Abstract
Chronic myeloid leukemia (CML) is a malignant myeloproliferative disease with a characteristic chronic phase (cp) of several years before progression to blast crisis (bc). The immune system may contribute to disease control in CML. We analyzed leukemia-specific immune responses in cpCML and bcCML in a retroviral-induced murine CML model. In the presence of cpCML and bcCML expressing the glycoprotein of lymphocytic choriomeningitis virus as a model leukemia antigen, leukemia-specific cytotoxic T lymphocytes (CTLs) became exhausted. They maintained only limited cytotoxic activity, and did not produce interferon-γ or tumor necrosis factor-α or expand after restimulation. CML-specific CTLs were characterized by high expression of programmed death 1 (PD-1), whereas CML cells expressed PD-ligand 1 (PD-L1). Blocking the PD-1/PD-L1 interaction by generating bcCML in PD-1–deficient mice or by repetitive administration of αPD-L1 antibody prolonged survival. In addition, we found that PD-1 is up-regulated on CD8+ T cells from CML patients. Taken together, our results suggest that blocking the PD-1/PD-L1 interaction may restore the function of CML-specific CTLs and may represent a novel therapeutic approach for CML.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder resulting from the neoplastic transformation of a hematopoietic stem cell.1 The disease is bi- or triphasic, comprising a chronic, an accelerated, and a terminal blast phase in which the patients develop an acute leukemia of either myeloid (AML) or, less often, lymphoid (ALL) cell type. More than 90% of all CML cases are associated with the presence of the Philadelphia chromosome, which results from a reciprocal translocation between chromosomes 9 and 22 forming the breakpoint cluster region/Abelson protein tyrosine kinase (BCR/ABL) fusion protein, a constitutively activated tyrosine kinase.2,3 Depending on the precise breakpoints in the BCR gene, different forms of BCR/ABL fusion protein with different molecular weights can be generated (p190BCR/ABL, p210BCR/ABL, and p230BCR/ABL). CML patients predominantly express p210BCR/ABL.1
Currently, BCR/ABL-selective tyrosine kinase inhibitors are the standard treatment for CML. However, resistant clones often develop during treatment. At present, the only curative treatment for CML is allogeneic hematopoietic stem cell transplantation.4
Several earlier studies suggested that the immune system plays an important role in the control of CML. CML cells are susceptible to lysis by CD8+ T cells5 and natural killer (NK) cells in vitro.6 For unknown reasons, CML is the most graft-versus-leukemia–sensitive leukemia.7 In addition, cytotoxic T lymphocytes (CTLs) directed against leukemia antigens are found in CML patients without hematopoietic stem cell transplantation, including CTLs specific for BCR/ABL, overexpressed self-proteins such as proteinase-3, and Wilms tumor 1 protein.5,8 However, the physiologic relevance of these leukemia-specific CTL responses in the control of CML is unknown. The presence of CTL escape mechanisms during CML disease progression to blast crisis suggests that CTLs are involved in the control of the chronic phase of the disease. Documented escape mechanisms include the expression of Fas ligand during blast crisis,9 the deletion of high-avidity CTLs specific for a leukemia-associated self-antigen,10 and the development of functional blocks in the caspase activation pathway in AML cells.11
Coinhibitory molecules are essential for the maintenance of T-cell homeostasis, self-tolerance, and tolerance to chronic infections. Programmed death 1 (PD-1) is a member of the immunoglobulin (Ig) superfamily, which is inducibly expressed on T cells, B cells, and activated monocytes.12 To mediate inhibitory signals through PD-1, the binding of either of the 2 ligands to the receptor is necessary. PD-ligand 1 (PD-L1, B7-H1) is expressed constitutively on resting T cells, B cells, dendritic cells (DCs), macrophages, mesenchymal stem cells, some parenchymal cells, and cultured bone marrow-derived mast cells. PD-L1 expression is further up-regulated after activation.13 PD-ligand 2 (PD-L2, B7-DC) is only inducibly expressed on DCs, macrophages, and cultured bone marrow-derived mast cells.13 Recently, PD-1 signaling has been identified as an important mechanism of antigen-specific T-cell dysfunction in chronic infections such as lymphocytic choriomeningitis virus (LCMV) infection in mice14 or HIV15 and hepatitis C virus (HCV)16 infections in humans. In addition, it has been shown that PD-1 leads to functional impairment of tumor-infiltrating T cells in solid tumors.17 In contrast, mechanisms determining the function of leukemia-specific CTLs have not yet been analyzed in detail.
In the present study, we analyzed the immunosurveillance of chronic phase CML (cpCML) and blast crisis CML (bcCML) in a murine retroviral bone marrow transduction and transplantation model.18 We found that leukemia-specific CTLs were functionally exhausted and phenotypically characterized by high expression of PD-1, in both cpCML and bcCML. Blocking PD-1 signal transduction by using PD-1–deficient recipient mice or by administration of αPD-L1 antibody reversed CML-specific T-cell tolerance, and the time to disease progression was increased.
Methods
Mice
C57BL/6 mice were purchased from Harlan. The p14 T-cell receptor (TCR) transgenic mice19 and H8 transgenic mice20 were obtained from the Institute for Laboratory Animals. CD45.1+ mice were obtained from C. Mueller (University of Berne). PD-1–deficient mice21 were provided by T. Honjo (Kyoto University). Animal experiments were performed with sex- and age-matched mice and approved by the Experimental Animal Committee of the Canton of Berne and performed according to Swiss laws for animal protection.
Viruses, peptide, and retroviral vectors
LCMV, strain WE, and Docile were provided by R. M. Zinkernagel (University Hospital, Zurich) and propagated, as described.22,23 The LCMV-GP, amino acids 33-41 (glycoprotein [gp]33, KAVYNFATM), was purchased from NeoMPS SA. The retroviral vectors pMSCV-p210 BCR/ABL-IRES-GFP, pMSCV-p210 BCR/ABL-pgk-neo, and pMSCV-NUP98/HOXA9-IRES-GFP (MSCV, mouse stem cell virus; IRES, internal ribosomal entry site; GFP, green fluorescent protein; neo, neomycin) were a gift from D. G. Gilliland (Havard Medical School), and the packaging vector pIK6 was purchased from Cell Genesys.24-26
51Cr-release assay
Spleens were isolated and analyzed after 5 days of in vitro restimulation in a standard 51Cr-release assay, as described.27 The cytotoxicity assay of ex vivo isolated p14 T cells was performed as follows: 6 × 105 CD45.1+ cells, purified by magnetic cell sorting (MACS; Miltenyi Biotec), were either used directly or restimulated for 5 days with 3 × 105 irradiated gp33-pulsed naive C57BL/6 splenocytes in the presence of 50 U/mL murine interleukin (IL)-2.
Cells and retroviral particle production
Retroviral particles were generated by transient cotransfection of 293-T cells with the respective MSCV vector and pIK6, using Superfect transfection reagent (Qiagen), according to the manufacturer's protocol. After 48 hours, virus-containing supernatant was harvested. For determination of retroviral titers, BA/F3 cells were infected with different amounts of retroviral supernatant using polybrene transfection reagent (10 μg/mL; Sigma-Aldrich). After 48 hours, retroviral titers were determined by enumerating GFP-positive cells by flow cytometry. Alternatively, to assess virus titers of the vector containing a neocassette, BA/F3 cells were infected and then cultivated for 5 days in the presence or absence of 0.8 mg/mL G-418 sulfate (Gibco). Surviving cells were assessed with trypan blue staining.
CML model
Bone marrow donor mice were pretreated with 150 mg/kg 5-fluorouracil intraperitoneally (Sigma-Aldrich). After 6 days, the bone marrow was flushed out from femurs and tibias. Erythrocytes were removed and the bone marrow cells were incubated in transplant media (RPMI/10% fetal calf serum with recombinant murine IL-3 [6 ng/mL; BD Biosciences], recombinant murine stem cell factor [10 ng/mL; Biocoba], and recombinant human IL-6 [10 ng/mL; BD Biosciences]) for 24 hours. A total of 4 × 106 cells was transfected twice on 2 consecutive days with the respective retroviral particles with polybrene (6.7 μg/mL) and 0.01 M HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) through spin infection (90 minutes at 1258g, 30°C). A total of 105 transduced bone marrow cells was injected intravenously into previously irradiated (4.5, 6.5, or 9.5 Gy) syngeneic recipient mice (transduction efficiency: 24.0% ± 9.8%).
Antibodies and flow cytometry
αCD8-allophycocyanin (APC), αCD4-biotin, αB220-biotin, αI-Ab-major histocompatibility complex (MHC) class II-biotin, αCD45.1-phycoerythrin (PE) and -APC, αCD11c-biotin, α interferon (IFN)-γ-PE, α tumor necrosis factor (TNF)-α-PE, streptavidin-PE and APC, αNK1.1-biotin, αPD-1-biotin, αPD-L1-biotin, αPD-L2-biotin, rat IgG2a isotype-biotin, and human αPD-1-PE were obtained from eBioscience. αGR-1-PE, αCD8-peridinin chlorophyll protein (PerCP)-Cy5.5, αCD4-PerCP-Cy5.5, αPD-1-PerCP-Cy5.5, αVα2-biotin and PE, αSca-1-APC, αMAC-1-PE-Cy7, αC-kit-PE-Cy7, mouse IgG1-PE isotype control, human αCD8-fluorescein isothiocyanate, and human αCD45-PerCP-Cy5.5 were obtained from BD Pharmingen. MHC class I (H-2Db) tetramer-PE complexed with gp33 or nuclear protein (np)396 was obtained from ProImmune and used according to the manufacturer's protocol. Intracellular staining was performed, as described.28 Relative fluorescence intensities were measured on a BD LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar). The frequency of IFN-γ– and TNF-α–producing CD8+ T cells was calculated as gp33-pulsed minus nonpulsed samples.
