 cells were transduced daily for 2 weeks, bulk fluorescence-activated cell sorter (FACS)–sorted for high expression of eGFP, and single-cell cloned by limiting dilution; supernatant from the expanded clones was titred on 293T cells to identify the highest titer producers. These stable producer lines were used to generate high-titer retroviral supernatants for transduction of EBV-CTLs.
Transduction of Jurkat cells and EBV-CTLs
Non–tissue-culture–treated 24 wp were coated for 2 hours at 37°C with 7 μg/mL Retronectin (TaKaRa, Bio USA) in phosphate-buffered saline (PBS), washed once with PBS, and coated with 0.5 mL retrovirus for 30 minutes at room temperature. A total of 0.5 × 106 Jurkat cells or CTLs per well were transduced with 2 mL of retroviral supernatant (100 IU/mL IL-2 was added to EBV-CTLs), spun for 40 minutes at 1000g, and incubated at 37°C. EBV-CTLs were transduced 3 days after their second stimulation. Transduction efficiency was determined by expression of eGFP at 3 days for Jurkat cells and 7 days for EBV-CTLs.
Stimulation of Jurkat cell line and measurement of IL-2
Jurkat cells cultured in RPMI (Gibco, Invitrogen) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin were split 1:10 on day −1. For stimulation, 5 × 105 Jurkat cells were plated per well of a 48-well plate with 20 ng/mL PMA and 1 μg/mL ionomycin. FK506/CsA were added at increasing concentrations (0.5-40 ng/mL and 50-800 ng/mL, respectively). Cells were incubated for 24 hours, and samples of supernatant were frozen at −20°C for enzyme-linked immunosorbent assay (ELISA). To measure IL-2 secretion, the IL-2 DuoSet ELISA was used according to manufacturer's instructions (R&D Systems) and read using a FLUOstar Optima.
Generation of EBV-CTL lines
EBV-CTLs were generated as previously published.18 In brief, EBV-CTLs were cultured in 45% Click medium (Irvine Scientific), 45% RPMI, and 10% FBS (both from HyClone) supplemented with 20 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. A total of 2 × 106 peripheral blood mononuclear cells (PBMCs) were plated per well of a 24-well plate and stimulated with 5 × 104 irradiated (40 Gy) autologous lymphoblastoid cell lines (LCLs). After 9 to 11 days, 106 viable cells per well were replated into 24-well plates and stimulated with 2.5 × 105 autologous irradiated LCLs. CTLs were restimulated in this way weekly thereafter. A total of 100 U/mL IL-2 (GenScript) was added on days 13 to 14 and changed at every stimulation and twice weekly.
Phenotyping EBV-CTL lines
Four days after the fifth stimulation with autologous LCLs, CTL lines were assessed for expression of surface markers using the following monoclonal antibodies: CD3, CD4, CD19, CD25 (Becton Dickinson), CD45RO (AbD Serotec), CD8, CD16, CD56, and CD62L (eBioscience). Appropriately matched isotype controls were included. Cells were analyzed on an LSRII flow cytometer (Becton Dickinson).
Antigen dependence
To confirm that transduced EBV-CTLs remained antigen dependent, after 4 stimulations with autologous LCLs, CTLs were cultured without further stimulation and cell growth was monitored thereafter. Some cells continued to receive 100 U/mL IL-2. Cells were counted weekly and replated at 106 viable cells per well of a 24-well plate.
3H-thymidine proliferation assays
On the day of stimulation 6, 105 CTLs were plated in triplicate in U-bottomed 96-well plates, with or without stimulation by 2.5 × 104 autologous irradiated LCLs, in the presence or absence of 10 ng/mL FK506 or 200 ng/mL CsA. After 4 days, wells were pulsed with 1 μL (37 Bq) of 3H-thymidine (Perkin Elmer) and incubated for a further 19 to 20 hours, when cells were harvested to a filter mat using a Tomtec MachIIIM Harvester 96. MeltiLex wax scintillation was applied (Perkin Elmer), and thymidine incorporation was measured using a Wallac 1450 MicroBeta trilux beta-counter (Perkin Elmer). Specific proliferation was calculated by subtracting mean counts per minute (cpm) of CTL- and LCL-alone control wells from those containing CTLs stimulated with LCLs.
Interferon-γ ELISA
At 24 hours after their sixth stimulation, samples of supernatant were taken from EBV-CTL cultures and frozen at −20°C. Interferon-γ (IFN-γ) concentration was measured using the IFN-γ Duo Set ELISA kit (R&D Systems).
51Cr release cytotoxicity assays
Cytotoxicity of EBV-CTLs was determined using a standard chromium (51Cr) release assay, as previously described.10 In brief, 2 × 106 target cells (autologous/allogeneic LCLs) were incubated at 37°C with 37 MBq (1 mCi) 51Cr-labeled sodium chromate (Perkin Elmer) for 1 hour and washed, and 5 × 103 targets were cocultured with EBV-CTLs in triplicate in V-bottomed 96-well plates at effector-target ratios of 30:1, 15:1, and 7.5:1 with or without 10 ng/mL FK506 or 200 ng/mL CsA. Target cells in complete media or lysed with 1% TritonX-100 determined spontaneous and maximum release, respectively. Supernatant was harvested after 4 to 6 hours, mixed with scintillation fluid (OptiPhase Supermix; Perkin Elmer) and read using a Wallac 1450 MicroBeta trilux. Percentage of specific lysis was calculated as follows: (specific release − spontaneous release) / (maximum release − spontaneous release).
Statistical analysis
Proliferation, IFN-γ secretion, and phenotype of transduced and untransduced (UT) CTLs in the absence of CN inhibitors were compared using a one-way analysis of variance (ANOVA). Proliferation and IFN-γ secretion of transduced CTLs in the presence of CN inhibitors were compared with that of UT CTLs in the absence of CN inhibitors using a one-way ANOVA with the Dunnett post hoc test. Enrichment of GFP+ CTLs was assessed using the paired t test. Cytotoxicity was compared between transduced and UT CTLs in the presence or absence of CN inhibitors using a 2-way ANOVA. Analysis was performed using the SPSS statistical package, version 16 (SPSS).
Results
Design and generation of CN mutants
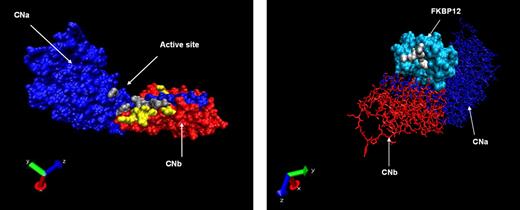
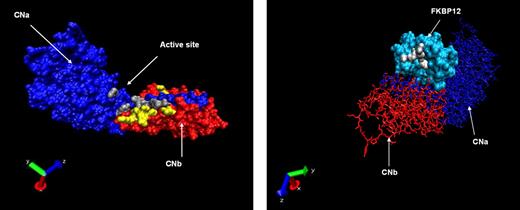
Using structural and crystallographic data from the CNa/b heterodimer in complex with FK506 or CsA, we identified critical amino acid residues involved in these interactions.27,29,34,35 These residues were targeted in the generation of CNa or CNb mutants designed to resist binding by FK506, CsA, or both CN inhibitors. A total of 22 CNa and 32 CNb mutants were generated; the location of these mutations is illustrated in Figure 1A, and details are shown in Tables 1 and 2. A parallel approach was also investigated, whereby mutants of the FK506 carrier protein FKBP12 were generated, as shown in Figure 1B.36 These were designed to bind FK506 but not CN, therefore sequestering the drug and preventing CN inhibition (Table 3).
