Abstract
Ischemia-reperfusion injury (IRI) triggers an inflammatory cascade that is initiated by the activation of CD1d-restricted iNKT cells. In sickle cell disease (SCD), misshapen erythrocytes evoke repeated transient bouts of microvascular IRI. Compared with C57BL/6 controls, NY1DD mice have more numerous and activated (CD69+, interferon-γ+ [IFN-γ+]) lung, liver, and spleen iNKT cells that are hyperresponsive to hypoxia/reoxygenation. NY1DD mice have increased pulmonary levels of IFN-γ, IFN-γ–inducible chemokines (CXCL9, CXCL10), and elevated numbers of lymphocytes expressing the chemokine receptor CXCR3. Treating NY1DD mice with anti-CD1d antibody to inhibit iNKT cell activation reverses baseline pulmonary dysfunction manifested as elevated vascular permeability, decreased arterial oxygen saturation, and increased numbers of activated leukocytes. Anti-CD1d antibodies decrease pulmonary levels of IFN-γ and CXCR3 chemokines. Neutralization of CXCR3 receptors ameliorates pulmonary dysfunction. Crossing NY1DD to lymphocyte-deficient Rag1−/− mice decreases pulmonary dysfunction. This is counteracted by the adoptive transfer of 1 million NKT cells. Like mice, people with SCD have increased numbers of activated circulating iNKT cells expressing CXCR3. Together, these data indicate that iNKT cells play a pivotal role in sustaining inflammation in SCD mice by a pathway involving IFN-γ and production of chemotactic CXCR3 chemokines and that this mechanism may translate to human disease.
Introduction
Persons with sickle cell disease (SCD) express a mutated form of β-globin referred to as hemoglobin S (HbS). Polymerization of deoxygenated HbS is the precipitating event in the molecular pathogenesis of SCD and results in a characteristic sickle erythrocyte morphology and reduced hemoglobin oxygen binding capacity. Since the pulmonary arterial circulation has low oxygen tension and pressure, low blood velocity, and constricts in response to hypoxia, the lung microenvironment is particularly conducive to the polymerization of HbS and is therefore highly vulnerable to ischemia-reperfusion injury (IRI).1 Consequently, pulmonary disease is the leading cause of morbidity and mortality in people with SCD.1-3
The hallmark of SCD is vaso-occlusive crisis (VOC), which is an acute and painful ischemic episode that eventually resolves, but lasts anywhere from minutes to days. Recent findings suggest that transient microvascular occlusion occurs chronically in a subclinical manner in SCD, and that end-organ damage and short life-span in SCD are due to the cumulative effects of repeated bouts of ischemic/hypoxic events followed by reperfusion.4,5 Historically, microvascular occlusion was thought to be caused solely by rigid sickled erythrocytes. More recently, it has been noted that VOC is associated with a proinflammatory, procoagulatory, and proadhesive hemodynamic phenotype in SCD that involves interactions between leukocytes, red blood cells (RBCs), vascular endothelial cells, and platelets that contribute to microvascular inflammation and injury.6,7
Natural killer T (NKT) cells have recently been shown to contribute to the pathology of hepatic and renal IRI.8,9 In this study, we investigated the role of NKT cells in contributing to pulmonary inflammation and IRI in SCD. In healthy persons, NKT cells comprise a relatively minor subset of lymphocytes (approximately 0.5% of the T-cell population in the blood and peripheral lymph nodes, approximately 2.5% of T cells in the spleen, mesenteric, and pancreatic lymph nodes, and up to 30% of T cells in the liver).10 In the normal immune response to pathogens, NKT cells are thought to bridge the innate and adaptive immune system by their ability to promptly release copious amounts of cytokines (Th1 or Th2) and interact with a variety of cells involved in innate immunity. For example, although the mechanism is unknown, it has been reported that NKT cells provide an early host protection against Streptococcus pneumonia by promoting the trafficking of neutrophils into airways.11 Also, NKT cells have been shown to participate in antitumor immunity and the balance between tolerance and autoimmunity.12
Invariant NKT (iNKT) cells, also known as type I, express a restricted T-cell receptor (TCR) [Vα14-Jα18 (murine) or Vα24-Jα18 (human)] that is activated by lipid antigen presentation by CD1d (a member of the CD1 family of major histocompatibility complex [MHC]–like molecules) found on antigen-presenting cells (APCs).12,13 CD1d is present on resting APCs at all times allowing CD1d and iNKT cells to function at early points during host response to infection or other challenges. While resting myeloid lineage cells express low levels of CD1d, circulating and splenic B cells express very high levels. CD1d is also expressed on epithelial cells, parenchymal cells, and vascular smooth muscle cells. Within minutes of presentation of CD1d-lipid to the invariant TCR and costimulation, iNKT cells become activated and secrete large amounts of cytokines.12 The best characterized CD1d-restricted ligand is α-galactosylceramide (α-GalCer), a marine sponge-derived glycolipid that specifically activates iNKT cells. CD1d activation has also been hypothesized to occur as a result of presentation of host lipids that are byproducts of the degradation of necrotic or apoptotic cells.14 Type II NKT cells express more diverse lipid-binding TCRs and are CD1d-restricted but unresponsive to α-GalCer; type III NKT cells have diverse TCRs.15
Upon TCR stimulation, iNKT cells rapidly release large quantities of cytokines including interleukin-4 (IL-4), IL-2, and interferon-γ (IFN-γ), which promote activation of dendritic cells (DCs), NK cells, B cells, and CD4+ and CD8+ T cells.16-19 Furthermore, IFN-γ can stimulate endothelial, epithelial, neuronal, and lymphoid cells to release IFN-γ–inducible CXC chemokines (MIG/CXCL9, IP-10/CXCL10, and ITAC/CXCL11), which are potent chemoattractants for mononuclear cells that express CXCR3 (ie, activated T cells and NK cells).20-23
A well-characterized model of moderate SCD is the NY1DD mouse (αHβS[βMDD]) that is homozygous for a spontaneous deletion of mouse βmajor-globin locus (βMDD) and carries a human α- and βS-globin transgene (αHβS).24,25 Similar to persons with SCD at baseline, NY1DD mice exhibit a proinflammatory phenotype that is believed to contribute to morbidity and mortality.6,26,27 In addition, the NY1DD model has been used to elucidate the role of ischemia-reperfusion injury in SCD. NY1DD mice exposed to 3 hours of hypoxia (10% O2), to induce RBC sickling and ischemia, followed by 4 hours of reoxygenation (room air) results in an inflammatory response that is not seen in C57BL/6 controls (no RBC sickling and thus no ischemia) nor in NY1DD mice exposed to hypoxia alone, suggesting that the proinflammatory state in NY1DD animals is due to reperfusion injury.4 In this study, we first subjected NY1DD mice to hypoxia/reoxygenation to investigate whether iNKT cells have an exaggerated inflammatory response to reperfusion injury in specific tissues. Then, we used the NY1DD mouse to investigate the role of iNKT cells in baseline pulmonary inflammation and dysfunction associated with moderate SCD.
Methods
Human subjects
Blood samples were obtained from persons with SCD (HbSS), ages 19 years and older, as well as age- and race-matched controls. Samples were taken during a routine office visit to the Adult Hemoglobinopathy Clinic at Washington University from persons with SCD who reported no more than typical pain. SCD diagnoses were confirmed by hemoglobin analysis. Age-matched African-American controls were healthy employees of Washington University, who were also appropriately consented for study participation. All human protocols were approved by the Institutional Review Board at Washington University and the University of Virginia and informed consent was obtained in accordance with the Declaration of Helsinki.
Animals
Twelve- to 16-week-old NY1DD mice (a gift from R. Hebbel, University of Minnesota Medical School, Minneapolis, MN), congenic C57BL/6 mice (The Jackson Laboratory), and UBC-GFP mice (The Jackson Laboratory) were used for experimentation. NY1DD × Rag1−/− mice were created by crossing NY1DD and Rag1−/− mice (The Jackson Laboratory) and identified by polymerase chain reaction (PCR) for the NY1DD human βS-globin transgene, mouse Βmajor deletion, and the Rag1−/− deletion. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Virginia.