Adoptive transfer experiments and reisolation of transferred CD8+ T cells
P14 × CD45.1 T cells were isolated and purified by MACS for CD8+Vα2+ T cells. A total of 2.5 to 4 × 106 CD8+Va2+CD45.1+ cells was injected intravenously into CML mice, naive C57BL/6 control mice, C57BL/6 mice that were infected with 104 plaque-forming units (pfu) of LCMV-WE, and C57BL/6 mice chronically infected with 107 pfu of LCMV Docile (all recipient mice were CD45.1−). CML disease progress was monitored by fluorescence-activated cell sorter (FACS) analysis of blood, and on the days indicated, CD45.1+ T cells from CML mice, naive C57BL/6 mice, and C57BL/6 mice infected with LCMV were isolated by MACS.
Proliferation assays
For ex vivo proliferation of transferred p14 T cells, 2 × 105 CD45.1+ cells were restimulated for 4 days with 2 × 105 irradiated gp33-pulsed or nonpulsed naive C57BL/6 splenocytes. For in vitro proliferation, naive p14 CD8+ T cells and p14 × PD-1−/− CD8+ T cells (2 × 105) were restimulated with irradiated (50 Gy) FACS-sorted H8-cpCML cells (BCR/ABL-GFP+), FACS-sorted H8-bcCML cells (NUP98/HOXA9-GFP+), H8 splenocytes, or C57BL/6 splenocytes (0.4 × 105). 3H-thymidine incorporation assay was performed, as described.22 The proliferation index was calculated as the ratio between gp33-pulsed and nonpulsed samples (ex vivo proliferation assay) and as the ratio between H8-CML cells or H8 splenocytes and C57BL/6 splenocytes used as stimulators (in vitro proliferation assay).
Cytometric bead array assay
A total of 1.5 × 105 CD45.1+ cells was restimulated for 18 hours with 3 × 105 irradiated gp33-pulsed or nonpulsed naive C57BL/6 splenocytes. Cytokine production in supernatant was analyzed with a mouse T helper cell 1/2 cytokine cytometric bead array assay according to the manufacturer's protocol (BD Biosciences).
PD-L1 blockade
bcCML mice were treated intraperitoneally every third day with 200 μg of αPD-L1 monoclonal antibody (clone 2G9; BioXCell) or IgG from rat serum (I8015; Sigma-Aldrich) starting on the day of transplantation.
CD8 depletion
bcCML mice were treated intraperitoneally on day 0 and day 2, and from then on weekly with 100 μg of αCD8 monoclonal antibody (YTS 169.4). The treatment depletes CD8+ T cells to below the detection limit of flow cytometry analysis (data not shown).
CML patients
Human blood sample collections were approved by the ethical committee of the Canton of Berne, Switzerland, and patients gave their written informed consent in accordance with the Declaration of Helsinki. The average age of healthy donors was 40.6 ± 13.8 years (3 [female] and 5 [male]) and of cpCML patients 46.0 ± 12.2 years (3 [female] and 5 [male]). All cpCML patients expressed p210BCR/ABL.
Statistical analysis
Data are presented as the mean plus or minus SEM. The significance of the differences in Kaplan-Meier survival curves was determined using the log-rank test (2-tailed). The significance between groups of human samples was determined by using unpaired Student t test (2-tailed) and the correlation with the Spearman correlation (2-tailed, α = .05).
Results
CML-like disease in immunocompetent mice
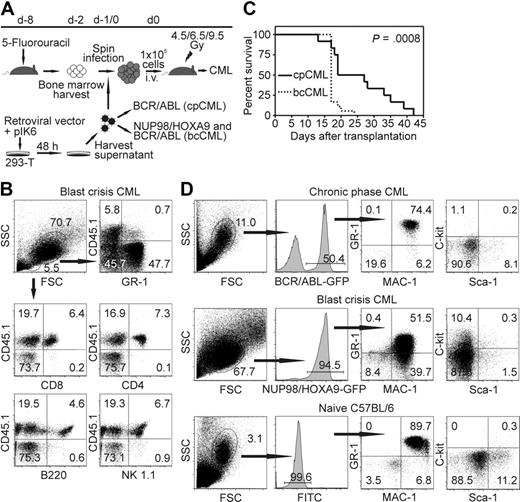
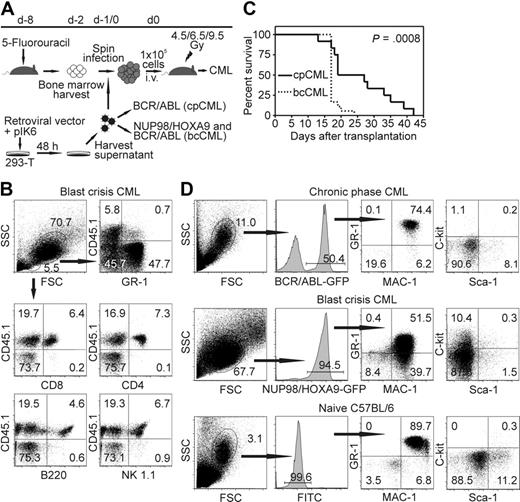
It has been shown previously that BCR/ABL expression in bone marrow cells leads to cpCML in mice.29 Coexpression of NUP98/HOXA9 leads to accumulation of immature myeloid cells and progression to blast crisis.18 Therefore, we transduced bone marrow from C57BL/6 mice with retroviral particles expressing p210BCR/ABL alone, or cotransduced bone marrow cells with p210BCR/ABL and NUP98/HOXA9 retroviral particles. Transduced bone marrow cells were adoptively transferred into irradiated syngeneic recipient mice (Figure 1A).
Model of CML-like disease in mice. (A) Schema of retroviral bone marrow transduction and transplantation to irradiated recipient mice. (B) Donor bone marrow (CD45.2+) was transferred into CD45.1+ recipient mice (irradiated with 4.5 Gy). Twenty days later, PBMCs were stained for GR-1, CD8, CD4, B220, and NK1.1, and analyzed by flow cytometry. Representative dot plots of 1 of 6 mice are shown. (C) Survival of cpCML (—, n = 12) and bcCML ( , n = 18) mice is shown in Kaplan-Meier plots. Statistical comparison was performed with the log-rank test. (D) Phenotypical characterization of GFP+ leukemic cells in cpCML and bcCML mice. Cells were gated on granulocytes in control C57BL/6 mice and on transduced granulocyte populations in cpCML and bcCML mice. One representative staining of 5 is shown.
, n = 18) mice is shown in Kaplan-Meier plots. Statistical comparison was performed with the log-rank test. (D) Phenotypical characterization of GFP+ leukemic cells in cpCML and bcCML mice. Cells were gated on granulocytes in control C57BL/6 mice and on transduced granulocyte populations in cpCML and bcCML mice. One representative staining of 5 is shown.
Model of CML-like disease in mice. (A) Schema of retroviral bone marrow transduction and transplantation to irradiated recipient mice. (B) Donor bone marrow (CD45.2+) was transferred into CD45.1+ recipient mice (irradiated with 4.5 Gy). Twenty days later, PBMCs were stained for GR-1, CD8, CD4, B220, and NK1.1, and analyzed by flow cytometry. Representative dot plots of 1 of 6 mice are shown. (C) Survival of cpCML (—, n = 12) and bcCML ( , n = 18) mice is shown in Kaplan-Meier plots. Statistical comparison was performed with the log-rank test. (D) Phenotypical characterization of GFP+ leukemic cells in cpCML and bcCML mice. Cells were gated on granulocytes in control C57BL/6 mice and on transduced granulocyte populations in cpCML and bcCML mice. One representative staining of 5 is shown.
, n = 18) mice is shown in Kaplan-Meier plots. Statistical comparison was performed with the log-rank test. (D) Phenotypical characterization of GFP+ leukemic cells in cpCML and bcCML mice. Cells were gated on granulocytes in control C57BL/6 mice and on transduced granulocyte populations in cpCML and bcCML mice. One representative staining of 5 is shown.
In previous studies, recipient mice were lethally irradiated.2,29,30 This completely prevented a leukemia-specific immune response by the host and made it impossible to study the immunosurveillance of CML. In this study, we titrated the irradiation dose and the number of retroviral particles NUP98/HOXA9 and BCR/ABL needed to generate cpCML and bcCML originating from donor bone marrow cells in a host with a largely intact immune system. To address whether immune cells for the control of the leukemia originate from recipient or donor bone marrow, transduced bone marrow cells were transferred into CD45.1+ recipient mice. CD8+ and CD4+ T cells, B cells, and NK cells were found to originate from donor bone marrow (CD45.1−) when recipient mice were irradiated with 9.5 Gy (data not shown). In contrast, when recipient mice were irradiated with 4.5 Gy (Figure 1B) or 6.5 Gy (data not shown), immune cells were found to originate from the recipient mouse, whereas the leukemia cells (GR-1+) originated from the cotransduced donor bone marrow.
