Abstract
Chromosomal translocations generating fusion proteins are frequently found in human leukemias. The fusion proteins play an important role in leukemogenesis by subverting the function of one or both partner proteins. The leukemogenic CALM-AF10 fusion protein is capable of interacting with the histone H3 lysine 79 (H3K79)–specific methyltransferase hDOT1L through the fused AF10 moiety. This interaction leads to local H3K79 hypermethylation on Hoxa5 loci, which up-regulates the expression of Hoxa5 and contributes to leukemogenesis. However, the long latency of leukemogenesis of CALM-AF10 transgenic mice suggests that the direct effects of fusion oncogene are not sufficient for the induction of leukemia. In this study, we show that the CALM-AF10 fusion protein can also greatly reduce global H3K79 methylation in both human and murine leukemic cells by disrupting the AF10-mediated association of hDOT1L with chromatin. Cells with reduced H3K79 methylation are more sensitive to γ-irradiation and display increased chromosomal instability. Consistently, leukemia patients harboring CALM-AF10 fusion have more secondary chromosomal aberrations. These findings suggest that chromosomal instability associated with global epigenetic alteration contributes to malignant transformation in certain leukemias, and that leukemias with this type of epigenetic alteration might benefit from treatment regimens containing DNA-damaging agents. This study is registered with www.clinicaltrials.gov as NCT00266136.
Introduction
The analysis of chromosomal translocations in leukemia has contributed greatly to our understanding of the pathogenesis of this disease.1 The translocation breakpoints pinpoint the location of genes that are critically altered in leukemogenesis. There are 2 principal mechanisms by which translocations alter gene function: (1) through the formation of fusion genes and (2) through deregulated expression of a gene close to the translocation breakpoint.2 It is well established that fusion genes act as oncogenes by ultimately activating signaling pathways that lead to proliferation and in many cases also to a block in differentiation.3,4 However, the results from many studies in which mouse models of fusion gene-driven leukemias were established showed that in most cases in animal models the expression of the fusion gene either did not result in the development of leukemia or showed a long latency until the animals developed leukemia (PML-RARA, ETV6-AML1). This observation can be explained by assuming that in addition to the expression of the fusion gene, other genetic alterations are required for the full leukemic phenotype. Whereas much work has focused on the direct mechanisms used by the various fusion proteins to activate or disrupt critical cellular pathways and on the identification of collaborating mutations, we now provide evidence that certain fusion proteins actively promote the acquisition of these additional mutations by causing genome-wide epigenetic changes that link to increased genomic instability
The AF10 gene is involved in 2 leukemia-associated fusion genes. The t(10;11)(p12;q23) translocation results in the MLL-AF10 fusion, and the t(10;11)(p12;q14) translocation results in the CALM-AF10 fusion.5,6 Whereas the MLL-AF10 fusion is only observed in acute myeloid leukemia (AML),7 CALM-AF10 fusions have been reported in both acute lymphoblastic and AML, as well as in lymphoma.8 The protein encoded by CALM (the clathrin assembly lymphoid myeloid leukemia gene) binds to clathrin and is involved in clathrin-mediated endocytosis and the trafficking of vesicles between the trans-Golgi network and endosomes.9 Mutations in the CALM gene are responsible for defects in the hematopoietic system and iron metabolism in fit1 mice.10
The gene product of AF10 (ALL-1 fused gene from chromosome 10, or MLLT10) is a putative transcription factor containing an N-terminal plant homeo domain zinc finger motif and an octapeptide motif-leucine zipper (OM-LZ) region close to its C terminus.11 Whereas little is known about the role of AF10 in transcriptional regulation, it has been identified as an interaction partner of the histone methyltransferase hDOT1L.12 DOT1 is the sole enzyme responsible for the methylation at lysine 79 of histone H3 (H3K79),13,14 a modification conserved in yeast, chicken, and mammals.13,15,16 The Dot1 gene was first identified in a genetic screen in yeast as a disrupter of telomeric silencing. Dot1 mutations affect telomere function due to the redistribution of SIR proteins from the telomeric heterochromatin to the euchromatin caused by a loss of H3K79 methylation.17 In addition, yeast cells deficient in Dot1 function show increased radiation sensitivity,18,19 suggesting that H3K79 methylation is also involved in DNA repair. In mammalian cells, the local level of H3K79 methylation correlates with transcriptional activity.20 Aberrant H3K79 hypermethylation is associated with the activation of oncogenic Hox genes in mouse bone marrow cells expressing CALM-AF10 and MLL-AF10 fusions.12,21 This process depends on the recruitment of hDOT1L to the Hox loci, and the small OM-LZ region of AF10, which mediates the interaction of AF10 with hDOT1L.
However, studies using mouse leukemia models often show an extended latency for the development of malignancy. CALM-AF10 transgenic mice developed acute leukemia at a median age of 12 months. The transgenic mice were clinically healthy and had normal blood counts for the first 9 months. Interestingly, the Hoxa genes were up-regulated in both clinically healthy and leukemic CALM-AF10 mice.22 In another study using retroviral transduction of CALM-AF10, followed by bone marrow transplantation, the recipient mice developed leukemia after a median of 3 to 4 months posttransplantation.23 The long latency to malignancy in various leukemia models suggests that the direct effects of the fusion oncogenes are not sufficient for the induction of leukemia, and that the accumulation of additional genetic alterations is necessary for leukemogenesis in vivo.3,24-28 There is evidence that fusion proteins involving oncogenic tyrosine kinases, such as BCR-ABL, induce genomic instability, which may facilitate the acquisition of secondary genetic alterations.29 In contrast, it is currently unknown whether fusion proteins (such as CALM-AF10) involving transcriptional regulators alter genomic stability.
In this study, we report that the leukemic fusion CALM-AF10 causes global H3K79 hypomethylation by disturbing the association of hDOT1L with chromatin in human and murine cells. Cells with CALM-AF10–induced H3K79 hypomethylation became hypersensitive to radiation and displayed increased chromosomal instability. Moreover, leukemia patients carrying the CALM-AF10 fusion have a higher frequency of additional chromosomal aberrations than patients with an MLL-AF9 or a PML-RARA fusion gene. Our findings suggest a novel pathway for CALM-AF10 to exert its leukemogenic effect partially by increasing genome instability through alterations in epigenetic marks, and indicate that leukemias with H3K79 hypomethylation might have increased sensitivity to treatment regimens using DNA-damaging agents.
Methods
Cell lines, constructs, and antibodies
The T-REx-293 cell line was purchased from Invitrogen. The U937T cell line (provided by Dr Gerard Grosveld, St Jude's Children's Hospital, Memphis) is a derivative of U937 harboring the tet-VP16 fusion gene under the control of a tetracycline-inducible promoter.30 For inducible expression in T-REx-293 cell lines, all genes were cloned into pcDNA4-Flag derived from pcDNA4/TO/myc-His B (Invitrogen) and stably transfected. For expression in U937T cell lines, all genes were cloned into pUHD10S-1 (gift from Dr Gerard Grosveld) and stably transfected. The methyl-K79–specific antibody was described.13 The methyl-H3-K4, -K9, -K27, -K36, and histone H3 antibodies were purchased from Upstate Biotechnology.