Vascular permeability
Pulmonary vascular permeability was evaluated by measurement of Evans blue dye (EBD) extravasation. EBD (30 mg/kg body weight, 200 μL) was injected intravenously in mice anesthetized with ketamine/xylazine and allowed to circulate for 30 minutes. The chest was opened, the inferior vena cava transected, and the pulmonary vasculature flushed with 10 mL saline via the right ventricle to remove excess intravascular dye. The lung was homogenized and incubated in 100% formamide at 37°C for 24 hours to extract EBD. The concentration of EBD extracted was analyzed by spectophotometry. Correction of optical densities (E) for contaminating heme pigments was performed as previously described, using the equation: E620(corrected) = E620 − (1.426 × E740 + 0.03).28 Data were calculated as micrograms EBD per gram lung.
Arterial oxygen saturation (% SO2)
Animals were anesthetized, and the skin and musculature over the left chest was dissected. A left ventricular heart puncture was performed with a heparinized syringe, and 150 μL arterial blood were collected for gas analysis (OPTI Critical Care Analyzer [CCA]; Osmetech).
Pulmonary immunohistochemistry and histopathologic grading
After perfusion, the lungs were inflation-fixed with 1% paraformaldehyde-lysine-periodate (PLP) at a height of 25 cm. Paraffin sections (5 μm) were stained with hematoxylin and eosin (H&E). Analysis was performed by a blinded pathologist using a modified scoring system as previously described.29,30 Two criteria were used for scoring: capillary congestion and alveolar wall thickness. Sections were graded by assigning a score: 0, absent; 1, mild; 2, moderate; 3, severe; and 4, very severe. The individual scores were combined to obtain an overall score ranging from 0 to 8. The mean was generated for each group of animals (3 sections from each lung, 4 lungs per group, n = 12) to generate a cumulative score.
Unrestrained whole body plethysmography
Frequency of breathing (FOB) and tidal volume (TV) were evaluated using unrestrained whole body plethysmography. Mice were placed into plexiglas chambers connected to a direct airflow sensor (Buxco Max II; Buxco Electronics). Airflow through the chambers was maintained at 70 mL/min. The flow signals were recorded using IOX software (EMKA Technologies). Respiratory function was recorded for 20 minutes and averaged after a 20-minute adjustment period.
Tissue preparation
Animals were anesthetized, the chest wall opened, and the inferior vena cava was transected. The mouse was perfused with 15 mL saline via the left ventricle to remove nonadhered intravascular cells. Organs were removed and minced. Lungs and livers were incubated in digestion buffer (1 mg/mL collagenase type Ia, 60 U/mL hyaluronidase type I-s, 60 U/mL DNase1, 2 μL/mL GolgiPlug [BD Biosciences]) for 45 minutes at 37°C. Single-cell suspensions were created by passing tissue through a 40-μm cell strainer.
Immunostaining of cells for flow cytometry and determination of absolute numbers of leukocytes
For more detailed information, please see supplemental methods (available on the Blood website; see the Supplemental Materials link at the top of the online article). Cells were resuspended at 106 cells/mL in 100 μL staining buffer (1% bovine serum albumin [BSA], 0.1% sodium azide in phosphate-buffered saline [PBS]). Cells were Fcγ receptor-blocked, stained with the α-GalCer-analog (PBS57) loaded CD1d tetramer, and stained with cell surface markers. A fixable LIVE/DEAD stain was used for viability (Invitrogen). Cells were fixed and permeabilized for intracellular staining with IFN-γ. Alternatively, 100 μL whole human blood were stained.
Fluorescence intensity was measured with a CyAn ADP LX 9 color analyzer (Dako), and data analyses were performed with FlowJo software (TreeStar). Murine live and CD45+ leukocytes were identified by the following staining: neutrophils: 7/4+, CD11b+, and Ly-6G+; NK cells: NKp46+ and CD3e−; iNKT cells: CD1d-tetramer+ and CD3e+; CD4 T cells: CD1d-tetramer−, CD3ϵ+, and CD4+; CD8 T cells: CD1d-tetramer−, CD3ϵ+, and CD8α+ (Supplemental Figure 1). Human iNKT cells were identified as live, CD45+, CD3+, CD1d-tetramer+ (Supplemental Figure 2). The absolute number of pulmonary leukocytes was determined from analysis with counting beads (Invitrogen).
Antibody treatments
Mice were injected with anti-CD1d mAb (1B1; 10 μg/g per day for 2 days) to inhibit CD1d-restricted NKT cell activation (controls were treated with rIgG2b). Antibodies were purified from hybridomas in the University of Virginia Lymphocyte Core. Mice were killed either 1 day or 5 days after the last injection. CXCR3 was neutralized by injections of anti-CXCR3 (1 mL/day for 7 days).31
Measurement of IFN-γ and IFN-γ–inducible chemokines
Lungs were perfused and snap-frozen. Tissue was homogenized and IFN-γ (eBioscience), MIG/CXCL9, IP10/CXCL10, and ITAC/CXCL11 were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems) according to the manufacturer's protocol.
Isolation and adoptive transfer of NKT cells
GFP-C57BL/6 animals were injected with polyclonal anti-Asialo-GM1 for 2 days (depletes NK cells). Splenocytes were passed over a T-cell enrichment column (R&D Systems), and eluted cells were incubated with anti-NK1.1-PE. Cells were incubated with magnetic anti-phycoerythrin (PE) beads (Miltenyi Biotec) and purified by magnetic isolation. By flow cytometric analysis, 85% of the resulting cells were NKT cells and not activated. One million cells were injected retro-orbitally. Successful transfer was confirmed by flow cytometric analysis for green fluorescent protein (GFP) positive cells.
Hypoxia/reoxygenation
Mice were subjected to 3 hours of hypoxia (8% oxygen). After hypoxic periods, mice were returned to normoxic conditions for 4 hours.4
Statistics
Prism software (GraphPad) was used for all statistical analyses. Unpaired Student t tests and one-way analysis of variance (ANOVA) with Neuman-Keuls posttesting were used to compare experimental groups. Two-way ANOVA with Bonferroni posttesting was used to compare experimental groups to each other over time. Histologic grading was analyzed with either a nonparametric t test (Mann-Whitney) or Kruskal-Wallis test with Dunn posttesting. A P value of less than .05 was accepted as being significant.
Results
NY1DD mice have increased numbers of activated iNKT cells in tissues that are hyperresponsive to hypoxia/reoxygenation
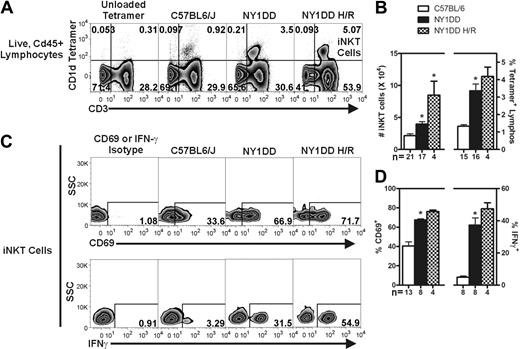
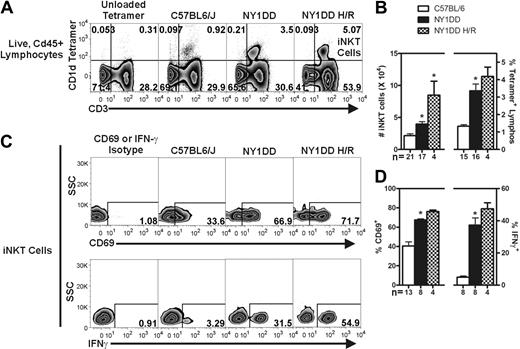
We used fluorescently labeled CD1d tetramers to selectively label iNKT cells (tetramer+ CD3+) in the lung, liver, and spleen of C57BL/6 and NY1DD mice, as well as NY1DD mice subjected to 3 hours of hypoxia followed by 4 hours of reoxygenation (Figure 1A and Supplemental Figure 3). Flow cytometric analysis and counting beads were used to determine the absolute numbers of iNKT cells and the percentage of iNKT cells among all lymphocytes (live, CD45+ low side scatter cells). Relative to C57BL/6 mice, NY1DD mice had increased numbers of iNKT cells in all tissues at baseline (Table 1, Figure 1B, and Supplemental Figure 3). Pulmonary iNKT cells were increased in number from 2.2 ± 0.3 × 104 to 3.9 ± 0.4 × 104 (P < .05) and as a percentage of all lymphocytes from 1.3 ± 0.09 to 3.4 ± 0.4% (P < .05; Figure 1A-B). Compared with normoxic NY1DD mice, NY1DD mice subjected to hypoxia/reoxygenation had significantly increased numbers of iNKT cells in all tissues as well as increased percentage of all lymphocytes (Table 1, Figure 1A-B, and Supplemental Figure 3). The number of pulmonary iNKT cells was increased to 8.5 ± 2.2 × 104 after hypoxia/reoxygenation, a 2.2-fold change over normoxic NY1DD mice (P < .05).
