Abstract
Under certain instances, factor VIII (FVIII) stimulates an immune response, and the resulting neutralizing antibodies present a significant clinical challenge. Immunotherapies to re-establish or induce long-term tolerance would be beneficial, and an in-depth knowledge of mechanisms involved in tolerance induction is essential to develop immune-modulating strategies. We have developed a murine model system for studying mechanisms involved in induction of immunologic tolerance to FVIII in hemophilia A mice. We used lentiviral vectors to deliver the canine FVIII transgene to neonatal hemophilic mice and demonstrated that induction of long-term FVIII tolerance could be achieved. Hemophilia A mice are capable of mounting a robust immune response to FVIII after neonatal gene transfer, and tolerance induction is dependent on the route of delivery and type of promoter used. High-level expression of FVIII was not required for tolerance induction and, indeed, tolerance developed in some animals without evidence of detectable plasma FVIII. Tolerance to FVIII could be adoptively transferred to naive hemophilia recipient mice, and FVIII-stimulated splenocytes isolated from tolerized mice expressed increased levels of interleukin-10 and decreased levels of interleukin-6 and interferon-γ. Finally, induction of FVIII tolerance mediated by this protocol is associated with a FVIII-expandable population of CD4+CD25+Foxp3+ regulatory T cells.
Introduction
Factor VIII (FVIII) can provoke a host immune response that is associated with the development of antibodies that inhibit FVIII's activity. FVIII-specific CD4+ T cells drive the synthesis of these antibodies, which are often referred to as inhibitors.1,2 FVIII inhibitors develop in approximately 25% of congenital hemophilia A patients, but the reason that the majority of patients remain inhibitor free is still unclear. The hemophilic genotype contributes to this risk, because patients with null mutations have the highest incidence of inhibitor development.3 However, other immunogenetic characteristics also contribute to this outcome.4,5
Most of the anti-FVIII antibodies that have been described to date target functional epitopes, and inhibit FVIII activity by steric hindrance.6 Other antibodies target nonfunctional epitopes to form immune complexes that accelerate FVIII clearance,7 or catalyze the hydrolysis of FVIII.8 The development of FVIII inhibitors poses significant challenges to clinical management, and therefore, immunomodulatory therapies that induce or re-establish immunologic tolerance to FVIII are an important therapeutic goal.
Immune tolerance induction to FVIII is successful in eradicating anti-FVIII antibodies in approximately 75% of treated patients.9,10 Most often, immune tolerance induction involves frequent intravenous infusions of high doses of FVIII administered more than many months. High doses of FVIII may inhibit the differentiation of FVIII-specific memory B cells into antibody-secreting plasma cells, and more than time, this results in the eradication of the anti-FVIII antibodies.11 There are, however, significant limitations to this approach, including the high cost of FVIII products, the requirement of frequent venous access, and the lack of affordable access to FVIII to induce tolerance in patients in developing countries.
Despite considerable clinical experience, our basic understanding of the pathogenesis of FVIII inhibitor development and the mechanisms involved in the induction of tolerance to FVIII remains fragmentary at best. A comprehensive understanding of these mechanisms is essential to develop effective strategies for immunomodulatory intervention. To this end, we have developed a relevant animal model for inducing and establishing long-term immune tolerance to FVIII. We chose to use the BALB/c12 as opposed to C57BL/613 hemophilia A mice because BALB/c mice have a biased T helper (Th)2 immune response, and reliably mount a robust anti-FVIII response. This would be a good model for inhibitor development in large hemophilic animals.12,14 The neonatal period provides a unique window of time to establish central tolerance to neoantigens, and we have used this opportunity and a lentiviral delivery system to introduce the canine FVIII (cFVIII) transgene into hemophilia A neonates. In this study, we demonstrate that long-term immunologic tolerance to FVIII is established in this model, and illustrate how the model can be used to evaluate mechanisms underlying tolerogenic responses to FVIII in hemophilia A mice.
Methods
Construction of lentiviral vectors
The vector backbone plasmid, plenti-cytomegalovirus (CMV)–cFVIII, contains a B-domain–deleted cFVIII cDNA15 and has previously been described.16 The chimeric apolipoprotein E locus control region/human α1-antitrypsin promoter (HCR/hAATp) element17 was polymerase chain reaction (PCR) amplified and subcloned into the vector backbone plasmid. cFVIII-expressing lentiviral vectors under the control of the CMV promoter or HCR/hAAT promoters are denoted as Lenti-CMV-cFVIII and Lenti-HCR/hAAT-cFVIII, respectively.
Lentiviral vector production
The vesicular stomatitis virus-G–pseudotyped lentiviral vectors were produced in 293T cells, and functional titers of the lentiviral vectors were determined, as previously outlined.16 Average lentivector titers of 2.88 ± 1.61 × 108 IU/mL were reproducibly achieved.
Endotoxin assay
All viral preparations and recombinant cFVIII were assayed for levels of endotoxin contamination using the QCL-1000 test kit (BioWhittaker), according to the manufacturer's recommendations. Previous modifications16 to our viral production methods resulted in an average 3-fold reduction in the assayed levels of endotoxin, as well as a significant reduction in contaminating proteins seen with earlier vector production protocols.
Animal procedures
Animals used in this study were derived from C57BL/6 FVIII exon 16-disrupted hemophilic mice13 that were crossed with normal BALB/c mice (Charles River Laboratories) for more than 10 generations.12,14 Unanesthetized mice, between 0 and 1 day of age, were administrated lentiviral vector intravenously through the temporal vein, intraperitoneally or subcutaneously. Peripheral vein blood samples were collected from the saphenous vein. All animal procedures were reviewed and approved by the Queen's University Animal Care Committee.
Assays for vector-mediated toxicity
For assessment of hepatotoxicity, alanine aminotransferase levels were assayed on plasma samples that were submitted to the Clinical Chemistry Laboratory at Kingston General Hospital.
FVIII and neutralizing anti-FVIII antibody assays
Measurement of plasma cFVIII functional activity was carried out by chromogenic bioassay (DiaPharma), as directed by the manufacturer for the microplate acid stop method. Normal human reference plasma (Precision Biologicals) was used to establish the FVIII standard curve. The sensitivity of FVIII detection with this assay, in our experience, is at least 10 mU/mL. Anti-cFVIII inhibitory antibodies were measured by the Bethesda assay.18 Various dilutions of sample plasma were mixed in a 1:1 ratio with normal canine-pooled plasma and incubated at 37°C for 2 hours, and residual FVIII activity was determined by a functional one-stage FVIII assay, using a canine-pooled plasma as standard. Total anti-cFVIII antibodies were measured, as previously described,19 except that the 96-well plates were coated with 0.1 μg of cFVIII and the secondary antibody was a goat anti–mouse immunoglobulin G-F(ab′) conjugated to horseradish peroxidase.
