Abstract
Haptoglobin, the haptoglobin-hemoglobin receptor CD163, and the heme oxygenase-1 are proteins with a well-established function in the clearance and metabolism of “free” hemoglobin released during intravascular hemolysis. This scavenging system counteracts the potentially harmful oxidative and NO-scavenging effects associated with “free” hemoglobin, and, furthermore, elicits an anti-inflammatory response. In the late primate evolution, haptoglobin variants with distinct functions have arisen, including haptoglobin polymers and the haptoglobin-related protein. The latter associates with a subspecies of high-density lipoprotein (HDL) particles playing a crucial role in the innate immunity against certain trypanosome parasites. Recent studies have elucidated this fairly sophisticated immune defense mechanism that takes advantage of a trypanosomal haptoglobin-hemoglobin receptor evolved to supply the parasite with heme. Because of the high resemblance between haptoglobin and haptoglobin-related protein, the receptor also takes up the complex of hemoglobin and the HDL-bound haptoglobin-related protein. This tricks the parasite into internalizing another HDL-associated protein and toxin, apolipoprotein L-I, that kills the parasite. In conclusion, variant human homologous hemoglobin-binding proteins that collectively may be designated the haptoglobins have diverted from the haptoglobin gene. On hemoglobin and receptor interaction, these haptoglobins contribute to different biologic events that go beyond simple removal from plasma of the toxic hemoglobin.
Introduction
Haptoglobin (Hp), an abundant acute phase plasma glycoprotein of the α2-globulin fraction, was initially reported in 1938 by Polonovski and Jayle to be a “plasma substance” that binds hemoglobin (Hb).1 In agreement with this initial characterization and its designation as “haptoglobin” (Greek: haptein, “to bind” + [hemo]globin), Hp has a well-established role in binding of “free” Hb released into the circulation during the process of intravascular hemolysis. By capturing the liberated Hb, Hp protects against toxic effects associated with Hb, and it facilitates Hb clearance through endocytosis by the macrophage scavenger receptor CD163.2 Later studies of human and primate plasma revealed variant forms of Hp as well as the existence of an Hp-related protein (Hpr) that recently has been shown also to bind Hb. As highlighted in this review, novel functions of Hp depend on high-affinity interactions with Hb and lipoprotein and subsequent receptor recognition of the resulting complexes.
Toxicity of hemoglobin released during intracellular hemolysis
Intravascular hemolysis, which accounts for approximately 10% to 20% of total red blood cell destruction,3 changes Hb from being an intracellular O2/CO2 transporter to a highly toxic substance in plasma. This toxicity arises from the heme iron, which can react with endogenous hydrogen peroxide to produce free radicals, which in turn may cause severe oxidative tissue damage,4 particularly in the kidney.5 In addition to the oxidative toxicity of Hb, Hb is a potent scavenger of nitric oxide (NO), a signaling molecule that functions as a critical regulator of smooth muscle relaxation, endothelial adhesion molecule expression, and platelet activation and aggregation.6,7 The reaction of Hb with NO is fast and irreversible, leading to the production of nitrate and methemoglobin. If allowed to occur, this Hb-mediated consumption of vascular NO, referred to as NO-scavenging, thus limits the bioavailability of NO and thereby impairs NO homeostasis.
Under normal physiologic conditions, Hp counteracts the Hb toxicity by capturing the released Hb and directing it to CD163-expressing macrophages, which internalize the complex.2 Furthermore, the rapid formation of the Hp-Hb complex reduces glomerular filtration of the Hb molecule, thereby limiting the loss of iron in the urine.8,9 However, in various situations, including infections, trauma, and hereditary sickle cell disease and thalassemia, intravascular hemolysis may accelerate and develop into a critical pathologic condition. During such excessive hemolysis, the Hp-CD163–mediated scavenging mechanism (as well as other Hb/heme scavenging systems) is saturated, and, eventually exhausted, because of the degradative pathway of Hp on endocytosis. The outcome is a pathologic state characterized by heme-mediated oxidative damage, as well as impaired NO homeostasis. The deficiency in vascular NO is suggested to be associated with the development of a series of clinical sequelae, including smooth muscle dystonias, endothelial dysfunction, and thrombosis.6,7 Indeed, it is suggested that up to 50% of sickle cell disease patients display endothelial dysfunction as a consequence of restricted NO bioavailability, due in large part to scavenging of NO by free plasma Hb.10
The haptoglobin genes
The human hp gene exists in 2 major allelic forms denoted hp1 and hp2.11-14 The latter allele arose by an apparent intragenic duplication of a 1.7-kb DNA fragment of the hp1 gene after the divergence of humans in late primate evolution.12,15 Accordingly, 3 major hp genotypes exist, hp1-1, hp2-1, and hp2-2. The frequency of these 3 hp genotypes varies markedly with geographic regions. For instance, in Southeast Asia, the hp1 allele frequency is only approximately 0.1, whereas in other regions it may become as high as approximately 0.8 (South America; for review, see Carter and Worwood16 ). The differences observed in the distribution of the hp genotypes are probably the result of both genetic drift and natural selection.16 Indeed, some studies indicate possible connections between specific hp genotypes/phenotypes and a broad range of clinical disorders (for review, see Langlois and Delanghe,17 Van Vlierberghe et al,18 and Carter and Worwood16 ). For instance, the Hp1-1 phenotype has been reported to be a susceptibility factor for infection and liver disease (eg, Louagie et al,19 Van Vlierberghe et al,20 Atkinson et al,21 and Cox et al22 ), whereas the Hp2-2 phenotype was reported to dominate in patients with diabetic atherosclerosis23 as well as in other autoimmune diseases.16,18 The Hp2-2 phenotype is moreover associated with higher average iron load.24 However, as results from other reports demonstrate lack of association between Hp phenotypes and particular disorders (reviewed by Carter and Worwood16 ), it seems that studies of larger cohorts are needed.