NY1DD mice have increased numbers of activated pulmonary iNKT cells that are hyper-responsive to hypoxia/reoxygenation. (A) Representative flow cytometry plots of pulmonary iNKT cells. (B) iNKT cells were identified from the live, CD45+ lymphocyte gate as CD1d-tetramer+ CD3+ cells. Compared with C57BL/6 mice, NY1DD mouse lungs have a higher number of pulmonary iNKT cells and hypoxia/reoxygenation amplifies this response. (C-D) iNKT cells from NY1DD mice are more activated as defined by higher percentage of surface CD69 and intracellular IFN-γ that is amplified by hypoxia/reoxygenation. Data were analyzed by one-way ANOVA with Neuman-Keuls posttesting (*P < .05 was considered significant). SSC, side-scatter; H/R, hypoxia/reoxygenation.
NY1DD mice have increased numbers of activated pulmonary iNKT cells that are hyper-responsive to hypoxia/reoxygenation. (A) Representative flow cytometry plots of pulmonary iNKT cells. (B) iNKT cells were identified from the live, CD45+ lymphocyte gate as CD1d-tetramer+ CD3+ cells. Compared with C57BL/6 mice, NY1DD mouse lungs have a higher number of pulmonary iNKT cells and hypoxia/reoxygenation amplifies this response. (C-D) iNKT cells from NY1DD mice are more activated as defined by higher percentage of surface CD69 and intracellular IFN-γ that is amplified by hypoxia/reoxygenation. Data were analyzed by one-way ANOVA with Neuman-Keuls posttesting (*P < .05 was considered significant). SSC, side-scatter; H/R, hypoxia/reoxygenation.
To determine the activation state of iNKT cells from the lung, liver, and spleen, we analyzed surface expression of CD69 and intracellular IFN-γ, which are both well-characterized markers of iNKT cell activation.32 At baseline, iNKT cells from all NY1DD mouse organs displayed significantly increased levels of both markers compared with controls (Table 1, Figure 1C-D, and Supplemental Figure 3). The percentage of pulmonary iNKT cells positive for IFN-γ increased from 5 in C57BL/6 mice to 37 in NY1DD mice (P < .05), a difference of 7.4-fold. iNKT cells from the lung, liver, and spleen of NY1DD mice were further activated by hypoxia/reoxygenation, as the expression of the activation markers CD69 and IFN-γ increased (Table 1, Figure 1C-D, and Supplemental Figure 3).
NY1DD mice display baseline pulmonary injury that is ameliorated by inhibition of iNKT cells
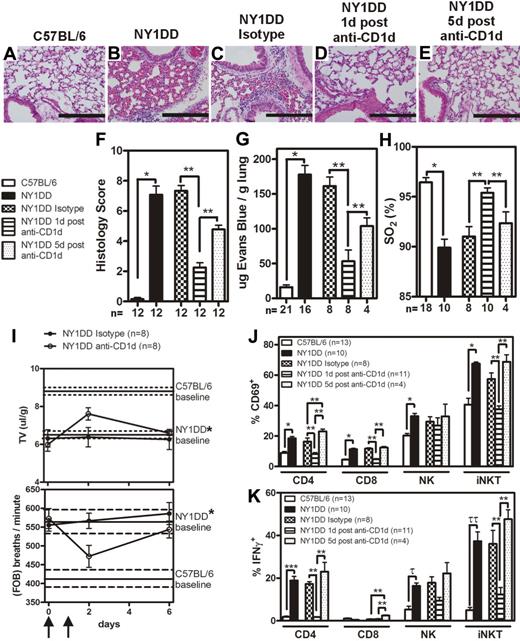
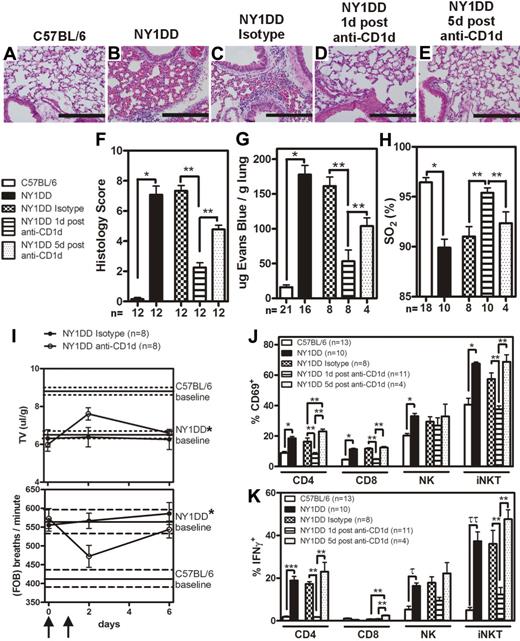
Although several SCD tissues displayed activated iNKT cells, we focused on the pulmonary system because it is associated with the highest morbidity and mortality in persons with SCD.2,3 Furthermore, since chronic low grade ischemia-reperfusion injury is believed to occur at baseline in SCD, we focused on measuring baseline pulmonary phenotype in NY1DD mice. To determine the baseline pulmonary phenotype, NY1DD mice were compared with C57BL/6. Quantitative analysis of lung histology revealed marked increases in capillary congestion and alveolar wall thickness in NY1DD mice compared with C57BL/6 mice (Figure 2A-B,F). In addition, at baseline, NY1DD mice had significantly increased vascular permeability (12.4-fold compared with C57BL/6 mice; P < .001) and decreased arterial oxygen saturation (90% compared with 96% in C57BL/6 mice; P < .001; Figure 2G-H). Furthermore, unrestrained whole body plethysmography revealed that NY1DD mice have an abnormally high rate of respiration and an abnormally low tidal volume, consistent with a restrictive ventilatory defect (Figure 2I).
Lungs of NY1DD mice have histologic evidence of inflammation and impaired function that can transiently be improved by anti-CD1d treatment. (A-E) Representative images of H&E-stained lungs. Scale bars represent 200 μm. (F) Histopathologic scores (1-8, see “Methods”) calculated by analysis of H&E-stained mouse lungs. (G-H) Vascular permeability is increased and arterial oxygen saturation is reduced in NY1DD mouse lungs at baseline compared with C57BL/6. Pulmonary dysfunction in SCD is inhibited 1 day, but not 5 days, after injection of anti-CD1d antibodies. (I) NY1DD mice have significantly impaired breathing (decreased tidal volume and increased frequency of breathing) compared with C57BL/6 mice (P < .001). The lines represent the mean (± SEM) baseline breathing parameters. In NY1DD mice, breathing parameters are improved 1 day, but not 5 days, after injection of anti-CD1d antibodies. Arrows indicate times of anti-CD1d injections. (J-K) NY1DD mice have increased CD69 surface expression and intracellular IFN-γ levels on pulmonary lymphocytes that decrease 1 day, but not 5 days, after treatment with anti-CD1d. The numbers on the x-axis (F-H,J-K) represent the number of animals for that group. C57BL/6 and NY1DD groups were evaluated by an unpaired Student t test (*P < .001; ***P = .001; τP = .002; ττP = .001). NY1DD-treated mice were analyzed by one-way ANOVA with Neuman-Keuls posttesting (**P < .05). Breathing parameters were analyzed by 2-way ANOVA with Bonferroni posttesting. Histologic grading was analyzed with either a nonparametric t test (Mann-Whitney; *P < .001) or a nonparametric Kruskal-Wallis test with Dunns posttesting (**P < .05). SO2 inidcates arterial oxygen saturation; FOB, frequency of breathing; and TV, tidal volume.