Metaphase chromosome immunofluorescence
Metaphase chromosome spreads were prepared, as described previously.31 Twenty-four hours posttransfection, cells were treated with 0.5 μg/mL nocodazole (Sigma-Aldrich) for 16 hours, harvested, washed with phosphate-buffered saline, and centrifuged (200g for 3 minutes). They were then resuspended and swollen in 75 mM KCl at 37°C for 20 minutes. The cells were recentrifuged (200g for 3 minutes) and resuspended in ice-cold fixative (freshly prepared methanol and glacial acetic acid at a 3:1 ratio) on ice for 5 minutes. Further fixation was for 25 minutes on ice. Chromosomes were spread on ice-cold slides coated with polylysine. When completely dried, slides were blocked in blocking solution at room temperature (RT) for 30 minutes. Anti-Flag monoclonal antibody (Sigma-Aldrich) diluted at 1/1000 and anti-hemagglutinin (HA) polyclonal antibody (Santa Cruz Biotechnology) diluted at 1/500 were used as primary antibodies. The slides were mounted with Gel/Mount (M01; Biomeda) and observed with a Nikon fluorescence microscope (E600). The objective lenses were ×40 and ×100.
Cell fractionation
Cell fractionation was performed as described.31 Two million cells were extracted in 200 μL cytoskeleton buffer on ice for 3 minutes, then centrifuged at 600g for 3 minutes to separate soluble proteins in supernatant from cytoskeletal matrix and chromatin. The pellet from the centrifugation was resuspended and treated with 0.25 U/μL DNase I (in cytoskeleton buffer with the NaCl concentration adjusted to 50 mM) for 30 minutes at RT. Then ammonium sulfate was added to a final concentration of 0.25 M. After incubation for 10 minutes at RT, the above centrifugation step was repeated to obtain the chromatin fraction in the supernatant and the cytoskeletal matrix in the pellet.
Quantification of H3K79 methylation by mass spectrometry
Relative quantification of H3K79 methylation was carried out by matrix-assisted laser desorption ionization–time of flight (Bruker, Autoflex) analysis of the peptides from the Arg-C digestion of histone H3. Histone H3 was purified from core histones by reverse-phase high-performance liquid chromatography using a C4 column, as previously reported.32
Short hairpin RNA-mediated stable inhibition of hDOT1L and AF10 expression in the T-REx-293 cell line
Plasmid constructs encoding a short hairpin RNA targeting the DOT1L or AF10 gene were prepared, as previously described.33 The sequences of 2 pairs of complementary DNA oligonucleotides encoding short hairpin RNA were as follows (both annealed oligonucleotide duplexes of each pair have 5′-BglII and 3′-HindIII compatible overhangs). For DOT1L, the sense strand is 5′-GATCCCCGGATGAAATGGTATGGAAATTCAAGAGA-TTTCCATACCATTTCATCCTTTTTA-3′; the antisense strand is 5′-AGCTTAAAAAGGATGAAATGGTATGGAAATCTCTTGAATTTCCA-TACCATTTCATCCGGG-3′. For AF10, the sense strand is 5′-GATCCCCGAAGATTAGAGGAACAAATTTCAAGAGAATTTGTTCCTCTA-ATCTTCTTTTTA-3′; the antisense strand is 5′-AGCTTAAAAAGAAGATTAGAGGAACAAATTCTCTTGAAATTTGTTCCTCTAATCTTCGGG-3′. Complementary oligonucleotides were cloned into pTER, and stable inducible knockdown cell lines were established in T-REx-293, as described.33
RNA isolation and reverse transcription–polymerase chain reaction
Total RNA was isolated with TRIzol reagent (Invitrogen). Reverse transcription into complementary DNA was performed using the Moloney murine leukemia virus reverse transcriptase (Invitrogen). Polymerase chain reactions (PCRs) were performed using the TaKaRa Taq Hot Start Version. Primer information is available upon request.
Establishment of stable cell lines
T-REx-293 stable cell lines were established by culturing in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS), 5 μg/mL blasticidin (Invitrogen), and 220 μg/mL Zeocin (Invitrogen). The establishment of U937T stable cell lines was as described previously.34 Cotransfected pEGFP-C1 (BD Clontech) was used as selection marker, and resistant clones were isolated by limited dilution. Positive clones were identified by Western blotting for expression of genes of interest upon tetracycline withdrawal. The established U937T stable cell lines were maintained in RPMI 1640 medium supplemented with 10% FBS, 1 μg/mL tetracycline, 0.5 μg/mL puromycin, and 1 mg/mL G418.
Colony formation assay
The assay was performed, as described.35 Six thousand cells were plated in triplicate into a 6-well plate with 5 mL/well of 1.3% (wt/vol) methylcellulose (Sigma-Aldrich) containing RPMI 1640 medium and 10% FBS. To examine the sensitivity to γ-irradiation, cells were plated and incubated at 37°C for 1 hour, and subsequently treated with the indicated dosages of 137Cs irradiation (Gammacell 3000Elan; MDS Nordion), followed by incubation for 10 days before colony counting.
Genomic instability assay
T-REx-293 cells were induced to express proteins (CALM-AF10 or CALM-AF10ΔOM-LZ) for 3 days before irradiation with 2 Gy of gamma radiation (137Cs). Colcemid was then added, and the cells were harvested 1 hour later by standard cytogenetic methods and stained with Giemsa. Images of metaphases were captured with an Ikaros karyotyping system (Metasystems), and the chromatid gaps and breaks were counted by 2 persons in a blinded fashion. The metaphases were scored for each experimental condition.
Patient data
Leukemia patient samples were analyzed by standard cytogenetic techniques (G-banding) and, if required, by multicolor fluorescence in situ hybridization. The CALM-AF10, MLL-AF9, and PML-RARA fusion transcripts were also verified by reverse transcription-PCR in every case. The samples were obtained under study protocols approved by the ethics committees of the participating centers, and all patients provided written informed consent, in accordance with the Declaration of Helsinki.
Statistical analysis
Data were presented as mean plus or minus SEM (n = 3 or more). Statistical analysis was performed by Student t test. Values were considered statistically significant when P values were less than .05.