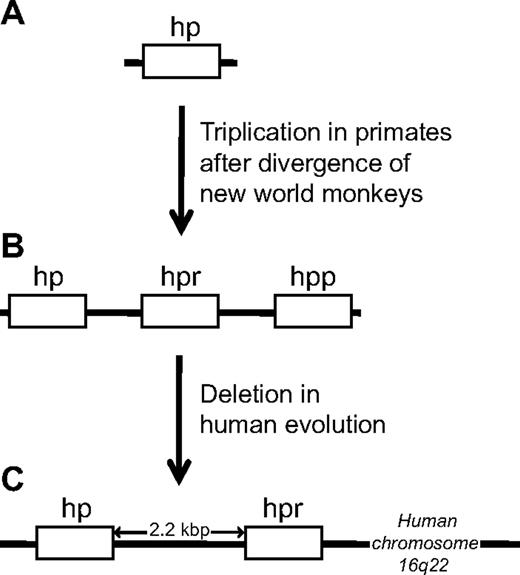
The existence of the other gene originating from the hp gene was defined by Bensi et al11 and Maeda13 in 1985, who discovered the gene encoding Hpr. The hpr and hp genes are highly similar (> 90% identity at the amino acid level)11,13 and are closely linked, with the hpr gene located only 2.2 kb downstream of the hp gene on chromosome 16.11,13,25,26 The hpr gene is only present in primates and appears to have arisen as a result of a gene triplication of the hp locus after the divergence of new world monkeys from other primates. Subsequently, one locus was deleted in humans, leaving only 2 genes in the current so-called hp gene cluster of humans (Figure 1).27 Curiously, in 1986 a small survey performed by Maeda et al28 revealed an apparent frequent occurrence of chromosomes with extra copies of the hpr gene among black descendents. Indeed, in one of the persons studied, as many as 6 tandemly arranged hpr genes were detected on a single chromosome. This early study has suggested that multiple hpr genes could be beneficial in some environments.
Schematic representation of the evolution of the hp gene cluster and the origin of the hpr gene in primates. The hpr gene arose by a triplication of the hp locus after the new world monkeys diverged from other primates. New world monkeys, such as the spider monkey (A), thus harbor only a single hp gene, whereas old world monkeys, exemplified by the chimpanzee (B), harbor an hp gene and an hpr gene, in addition to a so-called haptoglobin-primate (hpp) gene. In human evolution (C), one locus was deleted, leaving only 2 genes (hp and hpr) in the current “hp gene cluster.”
Schematic representation of the evolution of the hp gene cluster and the origin of the hpr gene in primates. The hpr gene arose by a triplication of the hp locus after the new world monkeys diverged from other primates. New world monkeys, such as the spider monkey (A), thus harbor only a single hp gene, whereas old world monkeys, exemplified by the chimpanzee (B), harbor an hp gene and an hpr gene, in addition to a so-called haptoglobin-primate (hpp) gene. In human evolution (C), one locus was deleted, leaving only 2 genes (hp and hpr) in the current “hp gene cluster.”
Expression and presence in plasma
The primary site of Hp expression is the liver (mRNA and protein detected), but expression has also been detected in several other organs, including lung (mRNA and protein detected), kidney (mRNA detected), spleen (mRNA detected), thymus (mRNA detected), and heart (mRNA detected).29-31 Circulating amounts of Hp (0.45-3 mg/mL in normal human serum) are well known to increase during the acute phase response because of an enhanced rate of synthesis in response to inflammatory cytokines, such as interleukin-6 (IL-6) and IL-1.32,33 Hp is therefore also a clinical marker of the acute phase response (high Hp levels) besides being a routine clinical marker of intravascular hemolysis (low Hp levels).