Quantitative reverse transcription–PCR analysis
In vivo expression from the cFVIII transgene was quantified by reverse transcription (RT)–PCR analysis. The cFVIII probe, 5′FAM-CATCCGCAGCACTCTTCGCATGG, and forward and reverse primers TTTGCACCCAACCCATTACA and ACTGTTGAAGTCACAGCCCAAGA, respectively, were designed from cFVIII mRNA sequence using the Primer Express software program. RNA was extracted from mouse organs using TRIzol (Invitrogen) and treated with RNase-free DNase. After inactivation of the enzyme with EGTA (ethyleneglycoltetraacetic acid), 0.5 μg of RNA was mixed with the probe, primers, RNase inhibitor, and TaqMan One-Step RT-PCR Mix, and loaded into the sequence detection system. The RNA copy number was determined using the vector plasmid pLenti-CMV-cFVIII as the DNA standard and β-actin as the endogenous control. The integrated vector copy number in transduced mouse tissue was also determined by quantitative RT-PCR, as described above, on DNA extracted from various tissues.20
Challenge of treated mice with recombinant cFVIII and isolation of splenocytes for in vitro and in vivo analysis
Before the termination of each experiment, mice were challenged with 4 intravenous infusions of recombinant His-tagged cFVIII protein21 (80 IU/kg) at weekly intervals. All preparations were assessed for endotoxin, and levels were undetectable. After each cFVIII challenge, anti-cFVIII–neutralizing antibodies were measured by the Bethesda assay.18 Within 48 hours of the last cFVIII challenge, mice were euthanized and splenocytes were isolated, teased into single-cell suspensions, and washed with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered saline solution. Red blood cells were lysed with Tris-buffered ammonium chloride. Isolated splenocytes were divided for 3 groups of studies, as follows: (1) cytokine release analysis and T-cell proliferation, (2) adoptive transfer, and (3) T regulatory cell analysis. Inhibitor-positive control mice received either temporal vein injection of the Lenti-CMV-cFVIII or subcutaneous injection of Lenti-HCR/hAAT-cFVIII as neonates. Naive control mice did not receive gene transfer as neonates and were not challenged with recombinant cFVIII.
Analysis of antigen-specific cytokine release and T-cell proliferation
Isolated splenocytes were cultured in triplicate in 96-well plates for 72 hours in 200 μL of X-vivo medium (Cambrex) in the absence or presence of recombinant cFVIII (0.1 IU, 1 IU, and 10 IU). For the cytokine release assays, 106 cells/well were used and 5 × 104 cells/well were used for the T-cell proliferation assays. Levels of interleukin (IL)–6, IL-10, and interferon (IFN)–γ in culture supernatants were assayed by enzyme-linked immunosorbent assay (ELISA; eBioscience). Results are expressed as pg/mL and represent the mean of triplicate values. T-cell proliferation was assessed using the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay kit (Promega), and results are expressed as raw data as a corrected absorbance at 490 nm.
Adoptive transfer of T cells
CD4+, CD4+CD25+, and CD4+CD25− T cells were separated from splenocytes using magnetic beads (MACS Cell Separation; Miltenyi Biotec). T cells (106) were injected intravenously via the tail vein into naive syngeneic sex-matched recipient BALB/c hemophilia A mice. Recipient mice were challenged within 48 hours with intravenous administration of recombinant cFVIII (80 IU/Kg) for 3 consecutive days and evaluated for anti-cFVIII inhibitors.
Analysis of regulatory T cells in immune-tolerized mice
Total splenocytes isolated from mice were analyzed directly for Foxp3. CD4+ T cells were separated using the magnetic beads, as described in “Adoptive transfer of T cells.” and surface stained with phycoerythrin cy5 anti–mouse CD25. A portion of the cells was stained intracellularly with phycoerythrin anti–mouse Foxp3 using a regulatory T cell staining kit (eBioscience). Flow cytometric analysis was performed on a FACScan. The remainder of the cells was cultured for 72 hours in the presence of FVIII, and then analyzed by flow cytometry for FoxP3, as described above.
Statistical analysis
All data are presented as the mean plus or minus SEM. Statistical comparisons of experimental groups were evaluated with a Student t test. A P value of less than .01 was considered statistically significant.
Results
cFVIII expression and inhibitor development after administration of lentiviral vectors to neonates
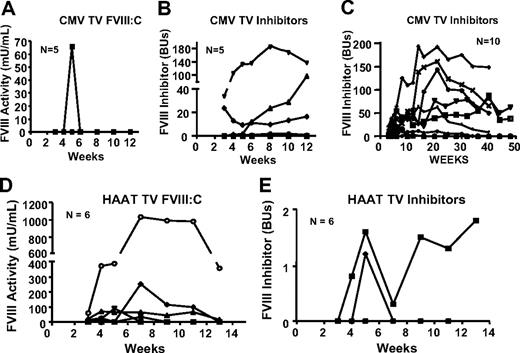
An initial cohort of 5 BALB/c hemophilia A neonates was injected intravenously via the temporal vein with 2 × 1010 IU/Kg Lenti-CMV-cFVIII. Sampling before 4 weeks is not feasible in neonates and, therefore, hepatotoxicity associated with lentiviral vector infusion cannot be evaluated. However, after infusion of the virus into adult mice, alanine aminotransferase levels were within the normal range (23.0 ± 9.3 IU/L),22 and no mice died as a result of injection of the lentiviral vector. Baseline FVIII activity in BALB/c hemophilia A mice is less than 10 mU/mL, and cFVIII inhibitor titers are less than 0.5 Bethesda Units (BU). Apart from one small spike of cFVIII activity at 5 weeks in one mouse, no detectible cFVIII was observed in any of these mice (Figure 1A), and at termination of the experiment at 12 weeks postgene transfer, 4 of the 5 mice had cFVIII inhibitor titers ranging from 0.7 to 137.0 BUs (Figure 1B). An additional 10 neonatal mice were infused in a similar manner, and because no plasma FVIII was detected (data not shown) and all developed cFVIII inhibitors with titers ranging from 1.2 to 193 BUs (Figure 1C), these treated mice were used as a positive control for an anti-cFVIII immune response.
