Abstract
von Willebrand factor (VWF) multimeric composition is regulated in plasma by ADAMTS13. VWF deglycosylation enhances proteolysis by ADAMTS13. In this study, the role of terminal sialic acid residues on VWF glycans in mediating proteolysis by ADAMTS13 was investigated. Quantification and distribution of VWF sialylation was examined by sequential digestion and high-performance liquid chromatography analysis. Total sialic acid expression on VWF was 167nmol/mg, of which the majority (80.1%) was present on N-linked glycan chains. Enzymatic desialylation of VWF by α2-3,6,8,9 neuraminidase (Neu-VWF) markedly impaired ADAMTS13-mediated VWF proteolysis. Neu-VWF collagen binding activity was reduced to 50% (± 14%) by ADAMTS13, compared with 11% (± 7%) for untreated VWF. Despite this, Neu-VWF exhibited increased susceptibility to other proteases, including trypsin, chymotrypsin, and cathepsin B. VWF expressing different blood groups exhibit altered ADAMTS13 proteolysis rates (O ≥ B > A ≥ AB). However, ABO blood group regulation of ADAMTS13 proteolysis was ablated on VWF desialylation, as both Neu-O-VWF and Neu-AB-VWF were cleaved by ADAMTS13 at identical rates. These novel data show that sialic acid protects VWF against proteolysis by serine and cysteine proteases but specifically enhances susceptibility to ADAMTS13 proteolysis. Quantitative variation in VWF sialylation therefore represents a key determinant of VWF multimeric composition and, as such, may be of pathophysiologic significance.
Introduction
von Willebrand factor (VWF) is a large multimeric plasma sialoglycoprotein that plays 2 essential roles in normal hemostasis.1 First, it mediates platelet adhesion to exposed subendothelial tissues at sites of vascular injury.2 Second, VWF acts as a carrier molecule for procoagulant factor VIII (FVIII), thereby protecting it from premature proteolytic degradation and clearance.3 In vivo expression of VWF is restricted to endothelial cells (ECs) and megakaryocytes only. VWF synthesized within ECs is either secreted constitutively into the plasma or alternatively stored in specific intracellular organelles known as Weibel-Palade bodies.4 In contrast, VWF synthesized within megakaryocytes is subsequently stored within the α granules of their platelet progeny. Consequently, plasma VWF is derived chiefly from ECs.5
Within ECs, VWF undergoes complex posttranslational modification before secretion.6 In the endoplasmic reticulum, individual VWF monomers are assembled into dimers through the formation of C-terminal disulphide bonds.7 Subsequently, VWF dimers form high-molecular-weight multimers in the Golgi after another round of N-terminal disulphide bond formation.8 The multimeric composition of plasma VWF is a critical determinant of its functional activity, because larger multimers bind both collagen and platelets with significantly higher affinities and are thus more efficient in inducing platelet aggregation under high-shear conditions.9,10 In normal plasma, VWF multimer distribution is regulated by ADAMTS13, a metalloprotease that specifically cleaves VWF at the Tyr1605-Met1606 peptide bond within the A2 domain.11
The posttranslational modification of VWF within ECs and megakaryocytes also includes significant glycosylation, with each VWF monomer containing 12 N-linked and 10 O-linked glycosylation sites.12 In the endoplasmic reticulum, high-mannose–containing oligosaccharide chains are added to the N-linked sites of VWF monomers before dimerization.13 After transport to the Golgi, the N-linked glycans of the dimers then undergo sequential modification to generate complex type carbohydrate structures, and O-linked glycosylation takes place. As a result, N- and O-linked oligosaccharide chains together account for almost 20% of the final monomeric mass.12 Analysis of the N-linked glycans of VWF has shown that monosialylated (56%) or desialylated (30%) bi-antennary and tri-antennary complex-type glycan chains constitute the predominant structures.14-16 Furthermore, terminal sialic acid residues on these N-linked glycans were predominantly α2-6–linked to penultimate galactose residues. In contrast, the O-linked glycans of VWF exist as short mucin-type carbohydrates. Indeed, a single sialylated tetrasaccharide structure (NeuAc(2-3)Gal(β1-3)[NeuAc(α2-6]GalNAc-ol) accounts for greater than 70% of the reduced O-linked glycans.17 Consequently, O-linked sialic acid may be either α2-6– or α2-3–linked.
