Abstract
Several recent reports have suggested that in vitro exposure of CD4+ T cells to rabbit antithymocyte globulin (rATG), which is commonly used to prevent and treat graft-versus-host disease and allograft rejection, is an effective method to induce CD4+CD25+FOXP3+ T regulatory cells (Tregs). We and others, however, have shown that FOXP3 is also expressed in activated T cells. We therefore investigated whether the induction of FOXP3 expression by rATG resulted in a stable population of suppressive Tregs. We found that exposure of peripheral blood mononuclear cells (PBMCs) or conventional T cells to rATG resulted in induction of transient rather than stable expression of CD25 and FOXP3. Furthermore, rATG-treated T effector cells acquired neither an immunosuppressive profile of cytokine production nor suppressive capacity, even at the time of maximal FOXP3 expression. These findings indicate that the notion that rATG can be used to induce Tregs in vitro for cellular therapy in vivo should be re-evaluated.
Introduction
Antithymocyte globulins (ATG) is a potent immunosuppressive agent used to prevent and treat allograft rejection1 and graft-versus-host disease.2 Historically, ATG activity was thought to result from the depletion of lymphocytes through complement-dependent lysis or Fas/Fas ligand–mediated activation-induced cell death.1,3 Emerging evidence suggests that the immunosuppressive activity of ATG may also result from its effects on CD4+CD25+FOXP3+ regulatory T cells (Tregs) because it was reported to preferentially deplete conventional T (Tconv) cells and spare Tregs.4,5 Moreover, in vitro exposure of T cells to ATG increased expression of 2 proteins characteristic of Tregs, CD256-10 and FOXP3,6,7,9,10 and resulted in acquisition of suppressive activity. These reports led to speculation that in vitro exposure to ATG might be an effective method to induce FOXP3+ Tregs for clinical applications.6-8,11-14
It is well established that human CD4+ Tconv cells express FOXP3 transiently as a normal consequence of activation15-17 ; only when FOXP3 expression is sustained at high levels do functional Tregs develop.18 Because previous reports on the effects of ATG on FOXP3 expression and Treg development did not take this into account, we investigated the kinetics and stability of rATG-induced expression of FOXP3 and the development of suppressive T cells.
Methods
Cell isolation and culture
Peripheral blood was obtained from healthy volunteers after approval by the University of British Columbia Clinical Research Ethics Board after obtaining written informed consent in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation (StemCell Technologies). CD4+ T cells were purified by negative selection (StemCell Technologies), and sorted into CD25− (Tconv) or CD25hi (Tregs) T cells on a FACSAria cell sorter (Becton Dickinson) to purity more than 95%.19 Contaminating CD25−FOXP3+ cells were less than 1%. Antigen-presenting cells (APCs) were CD3-depleted PBMCs (purity > 95%; StemCell Technologies). T cells (106/mL) were incubated in X-VIVO 15 medium (Cambrex) with 5% AB human serum (Cambrex), penicillin, streptomycin, glutamax (Invitrogen), and interleukin-2 (IL-2; 100 U/mL; Chiron) in the absence or presence of rATG (thymoglobulin [Genzyme] or control rabbit immunoglobulin G [rIgG; Sigma-Aldrich]) at concentrations from 1 μg/mL to 100 μg/mL. As a control Tconv cells were activated with anti-CD3/CD28 (Invitrogen)–coated beads at a 1:16 (bead-cell) ratio.
Flow cytometric analysis
All antibodies were from BD Pharmingen except for FOXP3-PE (eBiosciences) and goat anti–rabbit IgG (Invitrogen). Gates for FOXP3-positive or cytokine-positive cells (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article) were set on fluorescence minus 1 controls.17,20 For analysis of apoptosis, Tconv cells were cultured for 3 or 5 days as indicated and stained with annexin V and FOXP3. For analysis of IL-2, interferon γ (IFN-γ), and IL-10, CD4+CD25− Tconv cells were cultured for 3 days as indicated then stimulated with phorbol myristate acetate (PMA; 10 ng/mL; Sigma-Aldrich) and Ca2+ ionophore (500 ng/mL; Sigma-Aldrich) for 4 hours, with brefelden A (10 μg/mL; Sigma-Aldrich) added for the final 2 hours. Samples were acquired on a BD FACSCanto and analyzed using FCS Express Pro Software Version 3 (De Novo Software).