Location of mutated residues. (A) Crystallographic representation of the heterodimer of CNa (blue chain) and CNb (red chain). Mutated residues of CNa and CNb are shown in gray and yellow, respectively. (B) Reverse projection crystallographic representation of heterodimer of FKBP12 and FK506. CNa/b chains are included for orientation. Mutated residues of FKBP12 are shown in white.
Location of mutated residues. (A) Crystallographic representation of the heterodimer of CNa (blue chain) and CNb (red chain). Mutated residues of CNa and CNb are shown in gray and yellow, respectively. (B) Reverse projection crystallographic representation of heterodimer of FKBP12 and FK506. CNa/b chains are included for orientation. Mutated residues of FKBP12 are shown in white.
CN mutants confer resistance to CN inhibitors in transient luciferase reporter assay
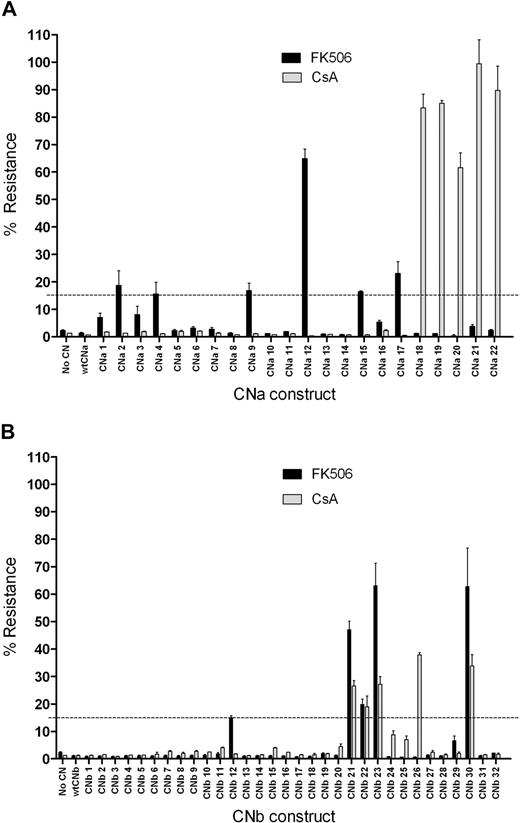
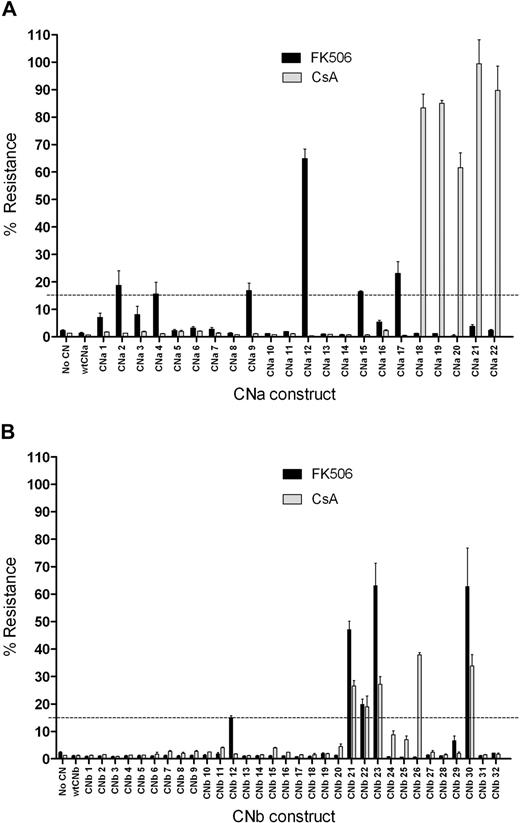
To assess the ability of these 54 CN mutants to confer resistance to CN inhibitors, mutants were initially assessed in a transient screening assay using Firefly and Renilla luciferase reporter genes. 293T cells were cotransfected with a CN mutant expression vector, an NFAT-driven Firefly luciferase (FLuc) reporter, and a constitutively expressed Renilla luciferase plasmid control. 293T cells were then activated with PMA and ionomycin in the presence or absence of CN inhibitors. FLuc expression was driven by an NFAT response element, and therefore indicated CN activity. Resistance to CN inhibitors was determined by comparing the FLuc signal from stimulated 293T cells in the presence of CN inhibitors to that in the absence of CN inhibitors. 293T cells not transfected with CN, or those transfected with wild-type (wt) CNa or wtCNb, express FLuc on activation in the absence of FK506 or CsA, but this expression is prevented by addition of either CN inhibitor (Figure 2). In contrast, transfection with CNa mutants 2, 4, 9, 12, 15, or 17 allowed some FLuc expression in the presence of FK506. Cells transfected with these mutants continued to express FLuc in the presence of FK506 at levels between 15% and 65% of that seen with stimulated 293T cells in the absence of FK506. Transfection with CNa mutants 18 through 22 resulted in between 62% and 99% resistance to CsA. Similarly, transfection with CNb mutants 12, 21, 22, 23, and 30 resulted in between 15% and 63% resistance to FK506 and transfection with CNb mutants 21, 22, 23, 26, and 30 resulted in between 18% and 38% resistance to CsA (Figure 2). We therefore selected these 11 CNa and 6 CNb mutants for further study in Jurkat T cells. Because only 6 FKBP12 mutants were generated, we proceeded directly to experiments in this cell line for these constructs.
Transfection of 293T cells with CN mutants allows luciferase expression in the presence of CN inhibitors. 293T cells were transfected with a CN mutant, a Firefly luciferase reporter gene driven by an NFAT response element, and an internal control Renilla luciferase plasmid. Cells were activated with PMA and ionomycin, and the expression of luciferase was assessed. Resistance was determined by comparing luciferase expression upon stimulation in the presence of CN inhibitors to luciferase expression upon stimulation in the absence of CN inhibitors. Mean and SEM of 3 experiments shown. (A) Screening of CNa mutants identifies 6 that confer resistance to 10 ng/mL FK506 (CNa2, 4, 9, 12, 15, and 17) and 5 that confer resistance to 200 ng/mL CsA (CNa18-CNa22). (B) Screening of CNb mutants identifies 5 that confer resistance to 10 ng/mL FK506 (CNb12, 21, 22, 23, and 30) and 5 that confer resistance to 200 ng/mL CsA (CNb21, 22, 23, 26, and 30). Of the CNb mutants identified, 4 confer resistance to both FK506 and CsA (21, 22, 23, and 30).