FVIII expression and inhibitor development after intravenous temporal vein infusion of Lenti-CMV-cFVIII and Lenti-HCR/hAAT-cFVIII into BALB/c neonatal mice. (A-B) An initial cohort of 5 BALB/c hemophilia A neonates was treated via temporal vein infusion with 100 μL of 2 × 107 IU of Lenti-CMV-cFVIII. (D-E) BALB/c hemophilia A neonates (n = 6) treated via the temporal vein with 2 × 107 IU of Lenti-HCR/hAAT-cFVIII. (A,D) Plasma levels of cFVIII activity were determined by a chromogenic assay. (B,E) Anti-cFVIII inhibitory antibodies were measured by the Bethesda assay. Throughout the duration of the study, hemophilia A mice that had been infused with 2 × 107 IU of Lenti-CMV-cFVIII via the temporal vein as neonates were used as controls for positive inhibitor development. The inhibitor titers for these mice are shown in panel C.
FVIII expression and inhibitor development after intravenous temporal vein infusion of Lenti-CMV-cFVIII and Lenti-HCR/hAAT-cFVIII into BALB/c neonatal mice. (A-B) An initial cohort of 5 BALB/c hemophilia A neonates was treated via temporal vein infusion with 100 μL of 2 × 107 IU of Lenti-CMV-cFVIII. (D-E) BALB/c hemophilia A neonates (n = 6) treated via the temporal vein with 2 × 107 IU of Lenti-HCR/hAAT-cFVIII. (A,D) Plasma levels of cFVIII activity were determined by a chromogenic assay. (B,E) Anti-cFVIII inhibitory antibodies were measured by the Bethesda assay. Throughout the duration of the study, hemophilia A mice that had been infused with 2 × 107 IU of Lenti-CMV-cFVIII via the temporal vein as neonates were used as controls for positive inhibitor development. The inhibitor titers for these mice are shown in panel C.
To evaluate whether the use of a liver-restricted promoter could prevent an anti-FVIII immune response, 6 BALB/c hemophilia A neonates were infused via the temporal vein with 2 × 107 IU of Lenti-HCR/hAAT-cFVIII. cFVIII activity was observed in 5 of the 6 mice, and in 3 of these mice, cFVIII activity was detected for more than 11 weeks after vector administration (Figure 1D). The maximum cFVIII activity was 1033.8 mU/mL. One mouse developed a persistent inhibitor, with a maximum titer of 1.8 BUs (Figure 1E). This mouse never expressed detectable levels of cFVIII. A second mouse developed a transient inhibitor at 5 weeks.
Quantification of vector copy number in mouse organs after temporal vein injection
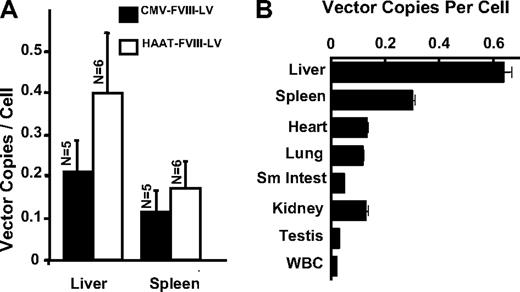
The number of integrated transgene copies in livers in mice treated with Lenti-CMV-cFVIII or Lenti-HCR/hAAT-cFVIII was 0.21 (± 0.07) and 0.40 (± 0.17) vector copies per cell, respectively, whereas spleen DNA contained 0.12 (± 0.05) and 0.17 (± 0.08) vector copies per cell, respectively (Figure 2A). To evaluate the tropism of vesicular stomatitis virus-G–pseudotyped Lenti-HCR/hAAT-cFVIII administered to neonates via the temporal vein, major organs were collected from a mouse that still expressed plasma cFVIII:C (1033 mU/mL) at 13 weeks (Figure 2B). DNA was also isolated from the 5 mice that received Lenti-HCR/hAAT-cFVIII, but that no longer expressed cFVIII, and no differences in integrated copy numbers were observed in any of the organs between the mouse that expressed cFVIII at 13 weeks and those that no longer expressed cFVIII at 30 to 32 weeks. Although lentiviral DNA sequences were detected in all of the organs analyzed, liver and spleen had the highest vector copy number per cell. A comparison of the levels of plasma cFVIII activity and the integrated vector copy number in liver samples isolated from 6 mice treated intravenously with Lenti-HCR/hAAT-cFVIII indicated that the level of cFVIII transgene integration does not correlate with the levels of plasma cFVIII activity (data not shown). When neonatal mice are treated with an integrating viral vector, there is clonal expansion of transduced hepatocytes with growth and development. Presumably, the site of vector integration and the efficiency of the neonatal temporal vein injection procedure influence levels of transgene expression.
Quantitation of viral copy number in mouse organs after lentivector transduction. Mice were killed at various time points, and the number of integrated viral copies was determined by quantitative PCR analysis. Integrated transgene copy number is represented as copies per cell using the copy number of the murine β-actin sequence as an endogenous reference. (A) The mean viral copy numbers in livers and spleens from the mice 12 to 13 weeks posttemporal vein infusion with Lenti-CMV-cFVIII (n = 5) or Lenti-HCR/hAAT-cFVIII (n = 6). Error bars represent the SEM. (B) Mice treated intravenously with Lenti-HCR/hAAT-cFVIII were killed at 13 (n = 1) or between 30 and 32 (n = 4) weeks after treatment. Organ distribution of the vector was studied in liver, spleen, heart, lung, kidney, and small intestine isolated from 5 mice, as well as testis and white blood cells (WBCs) isolated from a single mouse. Plasma cFVIII:C expression in the mouse that was killed at 13 weeks was 1033 mU/mL, whereas no plasma cFVIII:C could be detected in the mice killed at 30 to 32 weeks (n = 4).
Quantitation of viral copy number in mouse organs after lentivector transduction. Mice were killed at various time points, and the number of integrated viral copies was determined by quantitative PCR analysis. Integrated transgene copy number is represented as copies per cell using the copy number of the murine β-actin sequence as an endogenous reference. (A) The mean viral copy numbers in livers and spleens from the mice 12 to 13 weeks posttemporal vein infusion with Lenti-CMV-cFVIII (n = 5) or Lenti-HCR/hAAT-cFVIII (n = 6). Error bars represent the SEM. (B) Mice treated intravenously with Lenti-HCR/hAAT-cFVIII were killed at 13 (n = 1) or between 30 and 32 (n = 4) weeks after treatment. Organ distribution of the vector was studied in liver, spleen, heart, lung, kidney, and small intestine isolated from 5 mice, as well as testis and white blood cells (WBCs) isolated from a single mouse. Plasma cFVIII:C expression in the mouse that was killed at 13 weeks was 1033 mU/mL, whereas no plasma cFVIII:C could be detected in the mice killed at 30 to 32 weeks (n = 4).