Transduction of bone marrow with BCR/ABL retroviral particles alone (105 U) or cotransduction with NUP98/HOXA9 (106 U) regularly induced cpCML or bcCML, respectively. In contrast, transduction with NUP98/HOXA9 retroviral particles alone did not induce leukemia up to 3 months after transplantation. bcCML rapidly progressed and animals died within 3 weeks, whereas cpCML mice lived up to 6 weeks (Figure 1C). Because the retroviral vectors BCR/ABL in cpCML and NUP98/HOXA9 in bcCML coexpressed the fluorescent dye GFP, the development of CML-like disease was followed with flow cytometry of peripheral blood (Figure 1D). The majority of granulocytes were GFP positive, indicating that these cells expressed BCR/ABL in cpCML or NUP98/HOXA9 in bcCML. In addition, the granulocyte population in the blood of cpCML and bcCML mice was increased to 9 × 107 granulocytes/mL blood compared with the C57BL/6 control (< 2 × 106 granulocytes/mL blood; data not shown). Immature myeloid blast cells (MAC-1+, GR-1+, c-kit+) were detectable in bcCML. In contrast, all transduced GFP+ leukemic cells in cpCML phenotypically resembled mature granulocytes (MAC-1+, GR-1+, c-kit−) comparable with untransduced granulocytes in naive C57BL/6 mice (Figure 1D). Therefore, by reducing the dose of irradiation given to recipient mice (4.5 or 6.5 Gy) and by administering defined amounts of retroviral particles, it was possible to reproducibly induce cpCML and bcCML in recipient mice with a largely intact immune system.
Leukemia-specific CTLs are not detectable in CML disease progression
To analyze antigen-specific immune responses in vivo, bone marrow from H8 transgenic mice was transduced with the retroviral particles. In this experimental setup, all leukemia cells expressed the immunodominant CTL epitope gp33 of LCMV on MHC class I molecules as a model leukemia antigen (H8-CML mice). The leukemia-specific CTL response was analyzed in H8-CML mice at different time points after bone marrow transplantation. Transduction of bone marrow with 0.01 × 106 U of BCR/ABL or cotransduction with 0.01 × 106 U of BCR/ABL and 0.1 × 106 U of NUP98/HOXA9 retroviral particles induced a transient increase of GFP-positive granulocytes in recipient mice, but by day 30 all leukemia cells were eliminated (data not shown). Tetramer staining revealed that recipient mice that eliminated CML developed a LCMV-gp33–specific CTL response (Figure 2A). In contrast, if bone marrow cells were transduced with 0.1 × 106 U of BCR/ABL alone or together with 106 U of NUP98/HOX-A9 retroviral particles, H8-cpCML and H8-bcCML persisted and progressed to the death of the animals (disease kinetics comparable with Figure 1C). In H8-cpCML and H8-bcCML mice, LCMV-gp33–specific CTLs were not detectable by tetramer staining (Figure 2A) or by intracellular staining for IFN-γ or TNF-α after in vitro restimulation with gp33 (Figure 2B). In addition, CTLs isolated from cpCML and bcCML mice were not able to lyse gp33 peptide-pulsed target cells ex vivo after 5 days of in vitro restimulation (Figure 2C). Naive C57BL/6 mice and LCMV-immune mice that have been infected 8 weeks previously with 200 pfu of LCMV-WE were used as controls (Figure 2A-C).
Presence of leukemia-specific CTLs in CML. Bone marrow from H8 mice was transduced with BCR/ABL-GFP or cotransduced with NUP98/HOXA9-GFP and BCR/ABL-neo and injected into 4.5 Gy irradiated C57BL/6 mice. (A-B) The frequency of gp33-specific CTLs was analyzed by tetramer staining (A) and intracellular IFN-γ and TNF-α staining (B) in the blood of mice that eliminated H8-cpCML and H8-bcCML, in mice with progressive H8-cpCML and H8-bcCML, in naive C57BL/6, and in LCMV-immune mice. (C) Splenocytes of H8-cpCML and H8-bcCML mice were isolated 20 days after BMT and analyzed in a standard 51Cr-release assay (gp33-pulsed target cells [filled symbols]; nonpulsed target cells [open symbols]). CTL activity is given as mean ± SEM of 4 CML mice (●), naive C57BL/6 (♦), and LCMV-immune mice (■). (D) H8-cpCML and H8-bcCML mice and C57BL/6 controls were infected with 200 pfu of LCMV intravenously. Eight days after LCMV infection, the frequency of gp33-specific and np396-specific CTLs was analyzed in the blood and spleen by tetramer staining. One representative experiment of 2 is shown.
Presence of leukemia-specific CTLs in CML. Bone marrow from H8 mice was transduced with BCR/ABL-GFP or cotransduced with NUP98/HOXA9-GFP and BCR/ABL-neo and injected into 4.5 Gy irradiated C57BL/6 mice. (A-B) The frequency of gp33-specific CTLs was analyzed by tetramer staining (A) and intracellular IFN-γ and TNF-α staining (B) in the blood of mice that eliminated H8-cpCML and H8-bcCML, in mice with progressive H8-cpCML and H8-bcCML, in naive C57BL/6, and in LCMV-immune mice. (C) Splenocytes of H8-cpCML and H8-bcCML mice were isolated 20 days after BMT and analyzed in a standard 51Cr-release assay (gp33-pulsed target cells [filled symbols]; nonpulsed target cells [open symbols]). CTL activity is given as mean ± SEM of 4 CML mice (●), naive C57BL/6 (♦), and LCMV-immune mice (■). (D) H8-cpCML and H8-bcCML mice and C57BL/6 controls were infected with 200 pfu of LCMV intravenously. Eight days after LCMV infection, the frequency of gp33-specific and np396-specific CTLs was analyzed in the blood and spleen by tetramer staining. One representative experiment of 2 is shown.
To test whether gp33-specific CTLs can be activated in mice with CML-like disease, H8-cpCML, H8-bcCML mice, and control C57BL/6 mice were infected with 200 pfu of LCMV. Eight days later, the frequencies of CML-specific CTLs (gp33) and of CTLs specific for an unrelated viral epitope (np396) were analyzed by tetramer staining. In H8-cpCML and H8-bcCML mice, np396-specific CTLs, but not gp33-specific CTLs, were detectable (Figure 2D). In contrast, naive C57BL/6 mice infected with LCMV mounted gp33- and np396-specific CTL responses. These results demonstrate that (a) leukemia cells were able to induce a specific CTL response, and (b) in the presence of CML-like disease with high leukocytes counts, leukemia antigen-specific CTLs were not present.
CML-specific CTLs have residual effector functions
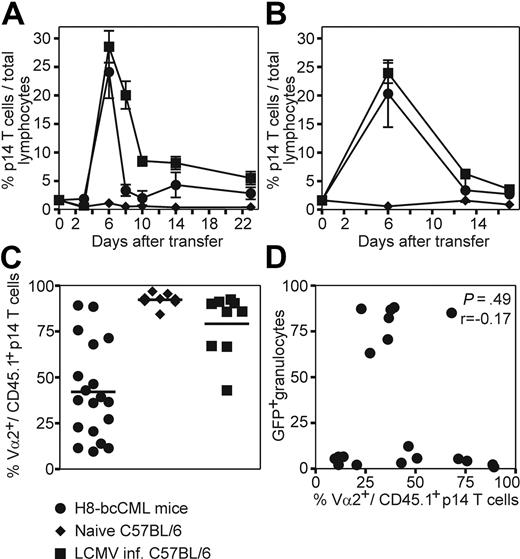
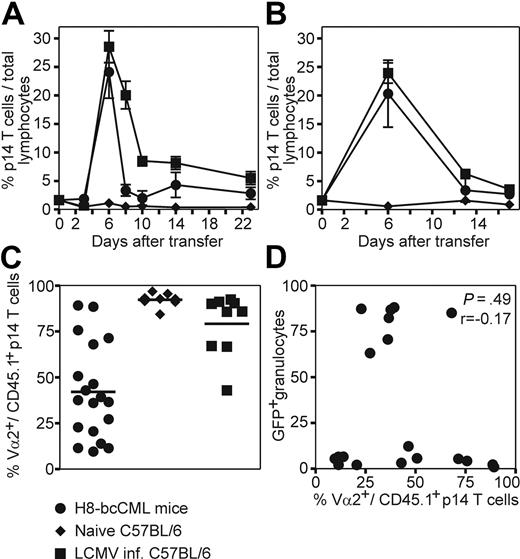
To study CML-specific CTL responses in more detail, purified p14 TCR transgenic CD8+ T cells (CD45.1+CD8+Vα2+) specific for LCMV-gp33 were adoptively transferred to H8-bcCML mice and to naive and LCMV-infected control mice. After transfer to H8-bcCML mice, p14 CD8+ T cells rapidly expanded in blood (Figure 3A) and spleen (Figure 3B). The specific CTLs reached a frequency of 25% of total lymphocytes 6 days after transfer. Thereafter, their number rapidly declined, reaching a stable frequency of approximately 2.8% (± 1.9%), which is slightly above the frequency of transferred p14 CTLs in naive C57BL/6 control mice. In addition, the leukemia-specific TCR chain Vα2 was down-regulated on CML-specific CTLs in blood (mean Vα2+ p14 CD8+ T cells: CML, 42.1% ± 25.8%; naive C57BL/6, 92.3% ± 3.5%; LCMV-infected, 79.2% ± 16.8%; Figure 3C) and spleen (data not shown), indicating TCR ligation and CTL activation. P14 CD8+ T cells transferred to C57BL/6 control mice did not proliferate or down-regulate the specific TCR, whereas p14 CD8+ T cells transferred to LCMV-infected mice rapidly expanded. Due to the large number of transferred specific CTLs, LCMV-WE was rapidly eliminated and Vα2 expression was already reconstituted in most animals at day 3 after transfer (Figure 3A-C). The down-regulation of the TCR Vα2 did not correlate with the percentage of leukemic GFP+ granulocytes when analyzed in blood (Spearman correlation, P = .49, r = −0.17; Figure 3D). We concluded that naive specific CTLs in H8-bcCML mice were initially activated after TCR ligation and expanded. However, the majority of these CML-specific CTLs were deleted, and only a small fraction persisted long-term.