Results
H3K79 methylation is reduced in leukemic cells from patients with a CALM-AF10 fusion
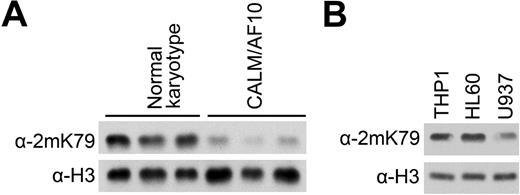
The leukemic fusion protein CALM-AF10 is known to interact with the histone H3K79 methyltransferase hDOT1L, which leads to local H3K79 hypermethylation at the Hoxa5 locus.12,21 To further analyze the impact of this fusion protein on hDOT1L function, we examined global H3K79 methylation levels in leukemic blasts from AML patients with the CALM-AF10 fusion. Western blotting with antibodies specific for dimethylated H3K79 revealed a drastic reduction in H3K79 methylation in samples from CALM-AF10–positive patients compared with samples from CALM-AF10–negative AML patients with a normal karyotype (Figure 1A). The reduction in H3K79 methylation was also obvious in the CALM-AF10–positive leukemia cell line U937,6 which shows local hypermethylation at the HOXA5 locus,21 but not in 2 other control cell lines (Figure 1B). Quantitative analysis of histone extracts using mass spectrometry confirmed the difference in methylation levels among these cell lines (Supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Both H3K79 dimethylation and monomethylation were the lowest in U937 cells, whereas the nonmethylated form of H3K79 was more abundant. H3K79 trimethylation was rare and below the sensitivity of mass spectrometric detection (supplemental Table 1). No significant changes were observed in the level of histone methylation at other sites, including H4K20 (Supplemental Figure 1B). These observations indicate that global H3K79 hypomethylation is a characteristic of cells expressing the CALM-AF10 fusion protein.
Global H3K79 hypomethylation in CALM-AF10 leukemic cells. (A) H3K79 methylation in leukemia cells with CALM-AF10 rearrangement. Whole-cell extracts were prepared from AML patient samples with normal karyotype or the CALM-AF10 translocation and analyzed by Western blotting with an antibody specific for dimethylated H3K79. Detection with α-H3 antibody (bottom row) provided a loading control. (B) H3K79 methylation in the U937 cell line that expresses the CALM-AF10 fusion. The other 2 monocytic cell lines, HL60 and THP1, were used for comparison.
Global H3K79 hypomethylation in CALM-AF10 leukemic cells. (A) H3K79 methylation in leukemia cells with CALM-AF10 rearrangement. Whole-cell extracts were prepared from AML patient samples with normal karyotype or the CALM-AF10 translocation and analyzed by Western blotting with an antibody specific for dimethylated H3K79. Detection with α-H3 antibody (bottom row) provided a loading control. (B) H3K79 methylation in the U937 cell line that expresses the CALM-AF10 fusion. The other 2 monocytic cell lines, HL60 and THP1, were used for comparison.
The CALM-AF10 fusion protein causes H3K79 hypomethylation
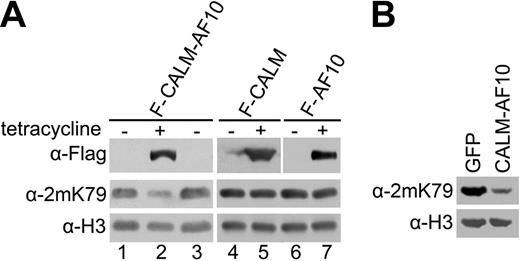
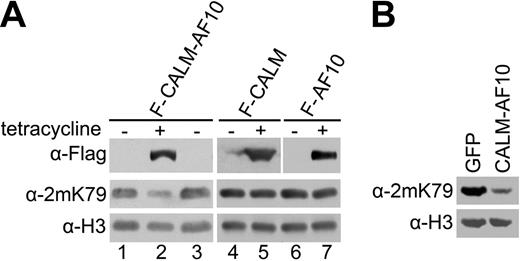
We next examined whether the reduction in H3K79 methylation is indeed caused by the expression of CALM-AF10. To this end, we established cell lines in which the ectopic expression of Flag-tagged CALM-AF10 can be controlled by tetracycline. Western analysis showed that H3K79 methylation was reduced substantially in cells expressing the fusion protein induced by tetracycline, whereas expression of CALM or AF10 alone had no apparent effect (Figure 2A). Moreover, the original H3K79 methylation levels were fully restored in the hypomethylated cells by inhibiting the expression of CALM-AF10 through tetracycline withdrawal (Figure 2A, lane 3). In addition, H3K79 hypomethylation was also detected in murine leukemia cells from our previously described CALM-AF10 leukemia mouse model, in which CALM-AF10 is expressed retrovirally in transplanted bone marrow cells23 (Figure 2B). The expression of CALM-AF10 did not affect the transcript level of endogenous hDOT1L in the cell lines (Supplemental Figure 2), which indicates that the H3K79 hypomethylation is not due to the down-regulation of the histone methyltransferase. These results demonstrate that the CALM-AF10 fusion causes global H3K79 hypomethylation in both human and murine cells.
CALM-AF10 fusion protein causes H3-K79 hypomethylation. (A) H3K79 methylation in human T-REx-293 cell lines stably transfected with the indicated expression constructs. The cells were not induced (−) or induced with tetracycline for 48 hours (+), or analyzed after 72-hour further culturing after the withdrawal of tetracycline (lane 3). (B) H3K79 methylation in blast cells from leukemic mice with retroviral expression of CALM-AF10. Whole-cell extracts were prepared from leukemic cells with CALM-AF10 expression (right lane) and bone marrow transduced with the retroviral vector only expressing GFP (left lane), and were probed with indicated histone antibodies. All the cells were from GFP-positive sorting by FACS.
CALM-AF10 fusion protein causes H3-K79 hypomethylation. (A) H3K79 methylation in human T-REx-293 cell lines stably transfected with the indicated expression constructs. The cells were not induced (−) or induced with tetracycline for 48 hours (+), or analyzed after 72-hour further culturing after the withdrawal of tetracycline (lane 3). (B) H3K79 methylation in blast cells from leukemic mice with retroviral expression of CALM-AF10. Whole-cell extracts were prepared from leukemic cells with CALM-AF10 expression (right lane) and bone marrow transduced with the retroviral vector only expressing GFP (left lane), and were probed with indicated histone antibodies. All the cells were from GFP-positive sorting by FACS.