Like Hp, Hpr is expressed in the liver. However, the Hpr transcript levels are apparently only approximately 6% of Hp transcript levels because Hpr expression is attenuated by a retroviral-like element present in intron 1.34 Accordingly, the Hpr levels (∼ 30-40 mg/L) are more than 10-fold lower than the Hp levels.35,36 Unlike Hp, the Hpr serum concentration seems rather resistant to intravascular hemolysis.35,36 The majority of Hpr is associated with 2 serum complexes referred to as trypanosome lytic factors 1 (TLF1) and 2 (TLF2), which both are able to induce lysis of Trypanosoma brucei brucei (T b brucei), an African protozoan parasite that is transmitted to the mammalian bloodstream through the bite of infected tsetse flies and is the causative agent of the lethal disease sleeping sickness in cattle and other animals.37-41 TLF1 is part of a minor subfraction of dense high-density lipoprotein (HDL) termed HDL3, whereas TLF2 is a lipid-poor complex that also contains IgMs.38,39,42,43 Apart from this, the 2 TLF species appear to compose the same protein components, including apolipoprotein A-I (apoA-I) and another primate-specific protein termed apolipoprotein L-I (apoL-I).38,42-44
Structural aspects
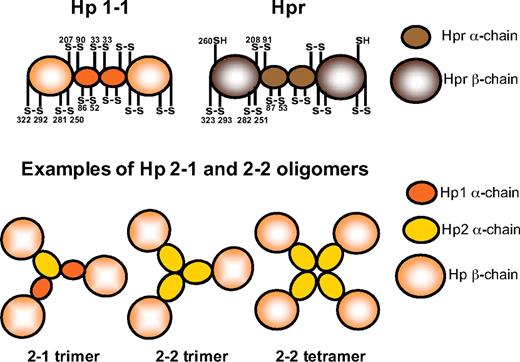
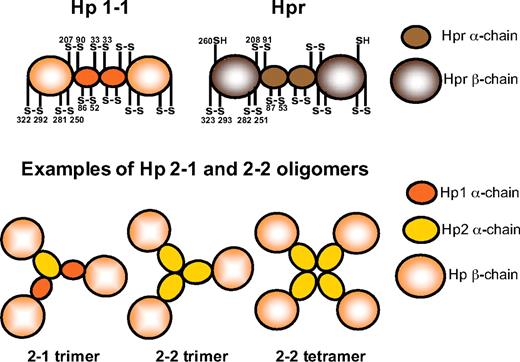
Hp and Hpr share more than 90% sequence identity at the protein level. Both proteins are composed of α- and β-subunits, representing a complement control protein domain (also known as a sushi domain) and a serine protease domain, respectively (Figure 2).45-49 These subunits are linked by disulfide binding.11,35,42,47
Schematic representation of the Hp and Hpr structures. Using electron microscopy, Wejman et al49 described the Hp1-1 molecule as a barbell-like elongated structure with 2 spherical head groups (the β-chains) connected by a thin filament with a central knob (the α-chains). The disulfide bridge pattern of Hp1-1 and Hpr is indicated. Examples of structures of the Hp2-1 and Hp2-2 molecules, as reported by electron microscopy by Wejman et al,48 are also shown.
Schematic representation of the Hp and Hpr structures. Using electron microscopy, Wejman et al49 described the Hp1-1 molecule as a barbell-like elongated structure with 2 spherical head groups (the β-chains) connected by a thin filament with a central knob (the α-chains). The disulfide bridge pattern of Hp1-1 and Hpr is indicated. Examples of structures of the Hp2-1 and Hp2-2 molecules, as reported by electron microscopy by Wejman et al,48 are also shown.
