Abstract
Severe combined immunodeficiency (SCID) is a syndrome of diverse genetic cause characterized by profound deficiencies of T, B, and sometimes NK-cell function. Nonablative human leukocyte antigen–identical or rigorously T cell–depleted haploidentical parental bone marrow transplantation (BMT) results in thymus-dependent genetically donor T-cell development in the recipients, leading to long-term survival. We reported previously that normal T-cell numbers, function, and repertoire developed by 3 to 4 months after transplantation in SCID patients, and the repertoire remained highly diverse for the first 10 years after BMT. The T-cell receptor diversity positively correlated with T-cell receptor excision circle levels, a reflection of thymic output. However, the fate of thymic function in SCID patients beyond 10 to 12 years after BMT remained to be determined. In this greater than 25-year follow-up study of 128 patients with 11 different molecular types of SCID after nonconditioned BMT, we provide evidence that T-cell function, thymic output, and T-cell clonal diversity are maintained long-term.
Introduction
Transplantation of human leukocyte antigen (HLA)–identical or haploidentical allogeneic-related, rigorously T cell–depleted bone marrow (BM) cells into humans with severe combined immunodeficiency (SCID) without pretransplantation chemotherapy or posttransplantation graft-versus-host disease (GVHD) prophylaxis results in immune reconstitution with a high survival rate.1,2 Within 3 to 4 months after transplantation, the number of genetically donor T cells and T-cell function gradually increase to normal levels in SCID recipients.1-3 Studies by this group have demonstrated that the T-cell reconstitution in SCID recipients of rigorously T cell–depleted allogeneic-related BM cells is the result of the development and maturation of donor stem cells in the infant's vestigial thymus.4,5 Within the SCID thymus, donor T-cell precursors undergo T-cell receptor (TCR) gene rearrangements by the junction of V(D)J gene segments and by the addition of N nucleotides. The process of TCR rearrangement generates extrachromosomal DNA episomes or TCR excision circles (TRECs), which can be detected in new T cells. The presence of TRECs in circulating T cells is an indication that rearrangement of their TCR genes has recently occurred in the thymus.6 Thymic function in normal persons (0-34 years of age), as measured by the number of TREC copies in peripheral blood mononuclear cells (PBMCs), ranges between 4 and 3 log10 TRECs/μg PBMC DNA.6 Infants with SCID who received HLA-identical or haploidentical T cell–depleted BM transplantations (BMTs) have the ability to generate T cells with newly rearranged antigen receptors.4,7 We reported 8 years ago that thymus-derived naive CD45RA+ T cells carrying TRECs become the predominant T cells in the circulation in SCID patients from 150 days after BMT.4,7 The number of CD45RA+ cells declined thereafter, but CD45RA+ T cells predominated over CD45RO+ memory phenotype T cells for 10 to 12 years. At the time of that publication, however, data were available on only a few patients who were more than 10 years after transplantation.
In other studies, we also demonstrated that maturation and selection of donor-derived precursor T cells from transplanted BM into SCID recipients result in the development of a diverse T-cell repertoire by 1 to 2 years after BMT, which correlates with the rise in the number of recent thymic emigrant (CD45RA+) T cells of donor origin and an increase in TRECs.5 We observed a direct correlation between TREC values and T-cell diversity that developed in the analyzed patients. A more skewed T-cell repertoire emerged 10 years after BMT5 that was predominantly found in the CD8 CD45RO+ T-cell subset, a physiologic phenomenon occurring with the development of immunologic memory and with aging in normal persons.
In our initial report,4 the fate of thymic function in SCID patients beyond 10 to 12 years after BMT remained to be determined. Some patients have now survived for nearly 26 years, have normal numbers of T, B, and NK cells, have normal T-cell function, and are generally healthy. In the present longitudinal study, we had the unique opportunity to perform a continued analysis beyond 10 to 12 years after transplantation of thymic reconstitution, T-cell clonal diversity, and T-cell function in 128 patients with 11 different molecular types of SCID.