The hDOT1L interaction region of AF10 is necessary for induction of H3K79 hypomethylation by the CALM-AF10 fusion
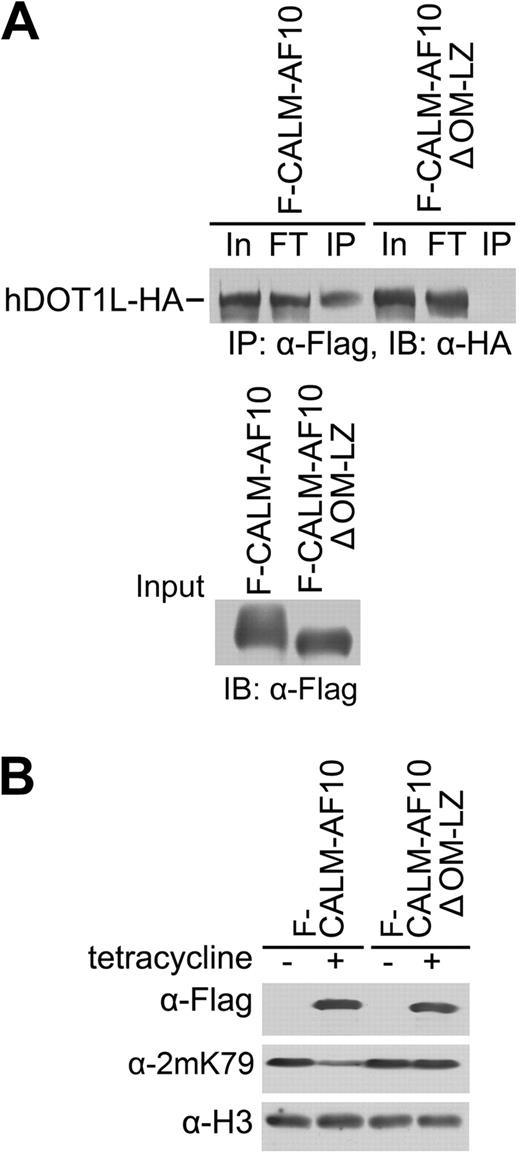
The conserved OM-LZ domain in AF10 mediates its interaction with hDOT1L and is necessary for the transforming activity of AF10 fusion proteins.12,21,36 To determine whether the interaction between CALM-AF10 and hDOT1L is required for H3K79 hypomethylation, Flag-tagged CALM-AF10 and its OM-LZ deletion mutant (CALM-AF10ΔOM-LZ) were coexpressed with hDOT1L-HA in 293T cells. As expected, CALM-AF10ΔOM-LZ was unable to coimmunoprecipitate hDOT1L-HA (Figure 3A). Significantly, expression of CALM-AF10ΔOM-LZ did not lead to reduced H3K79 methylation (Figure 3B). This observation strongly suggests that reduction of H3K79 methylation requires the interaction of the CALM-AF10 with hDOT1L.
The hDOT1L interaction domain of CALM-AF10 is required for the induction of H3K79 hypomethylation. (A) Coimmunoprecipitation of CALM-AF10 with hDOT1L depends on the OM-LZ domain of AF10. HA-tagged hDOT1L was coexpressed with Flag-tagged CALM-AF10 or its deletion mutant, as indicated in 293T cells, and the cell lysate was subjected to coimmunoprecipitation using Flag antibody. The presence of hDOT1L-HA in the immunoprecipitate was examined by Western blotting. One percent of the input cell lysate was loaded (In, input; FT, flow through; IP, immunoprecipitation). The expression of the AF10 fusion proteins in the input lysate was confirmed with Western blotting using a Flag antibody (bottom panel). (B) Expression of CALM-AF10 fusion protein lacking the OM-LZ domain failed to reduce H3K79 methylation in T-REx-293 cells. The tetracycline-induced expression of Flag-tagged fusion proteins was confirmed (top panel).
The hDOT1L interaction domain of CALM-AF10 is required for the induction of H3K79 hypomethylation. (A) Coimmunoprecipitation of CALM-AF10 with hDOT1L depends on the OM-LZ domain of AF10. HA-tagged hDOT1L was coexpressed with Flag-tagged CALM-AF10 or its deletion mutant, as indicated in 293T cells, and the cell lysate was subjected to coimmunoprecipitation using Flag antibody. The presence of hDOT1L-HA in the immunoprecipitate was examined by Western blotting. One percent of the input cell lysate was loaded (In, input; FT, flow through; IP, immunoprecipitation). The expression of the AF10 fusion proteins in the input lysate was confirmed with Western blotting using a Flag antibody (bottom panel). (B) Expression of CALM-AF10 fusion protein lacking the OM-LZ domain failed to reduce H3K79 methylation in T-REx-293 cells. The tetracycline-induced expression of Flag-tagged fusion proteins was confirmed (top panel).
AF10 regulates H3K79 methylation by targeting hDOT1L to chromatin, but fusion to CALM dramatically weakens the ability of this targeting
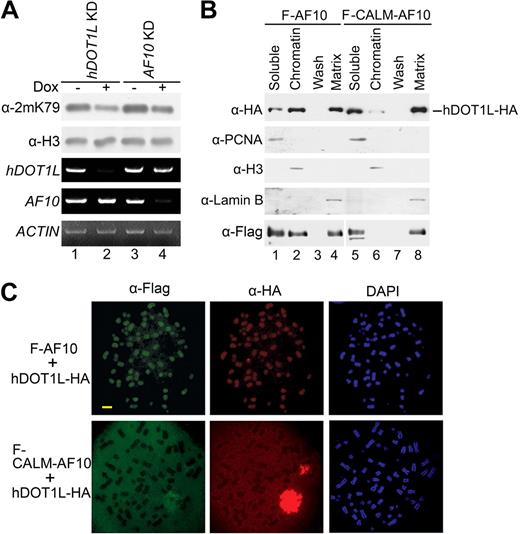
We have previously reported that AF10 can interact with hDOT1L,12 but the physiologic function of this interaction is still unknown. To evaluate the role of AF10 in hDOT1L-mediated H3K79 methylation, AF10 expression was knocked down in T-REx-293 cells using inducible small interfering RNAs (siRNAs; Figure 4A, compare lane 4 with lane 3). The knockdown of the hDOT1L methyltransferase was used as a positive control for H3K79 hypomethylation (Figure 4A, compare lanes 1 and 2). H3K79 hypomethylation occurred when the cells were treated with doxycycline to knock down the expression of either hDOT1L or AF10. This result indicates that AF10 is necessary for hDOT1L-mediated H3K79 methylation. We next examined how AF10 might regulate the function of hDOT1L and the effect of the AF10 fusion with CALM. To this end, Flag-AF10, Flag-CALM-AF10, and hDOT1L-HA were expressed in 293T cells alone or in combination, and the cellular proteins were separated into soluble, chromatin, and matrix fractions (Supplemental Figure 3A). Western analysis showed that a large portion of Flag-AF10, but not Flag-CALM-AF10, was located in the chromatin fraction when they were transfected separately (Supplemental Figure 3B). Interestingly, hDOT1L-HA alone failed to bind to chromatin even though it is a histone methyltransferase. This observation is consistent with a previous report for the yeast homolog of hDOT1L.37 However, when expressed together with Flag-AF10, hDOT1L-HA was largely (30%-50%) in the chromatin fraction (Figure 4B). In contrast, the fraction of hDOT1L in the chromatin was much smaller (∼1%) when Flag-CALM-AF10 was coexpressed (Figure 4B, compare lane 2 with lane 6). These observations suggest that AF10 can recruit hDOT1L to chromatin, and fusion to CALM dramatically reduces this recruitment. To further extend this finding, immunofluorescence staining was performed on metaphase chromosome spreads from transfected 293T cells. When transfected separately, Flag-AF10 was bound to chromosomes, whereas both hDOT1L-HA and Flag-CALM-AF10 were dispersed in the cell (Supplemental Figure 2C). However, hDOT1L-HA was bound to chromosomes when cotransfected with Flag-AF10, but was dispersed in the cells when cotransfected with Flag-CALM-AF10 (Figure 4C). These data suggest that AF10 is involved in hDOT1L-mediated methylation by targeting hDOT1L to chromatin, and that CALM-AF10 greatly reduces hDOT1L recruitment, leading to global H3K79 hypomethylation.