Adoptive transfer of leukemia-specific TCR transgenic CD8+ T cells to H8-bcCML mice. Purified p14 CD8+ T cells (CD45.1+CD8+Vα2+) were adoptively transferred to H8-bcCML mice (●), to naive C57BL/6 control mice (♦), and to C57BL/6 mice infected with 104 pfu of LCMV (■). (A-B) The expansion of the transferred p14 CD8+ T cells (CD45.1+) was analyzed in the blood (A) and spleen (B) by flow cytometry analysis. (C) The expression of the leukemia-specific TCR Vα2 on the transferred p14 CD8+ T cells (CD45.1+) was analyzed in blood 3 days after transfer by flow cytometry analysis. (D) The expression of the TCR Vα2 was compared with the percentage of leukemic granulocytes (GFP+) in the blood 3 days after transfer. Results are pooled from 5 independent experiments. Statistical comparison was made using the Spearman correlation.
Adoptive transfer of leukemia-specific TCR transgenic CD8+ T cells to H8-bcCML mice. Purified p14 CD8+ T cells (CD45.1+CD8+Vα2+) were adoptively transferred to H8-bcCML mice (●), to naive C57BL/6 control mice (♦), and to C57BL/6 mice infected with 104 pfu of LCMV (■). (A-B) The expansion of the transferred p14 CD8+ T cells (CD45.1+) was analyzed in the blood (A) and spleen (B) by flow cytometry analysis. (C) The expression of the leukemia-specific TCR Vα2 on the transferred p14 CD8+ T cells (CD45.1+) was analyzed in blood 3 days after transfer by flow cytometry analysis. (D) The expression of the TCR Vα2 was compared with the percentage of leukemic granulocytes (GFP+) in the blood 3 days after transfer. Results are pooled from 5 independent experiments. Statistical comparison was made using the Spearman correlation.
Memory CD8+ T cells in chronic infections with persisting antigen are either physically deleted or functional impaired, a process termed exhaustion.31 To analyze the degree of functional impairment of specific CD8+ T cells in the presence of H8-bcCML, adoptively transferred p14 CD8+ T cells were isolated from spleens of H8-bcCML mice and of naive and LCMV-infected control mice 6 days after transfer. P14 CTLs isolated from H8-bcCML mice produced hardly any proinflammatory cytokines such as IFN-γ, TNF-α, or IL-2 after in vitro restimulation (Figure 4A). In contrast, p14 CTLs isolated from LCMV-infected mice produced high amounts of IFN-γ and TNF-α (Figure 4A). In addition, p14 CTLs isolated from H8-bcCML mice did not lyse peptide-pulsed target cells directly ex vivo (data not shown), and had only limited lytic capacity after 5 days of in vitro restimulation (Figure 4B). Therefore, the cytokine profile and lytic function of LCMV-specific CD8+ T cells define effector CTLs, whereas the characteristic of CML-specific CD8+ T cells is consistent with partial exhaustion.
Functional characterization of transferred TCR transgenic CD8+ T cells. P14 CD8+ T cells were isolated 5 or 6 days after transfer from the spleens of H8-bcCML mice, C57BL/6 controls, and LCMV-infected mice. (A) The production of IFN-γ, TNF-α, and IL-2 by p14 CD8+ T cells was determined in the supernatant with a cytometric bead array assay. Results are given as mean ± SEM of 3 to 10 samples per group, pooled from 4 independent experiments. (B) Splenocytes were analyzed in a 51Cr-release assay (gp33-pulsed target cells [filled symbols]; nonpulsed target cells [open symbols]). CTL activity is given as mean ± SEM of 4 H8-bcCML mice (●), C57BL/6 controls (♦), and LCMV-infected mice (■). One representative experiment of 2 is shown. (C) FACS analysis of blood of H8-bcCML mice before and 6 days after adoptive transfer of p14 CD8+ T cells and control H8-bcCML mice. One representative staining of 5 independent experiments is shown. (D) Isolated p14 CD8+ T cells were restimulated in vitro. 3H-thymidine incorporation of isolated p14 CD8+ T cells is shown as proliferation index (mean ± SEM of 4-10 mice per group). Results are pooled from 3 independent experiments.
Functional characterization of transferred TCR transgenic CD8+ T cells. P14 CD8+ T cells were isolated 5 or 6 days after transfer from the spleens of H8-bcCML mice, C57BL/6 controls, and LCMV-infected mice. (A) The production of IFN-γ, TNF-α, and IL-2 by p14 CD8+ T cells was determined in the supernatant with a cytometric bead array assay. Results are given as mean ± SEM of 3 to 10 samples per group, pooled from 4 independent experiments. (B) Splenocytes were analyzed in a 51Cr-release assay (gp33-pulsed target cells [filled symbols]; nonpulsed target cells [open symbols]). CTL activity is given as mean ± SEM of 4 H8-bcCML mice (●), C57BL/6 controls (♦), and LCMV-infected mice (■). One representative experiment of 2 is shown. (C) FACS analysis of blood of H8-bcCML mice before and 6 days after adoptive transfer of p14 CD8+ T cells and control H8-bcCML mice. One representative staining of 5 independent experiments is shown. (D) Isolated p14 CD8+ T cells were restimulated in vitro. 3H-thymidine incorporation of isolated p14 CD8+ T cells is shown as proliferation index (mean ± SEM of 4-10 mice per group). Results are pooled from 3 independent experiments.
To analyze whether the lytic function of the transferred CTLs is sufficient to influence the leukemia load, we followed the frequency of GFP+ granulocytes before and 6 days after the transfer of p14 CD8+ T cells. In most animals, the frequency of GFP+ granulocytes was at least transiently reduced (Figure 4C). This indicates that the transferred naive p14 CD8+ T cells acquired effector function in vivo sufficient to transiently reduce the leukemia load.
To assess the proliferative capacity of the isolated p14 CD8+ T cells, 3H-thymidine incorporation was measured after in vitro restimulation. P14 CTLs from C57BL/6 control mice with a naive phenotype proliferated vigorously in contrast to effector p14 CTLs isolated from acute LCMV-infected mice (Figure 4A-B,D). P14 CTLs isolated from H8-bcCML mice neither expanded efficiently in vitro (Figure 4D) nor exhibited effector functions (Figure 4A-B). Thus, CML-specific CTLs are partially exhausted with limited residual effector functions.