Methods
Patients
We studied PBMCs from 128 SCID patients who were given unfractionated HLA-identical related BMT (N = 8), T cell–depleted HLA-identical related BMT (N = 8), or rigorously T cell–depleted HLA-haploidentical parental BMT (N = 112) between 1982 and 2007. None of the patients received pretransplantation chemotherapy or prophylactic drugs to prevent GVHD after transplantation. These 128 patients included all 123 of the survivors from a total of 158 SCID infants transplanted initially at Duke University Medical Center (DUMC) between May 19, 1982 and December 31, 2007, 2 more that had been transplanted elsewhere but given booster transplantations at DUMC and 3 that had been transplanted elsewhere but followed longitudinally at DUMC for most of their posttransplantation course. A majority of the 35 patients who died succumbed to viral infections present at the time of transplantation, but none died of GVHD. At the time of this report, patients ranged from 6 months to 25.6 years after transplantation, with 68 of them being more than 10 years after transplantation. Sixty of these surviving patients had X-SCID resulting from mutations in the gene encoding the common gamma-chain (γcDef),8 11 had mutations in the gene encoding Janus kinase 3 (Jak3Def),9 15 had IL-7R α-chain mutations (IL7RαDef),10 18 had ADA deficiency (ADADef), and 24 had mutations in other genes, including RAG-1 or RAG-2 (RAG1/2Def; N = 6),11 the genes encoding the various chains of CD3 (CD3Def; N = 4)12 the CD45 gene (N = 1),13 the Artemis gene (N = 1),14 autosomal recessive (AutoRec) SCID of unknown molecular type (N = 11), and one male SCID of unknown type (Table 1). Donor BM was depleted of T cells by agglutination with soybean lectin and 2 cycles of rosetting with sheep erythrocytes that had been treated with aminoethylisothiuronium bromide as previously described.3 The method of T-cell depletion was the same for all recipients over the more than 25 years of this study. Forty-three of these patients were treated with a nonablative booster BMT, and the outcome of the booster transplantations is described in a separate manuscript (R.H.B., manuscript in preparation). Four of the patients underwent gene therapy elsewhere (2 with ADA deficiency in Italy, 2 with γcDef SCID at the National Institutes of Health). Gene therapy was successful in 3 of the 4 but unsuccessful in one γcDef patient who subsequently received a matched unrelated donor (MUD) transplantation after reduced intensity conditioning. Four ADA-deficient patients are currently receiving polyethylene glycol-modified bovine adenosine-deaminase (PEG-ADA), and 1 received a MUD BMT elsewhere. One Artemis-deficient patient received a MUD BMT elsewhere.
Chimerism
Chimerism was detected by karyotyping; fluorescence in situ hybridization15 ; restriction fragment length polymorphism, HLA typing; flow cytometric detection of γc or CD45 on lymphocytes; or by the presence of ADA.
T-cell phenotype and mitogen/antigen stimulation
T-cell phenotypes were determined by 4-color immunofluorescent staining and flow cytometry on freshly isolated PBMCs or whole blood with labeled antibodies (Abs) to CD3, CD4, CD8, CD14, CD16, CD20, CD45, CD132, CD56 (clone NCAM16.2), TCRαβ (clone T10B9.1A-31), and TCRγδ (clone B1) purchased from BD Biosciences. Lymphocyte proliferation was assessed by measuring [3H] thymidine incorporation into PBMCs after culture with optimal concentrations of the indicated stimuli, as previously described.3
Real-time quantitative PCR assay for TRECs
Polymerase chain reaction (PCR) analysis of TCR episomes was performed as described elsewhere.5,16 Genomic DNA from unfractionated PBMCs was extracted using TRIzol (Invitrogen). Signal joint TCR rearrangement excision circles (sjTRECs) in PBMCs were quantified using real-time quantitative PCR with the 5′-nuclease assay with iCycler (Bio-Rad). PCR was performed using primers (5′cacatccctttcaaccatgct and 5′gccagctgcagggtttagg) and the probe FAM-acacctctggtttttgtaaaggtgcccactBHQ1 (IDT Integrated DNA Technologies). PCRs contained 0.5 U Platinum Taq polymerase (Invitrogen), 3.5 mM MgCl2, 0.2 mM deoxyribonucleoside triphosphates, 500 nmol/L of each primer, and 150 nmol/L probe. Amplification conditions were 95°C for 5 minutes, followed by 40 cycles of 95°C for 30 seconds and 60°C for 1 minute. Samples were analyzed in duplicate and corrected to reflect TREC/μg of PBMC DNA. The sjTREC values were calculated using a standard curve of TCR sjTREC plasmid DNA (107 to 102 molecules, generously provided by Dr Daniel Douek, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health). For consistency with previously published data from our group, results are reported as sjTREC per μg of PBMC DNA. The lower limit of confidence is 100 molecules of sjTREC per μg of PBMC DNA.
CDR3 size spectratyping
Spectratype analysis of the TCR Vβ chain used in these studies was performed as published.5,17 Briefly, total RNA was extracted from fresh or cryopreserved PBMC samples (2-10 × 106 cells/sample) using TRIzol and reverse transcribed to single-stranded cDNA with reverse transcriptase from avian myeloblastosis virus (Roche Diagnostics) using an oligo (dT) primer according to the manufacturer's protocol (Promega). The newly synthesized cDNA was then used as a template for 23 PCRs with 23 different unlabeled TCR Vβ- and 1 Cβ-specific primers. PCR amplifications were conducted to saturation (40 cycles). The products were visualized by an elongation reaction using a nested fluorescent Cβ primer. The elongation products, which contained the CDR3, were separated on a capillary system.