AF10 regulates H3K79 methylation by targeting hDOT1L to chromatin, but fusion to CALM reduces this targeting dramatically. (A) Confirmation of H3K79 hypomethylation in AF10 and hDOT1L knockdown T-REx-293 cells. siRNA expression was induced in the stable cell lines with doxycycline (Dox) for 48 hours (+), and the H3-K79 methylation levels were examined (top panel). The knockdown efficiency was confirmed by reverse transcription-PCR, and ACTIN was used as normalization control (3 bottom panels). (B) Expression of AF10 targeted the cotransfected hDOT1L to chromatin, but CALM-AF10 targeted only very little hDOT1L to chromatin. The 293T cells were cotransfected with expression constructs for hDOT1L-HA and Flag-tagged proteins indicated on the top and subjected to cell fractionation. The distribution of hDOT1L-HA in each fraction was examined by Western analysis with HA antibody (top panel). The efficiency of the cell fractionation was confirmed by the detection of proliferating cell nuclear antigen in the soluble fraction, histone H3 in chromatin, and lamin B in the matrix. (C) Expression of AF10, but not CALM-AF10, targeted cotransfected hDOT1L to chromosomes. Metaphase chromosome spreads were prepared from cotransfected 293T cells. DAPI (4,6 diamidino-2-phenylindole) was used as DNA counterstain. Scale bar indicates 1 μm.
AF10 regulates H3K79 methylation by targeting hDOT1L to chromatin, but fusion to CALM reduces this targeting dramatically. (A) Confirmation of H3K79 hypomethylation in AF10 and hDOT1L knockdown T-REx-293 cells. siRNA expression was induced in the stable cell lines with doxycycline (Dox) for 48 hours (+), and the H3-K79 methylation levels were examined (top panel). The knockdown efficiency was confirmed by reverse transcription-PCR, and ACTIN was used as normalization control (3 bottom panels). (B) Expression of AF10 targeted the cotransfected hDOT1L to chromatin, but CALM-AF10 targeted only very little hDOT1L to chromatin. The 293T cells were cotransfected with expression constructs for hDOT1L-HA and Flag-tagged proteins indicated on the top and subjected to cell fractionation. The distribution of hDOT1L-HA in each fraction was examined by Western analysis with HA antibody (top panel). The efficiency of the cell fractionation was confirmed by the detection of proliferating cell nuclear antigen in the soluble fraction, histone H3 in chromatin, and lamin B in the matrix. (C) Expression of AF10, but not CALM-AF10, targeted cotransfected hDOT1L to chromosomes. Metaphase chromosome spreads were prepared from cotransfected 293T cells. DAPI (4,6 diamidino-2-phenylindole) was used as DNA counterstain. Scale bar indicates 1 μm.
CALM-AF10 is a dominant-negative competitor of AF10 and affects H3K79 methylation by disrupting the AF10-mediated association of hDOT1L with chromatin
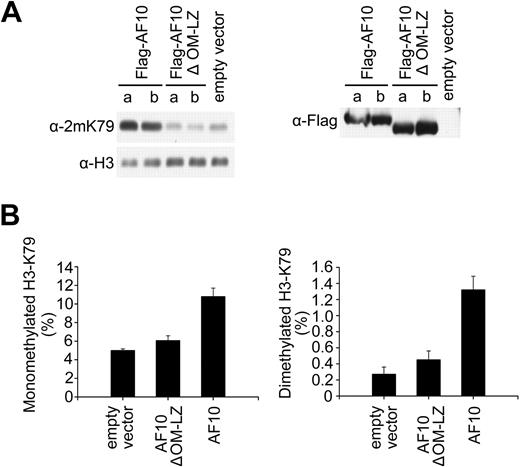
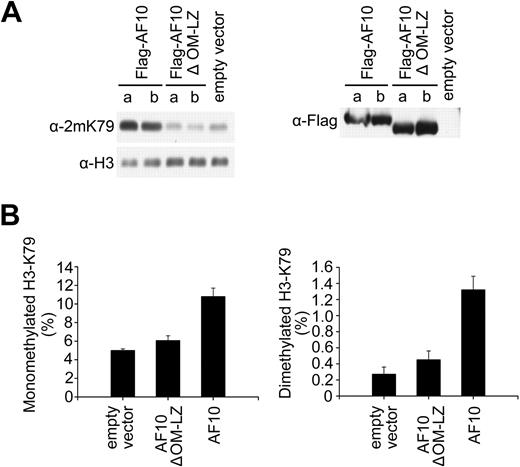
Our previous report has demonstrated that CALM-AF10 can interact with hDOT1L in the nucleus, and this interaction does not appear to weaken the activity of hDOT1L per se because local H3K79 methylation at the Hoxa5 gene is increased in CALM-AF10–expressing cells.21 In the presence of CALM-AF10, a small fraction of hDOT1L is still associated with chromatin, and this might account for local H3K79 hypermethylation (Figure 4B). We therefore reasoned that CALM-AF10 might compete with AF10 for hDOT1L interaction, and thus influence the recruitment of hDOT1L to chromatin. If the reduction of global H3K79 methylation in leukemia cells is really due to the hDOT1L-sequestering effect of the CALM-AF10 fusion protein, ectopic expression of AF10 might restore the function of the endogenous hDOT1L. To examine this, we established U937T cell lines (harboring the CALM-AF10 fusion) stably expressing Flag-AF10 or Flag-AF10ΔOM-LZ. H3K79 methylation analysis by Western blotting showed that the activity of endogenous hDOT1L was restored by AF10 expression, whereas expression of the AF10ΔOM-LZ mutant had no obvious effect (Figure 5A left panel), although Flag-AF10 and Flag-AF10ΔOM-LZ were expressed at similar levels (Figure 5A right panel). The changes in H3K79 mono- and dimethylation levels were confirmed by quantitative analysis of histone extracts using mass spectrometry (Figure 5B). These results suggest that endogenous hDOT1L, which had been dissociated from chromatin by the endogenous CALM-AF10 in U937T, was retargeted to chromatin by the overexpressed AF10, thus restoring H3K79 methylation levels.