Myeloid leukemia cells suppress T-cell proliferation through PD-1/PD-L1 interaction
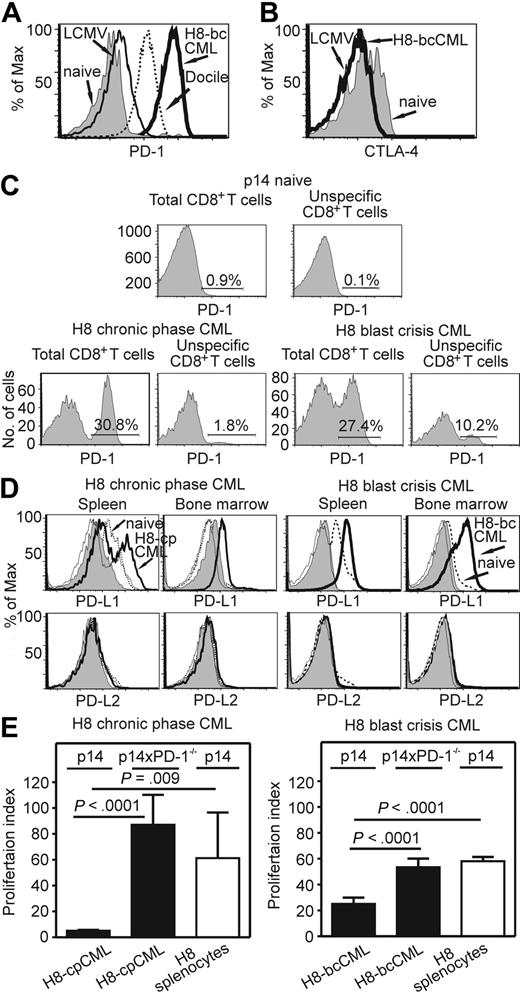
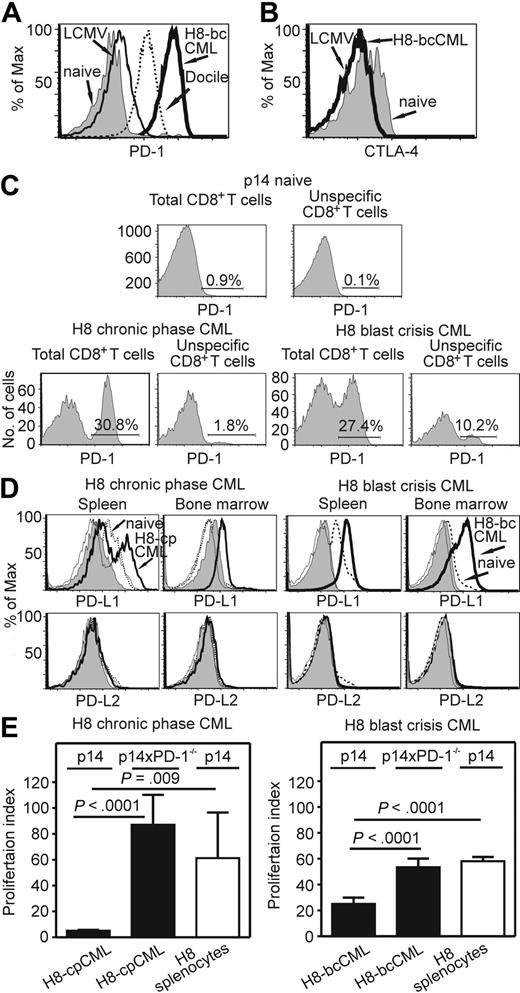
CTLA-4 and PD-1 are 2 well-known coinhibitory molecules that are of importance in the maintenance of peripheral tolerance.32 Therefore, we analyzed their expression on total and on CML-specific CD8+ T cells. In H8-bcCML mice, PD-1 was highly expressed on the transferred CML-specific p14 CTLs in the blood (data not shown) and spleen (Figure 5A). In contrast, PD-1 was not expressed on transferred p14 CD8+ T cells in C57BL/6 control mice, and was only expressed at low levels on p14 CTLs in acutely LCMV-WE–infected mice. However, as shown previously,14 PD-1 expression was high on p14 CTLs in mice chronically infected with LCMV Docile. The expression of PD-1 on transferred p14 CTLs in H8-bcCML mice was substantially higher than in mice with persistent LCMV infection (Figure 5A). Surprisingly, a large fraction of total CD8+ T cells expressed PD-1 (93.5% ± 1.8% in H8-CML mice; data not shown). In contrast, CTLA-4 was not expressed on transferred CML-specific CTLs in blood (data not shown) and spleen (Figure 5B) in H8-bcCML mice.
PD-1 and PD-L expression in H8-cpCML and H8-bcCML mice. (A) PD-1 expression on purified p14 CD8+ T cells (CD45.1+CD8+Va2+) isolated from the spleen 5 days after transfer. One representative histogram from 3 to 5 mice per group is shown. One representative experiment of 2 is shown. (B) CTLA-4 expression on purified p14 CD8+ T cells (CD45.1+CD8+Vα2+) isolated from the spleen 7 days after transfer. (C) cpCML and bcCML were generated from C57BL/6 donors in p14 recipient mice. PD-1 expression on total CD8+ T cells and on unspecific CD8+ T cells (Vα2+) in the spleen was analyzed 20 days after bone marrow transplantation. One representative FACS plot of 3 is shown. (D) PD-L1 and PD-L2 expression in the spleen and bone marrow of H8-cpCML, H8-bcCML, and naive C57BL/6 mice. Cells were gated on GFP-positive granulocytes (CML) and granulocytes (naive C57BL/6) in the forward/side scatter. Shaded area, isotype control H8-cpCML or H8-bcCML; thin line, isotype control naive C57BL/6. One representative experiment of 3 is shown. (E) Purified p14 CD8+ T cells or p14 × PD-1−/− CD8+ T cells were restimulated in vitro with granulocytes from H8-cpCML, H8-bcCML, or H8 mice. 3H-thymidine incorporation is shown as proliferation index (mean ± SEM of 3 mice per group).
PD-1 and PD-L expression in H8-cpCML and H8-bcCML mice. (A) PD-1 expression on purified p14 CD8+ T cells (CD45.1+CD8+Va2+) isolated from the spleen 5 days after transfer. One representative histogram from 3 to 5 mice per group is shown. One representative experiment of 2 is shown. (B) CTLA-4 expression on purified p14 CD8+ T cells (CD45.1+CD8+Vα2+) isolated from the spleen 7 days after transfer. (C) cpCML and bcCML were generated from C57BL/6 donors in p14 recipient mice. PD-1 expression on total CD8+ T cells and on unspecific CD8+ T cells (Vα2+) in the spleen was analyzed 20 days after bone marrow transplantation. One representative FACS plot of 3 is shown. (D) PD-L1 and PD-L2 expression in the spleen and bone marrow of H8-cpCML, H8-bcCML, and naive C57BL/6 mice. Cells were gated on GFP-positive granulocytes (CML) and granulocytes (naive C57BL/6) in the forward/side scatter. Shaded area, isotype control H8-cpCML or H8-bcCML; thin line, isotype control naive C57BL/6. One representative experiment of 3 is shown. (E) Purified p14 CD8+ T cells or p14 × PD-1−/− CD8+ T cells were restimulated in vitro with granulocytes from H8-cpCML, H8-bcCML, or H8 mice. 3H-thymidine incorporation is shown as proliferation index (mean ± SEM of 3 mice per group).
The observation of high rates of PD-1 expression on total CD8+ T cells prompted us to analyze the expression of PD-1 on non–leukemia-specific CTLs. Therefore, cpCML and bcCML from C57BL/6 bone marrow donors were induced in p14 recipient mice. In this experimental setup, 60% of CD8+ T cells express the TCR Vα2 and are non–leukemia specific. PD-1 was expressed on 3.4% (± 2.3%) of p14 CD8+ T cells in cpCML and on 7.9% (± 3.2%) in bcCML (Figure 5C). Therefore, PD-1 expression in cpCML and in bcCML is not restricted to leukemia-specific CTLs. However, the majority of leukemia-specific CTLs expressed PD-1 (Figure 5A).
We next investigated the expression of both PD-Ls on granulocytes in various organs, such as bone marrow, spleen, inguinal and mesenterial lymph nodes, thymus, and peripheral blood. PD-L1 was up-regulated on leukemic (GFP+) granulocytes in H8-cpCML and H8-bcCML in spleen (H8-cpCML, 38%; H8-bcCML, 100%) and bone marrow compared with the granulocyte population of naive C57BL/6 mice (Figure 5D). In contrast, PD-L2 was not expressed on leukemic cells in H8-cpCML and H8-bcCML mice (Figure 5D).
To functionally analyze the role of PD-L1 expressed on leukemic cells, H8-cpCML and H8-bcCML cells were used as stimulators in a 3H-thymidine incorporation assay with either p14 or p14 × PD-1−/− CD8+ T cells as responders. H8-cpCML and H8-bcCML cells stimulated only a limited expansion of p14 CD8+ T cells, whereas p14 CD8+ T cells proliferated efficiently when stimulated with splenocytes from naive H8 mice. In contrast, p14 × PD-1−/− CD8+ T cells efficiently expanded when stimulated with H8-cpCML and H8-bcCML cells (Figure 5E).
In summary, PD-1 is expressed on CML-specific CTLs and PD-L1 on leukemic cells with higher expression during bcCML than in cpCML.
Prolonged survival of bcCML mice in the absence of PD-1 signaling
Our data to date using the LCMV-gp33 as model leukemia antigen suggested that PD-1 may inhibit leukemia-specific CTLs and lead to disease progression. LCMV-gp33 is a foreign antigen that is expressed in the H8 transgenic mice under a relatively strong promoter. Therefore, the model leukemia antigen used has many similarities to the junction peptides derived of BCR/ABL, which are similarly expressed under a strong promoter and are novel antigens without preexisting self-tolerance. Nevertheless, the H8-CML model might overestimate the contribution of CD8+ T cells and the role of PD-1 in CML-specific tolerance induction. To test the physiologic role of PD-1 in CML control, bcCML was induced in PD-1–deficient mice and C57BL/6 control mice using transduced bone marrow of C57BL/6 mice (Figure 6A). In this experimental setup, CML cells did not express a model antigen, but endogenous leukemia antigens. C57BL/6 control mice died approximately 23 days after bone marrow transplantation. In contrast, PD-1–deficient mice with bcCML survived significantly longer (P = .004). Therefore, PD-1/PD-L1 signaling impairs the immune control of CML also in a physiologic setting without a model leukemia antigen.