The Hp protein found in most mammals consists of 2 such (αβ)-units linked by a disulfide bond between a cysteine of the 2 α-chains to form an (αβ)-dimer, (αβ)2, of approximately 90 kDa (Figure 2).47-49 In humans, this form corresponds to the Hp protein produced in persons carrying 2 hp1 alleles and is thus referred to as Hp1-1. However, as a consequence of the previously mentioned partial intragenic duplication giving rise to the hp2 allele, 2 additional Hp phenotypes are possible among humans, the Hp2-1 and Hp2-2 phenotypes. The duplicated region carried by the hp2 allele composes part of the sequence encoding the α-chain, and accordingly, the hp2 allele encodes a slightly larger α-chain but is otherwise identical to the hp1 allele.12,15 Because the duplicated section includes the cysteine involved in inter-α-chain disulfide bonding (cysteine 33 in humans), the Hp2-1 and Hp2-2 phenotypes constitute a complicated series of various (αβ)-multimers (Figure 2). This molecular heterogeneity of human Hp was originally reported in 1955 by Smithies50,51 who recognized the existence of the 3 Hp phenotypes based on their different migration patterns in starch gel electrophoresis. The Hp1-1 phenotype shows a single relatively fast-migrating protein band, whereas the Hp2-2 phenotype exhibits a series of slower migrating bands. The Hp2-1 phenotype displays a different series of slowly migrating bands in addition to a much fainter Hp1-1 band.50,51
The main function of the Hp α-chains appears to be connection of (αβ)-units to form dimers (Hp1-1 and Hp2-2 phenotypes) and multimers (Hp2-1 and Hp2-2 phenotypes),2,52 whereas the interaction with other proteins, including Hb and CD163, is mediated by the larger and more accessible β-chain.48,49,52-56 The latter chain is generally more conserved between species than is the α-chain57 and lacks the catalytic triad of genuine serine proteases.47 Accordingly, no proteolytic activity has been assigned to Hp. Apart from this evident difference, the most dramatic dissimilarity between the Hp β-chain and genuine serine proteases is located to the so-called loop 1 of the serine protease domain.45,47 This surface-exposed loop is considerably enlarged in Hp (and Hpr) compared with the corresponding loop in serine proteases and plays a critical function in Hb scavenging.52
The disulfide bridge pattern of the (αβ)-unit of Hpr is homologous to that of Hp (Figure 2).52 However, the inter–α-chain disulfide bond is missing in Hpr because the cysteine 33 found Hp1 is replaced by a phenylalanine in Hpr.11,13 Nevertheless, the Hpr α-chains are thought to associate in a noncovalent manner to form (αβ)-dimers.36,52 Unlike what is observed for Hp, the Hpr amino-terminal signal peptide thought to direct secretion of the protein remains uncleaved.38,42 This highly hydrophobic leader sequence may have a function in targeting Hpr to the lipoprotein complex TLF1 and to TLF2 that contains the amphipathic apoA-I protein.38
Haptoglobin: a hemoglobin scavenger facilitating the anti-inflammatory response
The complex formation between Hp and Hb has been studied for decades and represents one of the strongest noncovalent interactions reported in plasma,58 with only some minor variations in binding strength of the Hp phenotypes (Hp1-1 > Hp2-1 > Hp2-2).17 After its release into plasma, Hb rapidly dissociates from the α2β2 tetrameric structure into αβ-dimers with the ability to bind Hp. The Hp region involved in binding these Hb (αβ)-units was recently shown by deletion analysis to include Hp1 β-chain residues 243 to 258.52,59 Hence, the Hp1-1 molecule is able to bind 2 Hb αβ-dimers (Figure 3), whereas the Hp2-1 and Hp2-2 may be able to bind several Hb αβ-dimers.53-56,60-65 Besides having a direct inhibitory effect on the toxic properties of Hb, as well as preventing peroxidative modification of Hb,66 Hp highly expedites Hb clearance, leading to production of anti-inflammatory metabolites.67 The fast removal of Hp-Hb complexes is explained by the high-affinity binding to the 130-kDa transmembrane receptor CD163 expressed in monocytes and macrophages.2 Hp-Hb binds to the amino-terminal part of a scavenger receptor cysteine-rich domain region of CD163 and the cytoplasmic tail of the receptor conveys cellular uptake of the Hp-Hb complex by receptor-mediated endocytosis.2 Earlier studies have also reported uptake of Hp-Hb by a receptor-dependent mechanism in hepatocytes,8,68 but the crucial role of the macrophages in Hp-Hp uptake is indicated by the Hp-Hb accumulation in plasma of patients with depletion of the macrophage population because of treatment with the biologic drug gemtuzumab ozogamicin that targets CD33-positive myeloblastic cells.69
Binding of Hp to Hb and uptake of the complex by the CD163 receptor expressed on macrophages. Hp-Hb binds the macrophage scavenger receptor CD163. On endocytosis the protein moieties of the ligand are degraded, whereas CD163 recycles to the cell surface. Heme is converted to the overall anti-inflammatory molecules CO, Fe2+, and biliverdin/bilirubin by HO-1 and the biliverdin reductase in the cytosol. In contrast to the Hp-Hb complex, the Hpr/TLF1-associated Hb does not bind CD163.
Binding of Hp to Hb and uptake of the complex by the CD163 receptor expressed on macrophages. Hp-Hb binds the macrophage scavenger receptor CD163. On endocytosis the protein moieties of the ligand are degraded, whereas CD163 recycles to the cell surface. Heme is converted to the overall anti-inflammatory molecules CO, Fe2+, and biliverdin/bilirubin by HO-1 and the biliverdin reductase in the cytosol. In contrast to the Hp-Hb complex, the Hpr/TLF1-associated Hb does not bind CD163.