Quantitation of in vivo cFVIII-transgene expression
RNA was extracted from the livers and spleens from the single mouse that expressed 1033 mU/mL FVIII and from the 5 mice that no longer expressed FVIII. Levels of cFVIII mRNA in these tissues were evaluated by quantitative RT-PCR. The lowest limit of mRNA detection was approximately 100 copies/μg, and β-actin mRNA copy number was used as a control. In the treated mouse that still expressed cFVIII, 1.56 × 104 copies/μg cFVIII mRNA transcripts were detected in the liver and no cFVIII mRNA was detected in the spleen. This finding indicates that cFVIII expression mediated by the HCR/hAAT promoter appears to be liver specific. cFVIII mRNA was not detected in any of the livers or spleens from the 5 treated mice that no longer had detectable levels of plasma cFVIII.
Evaluation of route of transgene delivery and inhibitor development
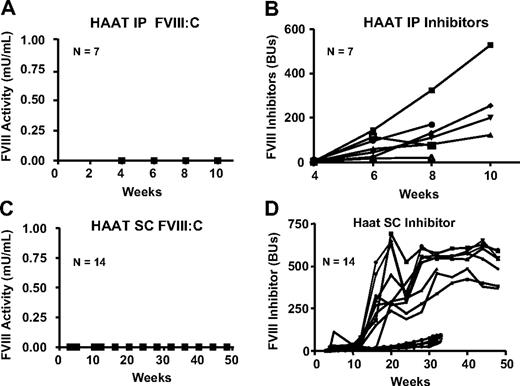
In adult mice, the route of vector delivery is a factor that contributes to triggering host immune responses,23 but the neonatal immune system is relatively immature.24 Because temporal vein infusions in neonatal mice are technically challenging, we wanted to evaluate alternate delivery routes. Seven and 14 hemophilia A BALB/c neonates (0-1 day old) were injected with 2 × 107 IU of Lenti-HCR/hAAT-cFVIII intraperitoneally or subcutaneously, and sampling was started after 3 weeks. No FVIII activity was detected in any of the mice (Figure 3A,C), and all mice developed neutralizing antibodies against cFVIII (Figure 3B,D). Long-term inhibitor titers were not available for mice injected intraperitoneally; however, high titer inhibitors persisted throughout the observation period in mice treated subcutaneously. Combined, these data demonstrate that, similar to adult mice, neonates are capable of mounting a robust anti-FVIII immune response after viral vector-mediated gene delivery, and the route of vector delivery and the type of promoter that regulates transgene expression influence the development of the anti-FVIII immune response.
FVIII activity and inhibitor titers after intraperitoneal or subcutaneous administration of lentiviral vectors to neonatal hemophilia A mice. Neonatal BALB/c hemophilia A mice were treated with 2 × 107 IU of Lenti-HCR/hAAT-cFVIII intraperitoneally (A-B) or subcutaneously (C-D). (A,C) Plasma levels of FVIII in mice treated via these delivery routes and (B,D) levels of neutralizing anti-cFVIII antibodies as measured by a Bethesda assay (note the different inhibitor axes). Five of the mice that were injected subcutaneously with Lenti-HCR/hAAT-cFVIII and which developed neutralizing anti-cFVIII antibodies that ranged between 23 and 95 BU were used as positive controls for inhibitor development.
FVIII activity and inhibitor titers after intraperitoneal or subcutaneous administration of lentiviral vectors to neonatal hemophilia A mice. Neonatal BALB/c hemophilia A mice were treated with 2 × 107 IU of Lenti-HCR/hAAT-cFVIII intraperitoneally (A-B) or subcutaneously (C-D). (A,C) Plasma levels of FVIII in mice treated via these delivery routes and (B,D) levels of neutralizing anti-cFVIII antibodies as measured by a Bethesda assay (note the different inhibitor axes). Five of the mice that were injected subcutaneously with Lenti-HCR/hAAT-cFVIII and which developed neutralizing anti-cFVIII antibodies that ranged between 23 and 95 BU were used as positive controls for inhibitor development.
Analysis of long-term induction of immune tolerance to cFVIII
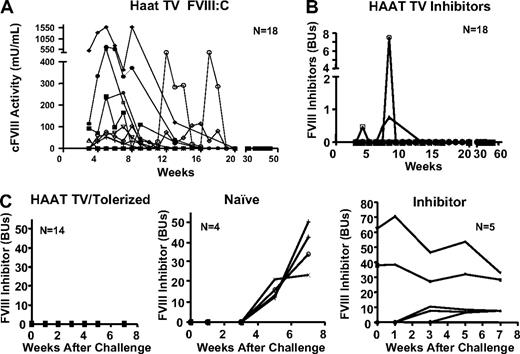
Subsequent to these initial studies, an additional 18 neonates were injected with Lenti-HCR/hAAT-cFVIII via the temporal vein. Five of these mice continued to express cFVIII beyond 13 weeks, with a peak cFVIII activity of 1587 mU/mL (Figure 4A). Similar to the initial cohort, levels of plasma FVIII did not persist and slowly began to drop in all mice. Loss of cFVIII expression began at different time points in the various treated mice, but in all mice cFVIII was undetectable by 20 weeks posttreatment. The loss of cFVIII expression was not associated with the development of anti-cFVIII inhibitors, although 3 mice did develop a low-level transient inhibitor (Figure 4B). In inbred mice, the Bethesda assay is indicative of an anti-FVIII immune response and was used because it provides a clinically relevant marker of this response. However, it does not measure total anti-FVIII antibodies. To ensure that no anti-FVII antibodies were present in the treated mice that no longer expressed cFVIII, plasma samples were isolated from 5 treated mice at 14 and 28 weeks postgene transfer, and total anti-cFVIII antibodies were measured by ELISA. No anti-cFVIII antibodies were detected (< 0.01 mg/mL) in any of these 5 mice. This is in contrast to inhibitor-positive (5.4-95.4 BU) controls (injected subcutaneously as neonates with Lenti-HCR/hAAT-cFVIII) that had total anti-cFVIII antibody levels of 0.3 to 2.2 mg/mL.
Evaluation of induction of tolerance to cFVIII in mice treated with Lenti-HCR/hAAT-cFVIII. (A) BALB/c neonatal mice (n = 18) were infused with 2 × 107 IU of Lenti-HCR/hAAT-cFVIII via the temporal vein, and levels of plasma cFVIII activity were assayed over the 58-week duration of the experiment. (B) Levels of anti-FVIII inhibitory antibodies were assayed. (C) Hemophilia A mice infused as neonates with Lenti-HCR/hAAT-cFVIII via temporal vein injection were challenged with 4 weekly intravenous infusions of recombinant cFVIII. These mice no longer expressed cFVIII at the time of challenge. Levels of anti-FVIII inhibitory antibodies were assayed. Hemophilia mice that received gene transfer as neonates are referred to as HAAT TV/Tolerized, and mice that did not receive gene transfer, but were challenged in a similar manner, are labeled naive. Hemophilia A mice treated via temporal vein injection with Lenti-CMV-cFVIII as neonates were challenged with the same intravenous cFVIII protocol and are labeled inhibitor. Inhibitor levels were measured at 0, 1, 3, 5, and 7 weeks after the first cFVIII injection.