Survival of bcCML mice in the absence of PD-1. (A) bcCML was induced in PD-1–deficient (—, n = 6) and naive C57BL/6 mice ( , n = 6) by transferring retroviral transduced C57BL/6 bone marrow cells. Survival of recipient mice is shown in a Kaplan-Meier plot. One representative experiment of 3 is shown. (B) bcCML was induced by transferring 105 (left) or 104 (right, lower leukemia load) retroviral transduced C57BL/6 bone marrow cells into irradiated C57BL/6 mice. Survival of recipient mice is shown in a Kaplan-Meier plot for αPD-L1–treated mice (—, n = 6 [left], n = 5 [right]) and ratIgG-treated mice (
, n = 6) by transferring retroviral transduced C57BL/6 bone marrow cells. Survival of recipient mice is shown in a Kaplan-Meier plot. One representative experiment of 3 is shown. (B) bcCML was induced by transferring 105 (left) or 104 (right, lower leukemia load) retroviral transduced C57BL/6 bone marrow cells into irradiated C57BL/6 mice. Survival of recipient mice is shown in a Kaplan-Meier plot for αPD-L1–treated mice (—, n = 6 [left], n = 5 [right]) and ratIgG-treated mice ( , n = 6 [left], n = 3 [right]). (C) bcCML was induced with 104 transduced bone marrow cells in irradiated recipient mice (lower leukemia load). Survival of bcCML mice treated with αCD8 (—, n = 5) or left untreated (
, n = 6 [left], n = 3 [right]). (C) bcCML was induced with 104 transduced bone marrow cells in irradiated recipient mice (lower leukemia load). Survival of bcCML mice treated with αCD8 (—, n = 5) or left untreated ( , n = 7) is shown in a Kaplan-Meier plot. Statistical comparison was performed with the log-rank test.
, n = 7) is shown in a Kaplan-Meier plot. Statistical comparison was performed with the log-rank test.
Survival of bcCML mice in the absence of PD-1. (A) bcCML was induced in PD-1–deficient (—, n = 6) and naive C57BL/6 mice ( , n = 6) by transferring retroviral transduced C57BL/6 bone marrow cells. Survival of recipient mice is shown in a Kaplan-Meier plot. One representative experiment of 3 is shown. (B) bcCML was induced by transferring 105 (left) or 104 (right, lower leukemia load) retroviral transduced C57BL/6 bone marrow cells into irradiated C57BL/6 mice. Survival of recipient mice is shown in a Kaplan-Meier plot for αPD-L1–treated mice (—, n = 6 [left], n = 5 [right]) and ratIgG-treated mice (
, n = 6) by transferring retroviral transduced C57BL/6 bone marrow cells. Survival of recipient mice is shown in a Kaplan-Meier plot. One representative experiment of 3 is shown. (B) bcCML was induced by transferring 105 (left) or 104 (right, lower leukemia load) retroviral transduced C57BL/6 bone marrow cells into irradiated C57BL/6 mice. Survival of recipient mice is shown in a Kaplan-Meier plot for αPD-L1–treated mice (—, n = 6 [left], n = 5 [right]) and ratIgG-treated mice ( , n = 6 [left], n = 3 [right]). (C) bcCML was induced with 104 transduced bone marrow cells in irradiated recipient mice (lower leukemia load). Survival of bcCML mice treated with αCD8 (—, n = 5) or left untreated (
, n = 6 [left], n = 3 [right]). (C) bcCML was induced with 104 transduced bone marrow cells in irradiated recipient mice (lower leukemia load). Survival of bcCML mice treated with αCD8 (—, n = 5) or left untreated ( , n = 7) is shown in a Kaplan-Meier plot. Statistical comparison was performed with the log-rank test.
, n = 7) is shown in a Kaplan-Meier plot. Statistical comparison was performed with the log-rank test.
To extend our observation to a therapeutic model, we analyzed whether blocking the PD-1/PD-L1 interaction by repetitive application of blocking αPD-L1 antibody would prolong survival. αPD-L1–treated bcCML mice survived longer than bcCML mice that have been treated with control antibody (Figure 6B left panel). The effects of blocking the PD-1/PD-L1 interaction in the reconstitution of a leukemia-specific CTL response require the reconstitution of the host immune response after irradiation. Therefore, the effect of blocking PD-1 signaling by αPD-L1 treatment was more pronounced after transferring a 10-fold lower number of transduced bone marrow cells (lower leukemia load), which results in a slower disease kinetic (Figure 6B right panel).
Blocking PD-1 improves not only the function of specific CTLs, but also of CD4+ T cells and possibly B cells.33 To study the influence of CD8+ T cells on disease control, bcCML mice were depleted of CD8+ T cells by monoclonal antibody after CML disease onset (> 5% GFP+ cells). bcCML was induced with 104 transduced bone marrow cells to reduce the initial leukemia load. Depletion of CD8+ T cells led to disease progression and death of 80% of the animals within 7 to 14 days (Figure 6C). In contrast, CML progression was slower in control mice, and 71% of the mice survived up to 40 days. These results suggest that CD8+ T cells are crucially involved in the control of CML disease progression. The fact that a minority of the CD8+ T cell–depleted animals still can control CML suggests that other effector mechanisms may contribute to the immunosurveillance of CML.
PD-1 expression on CD8+ T cells of CML patients
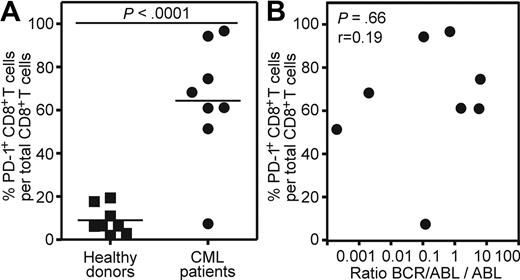
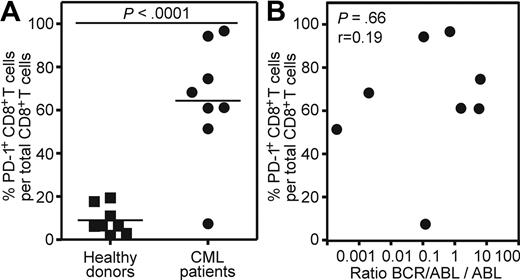
To extend our findings of the mouse model to human CML, we analyzed blood samples of CML patients in the chronic phase of the disease and of healthy donors. PD-1 expression was measured on total peripheral CD8+ T cells. The frequency of CML-specific CD8+ T cells is too low to be measured directly by tetramer staining or intracellular IFN-γ staining without previous in vitro restimulation.34 In addition, in vitro restimulation leads to PD-1 up-regulation.35,36 PD-1 expression could only be assessed reliably on blood samples analyzed directly ex vivo, because the freezing/thawing process reduced PD-1 expression approximately 5- to 10-fold (data not shown). Therefore, blood samples from 8 cpCML patients with detectable levels of BCR/ABL mRNA in quantitative reverse transcription-polymerase chain reaction (RT-PCR; BCR/ABL:ABL > 0.0001) were included in the analysis. Patients were either untreated (1 patient) or treated with tyrosine kinase inhibitors (imatinib, 6 patients; nilotinib, 1 patient). Patients treated with cytotoxic drugs were excluded from the analysis. PD-1 was expressed on an average of 64% of all CD8+ T cells in CML patients (Figure 7A). In contrast, PD-1 expression on CD8+ T cells of healthy donors was low (mean, 9%). None of the patients had clinical or laboratory signs of acute or chronic infection that might interfere with the PD-1 analysis. The level of PD-1 expression did not correlate with the quantitative level of BCR/ABL detected by RT-PCR in blood (P = .66; Figure 7B).
PD-1 is up-regulated on CD8+ T cells of CML patients in the chronic phase of the disease. (A) Percentage of PD-1 expression on total CD8+ T cells from cpCML patients (●, n = 8) compared with healthy donors (■, n = 8). Horizontal bars indicate the mean. Statistical comparison was made using unpaired 2-tailed Student t test. (B) Correlation between PD-1 expression on total CD8+ T cells from cpCML patients and quantitative levels of BCR/ABL:ABL mRNA levels determined by RT-PCR in blood (n = 8). Statistical comparison was made using the Spearman correlation.
PD-1 is up-regulated on CD8+ T cells of CML patients in the chronic phase of the disease. (A) Percentage of PD-1 expression on total CD8+ T cells from cpCML patients (●, n = 8) compared with healthy donors (■, n = 8). Horizontal bars indicate the mean. Statistical comparison was made using unpaired 2-tailed Student t test. (B) Correlation between PD-1 expression on total CD8+ T cells from cpCML patients and quantitative levels of BCR/ABL:ABL mRNA levels determined by RT-PCR in blood (n = 8). Statistical comparison was made using the Spearman correlation.
This descriptive dataset confirms high PD-1 expression on CD8+ T cells of CML patients comparable with our mouse model, and suggests that this inhibitory pathway may also be of importance in the regulation of CML-specific CTLs in humans.
Discussion
Clinical observations after allogeneic stem cell transplantations suggested that an effective CTL response is involved in the control of CML.37,38 This has in recent years been supported by laboratory results indicating the presence of CML-specific T cells in the lymphocyte repertoire of both healthy persons and disease-bearing patients.8 However, the functional role and phenotype of CML-specific CTLs in disease progression are not well defined. Therefore, we analyzed the immunosurveillance of cpCML and bcCML in a murine model. In some experiments, LCMV-gp33 was used as a model leukemia-specific antigen. LCMV-gp33, like the fusion junction peptides of BCR/ABL, is a novel foreign antigen that is not expressed in healthy tissue of the recipient mice. Therefore, self-tolerance mechanisms do not apply. The detection of BCR/ABL-specific CTLs in patients suggests an efficient processing and presentation of BCR/ABL-derived peptides on MHC class I molecules. Besides BCR/ABL, several other proteins such as proteinase-3, Wilms tumor 1 protein, and minor histocompatibility antigens have been described as potential leukemia-specific antigens.39,40
Our results revealed that leukemic cells are able to activate specific CTLs. However, in the presence of CML with high granulocyte counts, specific CTLs became functionally exhausted with impaired ability to expand and produce the proinflammatory cytokines, and with limited lytic activity. However, this limited lytic activity that was only detectable in a TCR-transgenic system and after in vitro restimulation was sufficient to at least transiently reduce the leukemia load.