Lungs of NY1DD mice have histologic evidence of inflammation and impaired function that can transiently be improved by anti-CD1d treatment. (A-E) Representative images of H&E-stained lungs. Scale bars represent 200 μm. (F) Histopathologic scores (1-8, see “Methods”) calculated by analysis of H&E-stained mouse lungs. (G-H) Vascular permeability is increased and arterial oxygen saturation is reduced in NY1DD mouse lungs at baseline compared with C57BL/6. Pulmonary dysfunction in SCD is inhibited 1 day, but not 5 days, after injection of anti-CD1d antibodies. (I) NY1DD mice have significantly impaired breathing (decreased tidal volume and increased frequency of breathing) compared with C57BL/6 mice (P < .001). The lines represent the mean (± SEM) baseline breathing parameters. In NY1DD mice, breathing parameters are improved 1 day, but not 5 days, after injection of anti-CD1d antibodies. Arrows indicate times of anti-CD1d injections. (J-K) NY1DD mice have increased CD69 surface expression and intracellular IFN-γ levels on pulmonary lymphocytes that decrease 1 day, but not 5 days, after treatment with anti-CD1d. The numbers on the x-axis (F-H,J-K) represent the number of animals for that group. C57BL/6 and NY1DD groups were evaluated by an unpaired Student t test (*P < .001; ***P = .001; τP = .002; ττP = .001). NY1DD-treated mice were analyzed by one-way ANOVA with Neuman-Keuls posttesting (**P < .05). Breathing parameters were analyzed by 2-way ANOVA with Bonferroni posttesting. Histologic grading was analyzed with either a nonparametric t test (Mann-Whitney; *P < .001) or a nonparametric Kruskal-Wallis test with Dunns posttesting (**P < .05). SO2 inidcates arterial oxygen saturation; FOB, frequency of breathing; and TV, tidal volume.
To determine whether iNKT cells were involved in the observed pulmonary injury, NY1DD mice were treated with anti-CD1d antibody (or isotype control antibody) to inhibit CD1d-restricted activation of iNKT cells. One treatment group was assessed 1 day after the last anti-CD1d injection, and another treatment group was assessed 5 days after the last dose. One day after anti-CD1d treatment, NY1DD mice displayed decreased pulmonary injury based on histology score (P < .05), vascular permeability (P < .05), arterial oxygen saturation (P < .05), and breathing parameters (Figure 2C-D,F-I). Five days after the secession of anti-CD1d antibody treatment, pulmonary parameters returned to baseline (Figure 2E-I).
We used flow cytometric analysis to define white blood cell (WBC) populations in digested whole lung tissue. Compared with C57BL/6 mice, NY1DD mice had significantly increased numbers of pulmonary leukocytes, which decreased to near control levels 1 day after anti-CD1d antibody treatment (Table 2). Five days after anti-CD1d antibody treatment, pulmonary leukocyte counts in NY1DD mice returned to pre-antibody treatment levels (Table 2). CD69 and intracellular IFN-γ were significantly increased on all NY1DD pulmonary lymphocyte subpopulations versus C57BL/6 mice (Figure 2J-K and Supplemental Figure 4). One day after anti-CD1d treatment, expression of CD69 and IFN-γ in NY1DD mice was significantly diminished compared with isotype control-treated NY1DD animals (Figure 2J-K and Supplemental Figure 2). Expression of CD69 and IFN-γ returned to pre-antibody treatment levels 5 days after anti-CD1d injections.
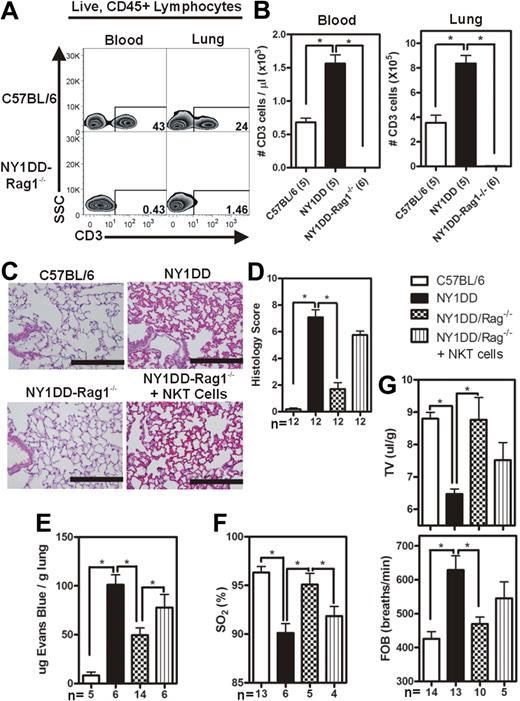
NY1DD-Rag1−/− mice are protected from developing pulmonary injury and the adoptive transfer of wild-type NKT cells reconstitutes injury
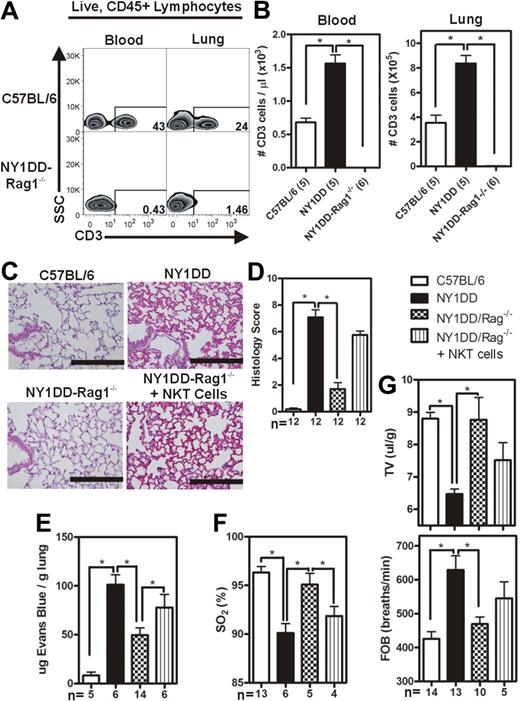
The experimental evidence suggesting the involvement of iNKT cells in the pathogenesis of pulmonary injury in the NY1DD mouse led us to hypothesize that NY1DD × Rag1−/− mice (lacking mature T cells and B cells) would be protected from developing pulmonary injury that was noted in NY1DD mice at baseline. The NY1DD × Rag1−/− genotype was confirmed by PCR, and the expected phenotype (lacking CD3+ cells) was confirmed by flow cytometric analysis (Figure 3A-B). Compared with NY1DD mice, NY1DD × Rag1−/− animals had decreased capillary congestion and alveolar wall thickness (Figure 3C-D). Furthermore, NY1DD × Rag1−/− animals had significantly decreased vascular permeability (P < .05; 2-fold) and increased arterial oxygen saturation (P < .05) compared with NY1DD mice at baseline (Figure 3E,F). NY1DD × Rag1−/− animals also had near normal breathing parameters (P < .05; increased tidal volume and decreased frequency of breathing) compared with NY1DD mice (Figure 3G). Interestingly, NY1DD × Rag1−/− animals had significantly increased pulmonary levels of NK cells (P < .05), perhaps as a compensatory adaptation to the lack of T cells and B cells (Table 3).
Reduced pulmonary injury caused by crossing NY1DD to Rag1−/− mice is reversed by the adoptive transfer of NKT cells. (A) Representative flow cytometry plots of CD3+ lymphocytes in blood and lungs of C57BL/6 and NY1DD × Rag1−/− animals. (B-C) Representative images of H&E-stained cells in C57BL/6 and NY1DD × Rag1−/− animals. Scale bar represents 200 μm. (D) Histopathologic scores (0-8, see Methods) by analysis of H&E-stained mouse lungs. (E-F) NY1DD × Rag1−/− animals have decreased vascular permeability and increased arterial oxygen saturation compared with NY1DD mice. The adoptive transfer of 106 NKT cells significantly increased vascular permeability and decreased arterial oxygen saturation to near NY1DD baseline levels. (G) NY1DD × Rag1−/− mice have significantly improved breathing (increased tidal volume and decreased frequency of breathing) compared with NY1DD mice. The adoptive transfer of 106 NKT cells decreased tidal volume and increased frequency of breathing to near NY1DD baseline levels. Data were analyzed by one-way ANOVA with Neuman-Keuls posttesting. Histologic grading was analyzed with a nonparametric Kruskal-Wallis test with Dunn posttesting (*P < .05). SO2 indicates arterial oxygen saturation; FOB, frequency of breathing; and TV, tidal volume.