Transfection of 293T cells with CN mutants allows luciferase expression in the presence of CN inhibitors. 293T cells were transfected with a CN mutant, a Firefly luciferase reporter gene driven by an NFAT response element, and an internal control Renilla luciferase plasmid. Cells were activated with PMA and ionomycin, and the expression of luciferase was assessed. Resistance was determined by comparing luciferase expression upon stimulation in the presence of CN inhibitors to luciferase expression upon stimulation in the absence of CN inhibitors. Mean and SEM of 3 experiments shown. (A) Screening of CNa mutants identifies 6 that confer resistance to 10 ng/mL FK506 (CNa2, 4, 9, 12, 15, and 17) and 5 that confer resistance to 200 ng/mL CsA (CNa18-CNa22). (B) Screening of CNb mutants identifies 5 that confer resistance to 10 ng/mL FK506 (CNb12, 21, 22, 23, and 30) and 5 that confer resistance to 200 ng/mL CsA (CNb21, 22, 23, 26, and 30). Of the CNb mutants identified, 4 confer resistance to both FK506 and CsA (21, 22, 23, and 30).
Selected CN but not FKBP12 mutants allow IL-2 secretion in the presence of supratherapeutic levels of CN inhibitors
To determine whether CN/FKBP12 mutants conferred resistance to CN inhibitors using a more physiologic readout, further experiments were performed in stably transduced Jurkat T cells. This cell line secretes high levels of IL-2 in response to stimulation with PMA and ionomycin, which is abrogated by the addition of FK506 or CsA. Jurkat cells were transduced with SFG retroviral constructs31 containing one of the 17 selected CN or 6 FKBP12 mutants, and were FACS-sorted to the same level of GFP reporter gene expression. Expression of CN/FKBP12 was comparable between cultures, as demonstrated by Western blot (supplemental figure 1). Stably transduced Jurkat cells were stimulated with PMA and ionomycin in the presence of FK506 (0.5-40 ng/mL) or CsA (50-800 ng/mL), IL-2 secretion was measured by ELISA, and levels in the presence or absence of CN inhibitors were compared. As shown in supplemental Figures 2 and 3, several of these CN mutants enabled Jurkat cells to secrete normal levels of IL-2 throughout the therapeutic range of FK506 or CsA, and indeed to concentrations well above this. In contrast, none of the FKBP12 mutants conferred significant resistance to FK506 (supplemental Figure 2C). Based on these data, we selected CN mutants that conferred the highest resistance to each CN inhibitor for further study. Compared with IL-2 secretion from untransduced Jurkat cells in the absence of CN inhibitors, CNa12 enabled 79% IL-2 secretion in the presence of 10 ng/mL FK506, CNa22 enabled 280% secretion in the presence of 200 ng/mL CsA, and CNb30 enabled both 70% secretion with FK506 and 38% secretion with CsA.
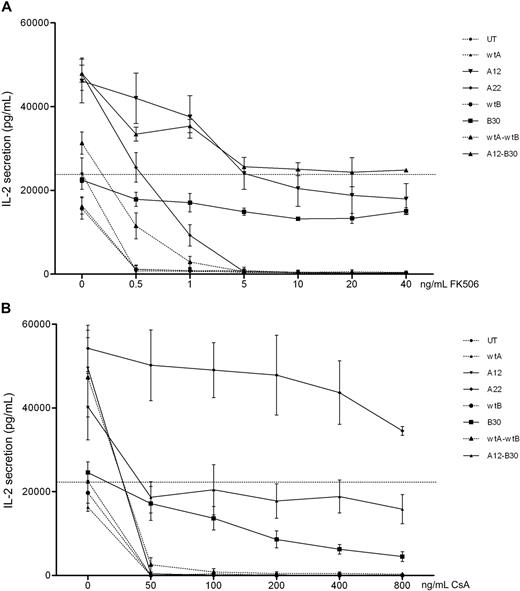
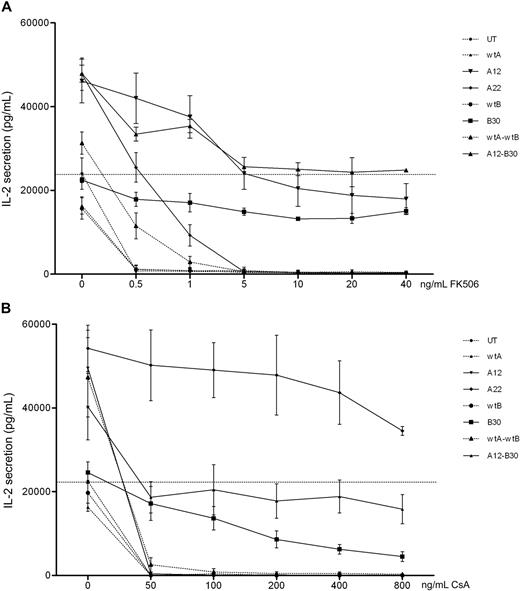
These mutants were then codon optimized for improved expression. Jurkat cells were transduced with codon-optimized constructs, FACS-sorted for comparable GFP expression, and retested. In addition, bicistronic constructs combining wild-type and mutant CNa and CNb were tested. Expression of CNa and CNb were once again assessed by Western blot and found to be comparable (supplemental figure 4). Figure 3 shows that codon-optimized CN mutants confer resistance to CN inhibitors in Jurkat cells. compared with IL-2 secretion by UT Jurkat cells without CN inhibitors, CNa12 transduced Jurkat cells were able to secrete IL-2 at 85% of this level in the presence of FK506. Similarly, transduction with CNa22 enabled IL-2 secretion in the presence of CsA (mean, 213%), and transduction with CNb30 enabled secretion of IL-2 with either FK506 (mean, 55%) or CsA (mean, 38%). The bicistronic construct of CNa12 with CNb30 allowed 101% secretion with FK506 and 79% with CsA. Based on these data, CNa12, CNa22, and CNb30 were selected for testing in EBV-CTL lines.
Jurkat cells transduced with codon-optimized CN mutants secrete IL-2 in the presence of CN inhibitors. Jurkat cells were retrovirally transduced with CN mutants and FACS sorted. Cultures were stimulated with PMA and ionomycin in the presence of increasing concentrations of FK506 (A) or CsA (B) and secretion of IL-2 assessed by ELISA. Mean and SEM of 3 experiments shown. (A) Jurkat cells transduced with CNa12, CNb30, or a bicistronic construct of these mutants were able to secrete IL-2 throughout the therapeutic range of FK506 (7-12 ng/mL). (B) Jurkat cells transduced with CNa22, CNb30, or the double construct CNa12-CNb30 were able to secrete IL-2 throughout the therapeutic range of CsA (100-250 ng/mL). Selected constructs CNa12, CNa22, CNb30, and CNa12-CNb30 are shown with solid lines; all other constructs are shown with dotted lines. The horizontal line indicates the amount of IL-2 secreted by untransduced Jurkat cells stimulated without CN inhibitors.