Suppression assays
CD4+CD25− T cells (50 000 cells/well) were stimulated with soluble anti-CD3 (1 μg/mL, OKT3) and irradiated (50 Gy) APCs (50 000 cells/well) in the absence or presence of increasing numbers of putative Tregs or control cells. As a positive control ex vivo, untreated CD4+CD25+ Tregs were also added. Suppression was assessed by measuring the amount of [3H]-thymidine incorporation in the final 16 hours of culture.
Statistical analysis
Results were analyzed using a 1-way analysis of variance test and Tukey multiple comparison test as a posttest (GraphPad Software Inc). For individual experiments error bars represent the SD of triplicates. For averaged data, error bars represent the SEM of independent experiments.
Results and discussion
rATG induces transient expression of CD25 and FOXP3
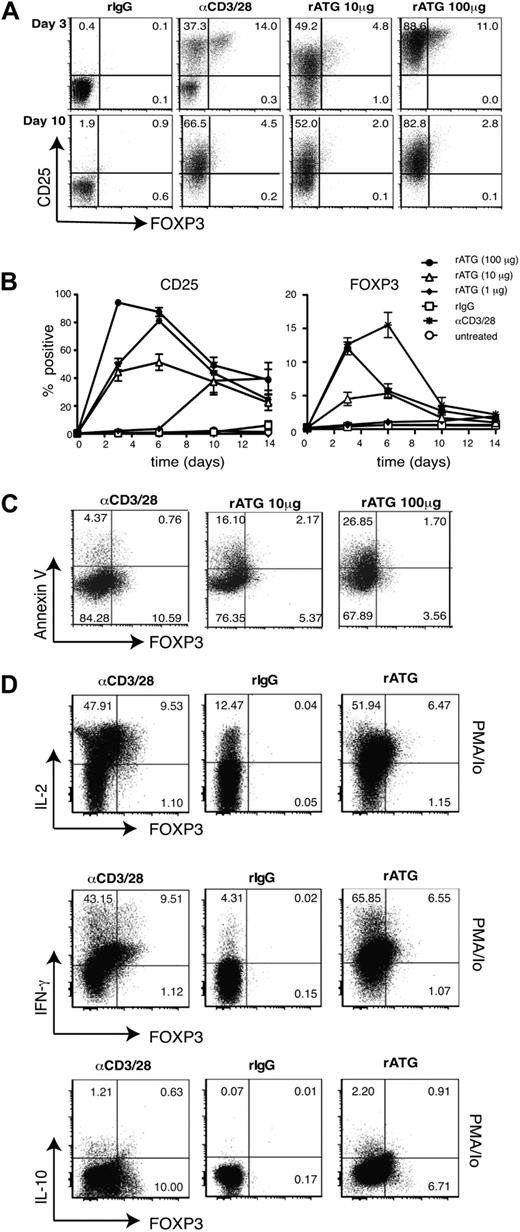
It has been shown that a short exposure of PBMCs to rATG induces expression of FOXP3 in 5% to 10% of CD4+ T cells.6,7,10 We confirmed these findings by incubating PBMCs with rATG or rIgG and analyzing FOXP3 and CD25 expression. Consistent with previous reports,6,7,10 rATG (10 μg/mL) increased the proportion of CD25+ (36.6% ± 3.9%, n = 6) and FOXP3+ (8.7% ± 1.5%, n = 6) T cells after 72 hours. The increase in FOXP3 expression, however, was transient, and by day 10 returned to baseline (data not shown). To exclude cell-extrinsic effects and determine whether ATG stimulated the de novo development of Tregs, we repeated these experiments with purified CD4+CD25− Tconv cells. Although rATG significantly increased the proportion of CD25highFOXP3+ T cells, similar to results with PBMCs, the increased CD25 and FOXP3 expression was transient (Figure 1A-B). Evidence that exposure of purified CD4+CD25hi cells to rATG did not significantly enhance their proliferation (data not shown), makes it unlikely that the FOXP3+ cells derived from expansion of the approximately 0.5% of CD25−FOXP3+ T cells in these cultures. The decrease in FOXP3 expression over time was not due to preferential death of the FOXP3-expressing cells, as we saw similar rates of