Evaluation of induction of tolerance to cFVIII in mice treated with Lenti-HCR/hAAT-cFVIII. (A) BALB/c neonatal mice (n = 18) were infused with 2 × 107 IU of Lenti-HCR/hAAT-cFVIII via the temporal vein, and levels of plasma cFVIII activity were assayed over the 58-week duration of the experiment. (B) Levels of anti-FVIII inhibitory antibodies were assayed. (C) Hemophilia A mice infused as neonates with Lenti-HCR/hAAT-cFVIII via temporal vein injection were challenged with 4 weekly intravenous infusions of recombinant cFVIII. These mice no longer expressed cFVIII at the time of challenge. Levels of anti-FVIII inhibitory antibodies were assayed. Hemophilia mice that received gene transfer as neonates are referred to as HAAT TV/Tolerized, and mice that did not receive gene transfer, but were challenged in a similar manner, are labeled naive. Hemophilia A mice treated via temporal vein injection with Lenti-CMV-cFVIII as neonates were challenged with the same intravenous cFVIII protocol and are labeled inhibitor. Inhibitor levels were measured at 0, 1, 3, 5, and 7 weeks after the first cFVIII injection.
When hemophilia A mice are challenged with repeated intravenous infusions of recombinant cFVIII, they invariably mount a robust anti-FVIII immune response. To determine whether the mice that received temporal vein (TV) infusion of Lenti-HCR/hAAT-cFVIII as neonates had established long-term immunologic tolerance to cFVIII, 14 mice were challenged with 4 intravenous infusions of recombinant cFVIII (80 IU/kg) at weekly intervals. Although none of these mice had detectable levels of plasma cFVIII:C, they did not develop inhibitors, which is in stark contrast to naive control hemophilia A mice (Figure 4C). Furthermore, 5 inhibitor-positive mice were also challenged with cFVIII, and in all mice levels of cFVIII inhibitors increased (Figure 4C). These results demonstrate that when hemophilia A neonates are infused intravenously with a lentiviral vector that encodes for liver-restricted expression of cFVIII, they develop long-term immunologic tolerance to cFVIII.
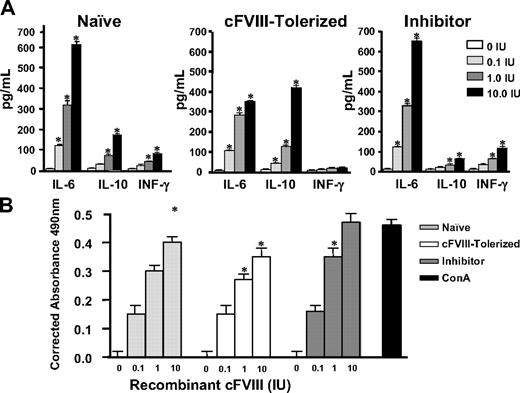
Shift in cytokine secretion profile when splenocytes from cFVIII-tolerized hemophilia A mice are stimulated in vitro with cFVIII
To gain insights into the mechanisms that facilitate the observed long-term tolerance to cFVIII, splenocytes were harvested within 48 hours after the last FVIII challenge from cFVIII-tolerized and inhibitor-positive hemophilia A mice. Naive control hemophilia A mice did not receive FVIII gene transfer as neonates and were not challenged with intravenous infusions of recombinant cFVIII. The splenocytes were cultured in the presence or absence of cFVIII, and cytokine production was assayed after 72 hours (Figure 5A). Increases in IFN-γ and IL-6 are associated with Th1 and Th2 T cell–mediated responses, respectively, whereas increases in IL-10 are associated with Th2 responses and are also characteristic of regulatory T-cell–mediated immune suppression. The results show that relative to the untreated naive control and inhibitor-positive mice, cFVIII-stimulated splenocytes isolated from cFVIII-tolerized mice secrete decreased amounts of IL-6 and IFN-γ and increased amounts of IL-10.
Cytokine secretion profile and relative proliferation of splenocytes from cFVIII-tolerized mice after in vitro cFVIII stimulation. (A) Within 48 hours of the last cFVIII challenge, splenocytes were isolated from mice that received temporal vein infusion of either Lenti-HCR/hAAT-cFVIII (n = 3) or Lenti-CMV-cFVIII (n = 3) as neonates, and the cells were cultured in vitro with or without cFVIII. Culture supernatants were evaluated for the presence of IL-6, IL-10, and IFN-γ by ELISA. The bar graphs represent the levels of assayed cytokines. Hemophilia A mice that received Lenti-HCR/hAAT-cFVIII are labeled cFVIII-tolerized, and those that did not receive gene transfer and were not challenged with intravenous infusions of recombinant cFVIII are labeled naive. Mice that were treated with Lenti-CMV-cFVIII and developed inhibitors are labeled inhibitor. Spleens isolated from 3 mice from each group were tested in triplicate with 4 different amounts of cFVIII (0, 0.1, 1.0, and 10 IUs). Results are the mean ± SEM. Significant differences in levels of the various cytokines relative to no FVIII stimulation are incidated: *P < .01. (B) In vitro proliferation analysis of isolated splenocytes cultured in various amounts of cFVIII. Spleens isolated from 3 mice from each group were tested in triplicate with 4 amounts of cFVIII (0, 0.1, 1, and 10 IUs). Results are the mean ± SEM. Significant differences in T-cell proliferation relative to the control are indicated: *P < .01.
Cytokine secretion profile and relative proliferation of splenocytes from cFVIII-tolerized mice after in vitro cFVIII stimulation. (A) Within 48 hours of the last cFVIII challenge, splenocytes were isolated from mice that received temporal vein infusion of either Lenti-HCR/hAAT-cFVIII (n = 3) or Lenti-CMV-cFVIII (n = 3) as neonates, and the cells were cultured in vitro with or without cFVIII. Culture supernatants were evaluated for the presence of IL-6, IL-10, and IFN-γ by ELISA. The bar graphs represent the levels of assayed cytokines. Hemophilia A mice that received Lenti-HCR/hAAT-cFVIII are labeled cFVIII-tolerized, and those that did not receive gene transfer and were not challenged with intravenous infusions of recombinant cFVIII are labeled naive. Mice that were treated with Lenti-CMV-cFVIII and developed inhibitors are labeled inhibitor. Spleens isolated from 3 mice from each group were tested in triplicate with 4 different amounts of cFVIII (0, 0.1, 1.0, and 10 IUs). Results are the mean ± SEM. Significant differences in levels of the various cytokines relative to no FVIII stimulation are incidated: *P < .01. (B) In vitro proliferation analysis of isolated splenocytes cultured in various amounts of cFVIII. Spleens isolated from 3 mice from each group were tested in triplicate with 4 amounts of cFVIII (0, 0.1, 1, and 10 IUs). Results are the mean ± SEM. Significant differences in T-cell proliferation relative to the control are indicated: *P < .01.