Jurkat cells transduced with codon-optimized CN mutants secrete IL-2 in the presence of CN inhibitors. Jurkat cells were retrovirally transduced with CN mutants and FACS sorted. Cultures were stimulated with PMA and ionomycin in the presence of increasing concentrations of FK506 (A) or CsA (B) and secretion of IL-2 assessed by ELISA. Mean and SEM of 3 experiments shown. (A) Jurkat cells transduced with CNa12, CNb30, or a bicistronic construct of these mutants were able to secrete IL-2 throughout the therapeutic range of FK506 (7-12 ng/mL). (B) Jurkat cells transduced with CNa22, CNb30, or the double construct CNa12-CNb30 were able to secrete IL-2 throughout the therapeutic range of CsA (100-250 ng/mL). Selected constructs CNa12, CNa22, CNb30, and CNa12-CNb30 are shown with solid lines; all other constructs are shown with dotted lines. The horizontal line indicates the amount of IL-2 secreted by untransduced Jurkat cells stimulated without CN inhibitors.
CN mutant transduced EBV-CTLs show a selective growth advantage in the presence of CN inhibitors
To evaluate the ability of CN mutants to confer resistance to CN inhibitors in primary cells, we tested the CN mutants identified here in EBV-CTL lines generated from 5 healthy donors. Stable retrovirus producer lines were generated to produce high-titer RD114 pseudotyped SFG retrovirus carrying the eGFP transgene alone or expressed with CNa12, CNa22, or CNb30, and supernatant from these cells was used to transduce CTLs.
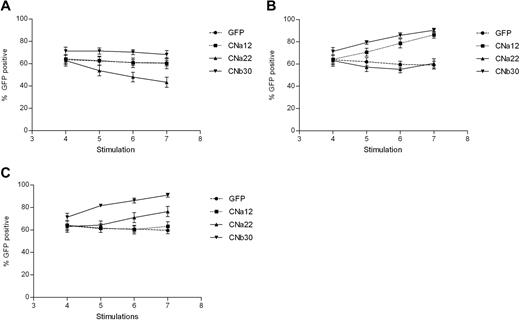
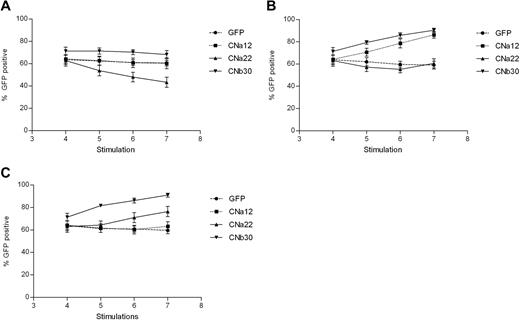
Transduction efficiency was assessed before the addition of CN inhibitors and at each stimulation thereafter. Transduction efficiencies were consistently high, between 50% and 85% in all 5 donors (GFP mean, 64% [range, 54%-77%]; CNa12 mean, 64% [52%-75%]; CNa22 mean, 63% [53%-81%]; and CNb30 mean, 71% [63%-82%]). As shown in Figure 4A, EBV-CTLs transduced with GFP, CNa12, and CNb30 vectors showed stable GFP expression throughout subsequent culture when grown with no CN inhibitors, indicating that transduced CTLs do not have an intrinsic proliferative advantage over UT CTLs (P = .239, .24, and .309, respectively). The proportion of transduced EBV-CTLs in cultures transduced with CNa22 gradually dropped from 63% to 43% (P = .004), which may suggest a toxic effect.
EBV-CTLs transduced with CN mutants show a selective advantage when cultured in FK506/CsA. Retrovirally transduced EBV-CTL lines were grown in the presence or absence of CN inhibitors for 3 weeks, and the percentage of cells expressing GFP were assessed by flow cytometry at various time points. Mean GFP expression ± SEM are shown (n = 5). (A) Expression of GFP when cultured without CN inhibitors. The proportion of transduced CTLs remains stable in all but the CNa22 culture, where the number of transduced cells drops. (B) Addition of 10 ng/mL FK506 to cultures results in an enrichment of GFP expressing CTLs in those that are resistant to FK506 (CNa12 and CNb30). (C) Addition of 200 ng/mL CsA to CTL cultures results in a rise in GFP-expressing CTLs in CsA-resistant cultures (CNa22 and CNb30).
EBV-CTLs transduced with CN mutants show a selective advantage when cultured in FK506/CsA. Retrovirally transduced EBV-CTL lines were grown in the presence or absence of CN inhibitors for 3 weeks, and the percentage of cells expressing GFP were assessed by flow cytometry at various time points. Mean GFP expression ± SEM are shown (n = 5). (A) Expression of GFP when cultured without CN inhibitors. The proportion of transduced CTLs remains stable in all but the CNa22 culture, where the number of transduced cells drops. (B) Addition of 10 ng/mL FK506 to cultures results in an enrichment of GFP expressing CTLs in those that are resistant to FK506 (CNa12 and CNb30). (C) Addition of 200 ng/mL CsA to CTL cultures results in a rise in GFP-expressing CTLs in CsA-resistant cultures (CNa22 and CNb30).
We hypothesized that in the presence of CN inhibitors, CN mutants providing resistance should confer a selective growth advantage to transduced T cells within the CTL line, leading to an enrichment of transduced cells. This is indeed what we observed. In the presence of 10 ng/mL FK506, GFP expression remained stable in GFP and CNa22 transduced CTL lines (Figure 4B). In contrast, the proportion of GFP+ cells rose with time in cultures transduced with CNa12 (mean, 64%-86%; P = .002) and CNb30 (mean, 71%-90%; P = .01). Similarly, as can be seen in Figure 4C in the presence of 200 ng/mL CsA, GFP expression in GFP and CNa12 transduced cultures remained stable, whereas GFP expression rose with time in CNa22 transduced (mean, 63%-76%; P = .107) and CNb30 transduced (mean, 71%-91%; P = .01) CTLs. These data suggest that CTLs transduced with CN mutants have a selective advantage in the presence of the appropriate CN inhibitor, but retain similar growth kinetics as that of untransduced CTLs when grown without CN inhibitors.