CALM-AF10 affects H3K79 methylation as a dominant-negative competitor of AF10. (A) Stable ectopic expression of AF10 in U937T cells containing CALM-AF10 restored H3K79 methylation. For each Flag-tagged expression construct, H3K79 methylation levels (left panel) after induction of the Flag-tagged proteins (right panel) were examined in 2 independent stable cell lines (denoted as a and b for each construct). (B) Mass spectrometry analysis of H3K79 methylation in total histone extracts prepared from stably transfected U937T cells with ectopic expression of AF10 or its OM-LZ deletion mutant.
CALM-AF10 affects H3K79 methylation as a dominant-negative competitor of AF10. (A) Stable ectopic expression of AF10 in U937T cells containing CALM-AF10 restored H3K79 methylation. For each Flag-tagged expression construct, H3K79 methylation levels (left panel) after induction of the Flag-tagged proteins (right panel) were examined in 2 independent stable cell lines (denoted as a and b for each construct). (B) Mass spectrometry analysis of H3K79 methylation in total histone extracts prepared from stably transfected U937T cells with ectopic expression of AF10 or its OM-LZ deletion mutant.
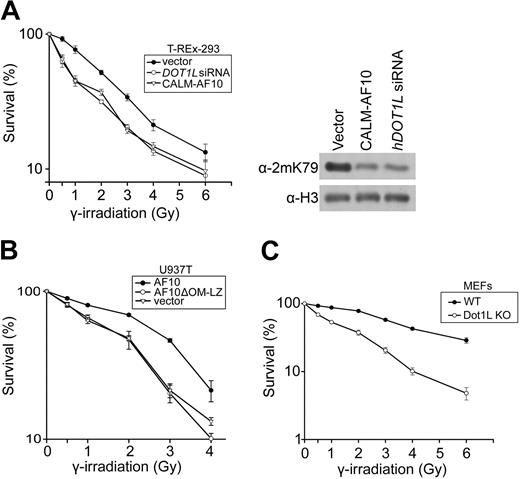
Increased γ-irradiation sensitivity and chromosomal instability in cells with H3K79 hypomethylation
Previous reports have demonstrated that Dot1 deletion or histone H3 lysine 79 substitution can lead to increased radiation sensitivity in Saccharomyces cerevisiae.18,19 To examine whether mammalian cells with hypomethylated H3K79 are more sensitive to radiation, T-REx-293 cell lines with inducible hDOT1L knockdown and inducible CALM-AF10 expression were used. The knockdown of hDOT1L and the expression of CALM-AF10 had similar effect on H3K79 methylation and radiation sensitivity (Figure 6A). In both types of hypomethylated cells, survival at low-dose γ-irradiation (0.5 Gy to 3 Gy) dropped by 15% to 30% compared with control cells. The correlation of γ-irradiation sensitivity with H3K79 hypomethylation was also observed in the U937T leukemia cell line (Figure 6B), in which increased methylation by ectopic expression of Flag-AF10 (Figure 5) conferred a higher resistance to γ-irradiation. In support of a direct link between H3K79 hypomethylation and sensitivity to γ-irradiation, Dot1L-knockout mouse embryonic fibroblasts (MEFs) showed a 20% to 40% lower viability after γ-irradiation treatment than wild-type cells (Figure 6C).
Cells with H3K79 hypomethylation show higher sensitivity to γ-irradiation. (A) Hypomethylated T-REx-293 cells show higher irradiation sensitivity. T-REx-293 cells expressing CALM-AF10 or hDOT1L knockdown siRNA were treated with different doses of γ-irradiation. After 7 days, the percentage of surviving cells was determined. The original T-REx-293 cell line stably transfected with empty vector was included for comparison. The H3K79 hypomethylation in T-REx-293 cells expressing the CALM-AF10 fusion protein or hDOT1L knockdown siRNA was confirmed by Western analysis (right panel). (B) Hypomethylated U937T cells show higher irradiation sensitivity. U937T cells expressing stably transfected AF10 or its deletion mutant were treated with different doses of γ-irradiation, and the number of surviving cells was determined by colony formation assay. (C) Irradiation survival test of MEF cells deficient in H3K79 methylation. Wild-type (WT) and Dot1L knockout (Dot1L KO) MEF cells were treated with different doses of γ-irradiation. The cell survival experiments were carried out 3 times in duplicate, and the percentage of cells not treated was set to 100%. The P values of survival percentage of normal methylation versus survival percentage of hypomethylation were lower than .01 at the radiation doses from 0.5 Gy to 3 Gy.
Cells with H3K79 hypomethylation show higher sensitivity to γ-irradiation. (A) Hypomethylated T-REx-293 cells show higher irradiation sensitivity. T-REx-293 cells expressing CALM-AF10 or hDOT1L knockdown siRNA were treated with different doses of γ-irradiation. After 7 days, the percentage of surviving cells was determined. The original T-REx-293 cell line stably transfected with empty vector was included for comparison. The H3K79 hypomethylation in T-REx-293 cells expressing the CALM-AF10 fusion protein or hDOT1L knockdown siRNA was confirmed by Western analysis (right panel). (B) Hypomethylated U937T cells show higher irradiation sensitivity. U937T cells expressing stably transfected AF10 or its deletion mutant were treated with different doses of γ-irradiation, and the number of surviving cells was determined by colony formation assay. (C) Irradiation survival test of MEF cells deficient in H3K79 methylation. Wild-type (WT) and Dot1L knockout (Dot1L KO) MEF cells were treated with different doses of γ-irradiation. The cell survival experiments were carried out 3 times in duplicate, and the percentage of cells not treated was set to 100%. The P values of survival percentage of normal methylation versus survival percentage of hypomethylation were lower than .01 at the radiation doses from 0.5 Gy to 3 Gy.