The results were analyzed using Gene Scan software (Applied Biosystems) and are shown as graphic distributions of size peaks centered on a peak corresponding to a CDR3 of 10 amino acids. The peaks are spaced by 3 nucleotides. Normal distributions of size peaks were obtained in our laboratory using RNA extracted from as few as 5 × 104 normal donor PBMCs. Comparison with size standards shows that the peaks correspond to in-frame transcripts. Normal profiles have an average of 6 to 10 peaks/Vβ family. Previously, we classified TCR Vβ peak profiles as oligoclonal, polyclonal skewed, and polyclonal Gaussian.5 Here (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), the Vβ density peak histograms were analyzed by a web-based automated program for the Kullback-Leibler divergence (DKL).18 The higher the DKL, the greater the divergence from a normal Gaussian profile and the more oligoclonal the distribution of peaks.19
Statistical analysis
Individual comparisons of the mean number of T, B, and NK cells at the latest transplantation date between pairs of molecular types of SCIDs and with normal controls were performed using a 2-sided Satterthwaite test.20 Because there were 7 molecular types of SCID, a total of 21 pairwise comparisons were performed for each cell type. To maintain an overall type 1 error rate near 0.1, a conservative Bonferroni21 0.005 (∼ 0.1/21) α level was used for all the pairwise comparisons; that is, only those pairwise comparisons with P values less than .005 were considered statistically significant. For pairwise comparisons of the T-cell proliferation at the latest transplantation date between pairs of molecular types of SCIDs and with normal controls, 7 pairwise comparisons were performed. To maintain an overall type 1 error rate near 0.1, a conservative Bonferroni 0.015 (∼ 0.1/7) α level was used for all these pairwise comparisons. In the analysis of TREC values, proliferation values, and T, B, and NK-cell counts over time after transplantation, the longitudinal change of these values over time was estimated after the effects between patients and transplantations per patient were removed from the model. For the analysis of the TREC values, 95% confidence intervals were placed around the predicted TREC line over time for times more than 1 year after transplantation. The prediction line is the line through the mean TREC value/mean time point with a slope equal to the predicted change in TREC over the time after transplantation.
Results
Chimerism
Table 1 shows the results of chimerism studies in the various molecular types of SCID after BMT. Ten patients did not achieve T-cell chimerism after transplantation. Seven of these were ADADef, 2 of whom later received successful gene therapy elsewhere, one received a BMT elsewhere, and 4 are currently on PEG-ADA. One was an AutoRec SCID, one a CD3ζ-chain–deficient SCID, and one was a γcDef SCID who received unsuccessful gene therapy and a subsequent MUD BMT elsewhere. Forty-one of the patients achieved B-cell chimerism, including one-third of the γcDef patients, although they did not receive pretransplantation chemotherapy. The clinically relevant data related to the patient population studied in this paper are reported in a parallel manuscript (M. D. Railey, Y. Lokhnygina, and R.H.B., manuscript submitted, January 2009), which also contains the Kaplan-Meier survival curve for the transplantation recipients.
Blood samples were obtained from healthy normal controls and from the patients before transplantation and at various intervals thereafter. Excess PBMCs were cryopreserved in RPMI 1640 medium containing dimethyl sulfoxide and 20% fetal bovine serum.3 All of the flow cytometric and proliferative assays were performed using fresh cells, whereas the TREC and spectratype assays, not available at the beginning of these studies, were performed using cryopreserved cells. All studies were performed with the approval of the DUMC Institutional Review Board and with the written informed consent of the patients or their parents in accordance with the Declaration of Helsinki.
Lymphocyte phenotypes and T-cell function before and at latest time tested after transplantation
Lymphocyte phenotypes.
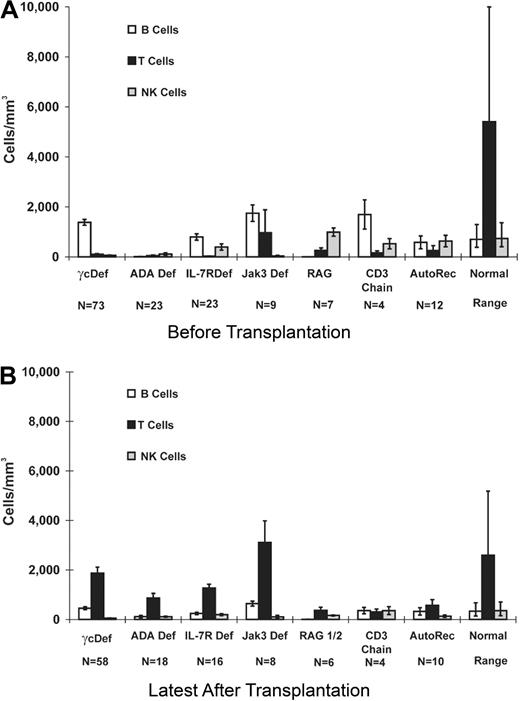
Patients with the various genetic types of SCID had distinct lymphocyte phenotypes before transplantation (Figure 1A).2 All patients had a profound deficiency of T cells and, when T cells were present, they were usually transplacentally transferred maternal T cells. In one Jak3Def patient, there were 8268 circulating maternal T cells/mm3 at presentation. At the latest time tested after transplantation, T-cell values (Figure 1B) were significantly (P < .001) higher than pretransplantation values in all molecular types of SCID. Before transplantation (Figure 1A), B cells were elevated in all categories except ADA and RAG deficiencies but were most elevated in γcDef and Jak3Def SCIDs (P < .001). At the latest time evaluated after transplantation (Figure 1A-B), B-cell values were significantly (P < .001) smaller than pretransplantation values and posttransplantation NK-cell values were significantly (P < .001) smaller than pretransplantation values. Given the sample sizes and variances of the patients and control groups, the mean numbers of T, B, and NK cells at the latest date after transplantation were different between some of the 7 SCID types and normal control values (Figure 1B), as determined by a conservative Bonferroni α level analysis for all of the pairwise comparisons.