Transduction of EBV-CTL lines with CN mutants allows growth in the presence of CN inhibitors
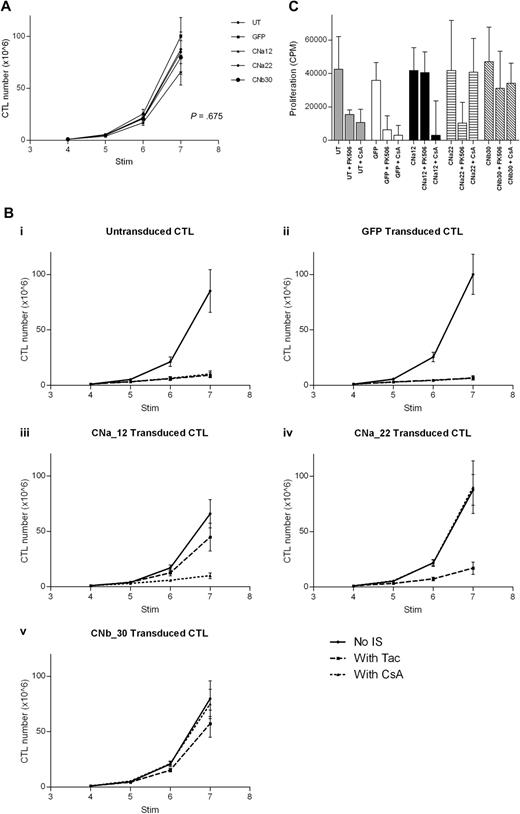
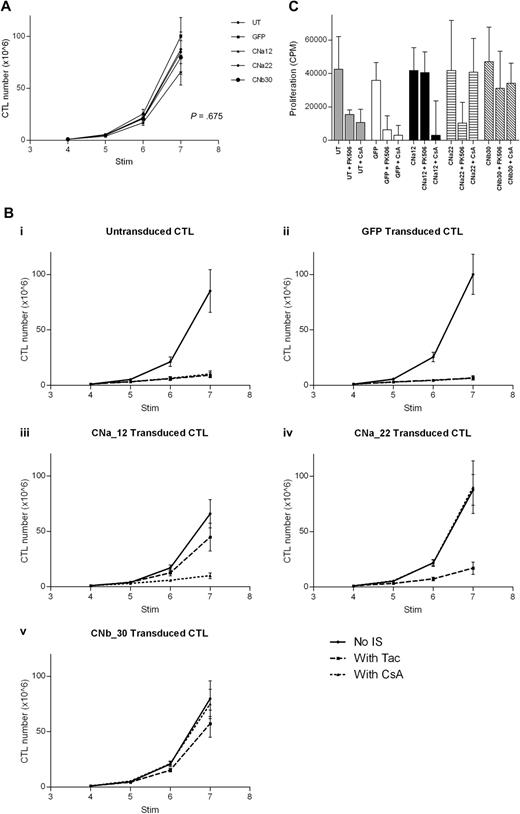
Proliferation of transduced EBV-CTL cultures was monitored for 3 weeks from addition of CN inhibitors at the fourth stimulation. In the absence of CN inhibitors, transduced CTL lines grew at a comparable rate to that of untransduced CTL lines (Figure 5A; P = .675). Importantly, this demonstrates that transduction with either GFP or CN mutants has no effect on the growth of EBV-CTLs.
Transduction with CN mutants allows EBV-CTL proliferation in the presence of CN inhibitors. EBV-CTLs were transduced with either a CN mutant or with GFP control after stimulation 2. CN inhibitors were added at stimulation 4, and CTL growth was assessed for 3 weeks (mean and SEM shown, n = 5). (A) In the absence of CN inhibitors, growth of transduced CTLs and untransduced CTLs is comparable over 3 weeks (P = .675). (B) Expansion of EBV-CTLs over time with or without FK506/CsA. (Bi) Untransduced CTLs are suppressed by either FK506 or by CsA. (Bii) GFP-transduced CTLs are suppressed by either CN inhibitor. (Biii) CNa12-transduced CTLs proliferate in the presence of FK506 but not CsA. (Biv) CNa22-transduced CTLs proliferate in the presence of CsA but not FK506. (Bv) CNb30-transduced CTLs proliferate in the presence of either FK506 or CsA. (C) 3H-thymidine uptake assay after stimulation with autologous LCLs in the presence or absence of CN inhibitors. CNa12 confers 90% resistance to FK506, CNa22 confers 103% resistance to CsA, and CNb30 confers both 83% resistance to FK506 and 86% resistance to CsA (median and interquartile range shown, n = 5).
Transduction with CN mutants allows EBV-CTL proliferation in the presence of CN inhibitors. EBV-CTLs were transduced with either a CN mutant or with GFP control after stimulation 2. CN inhibitors were added at stimulation 4, and CTL growth was assessed for 3 weeks (mean and SEM shown, n = 5). (A) In the absence of CN inhibitors, growth of transduced CTLs and untransduced CTLs is comparable over 3 weeks (P = .675). (B) Expansion of EBV-CTLs over time with or without FK506/CsA. (Bi) Untransduced CTLs are suppressed by either FK506 or by CsA. (Bii) GFP-transduced CTLs are suppressed by either CN inhibitor. (Biii) CNa12-transduced CTLs proliferate in the presence of FK506 but not CsA. (Biv) CNa22-transduced CTLs proliferate in the presence of CsA but not FK506. (Bv) CNb30-transduced CTLs proliferate in the presence of either FK506 or CsA. (C) 3H-thymidine uptake assay after stimulation with autologous LCLs in the presence or absence of CN inhibitors. CNa12 confers 90% resistance to FK506, CNa22 confers 103% resistance to CsA, and CNb30 confers both 83% resistance to FK506 and 86% resistance to CsA (median and interquartile range shown, n = 5).
The addition of either FK506 or CsA to EBV-CTL cultures markedly inhibited growth of both untransduced and GFP-transduced lines (Figure 5Bi and 5Bii; P = .001). Transduction of EBV-CTLs with CNa12 enabled growth in FK506 but not CsA (Figure 5Biii). CNa12 transduced CTLs expanded by a mean of 66-fold over 3 weeks in the absence of CN inhibitors, and 45-fold in the presence of FK506. compared with the growth of untransduced EBV-CTLs without CN inhibitors, which expanded by a mean of 85-fold, this represents 52% resistance to FK506 by CNa12 transduced EBV-CTLs (P = .216). CNa12 does not confer resistance to CsA (P = .001). As shown in Figure 5Biv, transduction with CNa22 confers resistance to CsA, allowing 90-fold expansion over 3 stimulations in the presence of CsA (106% resistance; P > .999) but not to FK506 (P = .004). Finally, transduction of EBV-CTLs with CNb30 confers resistance to both FK506 and CsA (Figure 5Bv). In the presence of FK506, CNb30 transduced EBV-CTLs expand 57-fold (67% resistance; P = .649), and in the presence of CsA, 75-fold expansion is seen (88% resistance; P > .999).
Proliferation of transduced EBV-CTLs in the presence or absence of CN inhibitors was also assessed using 3H-thymidine uptake assays after their sixth stimulation with LCLs, after 2 weeks of culture in CN inhibitors. In the absence of CN inhibitors, proliferation of all transduced CTLs was comparable with that of UT CTLs (P = .876). As shown in Figure 5C, the observed resistance profile in this assay was similar to that seen in Figure 5B. Untransduced or GFP transduced CTLs showed reduced proliferation in the presence of either FK506 or CsA, as previously reported.18 In contrast, CNa12 transduced CTLs were able to proliferate in the presence of FK506 but not CsA (mean proliferation compared with untransduced CTLs in the absence of CN inhibitors was 90%/23%; P > .999 and P = .02, respectively) and CNa22 transduced CTLs showed the converse pattern (mean proliferation compared with UT controls was 26%/103%; P = .03/P > .999, respectively). Once again, transduction with CNb30 enabled CTLs to proliferate in the presence of either FK506 or CsA (mean 83%/86%; P = .996/P = .999, respectively). Furthermore, CTLs transduced with CNb30 retained the ability to proliferate in supratherapeutic levels of FK506 (40 ng/mL) and CsA (800 ng/mL; supplemental Figure 5).