In contrast to the Ca2+-dependent high-affinity CD163 binding elicited by Hp-Hb complex formation,2 Hp alone does not bind the receptor.2,52,70 Hb, on the other hand, displays a low-affinity binding to CD16336,70 and is indeed endocytosed by CD163 in the absence of Hp, a clearance pathway that is thought to become operative if the amount of “free” Hb exceeds the binding capacity of Hp as is observed during massive hemolysis.70 The high-affinity interaction between Hp-Hb and CD163 critically depends on the scavenger receptor cysteine-rich domain 3 of CD16371 and 3 residues (glutamate 261, lysine 262, and threonine 264) of the Hp β-chain.52 Intriguingly, the latter 3 residues, which are thought to participate directly in receptor binding, are located in the previously mentioned unique extension of loop 1 of the Hp serine protease domain.52 This loop extension has thus conferred a completely novel function to the ancient serine protease domain. Another noteworthy aspect of the Hp-Hb-CD163 interaction is the finding that CD163 exhibits a higher functional affinity for the complexes generated between Hb and Hp(2-2) than it does for the smaller Hp(1-1)-Hb complex.2 We speculate that this is a result of the multiple receptor binding sites present in oligomeric Hp-Hb complexes. Somewhat paradoxically to this higher functional affinity for CD163, Hp(2-2)-Hb has been reported to exhibit a lower rate of CD163-mediated clearance than the complexes formed between Hp(1-1) and Hb.72
After CD163-mediated internalization of Hp-Hb, the globin moieties are degraded in the lysosome, whereas the Hb-derived heme is converted by the cytosolic heme oxygenase-1 (HO-1) into the less toxic compounds free iron (Fe2+), carbon monoxide (CO), and biliverdin.2,73 Biliverdin is subsequently converted into bilirubin by the biliverdin reductase (Figure 3). The Hp-CD163-HO-1 system for Hb clearance/metabolism and the anti-inflammatory response are linked by the metabolites generated.67 HO-1 is in the literature often regarded as having a protective function, but this function may be ascribed to the anti-inflammatory, antiapoptotic, and antiproliferative effects of one or more of its 3 products of heme catabolism, CO, biliverdin (or the downstream metabolite bilirubin), and Fe2+.67,74,75 In particular, CO, which alone has been shown to mimic the protective effect of HO-1 in rodent disease models, is thought to contribute to these overall protective effects.74 Biliverdin and bilirubin largely mediate their beneficial effect by their potent antioxidant properties.75 Fe2+, on the other hand, induces the iron-chelating protein ferritin, which mediates binding of Fe2+.74 The sequestering of Fe2+ by ferritin inhibits Fe2+-catalyzed generation of reactive oxygen species via the Fenton reaction and thereby protects against oxidative stress. Of interest, several therapeutic molecules, such as IL-10 and prostaglandin J2, function by activating HO-1; accordingly, it has been suggested that HO-1 may function as a so-called therapeutic funnel mediating the favorable effects of these therapeutic molecules.74
Hp, CD163, and HO-1 are all induced by the inflammatory cytokine IL-6.76 It thus seems likely and makes sense that these proteins are coordinately up-regulated during inflammatory conditions, thereby enhancing the capacity for Hb clearance and metabolism. Because of the antioxidative and anti-inflammatory effects of the heme metabolites, the overall outcome of the Hp-CD163–mediated delivery of Hb to monocytes/macrophages may be an anti-inflammatory response counteracting the inflammatory reaction.