Exhaustion has been reported for antigen-specific CD8+ T cells during human infections with HIV, hepatitis B virus, and HCV, and in murine models of chronic viral infection.41-45 In the presence of a chronic infection, CD8+ T cells with various levels of T-cell function coexist with viral antigens.31 These experiments documented that CD8+ T-cell exhaustion follows a pattern of progressive loss of function during chronic infection.31 Secondary expansion and the ability to produce IL-2 and TNF-α have been the first indications of partial exhaustion in the presence of a low amount of persisting antigen. In the presence of high antigen amounts, IFN-γ production and cytolytic activity were lost and resulted in complete exhaustion. Thus, our findings in the CML model are strikingly similar to the observations in chronic viral infections. Similarly, the intravenous infusion of high numbers of LCMV-gp33–transfected EL-4 lymphoma cells induced exhaustion of specific CTLs.46 However, due to the rapid death of the animals, a detailed analysis of the CTL function was not possible in this lymphoma model.
Until recently, the molecular basis for functional T-cell inactivation during chronic infections remained unknown. Barber et al have shown that PD-1 is up-regulated during chronic LCMV infection on exhausted virus-specific CTLs, and that blocking the PD-1/PD-L1 interaction reversed the decline in CTL numbers and function and improved the virus control.14 In addition, several studies have now highlighted a potential role for PD-1 in negatively regulating the CTL response to HIV15 and HCV.16 In the present study, we found an up-regulation of PD-L1 in cpCML and even at higher levels in bcCML. In addition, exhausted leukemia-specific CTLs expressed high levels of PD-1. The high frequency of PD-1 expression on 50% to 90% of total CD8+ T cells in CML mice and in patients was surprising and does not represent only CML-specific CTLs. We therefore analyzed the expression of PD-1 on non–leukemia-specific CTLs in a TCR transgenic system. We documented that a fraction of up to 8% of naive non–leukemia-specific CD8+ T cells expresses PD-1. This is reminiscent with a recent report that documented a higher expression of PD-1 on cytomegalovirus- and Epstein-Barr virus–specific CTLs in adult T-cell leukemia/lymphoma patients than in healthy controls.47 Therefore, PD-1 is not only up-regulated on leukemia-specific CTLs, but also on CTLs specific for unrelated antigens, suggesting a broadly compromised immune system.
The introduction of LCMV-gp33 as model tumor antigen allowed the functional characterization of CML-specific CTLs with the use of TCR transgenic T cells. However, to analyze the role of PD-1/PD-L1 signaling in a physiologic setting, survival of bcCML was analyzed using bone marrow cells from C57BL/6 mice that do not express a model antigen. PD-1–deficient mice and mice treated with αPD-L1 controlled the bcCML more efficiently than control mice, indicating that the improved CTL function resulted in prolonged disease control. Depletion experiments revealed that CD8+ T cells are one important effector mechanism in the control of CML in our model. However, it is likely that other effector mechanisms such as CD4+ T cells and possibly B cells are improved by blocking PD-1 signaling and contribute to disease control. Therefore, similar to chronic infections, the PD-1/PD-L1 interaction is one important mechanism in CML progression that induces specific CTL exhaustion. In addition to functioning as a coinhibitory molecule in the presence of a high leukemia load, PD-L1 was shown to mediate resistance against cytolytic T-cell destruction of dormant leukemia cells in the DA1-3b mouse model of acute myeloid leukemia.48
In accordance with our experiments in murine CML models, PD-L1 expression was documented previously on CML and AML patient samples.49,50 We consequently extended our findings to CML patients. In CML patients, a majority of total CD8+ T cells expressed PD-1, suggesting that the PD-1/PD-L1 interaction may be involved in the induction of CTL inhibition in humans. However, the magnitude of PD-1 expression on CD8+ T cells did not correlate with the quantification level of the BCR/ABL gene product as analyzed in peripheral blood mononuclear cells. This suggests that even after an excellent molecular response is reached (≥ 3 log reduction), for example, by treatment with imatinib, the remaining leukemia cells in bone marrow and in lymphoid organs may escape CTL control by expressing PD-L1. Therefore, blocking the PD-1/PD-L1 interaction is a promising strategy to treat CML at different stages of the disease, and may also be used in combination with tyrosine kinase inhibitors.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank T. Honjo for providing PD-1–deficient mice through RIKEN BioResource Center (BRC) and Jeannie Wurz for critical reading of the manuscript.
This work was supported by grants from the Swiss National Science Foundation (632-66020), Oncosuisse (OCS-01312-02-2003 and OCS-01627-02-2005), and the Bernische Krebsliga.
Authorship
Contribution: S.M. designed the experiments, performed experiments, analyzed data, and wrote the manuscript; C.S. performed experiments; J.S. provided reagents and contributed to overall research design; M.S. provided CML patient samples; and A.F.O. designed experiments, wrote the manuscript, and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adrian F. Ochsenbein, Institute for Medical Oncology, Inselspital, Freiburgstrasse 10, CH-3010 Berne, Switzerland; e-mail: adrian.ochsenbein@insel.ch.

![Figure 2. Presence of leukemia-specific CTLs in CML. Bone marrow from H8 mice was transduced with BCR/ABL-GFP or cotransduced with NUP98/HOXA9-GFP and BCR/ABL-neo and injected into 4.5 Gy irradiated C57BL/6 mice. (A-B) The frequency of gp33-specific CTLs was analyzed by tetramer staining (A) and intracellular IFN-γ and TNF-α staining (B) in the blood of mice that eliminated H8-cpCML and H8-bcCML, in mice with progressive H8-cpCML and H8-bcCML, in naive C57BL/6, and in LCMV-immune mice. (C) Splenocytes of H8-cpCML and H8-bcCML mice were isolated 20 days after BMT and analyzed in a standard 51Cr-release assay (gp33-pulsed target cells [filled symbols]; nonpulsed target cells [open symbols]). CTL activity is given as mean ± SEM of 4 CML mice (●), naive C57BL/6 (♦), and LCMV-immune mice (■). (D) H8-cpCML and H8-bcCML mice and C57BL/6 controls were infected with 200 pfu of LCMV intravenously. Eight days after LCMV infection, the frequency of gp33-specific and np396-specific CTLs was analyzed in the blood and spleen by tetramer staining. One representative experiment of 2 is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/8/10.1182_blood-2008-09-179697/4/m_zh89990938670002.jpeg?Expires=1770519430&Signature=S4o1JRA6MvGi2EjxKNT6YfIgdkCZ0irx6DuvZ7RCcWrFyYc9MHCGh52Pg2E3IJyH-AQZPmydC715KDaZaKrzmTnfSrb0UjDmpBBNaDtET41yTYsTBgi~tNZ5tLLIC877dsuTeptbmPTU0CGfzLOMwJbyO4WT4R4XUan5WMBNJ8xvZ19odyheUStnM6jF9hPMfOCH8KGV7rkfRDr67795ka7LKdTzmaEddmV7oGJIXuEVdHRy7UjCW-8kShff7-o9obcWrESdOiYXbbR5A0IA-1NBhtRzYhDQ1lO6EEKLoq3cMvIjF99Eq-401iQh2jKPfsSB3XQ6OpAzDAEn3k2cXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Functional characterization of transferred TCR transgenic CD8+ T cells. P14 CD8+ T cells were isolated 5 or 6 days after transfer from the spleens of H8-bcCML mice, C57BL/6 controls, and LCMV-infected mice. (A) The production of IFN-γ, TNF-α, and IL-2 by p14 CD8+ T cells was determined in the supernatant with a cytometric bead array assay. Results are given as mean ± SEM of 3 to 10 samples per group, pooled from 4 independent experiments. (B) Splenocytes were analyzed in a 51Cr-release assay (gp33-pulsed target cells [filled symbols]; nonpulsed target cells [open symbols]). CTL activity is given as mean ± SEM of 4 H8-bcCML mice (●), C57BL/6 controls (♦), and LCMV-infected mice (■). One representative experiment of 2 is shown. (C) FACS analysis of blood of H8-bcCML mice before and 6 days after adoptive transfer of p14 CD8+ T cells and control H8-bcCML mice. One representative staining of 5 independent experiments is shown. (D) Isolated p14 CD8+ T cells were restimulated in vitro. 3H-thymidine incorporation of isolated p14 CD8+ T cells is shown as proliferation index (mean ± SEM of 4-10 mice per group). Results are pooled from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/8/10.1182_blood-2008-09-179697/4/m_zh89990938670004.jpeg?Expires=1770519430&Signature=r54VYKANRm87RLZRzw79b6xaTVM0s2vb4FMZPoL7nUhnGeB8Y8KE74bIPg~rieVvYV8KLoOyWhQ9XZtsPGxb6R7XDXPwCgyb~eLBp4INFg1BegHY92h2Le-XYJ540uuRsn5xt9phYUmEr~in346UgY5FN-Hw0RXFDFe1NSmbcir8bzXfF0xnjNqOclynLzT64YK9bJnkgoDQwrUvYq4aln4xZIskdp-7H~yhhxjBctfBo~4X5VAPo7UDoFIiBqYz0F1V~rtwzyq41qYxz6NCyf4R72NQcHp61BFKtcTdWWTTZjcliWiYPhPg328i16d8Nc7IpbBB3in0asyYyYZxkg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Survival of bcCML mice in the absence of PD-1. (A) bcCML was induced in PD-1–deficient (—, n = 6) and naive C57BL/6 mice (, n = 6) by transferring retroviral transduced C57BL/6 bone marrow cells. Survival of recipient mice is shown in a Kaplan-Meier plot. One representative experiment of 3 is shown. (B) bcCML was induced by transferring 105 (left) or 104 (right, lower leukemia load) retroviral transduced C57BL/6 bone marrow cells into irradiated C57BL/6 mice. Survival of recipient mice is shown in a Kaplan-Meier plot for αPD-L1–treated mice (—, n = 6 [left], n = 5 [right]) and ratIgG-treated mice (, n = 6 [left], n = 3 [right]). (C) bcCML was induced with 104 transduced bone marrow cells in irradiated recipient mice (lower leukemia load). Survival of bcCML mice treated with αCD8 (—, n = 5) or left untreated (, n = 7) is shown in a Kaplan-Meier plot. Statistical comparison was performed with the log-rank test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/8/10.1182_blood-2008-09-179697/4/m_zh89990938670006.jpeg?Expires=1770519430&Signature=xhzVhTMF~9Ekl5q9conzRj2FMgR5CIVSYa0XSXx4ENr12t7Vtx6hT-nyQzDZKsBEeINs6nybWLUI7H5kOeJpP~ll2u-luURpvG~uZXXDdJYey6YqWWMLiBBcS~zRceHGYN11wE6hBUcNK3-CVRdWIjxENG54Lu70B9Df8Tb1fOsyPodVMKmA0aEXW5yEFZwGjJZVRv6dC3YeOCnDC7AfwXpet-pMd1tCM5Ht67cGQuMVwc6Q8cMolXavLgZ48uTiAJatJGaSeEBUQDiuuasYnxBnYR3duYDkZlqmKhMlesUTZe2i9AkGqJWGUCv9QvQ4cBM89~H-OHJNu2S-BBOAtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

 , n = 18) mice is shown in Kaplan-Meier plots. Statistical comparison was performed with the log-rank test. (D) Phenotypical characterization of GFP+ leukemic cells in cpCML and bcCML mice. Cells were gated on granulocytes in control C57BL/6 mice and on transduced granulocyte populations in cpCML and bcCML mice. One representative staining of 5 is shown.