Transduction with CN mutants enables cytokine secretion in the presence of CN inhibitors
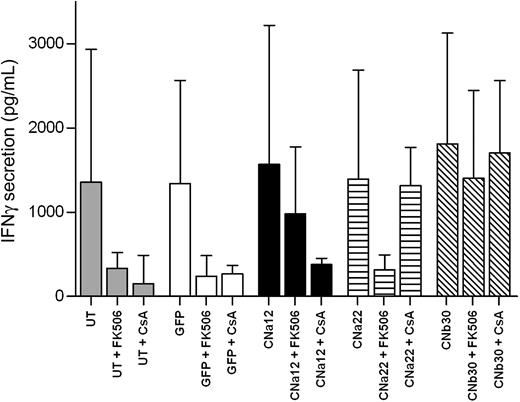
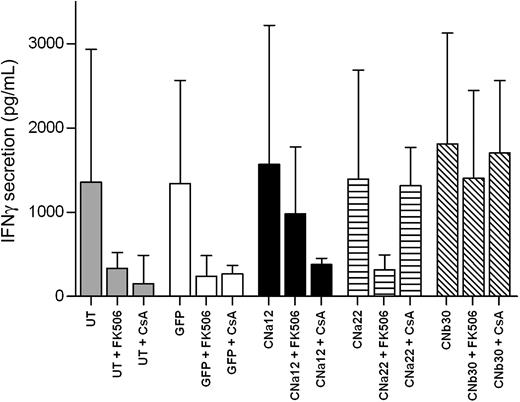
To further examine the functionality of EBV-CTLs transduced with our CN mutants, we assessed the ability of such CTLs to secrete the Th1 cytokine IFN-γ in the presence of CN inhibitors. IFN-γ secretion in culture supernatants was measured 24 hours after stimulation with autologous LCL in the presence/absence of FK506/CsA. As shown in Figure 6, transduced and UT CTLs secrete similarly high levels of IFN-γ in response to stimulation with autologous LCLs (P = .984). In UT/GFP-transduced CTLs, this is abrogated by the addition of either 10 ng/mL FK506 or 200 ng/mL CsA (P = .057 and P = .046, respectively). In contrast, EBV-CTLs transduced with CNa12 were able to secrete IFN-γ in the presence of FK506 (mean, 66% secretion compared with untransduced CTLs in the absence of CN inhibitors [P = .914]), but not CsA (mean, 17% secretion; P = .062). Similarly, CNa22-transduced CTLs secreted IFN-γ in the presence of CsA (mean, 66%; P = .917), but not FK506 (mean, 16%; P = .059). As expected, CTLs transduced with CNb30 were able to secrete IFN-γ in the presence of either FK506 (mean, 92%; P > .999) or CsA (mean, 100%; P > .999).
EBV-CTLs transduced with CN mutants secrete normal levels of IFN-γ in the presence of CN inhibitors. EBV-CTL lines cultured in the presence of CN inhibitors for 2 weeks were stimulated with autologous LCLs, and IFN-γ secretion was assessed by ELISA 24 hours after stimulation. Median and interquartile range are shown (n = 5). CTLs transduced with CNa12 were able to secrete IFN-γ in the presence of 10 ng/mL FK506 at comparable levels to that seen with untransduced CTLs in the absence of CN inhibitors (mean, 66%) and CNa22-transduced CTLs secreted IFN-γ in the presence of 200 ng/mL CsA (mean, 66%). CNb30-transduced CTLs secreted IFN-γ with either FK506 (mean, 92%) or CsA (mean, 100%).
EBV-CTLs transduced with CN mutants secrete normal levels of IFN-γ in the presence of CN inhibitors. EBV-CTL lines cultured in the presence of CN inhibitors for 2 weeks were stimulated with autologous LCLs, and IFN-γ secretion was assessed by ELISA 24 hours after stimulation. Median and interquartile range are shown (n = 5). CTLs transduced with CNa12 were able to secrete IFN-γ in the presence of 10 ng/mL FK506 at comparable levels to that seen with untransduced CTLs in the absence of CN inhibitors (mean, 66%) and CNa22-transduced CTLs secreted IFN-γ in the presence of 200 ng/mL CsA (mean, 66%). CNb30-transduced CTLs secreted IFN-γ with either FK506 (mean, 92%) or CsA (mean, 100%).
Transduced EBV-CTL lines remain dependent on autologous LCL stimulation for proliferation
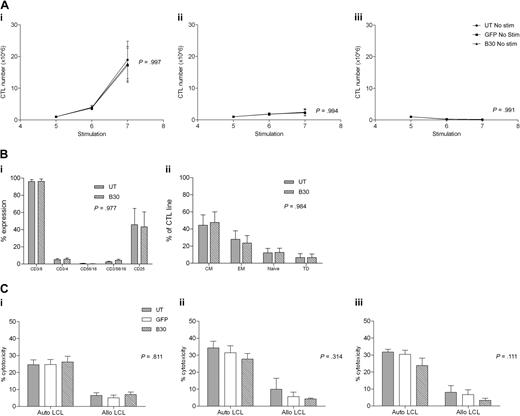
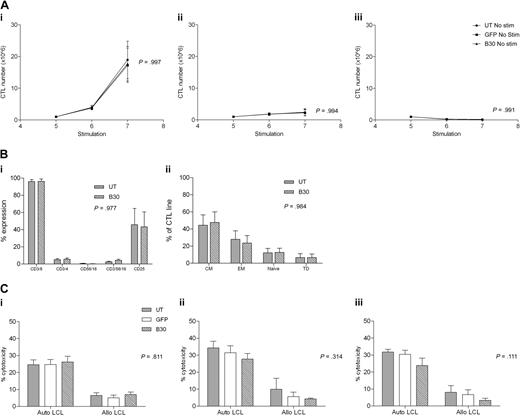
To ensure that CN transduced EBV-CTLs remain dependent on antigen stimulation, we examined whether transduced or UT CTLs were able to proliferate autonomously in the absence of LCL stimulation. CNb30 was chosen for these studies because it conferred resistance to both FK506 and CsA and hence is the mutant we plan to use in subsequent translational work. CTLs were cultured as normal for 4 stimulations from when LCL stimulation was withheld and CTL growth assessed. As demonstrated in Figure 7A, UT CTLs did not proliferate in the absence of stimulation with LCL. Similarly, GFP transduced and CNb30 transduced CTLs also did not grow without LCL stimulation, regardless of continued supplementation with 100 U/mL exogenous IL-2 (no LCL + IL-2, P = .994; no LCL/no IL-2, P = .991).