apoptosis in the FOXP3-positive and -negative populations (Figure 1C). This transient nature of FOXP3 suggested that rATG may not lead to the stable expression of FOXP3 that is necessary for conversion of Tconv cells into functional Tregs.15-17,21 Accordingly, rabbit anti–murine thymoglobulin treatment does not result in induction of FOXP3+ Tregs in mice.8
Effect of rATG on expression of CD25 and FOXP3, apoptosis and cytokine production. (A-B) CD4+CD25− T cells were untreated, incubated with 1, 10, or 100 μg/mL of rabbit antithymocyte globulin (rATG), rabbit immunoglobulin G (rIgG; 10 μg/mL), or anti-CD3/28–coated beads in the presence of IL-2 and analyzed for expression of CD25 or FOXP3 over 10 days. Percentage of FOXP3+ at day 3: rIgG, 0.38 ± 0.09; rATG 10 μg/mL, 4.52 ± 0.98; rATG 100 μg/μL, 12.02 ± 0.96 (P < .001; n = 10). (C) Apoptosis in cultures of CD4+CD25− T cells incubated with 10 or 100 μg/mL of rATG, or anti-CD3/28–coated beads in the presence of IL-2 for 5 days was evaluated by staining for annexin V. The average percentage of FOXP3+ annexin V+ cells (% FOXP3+ annexin V+/% FOXP3+ annexin V+ + FOXP3+ annexin V−) is as follows: anti-CD3/28, 14.81 ± 2.69; rATG 10 μg/mL, 13.21 ± 0.66; rATG 100 μg/mL, 22.37 ± 3.6. The average % annexin V+ cells in the FOXP3− gate is as follows: anti-CD3/28, 9.95 ± 2.79; rATG 10 μg/mL, 9.03 ± 1.07; rATG 100 μg/mL, 17.12 ± 5.23. n = 3; P = not significant (ns). (D) CD4+CD25− T cells were incubated with rabbit IgG (100 μg/mL), anti-CD3/28–coated beads, or rATG (100 μg/mL) in the presence of IL-2 and after 3 days cells were unstimulated or restimulated with PMA and ionomycin (Io) and analyzed for expression of IFN-γ, IL-2, IL-10, and FOXP3. The average percentage of cytokine-producing cells in the FOXP3+ gate is as follows: IL-2: anti-CD3/28, 89.2 ± 1.6; rATG 100 μg/mL, 92.7 ± 1.6; IFN-γ: anti-CD3/28, 94.1 ± 1.5; rATG 100 μg/mL, 90.8 ± 3.0, n = 6; IL-10: anti-CD3/28, 4.2 ± 0.9; rATG 100 μg/mL, 8.23 ± 2.2 (P = ns). Data are representative of 10 (A-B), 3 (C), and 6 (D, IL-2 and IFN-γ) and 3 (D, IL-10) experiments with different donors.