In addition, it has been suggested that intracellular signaling cascades activated by Hp-Hb binding to cell-surface CD163 may contribute even further to an anti-inflammatory response.77-79 Antibody-mediated cross-linking of CD163 at the cell surface was shown to trigger an intracellular signaling cascade resulting in Ca2+-mobilization, synthesis of inositol triphosphate, and secretion of IL-6 and granulocyte-macrophage colony-stimulating factor.78,79 In analogy, the binding of Hp-Hb to cell-surface CD163 has been reported to elicit Ca2+-mobilization,72 synthesis of inositol triphosphate,72 and secretion of IL-6,80 as well as IL-10.77,80 Secretion of the latter was accompanied by induction of HO-1.77 In view of the up-regulatory effect of IL-6 on Hp, CD163, and HO-1,76 and the up-regulatory effect of IL-10 on CD163 and HO-1,81-83 one could further speculate that IL-6 and IL-10 are involved in positive feedback mechanisms that enhance the capacity for Hb clearance and subsequent degradation of heme in response to red blood cell damage. Of interest, Levy and colleagues reported that secretion of IL-6 and IL-10 by macrophages was stimulated to a significantly higher degree by Hp(1-1)-Hb than by Hp(2-2)-Hb in a process that depended on Hp-Hb binding to CD163 and casein kinase activity.80
However, it should be stressed that Schaer et al73 were unable to detect any protein phosphorylation-dependent signaling on Hp-Hb binding to CD163. Furthermore, they found no evidence of IL-10 secretion when subjecting CD163-expressing cells to highly purified preparation of Hb complexed with Hp. In their hands, CD163 thus functions exclusively as an Hb transporter and the internalized Hb/heme triggers up-regulation of HO-1. Schaer et al suggested that the observed endotoxin contamination of commercially available Hb73 may have caused the secretion of IL-10 observed by others.77
Altogether, several functional differences among the Hp phenotypes in relation to its functional interaction with Hb have been reported, including the strength of the interaction with Hb17 and CD163,2 the rate of Hp-Hb clearance by CD163,72 and the outcome of the signaling pathways that may perhaps be initiated by Hp-Hb binding to CD163 on the cell surface.72,80,84 The ability to protect against Hb-mediated oxidation also appears to differ, with the Hp1-1 possessing superior antioxidant capacity compared with Hp2-2.59 It seems reasonable to speculate that one or more of these differences could explain the possible association of specific Hp phenotypes with particular pathologic states.16-18
A study by Levy et al showed that diabetic patients with the Hp2-2 phenotype are at a several-fold higher risk of developing cardiovascular disease.23 Further studies by the same group disclosed a possible mechanism of disease explaining such association between Hp2-2 and cardiovascular complications in diabetic persons. In the diabetic state, there is a marked increase in the fraction of glycosylated Hb. Importantly, it was shown that the antioxidant function of Hp is impaired against these glycosylated Hb species.72 Based on their finding that complexes of Hp2-2 and Hb are cleared less efficiently than Hp(1-1)-Hb, it has been hypothesized that diabetic patients with the Hp2-2 phenotype are exposed to higher amounts of oxidative stress.72
Haptoglobin-related protein: a part of the innate immune system combating trypanosome parasites
It has been known for long that Hpr is an essential component of the TLF1 particles causing lysis of the trypanosome parasite T b brucei.38,42-44,85,86 Initially, it was thought that Hpr exerted a direct effect in the killing of T b brucei, but later studies revealed the toxic effect to be possessed by the TLF1 component apoL-I.87 The lytic potential of apoL-I is explained by its structural relationship to colicin, a bacterial protein harboring a pore-forming domain. Accordingly, apoL-I was reported to elicit trypanolysis through an ability to create pores in the lysosomal membrane of T b brucei,87,88 subsequent to internalization of the TLF1 particle. This formation of pores leads to influx of chloride ions, osmotic swelling of the lysosome, and, ultimately, lysis of the parasite.87,88 Interestingly, the TLF1 has also been reported to combat Leishmania, but the molecular mechanism remains to be defined.89
The apparent controversies regarding the identity of the lytic factor90 were finally explained by Vanhollebeke et al,91 who used human sera lacking either Hpr or apoL-I to demonstrate that Hpr and apoL-I perform separate functions in the trypanolytic mechanism. According to this documented model, apoL-I accounts for the actual trypanolytic activity by its membrane-disruptive activity, whereas Hpr plays an indirect yet essential role in trypanolysis, by acting as a ligand for a receptor localized on the surface of the parasite. Hpr is thus crucial for efficient uptake of the associated apoL-I85,91,92 but possesses no direct lytic activity itself,91 as recently demonstrated in an apoL-I/Hpr transgenic mouse model.93