Antigen dependence, phenotype, and cytotoxicity of EBV-CTLs are unaffected by transduction with CNb30. (A) Transduced and untransduced EBV-CTLs were grown as normal for 4 stimulations. Proliferation was monitored from the day of stimulation 5. Mean and SEM shown (n = 5). (Ai) Growth of CTLs continuing to receive weekly stimulation with autologous LCLs and exogenous IL-2. (Aii) CTLs that receive no LCL stimulation do not proliferate, even with continuing supplement of 100 U/mL IL-2. (Aiii) CTLs that do not receive LCL stimulation or IL-2 do not proliferate. There is no difference in proliferation between transduced and untransduced CTL lines under any of these conditions. (B) EBV-CTLs were assessed for expression of surface markers 4 days after their fifth stimulation with autologous LCLs. Mean and SEM shown (n = 5). (Bi) Percentage of expression of CD3, CD4, CD8, CD16/56, and CD25 in untransduced and CNb30-transduced lines. (Bii) Distribution of memory subsets in untransduced and CNb30-transduced lines. CM indicates central memory (CD45RO+, CD62L+); EM, effector memory (CD45RO+, CD62L−); naive (CD45RO−, CD62L+); and TD, terminally differentiated (CD45RO−, CD62L−). (C) A 51Cr release cytotoxicity assay was performed to assess cytotoxicity against autologous or allogeneic LCL targets of EBV-CTL lines cultured for 2 to 3 weeks in the presence or absence of CN inhibitors (n = 5). Mean and SEM cytotoxicity at an effector-target ratio of 30:1 are shown. (Ci) After culture in the absence of CN inhibitors. (Cii) After 2 to 3 weeks of culture in 10 ng/mL FK506. (Ciii) After 2 to 3 weeks of culture in 200 ng/mL CsA. No effect of CN inhibitors was detected on cytotoxicity of transduced or untransduced EBV-CTLs. CN inhibitors were present during the cytotoxicity assay in panels Cii and Ciii.
Antigen dependence, phenotype, and cytotoxicity of EBV-CTLs are unaffected by transduction with CNb30. (A) Transduced and untransduced EBV-CTLs were grown as normal for 4 stimulations. Proliferation was monitored from the day of stimulation 5. Mean and SEM shown (n = 5). (Ai) Growth of CTLs continuing to receive weekly stimulation with autologous LCLs and exogenous IL-2. (Aii) CTLs that receive no LCL stimulation do not proliferate, even with continuing supplement of 100 U/mL IL-2. (Aiii) CTLs that do not receive LCL stimulation or IL-2 do not proliferate. There is no difference in proliferation between transduced and untransduced CTL lines under any of these conditions. (B) EBV-CTLs were assessed for expression of surface markers 4 days after their fifth stimulation with autologous LCLs. Mean and SEM shown (n = 5). (Bi) Percentage of expression of CD3, CD4, CD8, CD16/56, and CD25 in untransduced and CNb30-transduced lines. (Bii) Distribution of memory subsets in untransduced and CNb30-transduced lines. CM indicates central memory (CD45RO+, CD62L+); EM, effector memory (CD45RO+, CD62L−); naive (CD45RO−, CD62L+); and TD, terminally differentiated (CD45RO−, CD62L−). (C) A 51Cr release cytotoxicity assay was performed to assess cytotoxicity against autologous or allogeneic LCL targets of EBV-CTL lines cultured for 2 to 3 weeks in the presence or absence of CN inhibitors (n = 5). Mean and SEM cytotoxicity at an effector-target ratio of 30:1 are shown. (Ci) After culture in the absence of CN inhibitors. (Cii) After 2 to 3 weeks of culture in 10 ng/mL FK506. (Ciii) After 2 to 3 weeks of culture in 200 ng/mL CsA. No effect of CN inhibitors was detected on cytotoxicity of transduced or untransduced EBV-CTLs. CN inhibitors were present during the cytotoxicity assay in panels Cii and Ciii.
Transduction with GFP or CNb30 does not affect the phenotype or cytotoxicity of EBV-CTL lines
To determine whether transduction with CN mutants altered the phenotype of EBV-CTL, flow cytometric analysis was performed on untransduced or CNb30 transduced CTLs 3 weeks after transduction. As shown in Figure 7B, CTLs transduced with CNb30 showed a similar phenotype to untransduced CTLs, with a predominance of CD8+ CD45RO+ central or effector memory T cells.
Finally, we performed cytotoxicity assays to determine the effect of retroviral transduction with CNb30 on the cytotoxic activity of EBV-CTL lines. To assess this, 51Cr release cytotoxicity assays were performed after 2 to 3 weeks of culture in 10 ng/mL FK506 or 200 ng/mL CsA (6 days after stimulation 5 or 6). As illustrated in Figure 7Ci, in the absence of immunosuppression, cytotoxicity against autologous LCL targets was similar in untransduced, GFP transduced, and CNb30 transduced CTLs (mean, 24.6%, 24.7%, and 26.2%, respectively, at an effector-target ratio of 30:1; P = .811). Prior culture of CTLs in either FK506 (Fig 7Cii) or CsA (Fig 7Ciii), and inclusion of these in the cytotoxicity assay, did not significantly affect cytotoxic activity against autologous LCLs. Neither untransduced or transduced CTLs showed significant cytotoxicity against allogeneic LCLs, confirming that CTL lines are EBV specific and MHC restricted, and that transduction with our CN mutants does not increase alloreactivity (UT mean, 6.5%; GFP, 5%; CNb30, 6.9% at effector-target ratio of 30:1; P = .811). These data demonstrate that the cytotoxicity of EBV-CTL lines is not affected by either retroviral transduction with CNb30, or by the presence of therapeutic doses of CN inhibitors.
Discussion
Adoptive immunotherapy with EBV-specific CTLs has been given to more than 200 patients and is a highly effective strategy for both treatment and prophylaxis for PTLD after SCT.37 However, clinical studies using EBV-CTLs after SOT have been disappointing, most likely due to the inhibitory effect of ongoing pharmacologic immunosuppression on infused CTLs.19 To address this problem, we engineered EBV-CTLs to be resistant to suppression with CN inhibitors and have identified 3 CN mutants which enable EBV-CTLs to function in the presence of these drugs: CNa12 renders EBV-CTLs resistant to FK506 and CNa22 renders EBV-CTLs resistant to CsA, whereas CNb30 confers resistance to both.
Binding of FK506 and CsA to their chaperone proteins FKBP12 and CyPA, respectively, leads to docking of these small-molecule/protein complexes with the CN heterodimer, sterically blocking entry and subsequent activation of NFAT. The interactions between these complexes and CN occur at a common site at the CNa/CNb interface. This site is distinct from the CN active site, allowing design of mutations which inhibit docking of either or both FK506/FKBP12 and CsA/CyPA complexes, but which do not affect NFAT dephosphorylation. CNa12 was based on a novel combination of mutations first identified by Kawamura;28 we altered CNa by introducing substitutions T351E and L354A. These mutations should disrupt binding between CNa and the charged surface residues H87-P88 of FKBP12,38 but should not affect CsA/CyPA binding. CNa22 contains the mutations V314R, which directly disrupts CsA binding and Y341F,35 which could prevent FKBP12/CyPA binding by steric inhibition of close contact with the body of CNa. CNb30 has both the point mutation L124T39 and the insertion K125-LA in the latch region (based on the naturally occurring K125-VQ40 ) disrupting the folding structure, which is involved in binding of CNb to CNa but also in binding of FKBP12/CyPA to the CN heterodimer.39
Growth of transduced and untransduced CTL lines was assessed in the presence of therapeutic doses of either FK506 or CsA. As expected, addition of either to untransduced or control GFP transduced EBV-CTLs inhibited proliferation. We compared the growth of EBV-CTLs without CN inhibitors over a 3-week culture period with that of EBV-CTLs transduced with CN mutants in the face of therapeutic doses of either FK506 or CsA. CNa12 transduced CTLs proliferated at rates comparable with that of control CTLs in the presence of 10 ng/mL FK506, CNa22 transduced CTLs proliferated well in the presence of 200 ng/mL CsA, and EBV-CTLs transduced with CNb30 were resistant to suppression by either FK506 or CsA. Further, we have also demonstrated that these transduced EBV-CTLs retain functionality against EBV-infected target cells in the presence of CN inhibitors: whereas secretion of IFN-γ in response to LCL stimulation by untransduced EBV-CTLs was abrogated by either CsA or FK506, transduced CTLs showed equivalent secretion of IFN-γ in the presence or absence of CN inhibitors in the same pattern as seen with our proliferation data. Finally, we have shown that transduction with CNb30 (our favored construct) has no effect on either the phenotype or cytotoxic activity of EBV-CTLs. In particular, CNb30 transduced CTLs showed no increase in alloreactivity in cytotoxicity assays.