Reduced pulmonary injury caused by crossing NY1DD to Rag1−/− mice is reversed by the adoptive transfer of NKT cells. (A) Representative flow cytometry plots of CD3+ lymphocytes in blood and lungs of C57BL/6 and NY1DD × Rag1−/− animals. (B-C) Representative images of H&E-stained cells in C57BL/6 and NY1DD × Rag1−/− animals. Scale bar represents 200 μm. (D) Histopathologic scores (0-8, see Methods) by analysis of H&E-stained mouse lungs. (E-F) NY1DD × Rag1−/− animals have decreased vascular permeability and increased arterial oxygen saturation compared with NY1DD mice. The adoptive transfer of 106 NKT cells significantly increased vascular permeability and decreased arterial oxygen saturation to near NY1DD baseline levels. (G) NY1DD × Rag1−/− mice have significantly improved breathing (increased tidal volume and decreased frequency of breathing) compared with NY1DD mice. The adoptive transfer of 106 NKT cells decreased tidal volume and increased frequency of breathing to near NY1DD baseline levels. Data were analyzed by one-way ANOVA with Neuman-Keuls posttesting. Histologic grading was analyzed with a nonparametric Kruskal-Wallis test with Dunn posttesting (*P < .05). SO2 indicates arterial oxygen saturation; FOB, frequency of breathing; and TV, tidal volume.
One million NKT cells were adoptively transferred via retro-orbital injection into NY1DD × Rag1−/− animals, and pulmonary parameters were analyzed 4 days later. The adoptive transfer of NKT cells into the NY1DD × Rag1−/− mice resulted in significantly increased pulmonary levels of both NK cells (2-fold; P < .05) and polymorphonuclear leukocytes (PMNs; 4-fold; P < .05; Table 3). In addition, the adoptive transfer of NKT cells into NY1DD × Rag1−/− mice caused increased vascular permeability (P < .05) and decreased oxygen saturation (P < .05; Figure 3E-F).
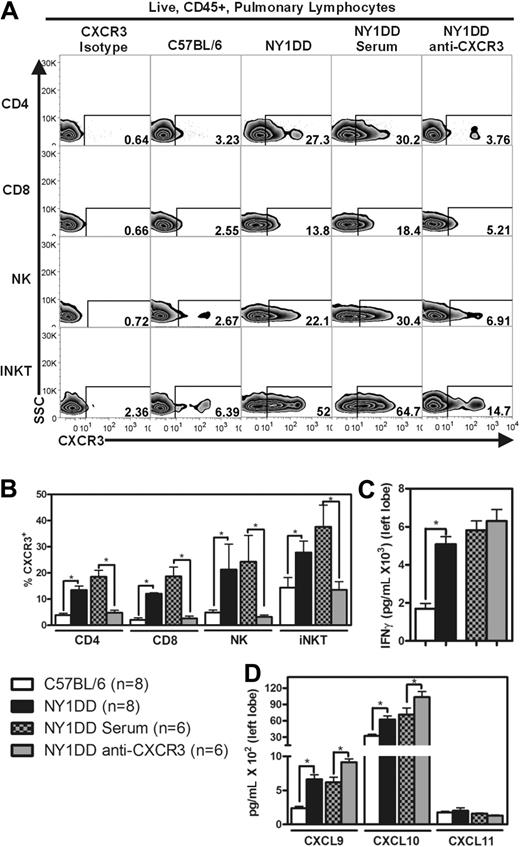
NY1DD animals have increased levels of pulmonary IFN-γ–inducible CXC chemokines and neutralization of CXCR3 ameliorates pulmonary injury
Our discovery of high levels of IFN-γ in pulmonary iNKT cells from NY1DD mouse lungs led us to hypothesize that the recruitment of pulmonary lymphocytes is through the IFN-γ–inducible CXC chemokine-CXCR3 axis. Flow cytometric analysis of pulmonary lymphocytes for CXCR3 revealed that the expression of this receptor is significantly higher on CD4 T cells (6-fold), CD8 T cells (7-fold), NK cells (4-fold), and iNKT cells (2-fold) from NY1DD animals compared with C57BL/6 animals (P < .05; Figure 4A-B). ELISAs of pulmonary tissue homogenate also revealed significantly increased levels of IFN-γ (P < .05) and the IFN-γ–inducible CXC chemokines CXCL9 and CXCL10 (P < .05) in NY1DD animals at baseline compared with C57BL/6 animals (Figure 4C-D).
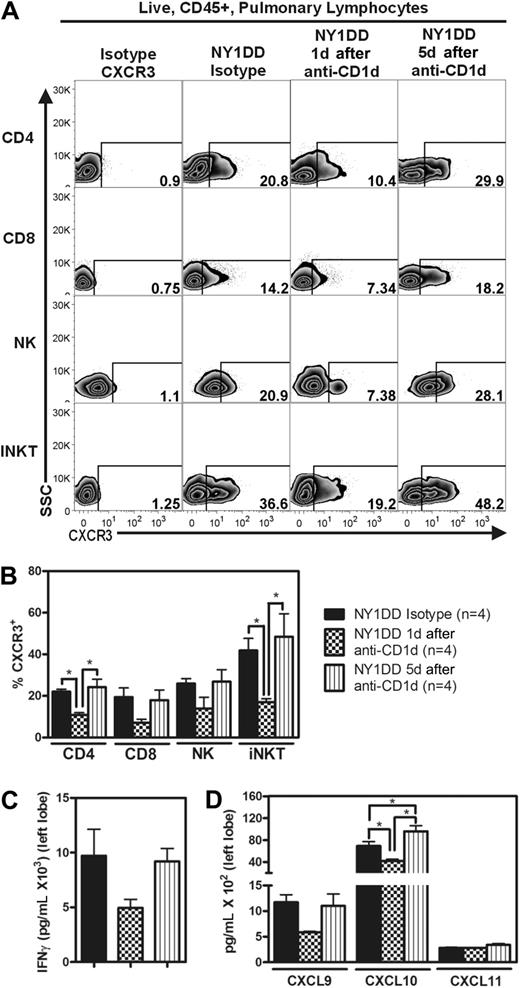
NY1DD mice have increased pulmonary lymphocyte expression of CXCR3 and increased whole lung expression of IFN-γ and IFN-γ–inducible chemokines. (A) Representative flow cytometry plots of CXCR3 expression on pulmonary lymphocytes from C57BL/6, NY1DD, NY1DD treated with goat serum, and NY1DD treated with goat anti-CXCR3 serum. (B) Pulmonary lymphocytes from NY1DD mice express significantly increased levels of CXCR3 compared with C57BL/6 mice. NY1DD animals treated with anti-CXCR3 display significantly decreased expression of CXCR3 on pulmonary lymphocytes. (C-D) Pulmonary homogenates from NY1DD animals have significantly increased levels of IFN-γ and IFN-γ–inducible chemokines (CXCL9 and CXCL10). NY1DD animals treated with anti-CXCR3 have significantly elevated levels of IFN-γ–inducible chemokines (CXCL9 and CXCL10). Data were analyzed by one-way ANOVA with Neuman-Keuls posttesting (*P < .05).
NY1DD mice have increased pulmonary lymphocyte expression of CXCR3 and increased whole lung expression of IFN-γ and IFN-γ–inducible chemokines. (A) Representative flow cytometry plots of CXCR3 expression on pulmonary lymphocytes from C57BL/6, NY1DD, NY1DD treated with goat serum, and NY1DD treated with goat anti-CXCR3 serum. (B) Pulmonary lymphocytes from NY1DD mice express significantly increased levels of CXCR3 compared with C57BL/6 mice. NY1DD animals treated with anti-CXCR3 display significantly decreased expression of CXCR3 on pulmonary lymphocytes. (C-D) Pulmonary homogenates from NY1DD animals have significantly increased levels of IFN-γ and IFN-γ–inducible chemokines (CXCL9 and CXCL10). NY1DD animals treated with anti-CXCR3 have significantly elevated levels of IFN-γ–inducible chemokines (CXCL9 and CXCL10). Data were analyzed by one-way ANOVA with Neuman-Keuls posttesting (*P < .05).