PBMC phenotype before and after BMT in SCID patients. Mean numbers (± SEM) of CD20 B, CD3 T, and CD16 NK cells were obtained by flow cytometric analysis of SCID PBMCs studied before (A; N = 151) and at the latest time after transplantation (B; N = 120). Results from 2 patients with Artemis deficiency (1 surviving), 1 with CD45 deficiency, 4 with SCID of unknown molecular type (1 surviving), and from the 5 patients who received transplants elsewhere for whom we do not have the original pretransplantation data, are not included in Figures 1 and 2. Values for normal infant (A) and pediatric to young adult (B) controls are shown for comparison. Using a conservative Bonferroni α level analysis for all of the pairwise comparisons, given the sample sizes and variances of the patients and control groups, differences in mean T-cell numbers (B) were found between γcDef, ADADef, IL7RαDef, RAG1/2Def, CD3Def, AutoRec, and the normal control range (P < .015); differences in mean B-cell numbers (B) were found between ADADef (P = .001) and RAG1/2Def (P < .001) and the normal control range; differences in mean NK-cell numbers (B) were found between γcDef (P < .001), ADADef (P < .001), IL7RαDef (P = .002), RAG1/2Def (P < .001), AutoRec (P = .001), and the normal control values.
PBMC phenotype before and after BMT in SCID patients. Mean numbers (± SEM) of CD20 B, CD3 T, and CD16 NK cells were obtained by flow cytometric analysis of SCID PBMCs studied before (A; N = 151) and at the latest time after transplantation (B; N = 120). Results from 2 patients with Artemis deficiency (1 surviving), 1 with CD45 deficiency, 4 with SCID of unknown molecular type (1 surviving), and from the 5 patients who received transplants elsewhere for whom we do not have the original pretransplantation data, are not included in Figures 1 and 2. Values for normal infant (A) and pediatric to young adult (B) controls are shown for comparison. Using a conservative Bonferroni α level analysis for all of the pairwise comparisons, given the sample sizes and variances of the patients and control groups, differences in mean T-cell numbers (B) were found between γcDef, ADADef, IL7RαDef, RAG1/2Def, CD3Def, AutoRec, and the normal control range (P < .015); differences in mean B-cell numbers (B) were found between ADADef (P = .001) and RAG1/2Def (P < .001) and the normal control range; differences in mean NK-cell numbers (B) were found between γcDef (P < .001), ADADef (P < .001), IL7RαDef (P = .002), RAG1/2Def (P < .001), AutoRec (P = .001), and the normal control values.
T-cell function.
Figure 2 shows in vitro responses to stimulation with phytohemagglutinin (PHA), concanavalin A (ConA), and pokeweed mitogen (PWM) by PBMCs from SCID patients according to their various molecular types, analyzed before (Figure 2A) and at the latest time after (Figure 2B) transplantation, compared with responses from normal controls. For responses to all 3 mitogenic stimuli, there was a significant difference (P < .001) between pretransplantation and posttransplantation proliferation. When analyzing posttransplantation proliferative responses to PHA and ConA using a conservative Bonferroni α level analysis for all of the pairwise comparisons, given the sample sizes and variances of the patients and control groups, there was a significant difference between some of the SCID molecular types and normal control values (Figure 2B). Finally, whereas T cells from all SCID molecular types responded poorly to allogeneic cells before transplantation, posttransplantation T cells from all SCIDs responded normally to allogeneic cells, tetanus antigens (data not shown), and Candida (Figure 2D).
PBMC function before and after BMT in SCID patients. [3H]Thymidine incorporation by proliferating lymphocytes in response to PHA, ConA, and PWM was evaluated before (A; N = 151) and at latest time studied after transplantation (B; N = 120). Values are mean responses in counts per minute (cpm; ± SEM). Using a conservative Bonferroni α level analysis for all of the pairwise comparisons, given the sample sizes and variances of the patients and control groups at the latest transplantation date (B), the proliferative response to PHA of γcDef, ADADef, RAG1/2Def SCIDs was different (P < .015) from that of normal controls. Mean proliferation to ConA stimulus was different from normal controls only for ADADef (P = .005), and for the response to PWM only the IL-7RαDef was different from normal control values (P < .001).
PBMC function before and after BMT in SCID patients. [3H]Thymidine incorporation by proliferating lymphocytes in response to PHA, ConA, and PWM was evaluated before (A; N = 151) and at latest time studied after transplantation (B; N = 120). Values are mean responses in counts per minute (cpm; ± SEM). Using a conservative Bonferroni α level analysis for all of the pairwise comparisons, given the sample sizes and variances of the patients and control groups at the latest transplantation date (B), the proliferative response to PHA of γcDef, ADADef, RAG1/2Def SCIDs was different (P < .015) from that of normal controls. Mean proliferation to ConA stimulus was different from normal controls only for ADADef (P = .005), and for the response to PWM only the IL-7RαDef was different from normal control values (P < .001).