, n = 18) mice is shown in Kaplan-Meier plots. Statistical comparison was performed with the log-rank test. (D) Phenotypical characterization of GFP+ leukemic cells in cpCML and bcCML mice. Cells were gated on granulocytes in control C57BL/6 mice and on transduced granulocyte populations in cpCML and bcCML mice. One representative staining of 5 is shown.![Figure 2. Presence of leukemia-specific CTLs in CML. Bone marrow from H8 mice was transduced with BCR/ABL-GFP or cotransduced with NUP98/HOXA9-GFP and BCR/ABL-neo and injected into 4.5 Gy irradiated C57BL/6 mice. (A-B) The frequency of gp33-specific CTLs was analyzed by tetramer staining (A) and intracellular IFN-γ and TNF-α staining (B) in the blood of mice that eliminated H8-cpCML and H8-bcCML, in mice with progressive H8-cpCML and H8-bcCML, in naive C57BL/6, and in LCMV-immune mice. (C) Splenocytes of H8-cpCML and H8-bcCML mice were isolated 20 days after BMT and analyzed in a standard 51Cr-release assay (gp33-pulsed target cells [filled symbols]; nonpulsed target cells [open symbols]). CTL activity is given as mean ± SEM of 4 CML mice (●), naive C57BL/6 (♦), and LCMV-immune mice (■). (D) H8-cpCML and H8-bcCML mice and C57BL/6 controls were infected with 200 pfu of LCMV intravenously. Eight days after LCMV infection, the frequency of gp33-specific and np396-specific CTLs was analyzed in the blood and spleen by tetramer staining. One representative experiment of 2 is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/8/10.1182_blood-2008-09-179697/4/m_zh89990938670002.jpeg?Expires=1770519431&Signature=nLXFxDwKqpxeMNI9S1mRyhjMlVQskG~UEF1lpP0iyMVMfy82LYdkAX9RHEH~-kBhUYbg3A2Q4f5riGRMjACDfzqqqVskZ~isglmUuigL5amlH~AOkD51BF6YWR7cmzxboIMcOo~-A3gHmkhyohtXT9DXP-NYgCGu9TQCgu8bu3jZwtqDD~VdTruzqYWfwScctB6UL42kFgZiCLaM61AQ2HuBYFttBySzAech7KH9FBR0y~hL21kcX2g2LSBKaHcpoqx2ii4fkRzNljiDb~ZIXQvYpaiW-fM37qBrdxltzJZeLXN~NPpnedy1QI~D42YWqC27n-KFYUufWJRP0MDr7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Functional characterization of transferred TCR transgenic CD8+ T cells. P14 CD8+ T cells were isolated 5 or 6 days after transfer from the spleens of H8-bcCML mice, C57BL/6 controls, and LCMV-infected mice. (A) The production of IFN-γ, TNF-α, and IL-2 by p14 CD8+ T cells was determined in the supernatant with a cytometric bead array assay. Results are given as mean ± SEM of 3 to 10 samples per group, pooled from 4 independent experiments. (B) Splenocytes were analyzed in a 51Cr-release assay (gp33-pulsed target cells [filled symbols]; nonpulsed target cells [open symbols]). CTL activity is given as mean ± SEM of 4 H8-bcCML mice (●), C57BL/6 controls (♦), and LCMV-infected mice (■). One representative experiment of 2 is shown. (C) FACS analysis of blood of H8-bcCML mice before and 6 days after adoptive transfer of p14 CD8+ T cells and control H8-bcCML mice. One representative staining of 5 independent experiments is shown. (D) Isolated p14 CD8+ T cells were restimulated in vitro. 3H-thymidine incorporation of isolated p14 CD8+ T cells is shown as proliferation index (mean ± SEM of 4-10 mice per group). Results are pooled from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/8/10.1182_blood-2008-09-179697/4/m_zh89990938670004.jpeg?Expires=1770519431&Signature=o0-QCHAIjtlcc07ei8skFypKfcqE8jNlj2F~TmunZ6CB2D~qV8toJ3fPxHitOJg9sNLXwZxME-ENEHJUXbVX-c~70IEpygnvu-WHDtKusJzItbAp89N18GqmCDP7nfngBWzFyTGgPonGo3ubA1WxEcjEOhKo7ark~qH7YQZYvqww8SCIy6HNrfKqrg3AGMVCDGjqREFhVjgam-Lq1JbY9F58cX55RaVDgszrwF8QMi5gZX7nwyjIBg8bphdc3i3RZAiZXxeIZKXbUlam8JkEW5fdghgq4DVkdcZKYslzfyQppZ0cwit2~sxb0mTsOF5oWhBo9555cbgEZ7ZeTg2uZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Survival of bcCML mice in the absence of PD-1. (A) bcCML was induced in PD-1–deficient (—, n = 6) and naive C57BL/6 mice (, n = 6) by transferring retroviral transduced C57BL/6 bone marrow cells. Survival of recipient mice is shown in a Kaplan-Meier plot. One representative experiment of 3 is shown. (B) bcCML was induced by transferring 105 (left) or 104 (right, lower leukemia load) retroviral transduced C57BL/6 bone marrow cells into irradiated C57BL/6 mice. Survival of recipient mice is shown in a Kaplan-Meier plot for αPD-L1–treated mice (—, n = 6 [left], n = 5 [right]) and ratIgG-treated mice (, n = 6 [left], n = 3 [right]). (C) bcCML was induced with 104 transduced bone marrow cells in irradiated recipient mice (lower leukemia load). Survival of bcCML mice treated with αCD8 (—, n = 5) or left untreated (, n = 7) is shown in a Kaplan-Meier plot. Statistical comparison was performed with the log-rank test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/8/10.1182_blood-2008-09-179697/4/m_zh89990938670006.jpeg?Expires=1770519431&Signature=YPWwhjGol9OrEvNTEyZ69be9KvN4foMiXsX~prGKbvR8f0UipM48C3qWdDcua-3J7IFKxzTACxpterfFMUoBA-tMsfH7MANGe8KyJRYaCXfAaRdBjjWjGsh8ksCD~uD5hvgJj9bDm1O~Ig1foPAz8YyC~H49I0oHlEn815GYq-iANNigqKeEnjK9hMmfuiYmxcX0EUOXEra4mdWasMRpZWrGCdITajexe297Bd0W3OsOVIyBzl~9y6DxyQ0IF7xq8ADqu0iHO48avVSts9UqQuRtdc7HrkwuriOYVflkPc4PxELNj4ilHIm4Hoa3r10AvGWRDIDM4BORAQOHx8feQA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)