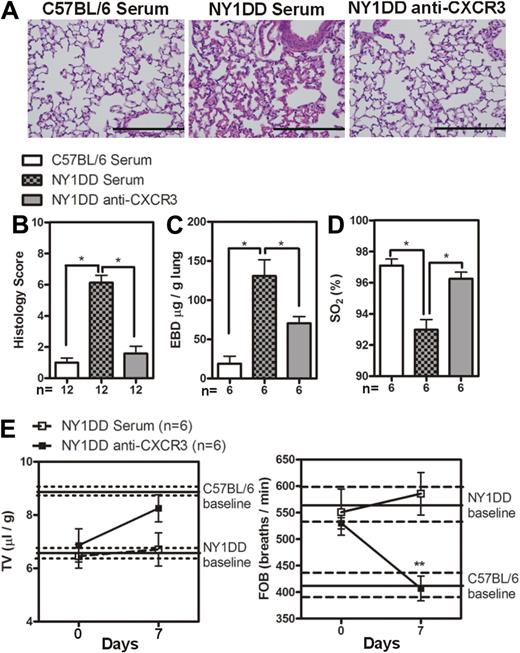
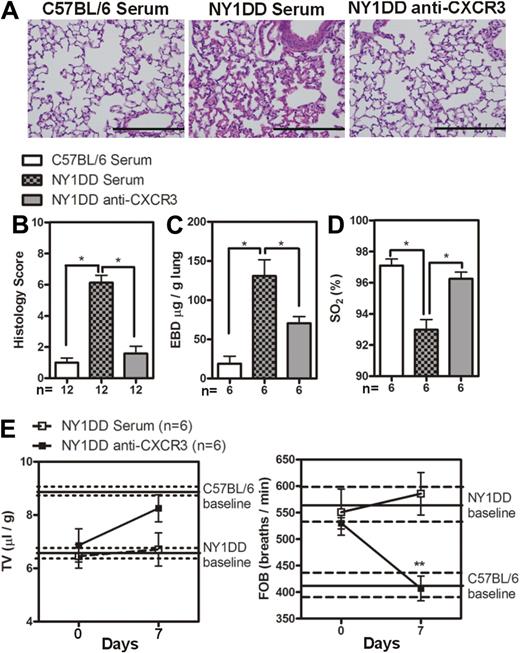
These data led us to hypothesize that neutralization of CXCR3 would ameliorate pulmonary dysfunction in NY1DD animals by blocking leukocyte trafficking to the lung. Treatment of NY1DD mice with goat anti-CXCR3 for 1 week resulted in significantly decreased levels of pulmonary lymphocyte CXCR3 expression on CD4 T cells, CD8 T cells, NK cells, and iNKT cells compared with NY1DD mice treated with goat serum (P < .05; Figure 4A-B). This treatment also resulted in a significant increase of CXCL9 and CXCL10 in NY1DD mice (P < .05; Figure 4C). The increase in chemokine concentration in mice with neutralized CXCR3 receptors can be attributed to the fact that chemokines are internalized and degraded after binding to chemokines receptors.33,34 Anti-CXCR3 treatment resulted in a decrease in total pulmonary leukocyte numbers (Table 4). Quantitative analysis of lung histology in the anti-CXCR3–treated animals revealed decreases in capillary congestion and alveolar wall thickness compared with goat serum–treated NY1DD mice (P < .05; Figure 5A-B). Furthermore, anti-CXCR3–treated NY1DD animals had significantly decreased vascular permeability (2-fold; P < .05) and increased arterial oxygen saturation compared with NY1DD mice (P < .05; Figure 5C-D). Anti-CXCR3–treated NY1DD animals also had improved breathing parameters (increased tidal volume and decreased frequency of breathing) compared with serum-treated NY1DD mice (Figure 5E).
Anti-CXCR3 treatment decreases pulmonary injury in NY1DD mice. (A) Representative images of H&E-stained lungs from C57BL/6 or NY1DD mice treated with goat serum and NY1DD mice treated with goat anti-CXCR3. Scale bar represents 200 μm. (B) Histopathologic scores (0-8, see “Methods”) by analysis of H&E-stained mouse lungs. (C-D) Anti-CXCR3-treated NY1DD animals have decreased vascular permeability and increased arterial oxygen saturation compared with serum-treated NY1DD mice. (E) Anti-CXCR3-treated NY1DD mice have improved breathing (increased tidal volume and decreased frequency of breathing) compared with serum-treated NY1DD mice. Data were analyzed by one-way ANOVA with Neuman-Keuls posttesting. Breathing parameters were analyzed by 2-way ANOVA with Bonferroni posttesting. Histologic grading was analyzed with a nonparametric Kruskal-Wallis test with Dunn posttesting (*P < .05). EBD, Evans blue dye; SO2 indicates arterial oxygen saturation; FOB, frequency of breathing; and TV, tidal volume.
Anti-CXCR3 treatment decreases pulmonary injury in NY1DD mice. (A) Representative images of H&E-stained lungs from C57BL/6 or NY1DD mice treated with goat serum and NY1DD mice treated with goat anti-CXCR3. Scale bar represents 200 μm. (B) Histopathologic scores (0-8, see “Methods”) by analysis of H&E-stained mouse lungs. (C-D) Anti-CXCR3-treated NY1DD animals have decreased vascular permeability and increased arterial oxygen saturation compared with serum-treated NY1DD mice. (E) Anti-CXCR3-treated NY1DD mice have improved breathing (increased tidal volume and decreased frequency of breathing) compared with serum-treated NY1DD mice. Data were analyzed by one-way ANOVA with Neuman-Keuls posttesting. Breathing parameters were analyzed by 2-way ANOVA with Bonferroni posttesting. Histologic grading was analyzed with a nonparametric Kruskal-Wallis test with Dunn posttesting (*P < .05). EBD, Evans blue dye; SO2 indicates arterial oxygen saturation; FOB, frequency of breathing; and TV, tidal volume.
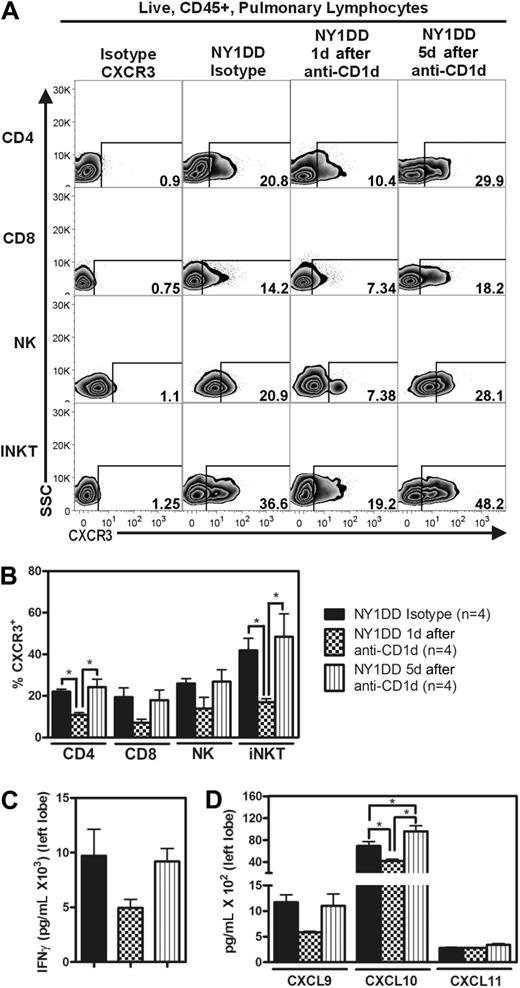
Anti-CD1d–treated NY1DD mice have decreased pulmonary levels of IFN-γ, IFN-γ–inducible chemokines, and CXCR3
We hypothesized that IFN-γ from iNKT cells in NY1DD mice was responsible for the increased levels of IFN-γ–inducible chemokines and the recruitment of CXCR3 positive lymphocytes. Blockade of iNKT activation by treatment of NY1DD mice with anti-CD1d for 2 days resulted in decreased whole lung levels of IFN-γ, CXCL9, CXCL10, and CXCR3 positive lymphocytes (Figure 6A-D). Five days after anti-CD1d treatment, all of these parameters returned to NY1DD isotype-treated levels (Figure 6A-D).