Longitudinal analysis of T-cell function after BMT in all SCIDs
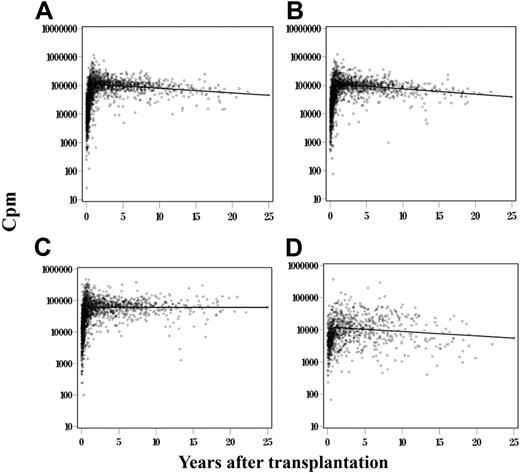
The data presented in Figure 2 represent a cross-sectional analysis of T-cell function in SCID PBMCs before and at the latest time after transplantation. In this section, PBMCs from 128 SCID patients who received BMT were also analyzed over time for their ability to proliferate to PHA, ConA, and PWM. A total of 6299 proliferative response results were collected on the 128 patients with the 3 stimuli over time (Figure 3). Because the longitudinal analysis was performed on each patient, it was necessary to remove patient-to-patient variation from the data before testing the changes in proliferation over time. In addition, changes over time were significantly different for proliferation observed before and 1 year after transplantation, when T-cell function is established. We analyzed the period 1 to 25.07 years after transplantation. The estimated change in the log10 proliferation value per year for the period 1 to 25.07 years after transplantation for the PHA stimulation was −0.0162 with an SD of 0.0043 with a P = .001 (Figure 3A), for the ConA response was −0.0187 with an SD of 0.0043 and a P < .001 (Figure 3B), for the Candida response the estimated change per year was −0.0136 with an SD of 0.0054 and a P = .011 (Figure 3D), whereas the change for the PWM response over time was not statistically significant with a P = .636 (Figure 3C).
Analysis of T-cell function over time after BMT in all SCIDs. Proliferation values (cpm; N = 6299) to PHA (A), ConA (B), PWM (C), and Candida (D) stimulations of PBMCs from 128 patients over time. Graphs represent the change in the log10 proliferation for PHA, ConA, and PWM stimulations, respectively, over time after the patient and transplantation effects were removed. Means for normal controls: PHA 187 073 cpm, SEM 2390 cpm; Con A 137 050 cpm, SEM 2289 cpm; PWM 92 191 cpm, SEM 2156 cpm; and Candida 48 793 cpm, SEM 2835 cpm.
Analysis of T-cell function over time after BMT in all SCIDs. Proliferation values (cpm; N = 6299) to PHA (A), ConA (B), PWM (C), and Candida (D) stimulations of PBMCs from 128 patients over time. Graphs represent the change in the log10 proliferation for PHA, ConA, and PWM stimulations, respectively, over time after the patient and transplantation effects were removed. Means for normal controls: PHA 187 073 cpm, SEM 2390 cpm; Con A 137 050 cpm, SEM 2289 cpm; PWM 92 191 cpm, SEM 2156 cpm; and Candida 48 793 cpm, SEM 2835 cpm.
Analysis of thymic function over time after BMT in all SCIDs
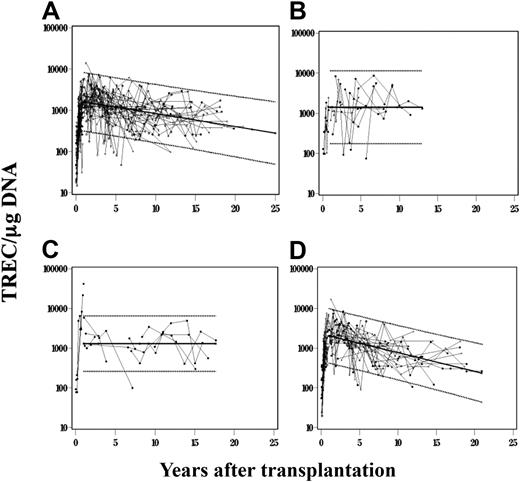
TREC values were determined over time in 115 of 128 total subjects with 11 different molecular types of SCID as a measure of thymic function. A total of 597 TREC observations were collected on the 115 patients over time. Thirty-one of the 115 patients had more than one transplantation. Because the longitudinal analysis was performed on each patient for each transplantation, it was necessary to remove patient-to-patient and transplantation-to-transplantation variation from the data before analyzing the changes in TREC over time on each patient. In addition, changes over time were significantly different for TREC observations collected less than 1 year after transplantation and greater than 1 year after transplantation. The main emphasis was to examine long-term changes in TREC values over time, after T-cell function was fully established (after 1 year after transplantation). Therefore, only the results associated with the period from 1 to 25.07 years after transplantation were analyzed. Figure 4A provides a graph of the log10 change in TREC values with time after transplantation for all molecular types of SCID, after the patient/transplantation variation was removed from the model. The change in the log10 TREC value per year for the period from 1 to 25.07 years after transplantation was −0.03155 with an SD of 0.0086 and P = .001. The solid trend-line passes through the mean years after transplantation for the combined data for all SCID molecular types, with the estimated slope of −0.03155. This TREC value change over time is within the value change in the normal population for the period 1 to 25 years of age (−0.033).4
Analysis of thymic function over time after BMT in all SCIDs. TREC values (N = 597) were observed over time in 115 patients for whom samples were available for analysis, each patient associated with 1 of 11 molecular types of SCID. (A) The combined results of all 115 patients. The points on the figure represent the predicted TREC value plus the residual after the patient and transplantation effects were removed. Lighter lines are then used to join points from the same patient before and 1 year after transplantation. The same analysis was performed for 15 IL7RαDef patients (B), 10 Jak3Def patients (C), and 57 γcDef patients (D); 95% confidence intervals were placed around the predicted TREC line over time for times more than 1 year after transplantation.