Effect of rATG on expression of CD25 and FOXP3, apoptosis and cytokine production. (A-B) CD4+CD25− T cells were untreated, incubated with 1, 10, or 100 μg/mL of rabbit antithymocyte globulin (rATG), rabbit immunoglobulin G (rIgG; 10 μg/mL), or anti-CD3/28–coated beads in the presence of IL-2 and analyzed for expression of CD25 or FOXP3 over 10 days. Percentage of FOXP3+ at day 3: rIgG, 0.38 ± 0.09; rATG 10 μg/mL, 4.52 ± 0.98; rATG 100 μg/μL, 12.02 ± 0.96 (P < .001; n = 10). (C) Apoptosis in cultures of CD4+CD25− T cells incubated with 10 or 100 μg/mL of rATG, or anti-CD3/28–coated beads in the presence of IL-2 for 5 days was evaluated by staining for annexin V. The average percentage of FOXP3+ annexin V+ cells (% FOXP3+ annexin V+/% FOXP3+ annexin V+ + FOXP3+ annexin V−) is as follows: anti-CD3/28, 14.81 ± 2.69; rATG 10 μg/mL, 13.21 ± 0.66; rATG 100 μg/mL, 22.37 ± 3.6. The average % annexin V+ cells in the FOXP3− gate is as follows: anti-CD3/28, 9.95 ± 2.79; rATG 10 μg/mL, 9.03 ± 1.07; rATG 100 μg/mL, 17.12 ± 5.23. n = 3; P = not significant (ns). (D) CD4+CD25− T cells were incubated with rabbit IgG (100 μg/mL), anti-CD3/28–coated beads, or rATG (100 μg/mL) in the presence of IL-2 and after 3 days cells were unstimulated or restimulated with PMA and ionomycin (Io) and analyzed for expression of IFN-γ, IL-2, IL-10, and FOXP3. The average percentage of cytokine-producing cells in the FOXP3+ gate is as follows: IL-2: anti-CD3/28, 89.2 ± 1.6; rATG 100 μg/mL, 92.7 ± 1.6; IFN-γ: anti-CD3/28, 94.1 ± 1.5; rATG 100 μg/mL, 90.8 ± 3.0, n = 6; IL-10: anti-CD3/28, 4.2 ± 0.9; rATG 100 μg/mL, 8.23 ± 2.2 (P = ns). Data are representative of 10 (A-B), 3 (C), and 6 (D, IL-2 and IFN-γ) and 3 (D, IL-10) experiments with different donors.
rATG-induced FOXP3 does not suppress cytokine production
A major difference between FOXP3+ Tregs and Tconv cells is cytokine secretion, because activation-induced FOXP3 does not suppress proinflammatory cytokine production.17 We therefore investigated the ability of ATG-induced FOXP3+ cells to produce IL-2 and IFN-γ at the peak of FOXP3 expression. CD4+CD25− T cells were cultured with rATG, rIgG, or anti-CD3/28 beads, or left unstimulated, for 3 days, then restimulated with PMA and Ca2+ ionophore. Consistent with previous reports that activation-induced FOXP3 does not suppress IL-2 and IFN-γ,15,17,21 the majority of rATG-induced FOXP3+ cells produced IL-2 and IFN-γ. In parallel we analyzed IL-10, but found no evidence for preferential induction of IL-10–producing cells compared with stimulation with anti-CD3/28 beads (Figure 1D).
rATG-treated cells do not acquire suppressive activity
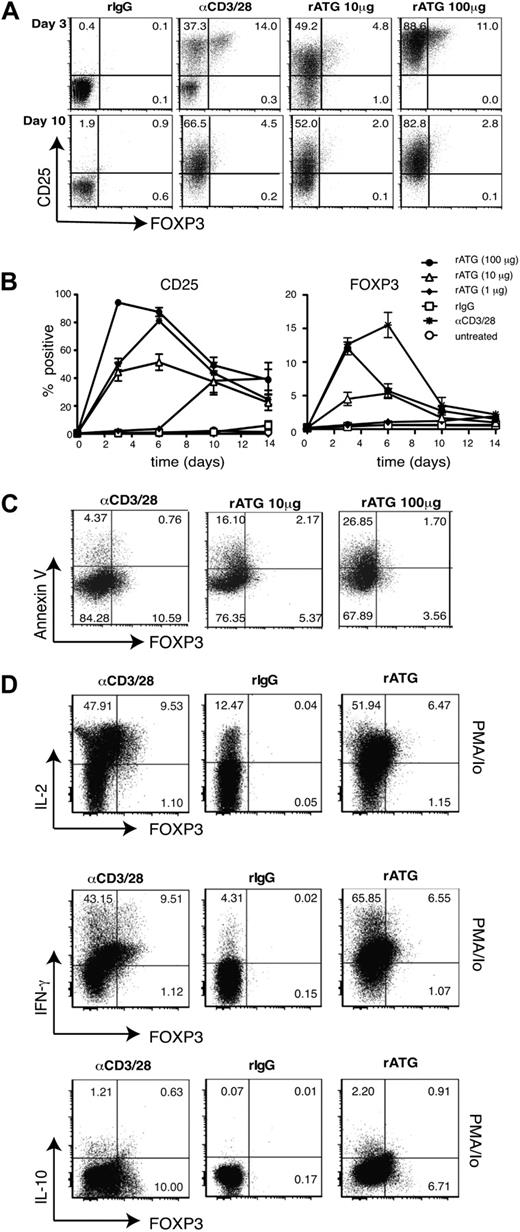
Finally we tested whether, despite the transient expression of FOXP3, rATG-treated Tconv cells acquired suppressive capacity. CD4+CD25− T cells were cultured as indicated and tested for suppressive activity after 3 or 10 days of culture. At day 3 when FOXP3 expression was maximal, there was no detectable suppressive activity of CD4+CD25− T cells treated with 1 or 10 μg/mL ATG even when added at a 1:1 ratio (Figure 2A). Similar results were obtained with cells that were cultured for 10 days (Figure 2B). Ex vivo CD4+CD25hi Tregs that were treated with anti-CD3/28 beads or 10 μg/mL rATG for 3 days did not lose their suppressive capacity, indicating that residual cell-surface bound rATG (supplemental Figure 2) does not nonspecifically inhibit suppression (Figure 2A).