Anti-CD1d treatment decreases the percentage of pulmonary lymphocytes expressing CXCR3 and reduces lung homogenate levels of IFN-γ and IFN-γ–inducible chemokines. (A) Representative flow cytometry plots of CXCR3 expression on pulmonary lymphocytes from NY1DD isotype-treated, NY1DD 1 day after anti-CD1d treatment, and NY1DD 5 days after anti-CD1d treatment. (B) Pulmonary lymphocytes from NY1DD mice express decreased CXCR3 when harvested 1 day, but not 5 days, after anti-CD1d. (C-D) Pulmonary tissues harvested from NY1DD animals have reduced levels of IFN-γ and IFN-γ–inducible chemokines when harvested 1 day, but not 5 days, after anti-CD1d. Data were analyzed by one-way ANOVA with Neuman-Keuls posttesting (*P < .05).
Anti-CD1d treatment decreases the percentage of pulmonary lymphocytes expressing CXCR3 and reduces lung homogenate levels of IFN-γ and IFN-γ–inducible chemokines. (A) Representative flow cytometry plots of CXCR3 expression on pulmonary lymphocytes from NY1DD isotype-treated, NY1DD 1 day after anti-CD1d treatment, and NY1DD 5 days after anti-CD1d treatment. (B) Pulmonary lymphocytes from NY1DD mice express decreased CXCR3 when harvested 1 day, but not 5 days, after anti-CD1d. (C-D) Pulmonary tissues harvested from NY1DD animals have reduced levels of IFN-γ and IFN-γ–inducible chemokines when harvested 1 day, but not 5 days, after anti-CD1d. Data were analyzed by one-way ANOVA with Neuman-Keuls posttesting (*P < .05).
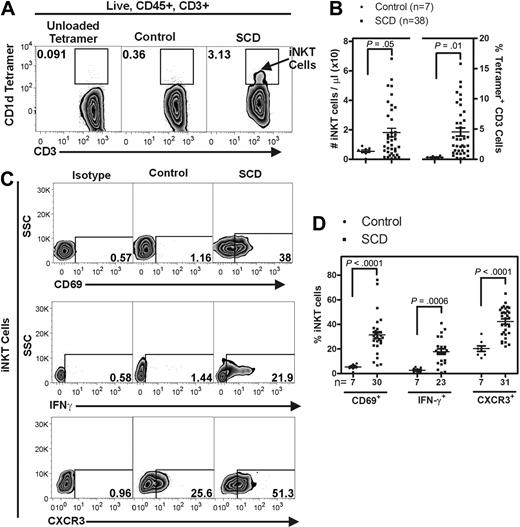
Persons with SCD have increased numbers of circulating activated iNKT cells.
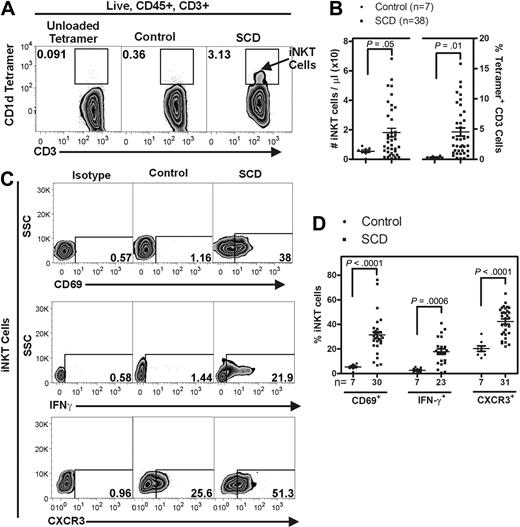
We used fluorescently labeled CD1d tetramers to selectively label iNKT cells (defined as tetramer+ CD3+) in the blood of 38 persons with SCD and 7 age- and race-matched controls (Figure 7A). The mean age of participants with SCD was 32 (range, 21-65), and 47.5% of the cohort were female. In comparison, the mean age of the controls was 41 (range, 36-49) years, and 85.7% of the controls were female. Flow cytometric analysis and counting beads were used to determine the absolute numbers of iNKT cells and the percentage of iNKT cells among all CD3+ lymphocytes (live, CD45+, CD3+) in the blood samples. Relative to controls, circulating iNKT cells from persons with SCD were increased in absolute number from 5.0 ± 1.0 to 18.0 ± 3.0 cells/μL (P = .05) and as a percentage of all CD3+ cells from 0.3 ± 0.06 to 4.5 ± 0.7% (P = .01; Figure 7A-B). In addition, iNKT cells from persons with SCD at steady state were found to be highly activated compared with controls (Figure 7C-D). CD69 and IFN-γ were both found to be increased on iNKT cells from persons with SCD (5.8- and 7.1-fold, respectively). Furthermore, a higher percentage of iNKT cells from persons with SCD were found to express CXCR3 (42.2% ± 2.1%) compared with controls (20.3% ± 2.3%; P < .001).
Persons with SCD have increased circulating iNKT cells that express activation markers. Fluorescently labeled CD1d tetramers were used to selectively label iNKT cells (defined as tetramer+ CD3+) in the blood of persons with SCD (HbSS) and appropriate age- and race-matched controls. (A) Representative flow cytometry plots of circulating human iNKT cells. (B) iNKT cells were identified from the live, CD45+ lymphocyte gate as CD1d-tetramer+ CD3+ cells. Compared with controls, persons with SCD have a higher number of circulating iNKT cells. (C-D) iNKT cells from persons with SCD are more activated as defined by higher percentage of surface CD69 and CXCR3 and intracellular IFN-γ. Data were analyzed by an unpaired Student t test. SSC indicates side scatter.
Persons with SCD have increased circulating iNKT cells that express activation markers. Fluorescently labeled CD1d tetramers were used to selectively label iNKT cells (defined as tetramer+ CD3+) in the blood of persons with SCD (HbSS) and appropriate age- and race-matched controls. (A) Representative flow cytometry plots of circulating human iNKT cells. (B) iNKT cells were identified from the live, CD45+ lymphocyte gate as CD1d-tetramer+ CD3+ cells. Compared with controls, persons with SCD have a higher number of circulating iNKT cells. (C-D) iNKT cells from persons with SCD are more activated as defined by higher percentage of surface CD69 and CXCR3 and intracellular IFN-γ. Data were analyzed by an unpaired Student t test. SSC indicates side scatter.
Discussion
The results of this study provide the basis for a new paradigm to understand the development of pulmonary and other tissue dysfunction in SCD. We demonstrate a pivotal role for CD1d-restricted iNKT cells in maintaining chronic pulmonary inflammation and dysfunction in SCD mice via the IFN-γ–inducible CXC chemokine-CXCR3 axis. The analysis of blood from persons with SCD suggests that this pathway may translate to human disease. We found iNKT cells from NY1DD mice to be increased in number and activation state in lung, liver, and spleen compared with C57BL/6 control mice. Furthermore, hypoxia/reoxygenation exaggerated this inflammatory response, indicating that iNKT cell activation may be involved with periodic painful crises experienced by persons with SCD. Although we focused on pulmonary inflammation and dysfunction for our studies, our findings indicate that this mechanism may be occurring in every organ system.