Analysis of thymic function over time after BMT in all SCIDs. TREC values (N = 597) were observed over time in 115 patients for whom samples were available for analysis, each patient associated with 1 of 11 molecular types of SCID. (A) The combined results of all 115 patients. The points on the figure represent the predicted TREC value plus the residual after the patient and transplantation effects were removed. Lighter lines are then used to join points from the same patient before and 1 year after transplantation. The same analysis was performed for 15 IL7RαDef patients (B), 10 Jak3Def patients (C), and 57 γcDef patients (D); 95% confidence intervals were placed around the predicted TREC line over time for times more than 1 year after transplantation.
Analysis of thymic function in 11 molecular types of SCIDs over time after BMT
Different cellular microenvironments and cell-cell interactions may develop in SCID of different molecular types, which could differentially affect the maintenance of thymic function over time. Therefore, we evaluated thymic function after BMT among the various molecular types of SCID. PBMCs from a total of 57 γcDef, 15 IL-7RαDef, 10 Jak3 Def, 13 ADADef, and 20 patients with other types of SCID (RAG1/2Def, CD3δDef, CD3ϵDef, CD3ζDef, CD45Def, AutoRec, and unknown) were subjected to TREC analysis. For all SCID molecular types except γcDef, TREC values measured within 20 years and beyond the first year of BMT remained constant. Figure 4 provides examples of these constant values over time, demonstrated by a horizontal trendline for IL7RαDef (Figure 4B) and Jak3Def SCID (Figure 4C) molecular types, respectively. For the γcDef SCIDs (Figure 4D), the change in the log10 TREC value per year for the period 1 to 21.91 years after transplantation was −0.0471 with an SD of 0.0099 and a P < .001. This significant negative TREC value change over time for γcDef is represented by a sloping trendline (Figure 4D), which was not statistically different (P = .078) from the slope of the trendline in the normal population for the period 1 to 25 years of age (−0.033).4
Ten or more years after transplantation: thymic output, T-cell phenotype, and function in BMT recipients
The results reported so far refer to composite groups of patients from the same molecular type of SCID or from the same in vitro stimulation. We next evaluated 68 individual SCID patients (Table 2) who were more than 10 years after transplantation, to determine whether, at the latest date after transplantation, they generated peripheral T cells with normal phenotype and function. These patients were monitored for their TREC levels, their naive versus memory phenotype (percentage of CD3+ T cells that expressed the CD45RA marker vs those that expressed the CD45RO marker22 ), and/or their ability to proliferate in response to stimulation with mitogens. For the majority (83%) of the patients, thymic output correlated well with T-cell phenotype and function. Only 3 of 53 patients failed to demonstrate thymic output and had poor T-cell function (patients 5, 35, and 50 in Table 2). A fraction of the 53 patients (N = 9: patients 10, 11, 17, 22, 34, 38, 43, 56, and 57) had negative TREC results, but normal T-cell phenotype and function.
Analysis of the TCR repertoire in SCIDs followed long-term post-BMT
In previous published studies, we showed that a fully diverse TCR Vβ repertoire developed in BM-transplanted SCIDs at peak thymus output, whereas a more skewed repertoire emerged in the few patients studied more than 10 years after BMT.5 In this study, we analyzed the TCR Vβ repertoire of 36 SCID patients of various molecular types before and up to 25 years after transplantation. The DKL statistical program was used to assess the diversity of the TCR Vβ repertoire in these patients, as determined by spectratype analysis. Using this program, the higher the DKL, the greater the divergence from a normal Gaussian profile and the more oligoclonal the distribution of peaks (Figure S1).
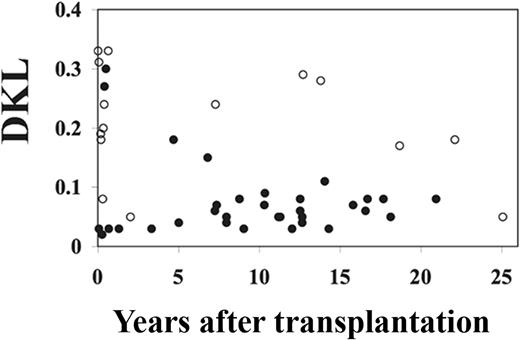
As shown in Figure 5, the DKL values decreased from more than 0.3 within the first year of BMT, when the TCR repertoire is still oligoclonal, to a range between 0.1 and 0.03, indicating that patients developed and maintained a diverse TCR Vβ repertoire. The 4 patients who had higher than normal (> 0.08) DKL values beyond 10 years after BMT did not show evidence of thymic function.