rATG-treated Tconv cells do not acquire suppressive capacity. (A-B) Responder (R) CD4+CD25− T cells were stimulated with soluble anti-CD3 (1 μg/mL) and APCs and cocultured with the indicated ratio of putative “suppressor” (S) CD4+CD25− T cells that had been incubated with different concentrations of rATG, rIgG (10 μg/mL), or anti-CD3/CD28–coated beads for either (A) 3 or (B) 10 days. Suppression was assessed by measuring the amount of [3H]-thymidine incorporation in the final 16 hours of a 4-day culture period. Left panels represent results from an individual experiment. Right panels represent the average % suppression at a 1:1 ratio from 7 independent experiments with different donors. Percentage suppression at day 3 with a 1:2 ratio (Treg/responder): −143.78 ± 32.30, untreated; −117.74 ± 40.87, treated with rIgG; −189.34 ± 78.0, with anti-CD3/28 beads; −178.74 ± 42.42, with 1 μg/mL rATG; −70.69 ± 21.76, with 10 μg/mL rATG; 15.17 ± 8.22, with 100 μg/mL rATG (P = ns); and 92.4 ± 7.5, with ex vivo Tregs; 73.41 ± 8.44, with Tregs treated with 10 μg/mL rATG (P = ns compared with ex vivo Tregs). (C) To further examine the suppressive capacity of CD4+CD25− T cells exposed to 100 μg of rATG, suppression was assessed at different time points by adding [3H]-thymidine after 1, 2, or 3 days. Assays were harvested 16 hours after [3H]-thymidine addition. Results are representative of % suppression. Representative data from 3 independent experiments with different donors are depicted.
rATG-treated Tconv cells do not acquire suppressive capacity. (A-B) Responder (R) CD4+CD25− T cells were stimulated with soluble anti-CD3 (1 μg/mL) and APCs and cocultured with the indicated ratio of putative “suppressor” (S) CD4+CD25− T cells that had been incubated with different concentrations of rATG, rIgG (10 μg/mL), or anti-CD3/CD28–coated beads for either (A) 3 or (B) 10 days. Suppression was assessed by measuring the amount of [3H]-thymidine incorporation in the final 16 hours of a 4-day culture period. Left panels represent results from an individual experiment. Right panels represent the average % suppression at a 1:1 ratio from 7 independent experiments with different donors. Percentage suppression at day 3 with a 1:2 ratio (Treg/responder): −143.78 ± 32.30, untreated; −117.74 ± 40.87, treated with rIgG; −189.34 ± 78.0, with anti-CD3/28 beads; −178.74 ± 42.42, with 1 μg/mL rATG; −70.69 ± 21.76, with 10 μg/mL rATG; 15.17 ± 8.22, with 100 μg/mL rATG (P = ns); and 92.4 ± 7.5, with ex vivo Tregs; 73.41 ± 8.44, with Tregs treated with 10 μg/mL rATG (P = ns compared with ex vivo Tregs). (C) To further examine the suppressive capacity of CD4+CD25− T cells exposed to 100 μg of rATG, suppression was assessed at different time points by adding [3H]-thymidine after 1, 2, or 3 days. Assays were harvested 16 hours after [3H]-thymidine addition. Results are representative of % suppression. Representative data from 3 independent experiments with different donors are depicted.