Neonatal temporal vein injection with Lenti-HCR/hAAT-cFVIII decreases the proliferative response of cFVIII-stimulated splenocytes
The isolated splenocytes were also assessed for in vitro proliferation upon cFVIII stimulation. Relative to naive mice, splenocytes obtained from the cFVIII-tolerized mice stimulated in vitro with cFVIII displayed decreased proliferation rates, and this is in contrast to splenocytes from inhibitor-positive mice that displayed increased proliferation rates when stimulated with cFVIII (Figure 5B). Concanavalin A was used as a positive control for splenocyte proliferation. Taken together, these results indicate that the induction of long-term cFVIII-specific tolerance by neonatal cFVIII gene transfer involves active peripheral suppression of an anti-cFVIII immune response.
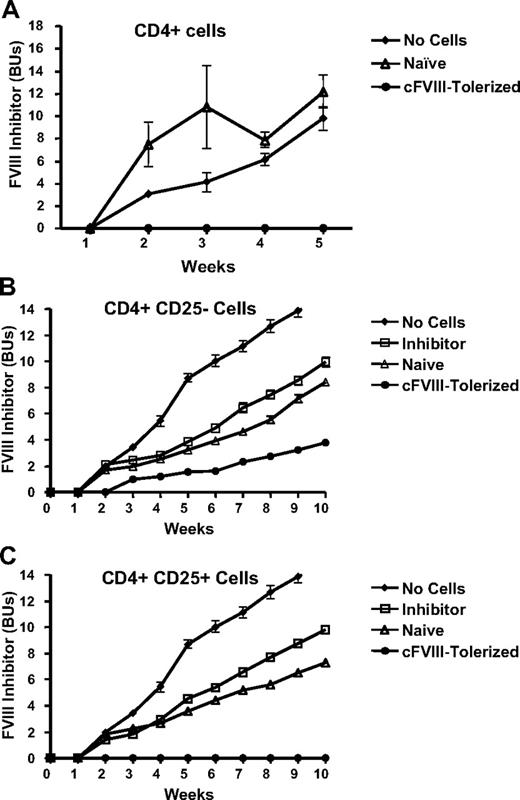
CD4+ T cells from cFVIII-tolerized mice are capable of transferring tolerance to naive recipient animals
With a view to determine whether the cFVIII-specific tolerance associated with neonatal intravenous delivery of the lentiviral vector is mediated by regulatory T cells, adoptive transfer experiments were carried out. CD4+ T cells were separated from the splenocytes isolated from cFVIII-tolerized, inhibitor-positive, and naive control hemophilia A mice and injected intravenously into naive syngeneic, sex-matched recipient hemophilia A mice. These recipient mice were subsequently challenged within 48 hours with 3 daily intravenous cFVIII injections (80 IU/kg per day) and evaluated for anti-FVIII inhibitor development. As a control for inhibitor development, 4 mice that did not receive CD4+ T cells were challenged with cFVIII in a similar manner. The results presented in Figure 6A clearly show that CD4+ cells isolated from cFVIII-tolerized mice protect naive recipient mice against anti-FVIII inhibitor development, whereas mice that did not receive CD4+ T cells, as well as mice that received CD4+ T cells from naive controls, all developed cFVIII inhibitors.
Splenocytes isolated from tolerized mice can adoptively transfer protection against anti-cFVIII inhibitor development to naive mice. (A) One million CD4+ splenocytes from tolerized mice that received Lenti-HCR/hAAT-cFVIII as neonates (cFVIII-Tolerized, n = 9) or mice that did not receive gene transfer and were not challenged with recombinant FVIII (Naive, n = 4) were injected intravenously into naive syngeneic sex-matched recipient hemophilia A mice. Mice that did not receive adoptive transfer of cells (No Cells, n = 4) were used as a control for inhibitor development. All recipient mice were subsequently challenged within 48 hours after cell transfer with 3 consecutive daily intravenous cFVIII injections (80 U/kg per day). Anti-FVIII inhibitor development was followed in the recipients for 5 weeks postadoptive transfer. (B-C) Splenocytes from hemophilia A mice that had received Lenti-HCR/hAAT-cFVIII as neonates (cFVIII-Tolerized, n = 5), mice that did not receive neonatal gene transfer or intravenous infusion of recombinant cFVIII (Naive, n = 5), and mice that were inhibitor positive after receiving subcutaneous infusion of Lenti-HCR/hAAT-cFVIII (Inhibitor, n = 5). One million CD4+CD25− (B) or CD4+CD25+ (C) were infused into naive syngeneic sex-matched recipient hemophilia A mice via the tail vein. Mice that did not receive adoptive transfer of cells (No Cells, n = 5) were used as a control for inhibitor development.
Splenocytes isolated from tolerized mice can adoptively transfer protection against anti-cFVIII inhibitor development to naive mice. (A) One million CD4+ splenocytes from tolerized mice that received Lenti-HCR/hAAT-cFVIII as neonates (cFVIII-Tolerized, n = 9) or mice that did not receive gene transfer and were not challenged with recombinant FVIII (Naive, n = 4) were injected intravenously into naive syngeneic sex-matched recipient hemophilia A mice. Mice that did not receive adoptive transfer of cells (No Cells, n = 4) were used as a control for inhibitor development. All recipient mice were subsequently challenged within 48 hours after cell transfer with 3 consecutive daily intravenous cFVIII injections (80 U/kg per day). Anti-FVIII inhibitor development was followed in the recipients for 5 weeks postadoptive transfer. (B-C) Splenocytes from hemophilia A mice that had received Lenti-HCR/hAAT-cFVIII as neonates (cFVIII-Tolerized, n = 5), mice that did not receive neonatal gene transfer or intravenous infusion of recombinant cFVIII (Naive, n = 5), and mice that were inhibitor positive after receiving subcutaneous infusion of Lenti-HCR/hAAT-cFVIII (Inhibitor, n = 5). One million CD4+CD25− (B) or CD4+CD25+ (C) were infused into naive syngeneic sex-matched recipient hemophilia A mice via the tail vein. Mice that did not receive adoptive transfer of cells (No Cells, n = 5) were used as a control for inhibitor development.