Thymic output correlates with TCR diversity. Shown are dot plots of the DKL values compared with TREC levels of samples taken from 36 SCID patients at multiple time points after BMT. DKL values from normal controls were < 0.08. ○ represents patients whose TREC value was less than 100; ●, patients whose TREC value was > 100.
Thymic output correlates with TCR diversity. Shown are dot plots of the DKL values compared with TREC levels of samples taken from 36 SCID patients at multiple time points after BMT. DKL values from normal controls were < 0.08. ○ represents patients whose TREC value was less than 100; ●, patients whose TREC value was > 100.
To examine the effect of DKL values on TREC values in these patients, we calculated the mean log10 TREC and DKL values per patient and analyzed these means through weighted least squares regression. Because of the small sample number, we did not evaluate the influence of DKL on TREC over time. The change in the log10 TREC value per DKL value was −4.728 with an SD of 1.607 with a P = .007, indicating a significant inverse correlation between DKL values and TREC values in these patients.
Discussion
In this longitudinal study, we analyzed a total of 128 surviving SCID patients for thymic output and T-cell reconstitution over a period of up to more than 25 years after BMT. Our results show that T-cell reconstitution, including thymic output and T-cell diversity, are maintained long-term to levels similar or equal to normal control values.
T-cell chimerism was achieved in 92% of the transplanted patients, and 32% also developed B-cell chimerism, although these patients did not receive pretransplantation chemotherapy. The T-cell numbers after BMT were significantly greater than before transplantation, whereas the numbers of B and NK cells, which appeared elevated before BMT, significantly decreased to levels similar to normal controls at the latest time tested after transplantation. T-cell function, measured as proliferative responses to T-cell mitogens (PHA, ConA, PWM) was significantly increased in patients analyzed at their latest study after transplantation compared with responses before transplantation.
Ninety-three (81%) of the 115 patients analyzed (Table 1) developed detectable levels of TREC, and 14 of the patients who did not, received one or more booster BMT. The lack of thymic function in 5 SCIDs was attributed to the fact that they were tested at less than 1 year after BMT. Nine SCIDs who lacked TREC function are long-term survivors (> 10 years after BMT). One is RAG1/2Def, 4 are γcDef, 2 are ADADef, one is IL-7RαDef, and one is AutoRec recessive SCID of unknown molecular type, all of whom are in good health at times up to 25.6 years after transplantation. Those with ADA deficiency or with VDJ recombination defects have lower T-cell numbers, function and thymic output after transplantation than SCIDs of other molecular types. Whether this is the result of intrinsic thymic defects in these SCIDs or to postthymic influences is unknown. Defective double-negative thymocytes (in RAG1/2 Def SCIDs, for example) present in thymic stromal niches could compete for space with incoming progenitor T cells of donor origin derived from BMT as has been reported for RAG SCID mice.23 This could result in poor maturation of donor stem cells and insufficient peripheral T-cell reconstitution after BMT. By contrast, we did not see any evidence of defects in early or late thymopoiesis in the IL-7RαDef, γc-def SCIDs, or Jak3Def SCIDs, all of whose pretransplantation T-cell defects are caused by failure of IL-7 signaling. The ADA and RAG-deficient patients, although long-term survivors, are not as healthy as those with the other molecular types of SCID. It is possible that their inferior immune reconstitution or other consequences of their underlying molecular defects may result in even poorer general health over longer follow-up. Further investigation of this issue is forthcoming in our laboratory.
When an extensive TREC analysis was performed on 115 patients over time in the present study (Figure 4A), the estimated change in TREC values per year for the period of 1 year to more than 25 years after transplantation demonstrated a negative slope, which was within the level of change in slope for normal controls noted previously.4 TREC values change in the normal population between 4 and 3 log10 within the first 25 years of life. When the TREC analysis was performed separately for the various molecular types of SCID (Figure 4B,C), TREC values measured beyond the first year of BMT remained constant, except for γcDef, which demonstrated a significant negative slope (Figure 4D). However, this negative slope was not statistically different from the change in slope for normal controls noted previously.4
The proliferative response to PWM over a period of 1 to more than 25 years of transplantation remained constant over time with responses to PHA and ConA producing a small but statistically significant deviation from a horizontal line. Studies by others have previously reported a decrease in proliferative responses of T cells to mitogens in the normal population over time.24
Our group previously reported that thymic output appeared to decline at more than 10 years after BMT,4 a finding that has been frequently cited in the literature as a concern that nonablative marrow transplantations could not maintain sufficient T-cell function long-term to prevent infections.25,26 At the time of this earlier study,4 only a few of our patients were more than 10 years after transplantation. We now have many more (N = 68) patients beyond 10 years after transplantation; and, as shown in the present study, thymic output and T-cell function were retained in a majority (83%) of these patients. Patients were analyzed beyond 10 years of BMT for their thymic function, T-cell phenotype, and responsiveness. In 83% of the patients, the TREC values were positive and the CD45RA/RO ratio was more than 1 in 48% of the subjects, suggesting that many of the circulating T cells had a naive phenotype. Responses to mitogens were within or above normal ranges in 90% of the subjects. Interestingly, 3 of the patients who showed a decline in thymic function (J-1, J-2, and X-5) in our previous report5 were followed beyond 10 years of BMT in the present study and subsequently had improved thymic function. Therefore, the supposition that TREC function declines with time does not appear to be a general finding applicable to all SCIDs treated with nonablative BMT. The data in the present study also refute the hypothesis that there is an intrinsic inability of the SCID thymus to maintain thymic output beyond the first decade of life.