The CN heterodimer is a pivotal signal transduction molecule in mediating transcriptional activation of T cells after calcium flux from T-cell receptor (TCR) engagement. One potential unwanted consequence of our engineering is autonomous or aberrant activity of a mutant CN. Alternatively, simple overexpression of CN might lead to hyperactivation of transduced cells after stimulation. In transduced Jurkat cells, none of the 54 mutant CNs allowed autonomous IL-2 secretion; however some increased production of IL-2 from CN-transduced cultures was observed upon stimulation with PMA and ionomycin compared with UT control. This effect was more pronounced in cells transduced with CNa than CNb, which is likely to reflect CNa as the limiting component of the CNa/CNb heterodimer39 (supplemental figure 2). However, in primary EBV-CTLs, expression of CN mutants had no effect on cell proliferation in the absence of CN inhibitors (Figure 5A) with or without stimulation. Importantly, no EBV-CTL expansion was observed upon removal of LCL stimulation in transduced lines even when supplemented with exogenous IL-2, confirming that proliferation remains entirely antigen-dependent.
Generation of CTLs that can function in the presence of immunosuppression represents a considerable advance for the immunotherapy of PTLD and may enable extension of the benefits of this approach to the SOT setting. Potentially, adoptive transfer of autologous CN inhibitor–resistant EBV-specific CTLs could be used as prophylaxis for PTLD in high-risk groups, such as in patients undergoing pediatric small-bowel transplantation, where the risk of PTLD may be as high as 30%.41 In cohorts at lower risk of PTLD, resistant EBV-CTLs could be used as adjunctive therapy for established PTLD with rituximab. In this situation, first-line therapy with rituximab could be used to establish disease control during the time required for generation of the EBV-CTLs, with subsequent transfer of resistant CTLs to maintain remission and overcome the significant rates of partial response and relapse associated with rituximab monotherapy.42-44 Critically, such a strategy would obviate the need for reduction in immunosuppression with calcineurin inhibitors, which, as noted, is a frequent cause of rejection and treatment failure.4 Importantly, the methodology we used has previously been used to generate therapeutic cell products for more than 100 patients; therefore, we are confident these EBV-CTLs will have clinical efficacy. As a prelude to such clinical studies, we are currently testing the efficacy of EBV-CTLs transduced with CNb30 in preventing or treating EBV–lymphoproliferative disease (LPD) in the presence of CN inhibitors in a xenogeneic γ-chain−/RAG2−/C5− mouse model.45,46
Other factors may limit the effectiveness of resistant EBV-CTLs to treat PTLD in the SOT setting. Firstly, the efficacy of infused EBV-CTLs after SCT may have been enhanced by the availability of an immunologic niche due to the lymphodepletion caused by conditioning, a situation clearly different from SOT. However, EBV-CTLs have proven functional without prior lymphodepletion.47 In addition, the selective advantage-resistant EBV-CTLs have in an immunosuppressed host may result in effects equivalent to that of pharmacologic conditioning. Second, the requirement for continued antigen stimulation to promote survival of some types of adoptively transferred T cells may limit their use as long-term prophylaxis or treatment. In fact, EBV-CTLs generated as we have have shown remarkable long-term survival in vivo: gene-marked EBV-CTLs have been detected in peripheral blood of patients many years after infusion, often increasing in response to rise in EBV viral load.48 Finally, although CN inhibitors form the cornerstone of immunosuppressive regimens in SOT, these agents are often combined with others, particularly mycophenolate (MMF). This agent is likely dispensable in the face of PTLD. If resistant EBV-CTLs are to be given as prophylaxis in a clinical setting where MMF is indispensible, it should be possible to coexpress a previously described mutant IMPDH2 that will render T cells resistant to MMF49 in addition to FK506/CyA.
Our approach represents a generic means of enabling adoptively transferred T cells to function in the face of ongoing immunosuppression and has important broader applicability. Potentially, this could be used to enable adoptively transferred T cells with specificity for other viruses to function despite ongoing immunosuppression in either the SCT or SOT settings. Similarly, our approach may enable T cells redirected to recognize leukemia-associated antigens or cell-surface molecules by TCR50 or chimeric antigen receptor51 (CAR) transfer to be administered early after SCT, without the need for withdrawal of immunosuppression and the attendant risk of graft-versus-host disease (GVHD). In this regard, the small size of our preferred mutant CNb30 (approximately 500 bp) will allow coexpression of other useful transgenes, including TCRs, CARs, other resistance genes (eg, IMPDH2),49 and suicide genes.52 Potentially, administration of calcineurin inhibitors after transfer of T cells transduced with our mutants may enable in vivo selection for transduced cells expressing these other transgenes. Further, there may be clinical utility to using a CN mutant which renders EBV-CTLs resistant to only one CN inhibitor, because these CTLs could be suppressed using the alternative CN inhibitor if adverse effects were observed.
In summary, we report the generation of CN mutants that confer resistance to either or both FK506 and CsA. EBV-CTLs transduced with these mutants retain the capacity to proliferate, secrete cytokines, and kill target cells in the presence of therapeutic levels of CN inhibitors. These resistant T cells could increase the efficacy of immunotherapy for PTLD after SOT, and may have wider applications for immunotherapy in other settings.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is supported by the British Heart Foundation (J.B.), the Leukaemia Research Fund (C.M.), and the Medical Research Council (M.P.). The authors are grateful to the Institute of Child Health statistics support service for statistical advice.
Authorship
Contribution: J.B., M.P. and P.J.A. designed the work; J.B. and M.P. designed and generated the CN mutants; M.P. codon-optimized CNa and CNb; J.B. performed experiments and analyzed data; C.M., K.S., H.K., and S.S. gave assistance in the laboratory; J.B., M.P., and P.J.A. wrote the manuscript; and all authors read and commented on the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. Pule, Department of Haematology, UCL Cancer Institute, 72 Huntley St, London, United Kingdom WC1E 6BT; e-mail: m.pule@ucl.ac.uk.
References
Author notes
M.P. and P.J.A. contributed equally to this work.