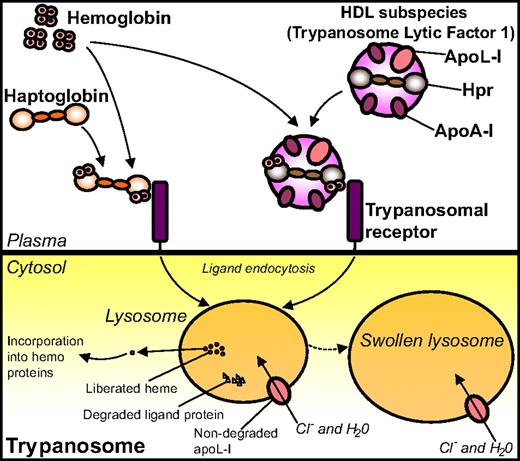
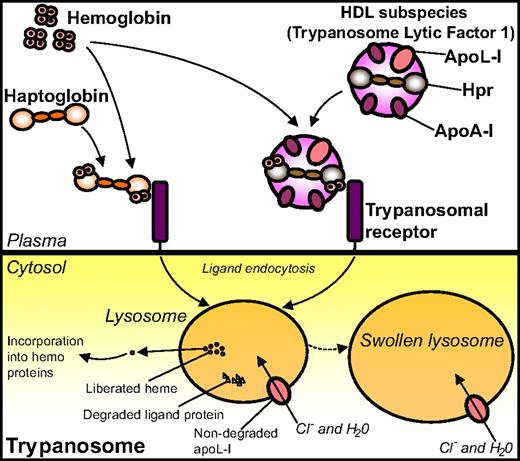
Later, Hb came into play when it was somewhat surprisingly demonstrated that Hpr binds Hb with high affinity,36 thus contradicting previous assumptions of Hpr being inactive in terms of Hb binding.35,94 This high-affinity interaction between Hpr and Hb hence enables TLF1 to associate with Hb.36,92 Based on these findings, together with studies indicating the involvement of an Hp-like receptor in the trypanosome uptake of TLF1,85,91 Vanhollebeke et al used an Hp-Hb-affinity–based strategy in search for the receptor involved in TLF1 uptake.95 Using this approach, the gene product of Tb927.6.440, a 403–amino-acid putative glycosyl-phosphatidyl inositol-anchored protein with no homology to any protein of known function, was identified as an Hp-Hb binding protein. Subsequent experiments using gene knockdown and knockout, as well as gene reintroduction techniques and competition assays with excess recombinant TbHpHbR, confirmed the presumed role of this protein as a receptor for uptake of Hp-Hb complexes. The trypanosomal receptor was named T brucei Hp-Hb receptor (TbHpHbR). Both Hp-Hb and Hpr-Hb complexes are bound by TbHpHbR with high affinity (Kd values of 13 × 10−9 M and 17 × 10−9 M, respectively) in surface plasmon resonance binding experiments, whereas neither Hp/Hpr alone nor Hb by itself binds the receptor. Importantly, it was demonstrated by affinity precipitation experiments that TbHpHbR also recognizes Hpr-Hb when embedded in TLF1. In agreement with the data obtained from the binding analyses, Hp/Hpr was shown to be taken up by the parasite only in the presence of Hb; conversely, Hb needed Hp/Hpr to be internalized itself (Figure 4).95
Binding of Hp and Hpr to Hb and uptake of the complexes by the trypanosomal receptor. Complexes of Hp-Hb and Hpr/TLF1-Hb bind to, and are internalized by, the TbHpHbR positioned on the trypanosome surface. On internalization, the heme moiety liberated from Hb is incorporated into hemoproteins of the parasite. The internalized TLF1 component apoL-I creates pores in the lysosomal membrane, thus leading to influx of chloride ions, osmotic swelling of the lysosome, and, ultimately, lysis of the parasite.
Binding of Hp and Hpr to Hb and uptake of the complexes by the trypanosomal receptor. Complexes of Hp-Hb and Hpr/TLF1-Hb bind to, and are internalized by, the TbHpHbR positioned on the trypanosome surface. On internalization, the heme moiety liberated from Hb is incorporated into hemoproteins of the parasite. The internalized TLF1 component apoL-I creates pores in the lysosomal membrane, thus leading to influx of chloride ions, osmotic swelling of the lysosome, and, ultimately, lysis of the parasite.
It was further discovered that the TbHpHbR-internalized heme originating from Hb is incorporated into endogenous hemoproteins, and when knocking out the TbHpHbR gene, the growth of the trypanosomes was severely compromised.95 It thus seems likely that TbHpHbR has evolved to supply the parasites with heme/porphyrin from the internalized Hp-Hb as a way of overcoming heme auxotrophy. However, as TbHpHbR lacks the ability to distinguish between Hp-Hb and Hpr-Hb, the parasite is elegantly tricked into taking up the lytic apoL-I–containing TLF1 in complex with Hb. Evolvement of the hpr gene from the hp gene in primates has thus resulted in a clever mechanism of combating the T b brucei subspecies, a mechanism that is clearly reminiscent of a Trojan horse–like strategy to fight off the enemy. The T brucei family members T b rhodesiense and T b gambiense are, however, resistant to the lytic activity of apoL-I and thus cause sleeping sickness in primates, including humans.96 In T b rhodesiense the resistance is explained by the presence of an apoL-I–interacting protein, which neutralizes the lytic activity of apoL-I.87 The mechanism underlying the resistance of T b gambiense remains yet enigmatic.90
In contrast to TbHpHbR, the mammalian Hp-Hb receptor CD163 does not recognize Hpr-Hb complexes36,52 because of a slight sequence difference in the loop 1 of Hpr compared with the corresponding loop of Hp.52 It is likely that nature's rationale behind this ability of CD163 to discriminate between Hp-Hb and Hpr-Hb is to protect the macrophages/monocytes from entrance of the membrane-disruptive protein apoL-I and retain that protein in plasma for the immune defense against parasites. On the other hand, this leaves the question of how Hpr-Hb complexes are removed from plasma in the absence of trypanosomes in the bloodstream. The presence of a high-affinity receptor is contradicted by the finding that the Hpr plasma concentration appears largely unaffected by intravascular hemolysis.35,36