The TCR Vβ repertoire studies extended beyond 10 years of BMT also demonstrated that DKL values inversely correlated with TREC values (low DKL indicating polyclonality with high TREC values). The inverse association between TREC and DKL was statistically significant. For 4 patients who failed to develop TREC over time, the DKL values remained high, indicating oligoclonality. Failure to develop a diverse repertoire in those 4 patients was not associated with a specific molecular type.
Few studies have been reported to date on long-term T-cell reconstitution after hematopoietic stem cell transplantation (HSCT) in SCID patients.25-31 The findings described in the present study are in keeping with those of Borghans et al,27 who found that 11 of 19 SCID patients followed up to 25 years after conditioned BMT had no evidence for an accelerated decline of T-cell immunity or thymus output; however, those SCIDs who had low thymic output soon after transplantation continued to have decreased long-term T-cell reconstitution. Mazzolari et al25 reported on the long-term immune reconstitution and clinical outcome of 40 patients with severe T-cell immunodeficiency who survived for up to 16.3 years after HSCT. A total of 35% of the 40 patients had low TREC levels at their last follow-up, and oligoclonality of the T-cell repertoire was demonstrated at the last follow-up in 27.5% of the patients. Cavazzana-Calvo et al26 and Friedrich et al28 have reported findings similar to those of Mazzolari et al25 and speculated that the use of a conditioning regimen before HSCT for SCID was necessary for thymic output at greater than 10 years after transplantation. However, Patel et al30 have recently reported sustained T-cell function in 15 long-term SCID survivors (up to 26 years) of nonconditioned BMTs. Our data confirm that finding and provide evidence for continued thymic output at more than 10 years after transplantation in the absence of both pretransplantation conditioning and posttransplantation GVHD prophylaxis.
In conclusion, these results indicate that transplantation of HLA-identical or haploidentical allogeneic-related, T cell–depleted BM into SCID infants in the absence of pretransplantation chemotherapy or posttransplantation GVHD prophylaxis is successful at reconstituting long-term T-cell function. The ability of these patients to generate new T cells at these late time points is indirect evidence for stem cell engraftment.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families, the physicians who referred them and cared for them, the members of the Duke University DNA Analysis Facility, and the Duke Comprehensive Cancer Center Flow Cytometry Facility.
This work was supported by the National Institutes of Health (grants AI47605 and AI042951) and in part by the Duke Regional Biocontainment Laboratory (AI58607) and the Duke Center for Translational Research (AI51445).
National Institutes of Health
Authorship
Contribution: M.S.-K. designed research, collected and analyzed data, and wrote the manuscript; C.M.W., R.E.P., and M.C. performed research and collected data; J.L.R. contributed research discussions and analyzed data; B.K.M. performed complete statistical analysis of data; G.D.S. contributed vital reagents and analytical tools; and R.H.B. designed research, collected and analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcella Sarzotti-Kelsoe, Duke University Medical Center, 2812 Erwin Rd, Erwin Terrace II, Ste 301, Durham, NC 27710; e-mail: msarzott@duke.edu.

![Figure 2. PBMC function before and after BMT in SCID patients. [3H]Thymidine incorporation by proliferating lymphocytes in response to PHA, ConA, and PWM was evaluated before (A; N = 151) and at latest time studied after transplantation (B; N = 120). Values are mean responses in counts per minute (cpm; ± SEM). Using a conservative Bonferroni α level analysis for all of the pairwise comparisons, given the sample sizes and variances of the patients and control groups at the latest transplantation date (B), the proliferative response to PHA of γcDef, ADADef, RAG1/2Def SCIDs was different (P < .015) from that of normal controls. Mean proliferation to ConA stimulus was different from normal controls only for ADADef (P = .005), and for the response to PWM only the IL-7RαDef was different from normal control values (P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/7/10.1182_blood-2009-01-199323/4/m_zh89990938900002.jpeg?Expires=1767732685&Signature=TGXcVaJE5otHhUwoJ-ZORyT7JcTDHI28ZLcyTxEYpuksOL4Gh02iFAHJMbiny0GqIV3YCDeyivauppYy3MruYCUB3Kxtd3qsRIRKee2bUxyglwKq5CWFHXZRLElJAnXIm3ZiCNDVKIgE64YYPKzk69REVm~RKArevrO-08rq1dygnjKwBViZ3e6w~9PkdsCa6ZfZNOUDDxnB78RG1~G8F-9elwXnD5bXtDvewR34j7rjAcQIRNlCYpHaqSlKvp3UyDKQzJSzOXzcXF52ynFxRDq0ab13miUP7VY9ljGBriWGloLgzopX0RAMkC3tdFind7RRk5zYTmZvUKy06GdReA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)