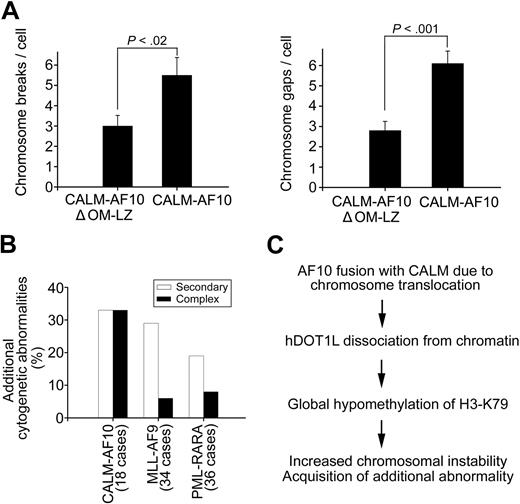
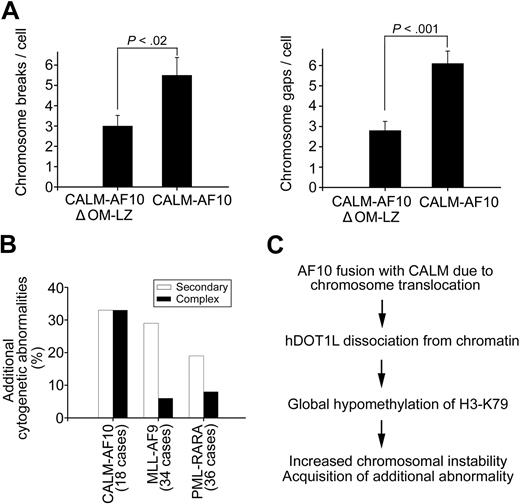
To examine whether the radiation sensitivity in H3K79-hypomethylated cells is related to genomic instability, we examined the occurrence of chromosome breaks and gaps in T-REx-293 cells expressing the CALM-AF10 or CALM-AF10ΔOM-LZ fusion protein. In addition to reduced viability, cells with H3K79 hypomethylation showed an increased number of chromosome breaks and gaps after γ-irradiation. The mean number of breaks and gaps was 60% and 85% higher, respectively, in cells expressing CALM-AF10 (hypomethylation) compared with cells expressing CALM-AF10ΔOM-LZ (normal methylation) (Figure 7A and supplemental Table 2). These observations suggest that CALM-AF10–mediated H3K79 hypomethylation might be related to chromosomal instability. Consistent with this hypothesis is the observation that CALM-AF10–positive patients have a much higher frequency of additional chromosomal aberrations than patients with an MLL-AF9 or PML-RARA fusion gene. Chromosomal analysis of 18 cases of CALM-AF10–, 34 cases of MLL-AF9–, and 36 cases of PML-RARA–positive leukemias demonstrated that CALM-AF10 patients had the highest frequency of additional chromosomal abnormalities (Figure 7B). Most strikingly, the occurrence of 3 and more rearrangements (a so-called complex aberrant karyotype) in CALM-AF10 patients was approximately 3- to 4-fold more frequent than in the other 2 types of patients. Collectively, our data suggest that histone H3K79 hypomethylation caused by CALM-AF10 links to an increased radiation sensitivity and subsequent chromosomal instability in cell lines, and that this increased chromosomal instability is reflected in a higher prevalence of complex aberrant karyotypes in patients with CALM-AF10–positive leukemias (Figure 7C).
CALM-AF10 fusion induces chromosomal instability. (A) Hypomethylated cells display increased chromosomal instability. T-REx-293 cells expressing CALM-AF10 (H3K79 hypomethylation) or CALM-AF10ΔOM-LZ (normal H3K79 methylation) were irradiated at 2 Gy, and the average number of chromosome breaks (left panel) and chromosome gaps (right panel) per metaphase spread was determined 1 hour after irradiation. (B) CALM-AF10–positive patients show more additional cytogenetic abnormalities than patients with an MLL-AF9 or a PML-RARA fusion. Percentages of cases with a secondary (one additional rearrangement to the primary translocation) and complex (2 or more additional rearrangements) cytogenetic abnormalities were calculated. (C) Model for chromosomal instability in association with CALM-AF10 translocations.
CALM-AF10 fusion induces chromosomal instability. (A) Hypomethylated cells display increased chromosomal instability. T-REx-293 cells expressing CALM-AF10 (H3K79 hypomethylation) or CALM-AF10ΔOM-LZ (normal H3K79 methylation) were irradiated at 2 Gy, and the average number of chromosome breaks (left panel) and chromosome gaps (right panel) per metaphase spread was determined 1 hour after irradiation. (B) CALM-AF10–positive patients show more additional cytogenetic abnormalities than patients with an MLL-AF9 or a PML-RARA fusion. Percentages of cases with a secondary (one additional rearrangement to the primary translocation) and complex (2 or more additional rearrangements) cytogenetic abnormalities were calculated. (C) Model for chromosomal instability in association with CALM-AF10 translocations.
Discussion
Although many leukemia-associated fusion genes involve chromatin-modifying genes, we report for the first time that a fusion protein can alter genome-wide histone modification (H3K79 methylation) levels, and that this alteration (hypomethylation) might relate to chromosomal instability.
We previously described that the CALM-AF10 and MLL-AF10 fusion proteins can recruit mouse Dot1L to Hoxa loci, causing local H3K79 hypermethylation and increased Hoxa expression, which was proposed to lead to leukemic transformation of mouse bone marrow progenitor cells.12,21 The importance of local H3K79 hypermethylation was recently shown to be very important for MLL-AF4–positive leukemias as well.38 We now show that the CALM-AF10 fusion protein, in addition to Hox gene up-regulation, causes global H3K79 hypomethylation and links to chromosomal instability. Global H3K79 hypomethylation as well as an increased number of complex chromosomal abnormalities were seen in CALM-AF10–positive patient samples as well. Interestingly, global hypomethylation and an increased number of complex chromosome aberrations were also found in MLL-AF10 patients (Supplemental Figure 4). Considering that the transcriptional orientation of the CALM gene is the same as that of AF10 (both are transcribed telomere to centromere), the complex chromosomal abnormalities in CALM-AF10–positive patients should be not due to the additional rearrangements that have to occur to generate a functional fusion protein as in the case of the MLL-AF10 (the MLL and AF10 are transcribed in opposite orientations). Paradoxically, local hypermethylation and global hypomethylation of histone H3K79 coexist in leukemia cells, a situation reminiscent of the pattern of DNA methylation in tumors. Genome-wide DNA hypomethylation and local hypermethylation in the promoter regions of certain tumor suppressor genes are frequently found in various cancers.39 To confirm the coexistence of local hypermethylation and global hypomethylation of histone H3K79 in the same cells, chromatin immunoprecipitation experiments were performed in U937 (global H3K79 hypomethylation) and HL60 cells (normal H3K79 methylation). Consistent with our previous report,21 the HOXA5 gene was found to be up-regulated in U937 cells (CALM-AF10 positive) comparing with HL60 cells (CALM-AF10 negative; Supplemental Figure 5A). Chromatin immunoprecipitation data showed that some regions (amplicon b and e) of the HOXA5 locus have much more H3K79 methylation in U937 cells than those in HL60 cells (Supplemental Figure 5C). This result directly supports the hypothesis that local hypermethylation and global hypomethylation of histone H3K79 can indeed coexist in the same cells.