Interestingly, and in contrast to TLF1-mediated lysis of T b brucei, TLF2-mediated trypanolysis is not decreased by addition of exogenous Hp,38,40 thereby suggesting that TLF2 enters the parasite independently of Hpr. Indeed, the role played by Hpr in relation to TLF2-mediated trypanolysis remains presently unresolved. Likewise, it is unclear whether Hpr possesses functions in addition to its critical role in the innate immune response against T b brucei. Because of the considerably lower concentration of Hpr compared with Hp in the circulation,35,36 very little, if any, Hpr is expected to associate with Hb under normal circumstances. However, in situations with extensive hemolysis leading to exhaustion of Hp from plasma, Hpr may become the only Hb binding protein of plasma. However, the low concentration of Hpr may have limited effect on protection against Hb toxicity. Instead, hemopexin that binds heme released from Hb and other hemoproteins is regarded as the essential “back-up” mechanism when Hp is depleted from plasma.97,98 It is unknown whether Hb associated with circulating Hpr/TLF1 constitutes a toxic threat to the surrounding environment. Likewise, it remains to be determined whether the sequestration of Hb by Hpr/TLF1 impedes NO scavenging.
Conclusions and future aspects
In conclusion, Hp and Hpr are both haptoglobins in the sense that they both bind Hb. Despite their striking overall common structural characteristics and similar basic functionality, Hp and Hpr appear to have completely different Hb-dependent biologic roles. Hp collaborates with the receptor CD163 to clear Hb from the circulation, thus protecting against the toxic effects of “free” Hb/heme. This mechanism for removal of Hb furthermore elicits an overall anti-inflammatory response because of the HO-1–mediated production of anti-inflammatory heme metabolites, and additionally, various signaling cascades triggered by Hp-Hb binding to CD163 may be speculated to contribute to and fine-tune such response. Hpr, on the other hand, does not target Hb to CD163, but plays a crucial role in a sophisticated defense mechanism against the parasite T b brucei. In this mechanism, Hpr-Hb mimics the Hp-Hb complex by serving as the ligand for a parasite Hp-Hb receptor (originally evolved to supply heme from Hp-Hb to the heme-auxotrophic parasite), thereby tricking the parasite into taking up a toxic Hpr-associated protein apoL-I, leading to killing of the parasite.
The potential medical application of the Hp/Hpr-Hb systems merits deeper investigation. From a diagnostic point of view, Hp is an established marker for hemolysis and acute phase conditions. However, it is tempting to speculate that the determination of Hp phenotype may be of value in future disease risk estimations in combination with other markers. In addition, molecules related to this system, such as CD163, which is present in plasma in a soluble form at concentrations that positively correlate with macrophage activity,99 might be promising biomarkers of infections/inflammatory disease.
In a therapeutic perspective, the selective expression of CD163 on cells of the monocytic lineage might be used for specific targeting of drugs to monocytes/macrophages, an approach that could be achieved by chemical linkage of the drug in question to Hp-Hb complexes. Such treatment strategy could be relevant in a large variety of diseases, such as anti-inflammatory diseases, many infections, and certain cancers, which all have CD163-expressing macrophages and/or malignant derivatives as the key cell type involved in pathogenesis.
A similar targeting strategy might be applied to improve the treatment possibilities in relation to human sleeping sickness caused by T b rhodesiense and T b gambiense. Administration of the currently used trypanocidal drugs does not provide a satisfactory treatment because of serious problems with side effects and resistance.100 However, with the recent identification of TbHpHbR (which is also expressed in T b rhodesiense and T b gambiense) as a receptor recognizing Hp/Hpr-Hb with high affinity, it might be possible to overcome some of these obstacles using more specific drug targeting.
Acknowledgments
This work was supported by grants from the Danish Medical Research Council and the Novo Nordisk Foundation.
Authorship
Contribution: M.J.N. and S.K.M. wrote the manuscript.
Conflict-of-interest disclosure: S.K.M. has declared ownership interests in Cytoguide, a spinoff company of the University of Aarhus. M.J.N. declares no competing financial interests.
Correspondence: Søren Kragh Moestrup, Department of Medical Biochemistry, University of Aarhus, Ole Worms Allé, Bldg 1170, 8000 Aarhus C, Denmark; e-mail: skm@biokemi.au.dk.