Surprisingly, CD4+CD25− T cells treated with 100 μg/mL rATG for 3 or 10 days consistently resulted in a minor reduction in proliferation in these cocultures, which could be interpreted as suppression, possibly due to consumption of IL-2.22 We speculated that the apparent suppressive capacity of the cells could be due to altered kinetics of proliferation and evaluated the proliferation of these cocultures at different time points. Indeed, the peak of proliferation of cocultures with cells treated with 100 μg/mL rATG occurred after 1 day, whereas the proliferation of cocultures with rIgG-treated cells peaked after 3 days, the time point at which in vitro suppression assays are typically evaluated (Figure 2C). Therefore, cells treated with 100 μg/mL rATG are not suppressive, and the apparent “suppressive” activity at day 3 is related to the fact that highly activated cells proliferate rapidly upon restimulation. Because live rATG-induced FOXP3+ cells could not be isolated and tested directly for their suppressive capacity, we cannot rule out that their suppressive potential might be masked in these “mixed” cell assays. However, because the capacity to produce proinflammatory cytokines is a reliable surrogate measure for lack of suppressive function,17 the finding that rATG-induced FOXP3+ cells uniformly produce IL-2 and IFN-γ makes this possibility seem unlikely.
In conclusion, exposure of T cells to rATG resulted in transient induction of FOXP3, and treated cells acquired neither an immunosuppressive cytokine profile nor suppressive capacity. These results support the conclusion that although rATG induces FOXP3 expression in Tconv cells, it does not convert to Tregs, at least in vitro. Thus the suggestion that exposure of Tconv cells to rATG in vitro is a clinically relevant to approach to induce FOXP3+ Tregs should be re-evaluated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Paul Orban for critical reading of the manuscript.
This study was supported by the Roche Organ Transplant Research Foundation (867692131), the Hematology Clinical Trials Unit, the Leukemia & Lymphoma Society of Canada, and StemCell Technologies Inc. Core support for flow cytometry was funded by the Michael Smith Foundation for Health Research Immunity and Infection Research Center Unit. R.B. holds a University of British Columbia Academic Enhancement Award; M.K.L. holds a Canada Research Chair in Transplantation and is a Michael Smith Foundation for Health Research Scholar. We gratefully acknowledge the support of the Cell Separator Unit at Vancouver General Hospital for providing PBMCs.
Authorship
Contribution: R.B. designed the research, analyzed data, and wrote the paper; J.Y. performed the experiments and analyzed data; and M.K.L. designed the research, analyzed data, and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raewyn Broady, Division of Haematology, University of British Columbia, 2775 Laurel St, Vancouver, BC, Canada V5Z 1M9; e-mail: rbroady@interchange.ubc.ca.