CD4+ T regulatory cells consist of both CD25+ and CD25− cells. To determine whether either or both of these regulatory T cells participate in suppressing the anti-FVIII immune response in the tolerized hemophilia A mice, we carried out similar adoptive transfer experiments using CD4+CD25− and CD4+CD25+ T cells (Figure 6B-C, respectively). Naive hemophilia mice that received either CD4+CD25+ or CD4+CD25− T cells from the inhibitor-positive or naive control mice produced similar levels of neutralizing anti-cFVIII antibodies when challenged with cFVIII. In contrast, when challenged with cFVIII, the CD4+CD25+ T cells isolated from the cFVIII-tolerized mice completely suppressed the anti-cFVIII immune response in the recipient hemophilia A mice. Although the CD4+CD25− T cells from the FVIII-tolerized mice did not completely suppress the anti-cFVIII immune response, levels of neutralizing anti-cFVIII antibodies are lower and appear later compared with recipient mice that got CD4+CD25− cells from either inhibitor-positive or naive control mice. The fact that CD4+CD25+ T cells adoptively transferred from cFVIII-tolerized mice prevent anti-FVIII inhibitor development suggests that active peripheral suppression is playing a significant role in the maintenance of cFVIII tolerance in this model.
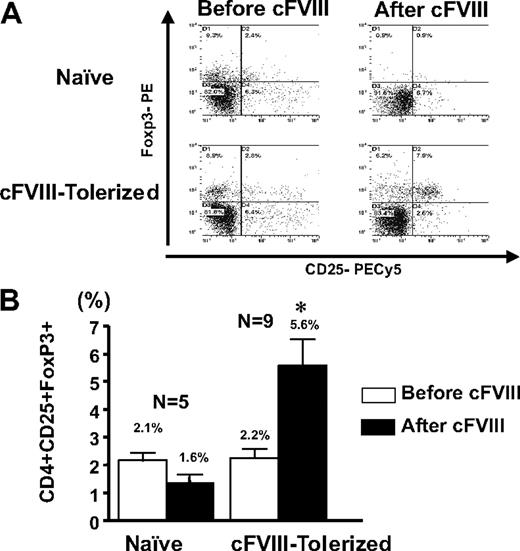
Immune tolerance to cFVIII is regulated by CD4+CD25+Foxp3+ T cells
To determine whether the CD4+CD25+ regulatory T cells that participate in the observed active suppression of the anti-FVIII immune response are Foxp3+, splenocytes were isolated from cFVIII-tolerized mice that were challenged with cFVIII. Naive control mice did not receive cFVIII gene transfer and were not challenged with recombinant cFVIII. Flow cytometric analysis was performed on CD4+ T cells before and after in vitro cFVIII stimulation. The results presented in Figure 7 clearly show that in both groups of mice there are CD4+CD25+Foxp3+ T cells within the total CD4+ compartment, but it is only in the mice that received neonatal cFVIII gene delivery that the CD4+CD25+Foxp3+ T cells expand from 2.2% to 5.6% after stimulation with FVIII. Furthermore, the increase in Foxp3+ T cells was specific for cFVIII because in vitro stimulation with albumin did not result in an increase in Foxp3+ T cells (data not shown). These results, combined with the data from both the in vitro cytokine assays and the adoptive transfer study, indicate that the long-term FVIII-specific immune tolerance in these mice is mediated, at least in part, through cFVIII-specific CD4+CD25+Foxp3+ T regulatory cells.
Immune tolerance to cFVIII is associated with a cFVIII-expandable population of CD4+CD25+Foxp3+ T cells. (A) A representative flow cytometric analysis obtained with CD4+ T cells, derived from the spleens of hemophilia A mice that received Lenti-HCR/hAAT-cFVIII as neonates (cFIII-Tolerized) or did not receive gene transfer and were not challenged intravenously with cFVIII (Naive) and that were challenged with FVIII. Isolated splenocytes were stained directly (Pre-cFVIII) for CD4+CD25+Foxp3+ or after 72 hours in vitro stimulation with cFVIII (Post-cFVIII). (B) Comparison of the mean percentage of CD4+CD25+Foxp3+ T cells within the CD4+ cell population from the tolerized and naive mice before and after in vitro cFVIII stimulation. Error bars represent SEM, and * indicates a significant (P < .01) increase in CD4+CD25+Foxp3+ T cells.
Immune tolerance to cFVIII is associated with a cFVIII-expandable population of CD4+CD25+Foxp3+ T cells. (A) A representative flow cytometric analysis obtained with CD4+ T cells, derived from the spleens of hemophilia A mice that received Lenti-HCR/hAAT-cFVIII as neonates (cFIII-Tolerized) or did not receive gene transfer and were not challenged intravenously with cFVIII (Naive) and that were challenged with FVIII. Isolated splenocytes were stained directly (Pre-cFVIII) for CD4+CD25+Foxp3+ or after 72 hours in vitro stimulation with cFVIII (Post-cFVIII). (B) Comparison of the mean percentage of CD4+CD25+Foxp3+ T cells within the CD4+ cell population from the tolerized and naive mice before and after in vitro cFVIII stimulation. Error bars represent SEM, and * indicates a significant (P < .01) increase in CD4+CD25+Foxp3+ T cells.
Discussion
The neonatal immune system is a unique developmental stage that is characterized by lymphopenia, quantitative and qualitative differences in antigen-presenting cells (APCs), and a notable bias toward Th2 polarization of immune responses.24 It is in this early stage of life that central tolerance to both self and exogenously introduced antigens is established. We chose to capitalize on this to develop a murine model for induction of long-term immunologic tolerance to cFVIII with a view to better understand the mechanisms associated with tolerance to cFVIII. In this study, we show that lentiviral delivery of a cFVIII transgene to BALB/c hemophilia A neonates can induce long-term tolerance to FVIII. We have further evaluated the mechanisms underlying this tolerance and have shown that it is associated with 4 important phenomena. Immunologic tolerance to cFVIII can be established and maintained in the absence of detectable plasma cFVIII. Secondly, there is a deviation in cytokines secreted from FVIII-stimulated splenocytes, with a reduction in proinflammatory IFN-γ and IL-6, and an increase in the immunomodulatory IL-10 levels. Next, cFVIII tolerance can be transferred to naive recipient hemophilic animals with CD4+CD25+ T cells from tolerized donors. Finally, there is a population of CD4+CD25+Foxp3+ cells that undergo expansion upon cFVIII stimulation in vitro.