In the lung, compared with C57BL/6 mice, NY1DD mice have moderately increased numbers of iNKT cells (increased from 2.2 to 3.9 × 104 cells/lung). A much greater difference is noted in the fraction of iNKT cells that are judged to be activated, based on intracellular IFN-γ production, which increases from 5% in C57BL/6 mice to 35% in NY1DD mice. In addition, our data demonstrate that activation of iNKT cells plays an important role in pulmonary dysfunction of SCD. Pulmonary dysfunction and inflammation in SCD mice is largely reversed by inhibition of CD1d-restricted NKT activation, and this protection was observed to be transient. Five days after anti-CD1d treatment, NY1DD mice were observed to return to baseline levels of pulmonary inflammation and dysfunction. In addition, pulmonary dysfunction and inflammation in NY1DD mice is largely improved by lymphocyte deletion produced by crossing NY1DD mice to Rag1−/− mice. The beneficial effects on pulmonary function in response to lymphocyte deletion in NY1DD × Rag1−/− are transiently reversed by adoptive transfer of 1 million NKT cells. These findings indicate that although NKT cells represent only a minor subset of the total lymphocyte population, they play a pivotal role in sustaining the pulmonary pathophysiology in a mouse model of SCD.
Several murine models of SCD with phenotypes that vary in severity have been developed to model human SCD. In this study, we used the well-characterized NY1DD model that expresses 75% human βS-globin and 56% human α-globin.24 While these mice have been previously described as having a relatively mild hematologic pathology (ie, they have a normal hematocrit), they have been shown to exhibit baseline organ damage to lung, liver, spleen, and kidney.24 In patients, baseline SCD with various degrees of organ dysfunction is often punctuated by periodic exacerbations referred to as sickle “crises.” A disease exacerbation, or crisis, can also be produced in mice exposed to endotoxin or transient hypoxia.25,35 We chose to focus our study on baseline disease rather than crisis for several reasons: (1) recent findings suggest that transient microvascular occlusion occurs chronically in a subclinical manner in SCD patients, and that end-organ damage and short life span in SCD are often due to the cumulative effects of repeated bouts of minor ischemic events4,5 ; (2) relatively little attention has been paid to evaluating the natural progression of chronic organ injury in this model; and (3) the baseline SCD pulmonary phenotype is more stable and amenable to investigation than are crisis phenotypes. Our findings confirm that at baseline, NY1DD mice display substantial pulmonary inflammation and pathophysiology that is manifested by increased numbers of pulmonary leukocytes, impaired endothelial integrity (increased vascular permeability), and microvascular occlusion. Histologic examination of lungs from NY1DD animals revealed striking inflammatory changes. Similar findings have been noted in pulmonary autopsy samples.36 We also found a significant decrease in arterial oxygen saturation (% SO2) in NY1DD mice. Low oxygen saturation has been associated with increased pulmonary artery pressures in persons with SCD and is believed to be a risk factor for the development chronic lung disease.37,38
Recent studies indicate that iNKT cells are activated by IRI.8,9 One possible route for iNKT cell activation is through self-lipid presentation to invariant TCRs on NKT cells. The existence of such lipids is suggested by the fact that deletion of CD1d in mice results in depletion of type I and type II NKT cells in the thymus. The identification of putative endogenous lipid(s) that may be responsible for iNKT activation has been controversial. In 2000, Gumperz et al demonstrated that self lipids could stimulate iNKT cells, and in 2004, Zhou et al suggested that isoglobotrihexosylceramide (iGb3) was a self lipid that mediates iNKT activation.19,39,40 However, a recent study demonstrates that iGb3 is not produced in human tissues, and endogenous ligand(s) have yet to be identified.41 In addition to lipid antigens, iNKT cell activation also is regulated by cytokine stimulation. In certain instances, it has been shown that IL-12 in combination with IL-18 is sufficient to cause iNKT cell activation.42 In the current study, we demonstrate a role for CD1d-restricted NKT cells, as treatment of NY1DD mice with anti-CD1d antibodies ameliorates pulmonary pathophysiology. One interpretation of these data is that host lipid antigens for iNKT cells are elevated in SCD. Another possibility is that lipid antigens remain constant, while cytokines that facilitate activation of iNKT cells are enhanced. The most likely possibility is that pulmonary IRI in response to microvascular occlusion with sickled RBCs leads to increased release or oxidation of self-lipids that are presented via CD1d to activate iNKT cells and also triggers the release of cytokines that facilitate activation of iNKT cells. A resulting inflammatory cascade leads to the release of IFN-γ–inducible and hypoxia-inducible chemokines from pulmonary resident epithelial or endothelial cells resulting in the recruitment and activation of CXCR3-positive lymphocytes, which further exacerbate inflammation and vaso-occlusion (Supplemental Figure 5). Our data indicate that compared with C57BL/6 lung lymphocytes, the fraction of lung lymphocytes positive for CXCR3 is greatly enhanced in NY1DD mice. This is likely due in part to enhanced production in the lung of CXCL9 and CXCL10, which are chemotactic ligands for CXCR3. In addition, activated iNKT cells release IL-2, which is known to induce expression of CXCR3 on lymphocytes. Consistent with the idea that IL-2 and IFN-γ–inducible chemokines stimulate lymphocyte accumulation in the NY1DD lung via CXCR3, we show that blockade of CXCR3 receptors inhibits lung inflammation and injury. In addition, our data demonstrate that circulating iNKT cells from persons with SCD express increased CXCR3, suggesting that chemotaxis mediated by this receptor may play a role in human disease.
While our results suggest a novel mechanism for vaso-occlusion and subsequent inflammation, we cannot exclude the fact that other factors also contribute to inflammation in SCD. Over the past decade, many studies have described dysregulation of nitric oxide–mediated blood flow in SCD. It is hypothesized that the hemolysis of sickled RBCs contributes to a state of nitric oxide resistance and limited bioavailability of l-arginine, the substrate for nitric oxide synthesis. Hemolysis contributes to reduced nitric oxide bioavailability and endothelial dysfunction via release of erythrocyte arginase, which limits arginine bioavailability, and release of erythrocyte hemoglobin, which scavenges nitric oxide.43 Abnormal nitric oxide–dependent regulation of vascular tone, adhesion, platelet activation, and inflammation is believed to contribute to the pathophysiology of vaso-occlusion.44 Furthermore, treatment of patients with SCD with inhaled nitric oxide gas has been shown, via its vasodilatory effects, to improve pulmonary ventilation-perfusion mismatch and hemodynamics thereby increasing arterial oxygen tension and decreasing inflammation.45,46 Nitric oxide dysregulation may coexist and work in concert with iNKT cell–mediated immune activation described in this study.
In conclusion, our results suggest a new paradigm for understanding the pathogenesis of pulmonary inflammation and vaso-occlusion in SCD. Lung iNKT cells are activated in the NY1DD mouse and trigger an inflammatory cascade resulting in increased vascular permeability, decreased arterial oxygen saturation, and abnormal breathing parameters. Furthermore, we show that CD1d-restricted NKT cells are important for recruiting other inflammatory cells to the lung via the IFN-γ–inducible chemokines-CXCR3 axis. In addition, we demonstrate that circulating iNKT cells from persons with SCD are increased in number and highly activated. The results of this study have important therapeutic implications. By inhibiting CD1d-restricted NKT cell activation or neutralizing CXCR3 on lymphocytes, it may be possible to reduce vascular occlusion and tissue damage associated with acute and chronic IRI in SCD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Robert Hebbel of the University of Minnesota for his gift of NY1DD mice, Mitch Kronenberg of the La Jolla Institute of Allergy and Immunity for providing an anti-CD1d hybridoma, Nicole White of Washington University of St Louis for collection and preparation of patient samples, and Vanessa Hejus at the University of Virginia for breeding the NY1DD-Rag1−/− animals. We are grateful to Michael Solga of the University of Virginia Flow Cytometry Core for help with flow cytometric analysis, the University of Virginia Lymphocyte Culture Core for antibody production and purification, and the National Institutes of Health Tetramer facility at Emory University for their gifts of loaded and unloaded CD1d tetramer.
This work was supported by grant P01 HL073361 from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: K.L.W. designed, performed, and analyzed all experiments and prepared the manuscript; M.A.M. provided technical assistance; S.I.R. performed all histologic preparation and staining; J.A.L. provided assistance with flow cytometry; J.J.F., a hematologist, provided human blood samples and demographic information; R.M.S., a pulmonologist, graded the histology; and J.L. provided experimental design and manuscript review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joel Linden, University of Virginia, Cardiovascular Research Center, MR5 Box 801394, Charlottesville, VA 22908; e-mail: jlinden@virginia.edu.