![Figure 2. rATG-treated Tconv cells do not acquire suppressive capacity. (A-B) Responder (R) CD4+CD25− T cells were stimulated with soluble anti-CD3 (1 μg/mL) and APCs and cocultured with the indicated ratio of putative “suppressor” (S) CD4+CD25− T cells that had been incubated with different concentrations of rATG, rIgG (10 μg/mL), or anti-CD3/CD28–coated beads for either (A) 3 or (B) 10 days. Suppression was assessed by measuring the amount of [3H]-thymidine incorporation in the final 16 hours of a 4-day culture period. Left panels represent results from an individual experiment. Right panels represent the average % suppression at a 1:1 ratio from 7 independent experiments with different donors. Percentage suppression at day 3 with a 1:2 ratio (Treg/responder): −143.78 ± 32.30, untreated; −117.74 ± 40.87, treated with rIgG; −189.34 ± 78.0, with anti-CD3/28 beads; −178.74 ± 42.42, with 1 μg/mL rATG; −70.69 ± 21.76, with 10 μg/mL rATG; 15.17 ± 8.22, with 100 μg/mL rATG (P = ns); and 92.4 ± 7.5, with ex vivo Tregs; 73.41 ± 8.44, with Tregs treated with 10 μg/mL rATG (P = ns compared with ex vivo Tregs). (C) To further examine the suppressive capacity of CD4+CD25− T cells exposed to 100 μg of rATG, suppression was assessed at different time points by adding [3H]-thymidine after 1, 2, or 3 days. Assays were harvested 16 hours after [3H]-thymidine addition. Results are representative of % suppression. Representative data from 3 independent experiments with different donors are depicted.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/24/10.1182_blood-2009-04-214437/4/m_zh89990945480002.jpeg?Expires=1768326050&Signature=iRDHNYsHpEHA0nvMvFP7vK5AcxKSw~K9e0jRY1QNZQ4hwIO1wXTtJ0hpSP2tdVYr9ERZi0KEUZoTbRGvOjY-bYDWvcCckl5piLhh8vpTAfdu0BVAPexnH29zmyQK8OdLL4p2stehnlTN3tSPjcSQPu39Pnbx~EYoYKsWqGeEnvlaqMPpSPQtHwclbOb4D3wU1s9WXHFgKc4pc6a2ePrDO1DmTadB~1gOWpQxvQIuVcq9eVEhmMrYD42DkxnYrj35ZhhiNhT9ucktBHOsG7nEvOae3JAmWR8e3ofZd3wdoDMCq1X-F981w-iRqG6Fj1pqfc~7-fOQL75UChyLAAr5zQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. rATG-treated Tconv cells do not acquire suppressive capacity. (A-B) Responder (R) CD4+CD25− T cells were stimulated with soluble anti-CD3 (1 μg/mL) and APCs and cocultured with the indicated ratio of putative “suppressor” (S) CD4+CD25− T cells that had been incubated with different concentrations of rATG, rIgG (10 μg/mL), or anti-CD3/CD28–coated beads for either (A) 3 or (B) 10 days. Suppression was assessed by measuring the amount of [3H]-thymidine incorporation in the final 16 hours of a 4-day culture period. Left panels represent results from an individual experiment. Right panels represent the average % suppression at a 1:1 ratio from 7 independent experiments with different donors. Percentage suppression at day 3 with a 1:2 ratio (Treg/responder): −143.78 ± 32.30, untreated; −117.74 ± 40.87, treated with rIgG; −189.34 ± 78.0, with anti-CD3/28 beads; −178.74 ± 42.42, with 1 μg/mL rATG; −70.69 ± 21.76, with 10 μg/mL rATG; 15.17 ± 8.22, with 100 μg/mL rATG (P = ns); and 92.4 ± 7.5, with ex vivo Tregs; 73.41 ± 8.44, with Tregs treated with 10 μg/mL rATG (P = ns compared with ex vivo Tregs). (C) To further examine the suppressive capacity of CD4+CD25− T cells exposed to 100 μg of rATG, suppression was assessed at different time points by adding [3H]-thymidine after 1, 2, or 3 days. Assays were harvested 16 hours after [3H]-thymidine addition. Results are representative of % suppression. Representative data from 3 independent experiments with different donors are depicted.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/24/10.1182_blood-2009-04-214437/4/m_zh89990945480002.jpeg?Expires=1768326051&Signature=vXX5nFkaGsjvIPTkn-6WemT6Olsg3woWLVlCLlofJZUFpBEI1v3ZMdhqlwtPgsf9RlG3S7Az4FF8T8wjqJKTt2E3o~eDpUzzYd~OJYp6Q6S2kL-LlJbKo0i8JfXBCv32MvRM7963Pgkp-oS05SF-f0Li~jfZc1-IZNgtsRT8WtdYB8u6LdrhnilNoaZz4JOuZSX5PqasUbcHaoMwis2FsekPpsr34IKxmbJBaHWIIZX-15UrD3~yfdb24Qc~jgV52p1WkquKzXBLSeHFVhBgfgfGlpBtETQki73npwThDKcoXRt-kxx66yEM1HB5lpq35ltRlbwM1P85vokGu3S~JQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)