The results of our study clearly demonstrate that neonatal mice can mount an adult-like anti-FVIII immune response. Lentivector delivery via the subcutaneous and intraperitoneal routes and transgene regulation from the CMV promoter were always associated with neutralizing anti-FVIII antibodies. In adult mice, switching from a ubiquitously expressing CMV promoter to a liver-restricted construct limits immune responses.25 However, we continued to see an anti-FVIII immune response even when we used the liver-specific HCR-hAAT–directed transgene delivered subcutaneously or intraperitoneally. This observation implies that there must be some “off-target” cFVIII that is synthesized and presented to the immune system. Lentiviral vectors are very efficient at transducing hematopoietic cells and, in general, integrate in the vicinity of promoters. Despite using tissue-specific promoters, promoter trapping in transduced hematopoietic cells results in transgene expression and presentation of the transgene product by APCs.26 Viruses delivered subcutaneously or intraperitoneally traffic to draining lymph nodes, whereas viruses delivered intravenously traffic to the liver and spleen. There are marked phenotypic differences between APCs present in the different secondary lymphoid organs during the neonatal period.24 Therefore, the route of vector administration ultimately determines where the virus will traffic, and the phenotypic differences in APCs within the different organs influence the balance between tolerogenic and immunogenic presentation of cFVIII.
The tolerance to cFVIII achieved in these animals persisted for the duration of the study; although when tolerance was assessed, cFVIII expression was not detectable. This tolerance was mediated, at least in part, through the generation of CD4+CD25+FoxP3+ cells, and these cells persisted even in the absence of detectable levels of plasma FVIII. Furthermore and somewhat surprisingly, cFVIII tolerance was established in several treated mice in which plasma cFVIII was never detected. Notably, in 2 mice without detectable cFVIII, this tolerance persisted for 50 weeks. This appears to contradict studies that used oncoretroviral vectors to deliver FVIII to neonates27,28 and in which long-term tolerance was associated with high levels of FVIII expression.29,30 Furthermore, in adult mice, higher levels of factor IX transgene expression, restricted to hepatocytes, favor tolerance induction.31 The tolerance in this case is also mediated by CD4+CD25+Foxp3+ cells.32 However, none of these studies, including the one we present in this work, investigates early events and mechanisms responsible for the development of long-term tolerance. In general, central tolerance is established by negative selection of antigen-specific thymocytes and induction of Foxp3+ T regulatory cells33 that migrate into the periphery to actively suppress antigen-specific immune responses.34 Peripheral dendritic cells that migrate into the thymus, as well as resident intrathymic dendritic cells and thymic epithelial cells, together or individually, mediate the development of antigen-specific central tolerance.35 Additional studies are required to determine what cells are transduced after neonatal gene delivery and to understand the mechanisms involved in how these cells participate in the induction of central tolerance to FVIII.
Induction of immune tolerance to FVIII, in the absence of FVIII expression, has been investigated in immunocompetent adult hemophilia A mice. These strategies involve the use of cells that have been exposed to FVIII for antigen presentation or have been genetically modified to express to FVIII.36-38 One such early study infused syngeneic bone marrow cells transduced with a retroviral vector that contained a FVIII transgene into conditioned hemophilia A mice.36 Plasma FVIII levels were undetectable, but when challenged with FVIII, 50% of the mice have a reduced immune response to FVIII. In these mice, immune modulation cannot be attributed to secreted FVIII. Rather, the immune modulation may result from MHC class II–mediated antigen presentation of FVIII by transduced APCs.39 Infusion of dendritic cells that presented FVIII in the context of MHC class II into adult hemophilia A mice before FVIII challenge also significantly reduced the anti-FVIII immune response.38 B cells transduced with a retroviral vector encoding the A2 and C2 domains of FVIII fused to a mouse immunoglobulin G were very effective at eliminating an anti-FVIII immune response in adult hemophilia A mice.37 Interestingly, similar to the results that we present in this study, this modulation of the anti-FVIII immune response was also mediated by CD25+ T regulatory cells.
The mechanisms responsible for the gradual loss of cFVIII expression in the tolerized mice that received intravenous delivery of Lenti-HCR/hAAT-cFVIII require further investigation. We have no evidence to suggest that either a cellular or humoral immune response accounts for this loss of expression. In our experience, loss or absence of plasma FVIII after gene therapy in adult mice is associated with inhibitory antibodies and a rapid loss of plasma cFVIII that occurs by the third to fourth week posttreatment.12,14 The loss of cFVIII expression that we observed in this study occurs much later and without detectable anti-cFVIII antibodies. Furthermore, in the mice that no longer expressed cFVIII, we have evidence for the persistence of lentiviral DNA. It was therefore surprising that no cFVIII mRNA could be detected. This was in contrast to the mouse that had detectable levels of plasma cFVIII and in which we could demonstrate significant levels of cFVIII mRNA. Taken together, these results suggest that transcriptional silencing of the cFVIII transgene may be responsible for the loss of expression in the tolerized mice.
The other mechanistic issue raised by our results is the evidence that IL-10 from antigen-stimulated splenocytes may play a role in mediating FVIII tolerance. Current evidence indicates that although CD4+CD25+Foxp3+ regulatory T cells produce IL-10, they do not mediate their suppressive effects on T-effector cells through IL-10 secretion, but through direct cell-cell contact and possibly through the elaboration of TGF-β.40 T regulatory type 1 cells are CD4+CD25−FoxP3− cells that mediate their suppressive effects through IL-10 secretion,41 and therefore, we cannot rule out the possibility of their additional contribution to the tolerogenic process. Studies will be required to determine whether the CD4+CD25− cells that suppress the anti-FVIII immune response are indeed T regulatory type 1 cells and whether they mediate their suppressive effect through secretion of IL-10.
In conclusion, we have developed a relevant animal model system to study mechanisms involved in long-term immunologic tolerance to FVIII. These studies have provided novel insights into the influence of the route of antigen administration and antigen concentration on tolerance induction, and have provided evidence of the involvement of IL-10 and Foxp3+ regulatory T cells in maintaining prolonged tolerance to this antigen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was funded by an operating grant from the Canadian Institutes of Health Research (Ottawa, ON; MOP10912). D.L. is the recipient of a Canada Research Chair in Molecular Hemostasis and a Career Investigator Award from the Heart and Stroke Foundation of Ontario (Toronto, ON). H.M. holds an Industry-Partnered Fellowship with the Canadian Institutes of Health Research.
Authorship
Contribution: H.M. and M.S. performed research, interpreted data, and wrote the paper. B.B., A.L., C Hegadorn, and C.A. performed research. M.C., T.V., and C.H.M. provided advice and important reagents. C Hough and D.L. designed research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Lillicrap, Department of Pathology and Molecular Medicine, Richardson Laboratory, 88 Stuart St, Queen's University, Kingston, ON, Canada K7L 3N6; e-mail: lillicrap@cliff.path.queensu.ca.
References
Author notes
*H.M. and M.S. contributed equally to this work.