In our previous report,21 we found that CALM-AF10 and hDOT1L could colocalize in the nucleus when they were coexpressed. Yet, this colocalization apparently does not allow CALM-AF10 to target hDOT1L to chromatin as efficiently as AF10, due to the poor chromatin-binding ability of CALM-AF10 itself (Figures 4B-C and Supplemental Figure 2). But CALM-AF10 might still be able to target hDOT1L to a limited number of chromatin sites (Figure 4B, lane 6) through binding to specific target genes leading to local H3K79 hypermethylation.21 However, the majority of CALM-AF10 proteins in the nucleus could have a dominant-negative effect in competing with the endogenous AF10 for hDOT1L binding to cause disruption of chromatin association of hDOT1L, and thus global H3K79 hypomethylation. One has to be cautious about the molecular mechanism leading to H3K79 hypomethylation because most of our observations are based on experiments with overexpressed proteins and may not be easily extrapolated to the leukemogenesis process in vivo. For example, one contributing factor in vivo could be that the CALM-AF10 fusion moves endogenous hDOT1L out of nucleus (because the CALM-AF10 shuttles between nucleus and cytoplasm40 ), which would cause the transcription elongation complex to function less efficiently and lead to reduced H3K79 methylation because of a smaller nuclear hDOT1L pool.
In the present study, we demonstrated that global histone H3K79 hypomethylation clearly increases γ-irradiation sensitivity in association with chromosomal instability. Consistent with our findings of a high proportion of CALM-AF10 patients with complex aberrant karyotypes, it was reported recently that Dot1L-deficient mouse embryonic stem cells lack H3K79 methylation and also show chromosomal instability (chromosome aneuploidy).41 However, the mechanism linking genome instability to H3K79 hypomethylation is not firmly established at the moment. Especially the controversy regarding the binding of the damage sensor protein 53BP1 to methylated H3K79 or methylated H4K20 at sites of DNA damage still needs to be resolved.42,43 Because our Western blot analysis and mass spectrometry data indicate that the expression of CALM-AF10 does not alter H4K20 methylation levels (Supplemental Figure 1B and data not shown), the radiation sensitivity of cells with global H3K79 hypomethylation is unlikely to be due to altered H4K20 methylation. The relevance of H3K79 hypomethylation in the control of genome stability warrants further investigation.
Because H3K79 methylation is important for transcriptional elongation,44 global hypomethylation could also lead to a reduced expression of a great number of genes.45 Thus, an interesting scenario about the contribution of CALM-AF10 in leukemogenesis can be envisioned whereby the CALM-AF10 fusion, in addition to up-regulating specific oncogenes (eg, HOXA5 genes) via local hypermethylation, might promote leukemogenesis by interfering with multiple cellular pathways through global hypomethylation of H3K79.
The work presented in this study suggests that CALM-AF10 fusion-mediated leukemogenesis is not only dependent on the transcriptional deregulation of specific target genes (eg, HOXA5), but that a second mechanism, namely chromosomal instability, might be involved in leukemogenesis. Chromosomal instability is well known to play an important role in both tumor initiation and progression,46,47 and its induction has been suggested for fusion proteins involving oncogenic tyrosine kinases such as BCR-ABL.29 However, the induction of genomic instability has never previously been linked to a fusion with proteins involved in transcriptional regulation. Recent work with a CALM-AF10 transgenic mouse model supports our hypothesis, demonstrating that the transgenic mice were clinically healthy and had normal blood counts for the first 9 months of their lives, but that the Hoxa genes were up-regulated in both clinically healthy and leukemic CALM-AF10 mice. The long latency in these transgenic mice suggests that the mere up-regulation of Hox genes is not sufficient for leukemic transformation, and additional genetic lesions are necessary for this process.22 Our data suggest that the increased chromosomal instability associated with H3K79 hypomethylation caused by the CALM-AF10 fusion might accelerate the acquisition of additional genetic abnormalities required for leukemogenesis.
It has become quite obvious in recent years that multiple genetic hits are required to achieve full transformation of a normal cell. In a study in which the coding exons of 11 breast cancer and 11 colorectal cancer cell lines were sequenced, an average of 11 genes per tumor was found to be mutated.48 In a very recent study, Ley et al49 were able to identify 8 missense and nonsense mutations in the genome of a single case of AML with normal karyotype. Considering the apparently large number of mutations required for tumorigenesis, one has to assume that the well-known leukemia-associated translocations are highly selected for their malignant transformation efficiency. This seems to be indeed the case. For example, in childhood acute lymphoblastic leukemia cases with a t(12;21)(p12;q22) translocation leading to an ETV6-RUNX1 fusion, one frequently finds deletions of the nonrearranged ETV6 allele. There is ample evidence that ETV6 is a tumor suppressor gene. Thus, the t(12;21) does not only lead to the formation of a fusion oncogene (EVT6-RUNX1), but at the same time leads to the destruction of one allele of a tumor suppressor gene (ETV6).50 Similarly, AF10 fusion proteins seem to use at least 2 mechanisms that promote leukemogenesis: (1) deregulation of target genes resulting from local epigenetic changes, and (2) increasing genomic instability due to global epigenetic changes.
Moreover, our findings may have important therapeutic implications. CALM-AF10–expressing cells and most likely cells with H3K79 global hypomethylation have an increased radiosensitivity, suggesting that irradiation or chemotherapeutic drugs that cause DNA damage might be of benefit for patients with H3K79 hypomethylation leukemias.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Gerard Grosveld for the U937T cell line and pUHD10S-1 vector; Yoshihito Taniguchi for the protocol of colony formation assay; Junjie Chen for Dot1L−/− MEFs; Hans Clevers for pTER vector; Brian Huntly and Pentao Liu for manuscript reading and comments; and Lorelyn Bisa and Natalia Kerber for technical assistance.
This work was supported by grants from the Ministry of Science and Technology of China (2005CB522400, 2006CB943900, and KSCX2-YWN-019), the Max-Planck-Gesellschaft of Germany, and the National Science Foundation of China to G.-L.X., and by grants from the German Ministry of Research and Education (Bundesministerium fur Bildung, Wissenschaft, Forschung und Technologie; 01GS0876) and the Deutsche Forschungsgemeinschaft (SFB 684-A6) to S.K.B.
Authorship
Contribution: G.-L.X., S.K.B., Y.Z., and Y.-H.L. designed the experiments and the study; G.-L.X., S.K.B., and Y.-H.L. wrote the manuscript; Y.-H.L., P.M.K., S.K.B., and K.-L.Z. performed experiments and collected and analyzed data; and Y.C., Y.-Q.L., A.J.D., C.B., and Y.Z. provided important reagents and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan K. Bohlander, Medizinische Klinik und Poliklinik III, Klinikum Grosshadern Raum 003, KKG Leukemia Haematologikum, GSF Marchioninistr 25, Munich 81377, Germany; e-mail: stefan.bohlander@med.uni-muenchen.de, or Guo-Liang Xu, Institute of Biochemistry and Cell Biology, 320 Yueyang Rd, Shanghai 200031, China; e-mail: glxu@sibs.ac.cn.