Recent studies have shown an important role for VWF carbohydrate structures in determining susceptibility to ADAMTS13 proteolysis.18-20 In particular, ABO(H) blood group carbohydrate antigens expressed on the N-linked glycans of VWF have been shown to significantly influence rate of cleavage by ADAMTS13, although the absolute differences in susceptibility between each blood group were minor.18,20 These observations are interesting, given that ABO(H) determinants differ only with respect to a single terminal monosaccharide moiety.21 Furthermore, ABO(H) blood group determinants are only expressed on a minority (13%) of the complex N-linked glycans of plasma VWF.15 In contrast, as discussed earlier, most of the N- and O-linked glycan chains of VWF are actually capped by negatively charged sialic acid residues.15-17 It is well established that expression of sialic acid on many other glycoproteins plays a critical role in protection from both intracellular and extracellular proteolytic degradation.22 Moreover, previous studies have shown that sialylation plays an important role in protecting VWF against a variety of plasma proteases.23 In this study, we sought to determine whether terminal N-linked, O-linked, or both sialic acid residues play a role in determining VWF susceptibility to proteolysis by ADAMTS13. Herein, we report the novel observation that, although sialic acid expression protects VWF against other serine and cysteine proteases, expression of α2-6–linked sialic acid on VWF specifically enhances proteolysis by ADAMTS13 in a dose-dependent manner. We further demonstrate that this α2-6–linked sialic acid is predominantly expressed on the N-linked glycans of VWF and that its effect on regulating ADAMTS13 proteolysis is significantly more marked than that previously ascribed to N-linked ABO(H) blood group antigenic determinants.
Methods
Purification of human plasma–derived VWF
Plasma-derived VWF (pd-VWF) was purified from the VWF-containing concentrate Haemate P (ZLB Behring) as previously described.19 On resuspension, Haemate P was applied to a Sepharose CL-2B XK 16/70 gel filtration column (GE Healthcare Life Sciences) preequilibrated with a buffer containing 20mM Tris-HCl and 100mM NaCl, pH 7.4. VWF-containing fractions were then eluted with the same buffer. Eluate fractions were assessed for VWF content, multimer distribution, and purity with the use of previously described assays.19,20 In addition, pd-VWF was also purified from human blood group–specific (AB, A, and O) plasmas. In brief, group-specific plasma was first cryoprecipitated. The pellet was then resuspended in 20mM Tris-HCl, 10mM sodium citrate (pH 7.4), and passed through a Sepharose CL-2B column as described earlier. Commercial purified human VWF (FVIII:C content, < 0.01 IU/mL) was also purchased from Haematologic Technologies.
In vitro modification of pd-VWF glycan structures
To remove both α2-3– and α2-6–linked sialic acid, purified pd-VWF (final concentration, 65 μg/mL) was incubated with α2-3,6,8,9 neuraminidase (from Arthrobacter ureafaciens; Calbiochem) overnight at 37°C under nonreducing conditions. Neuraminidase (8-1250 mU) was used to generate VWF preparations with various residual sialic acid expressions. To remove specifically α2-3–linked sialic acid, pd-VWF was treated overnight with α2-3 neuraminidase (final concentrations, 10-156 mU; from Streptococcus pneumoniae; Sigma) as before. Overnight treatment with peptide N glycosidase F (PNGase F) from Flavobacterium meningosepticum (concentration, 500 U; New England Biolabs) or O-glycosidase (from S pneumoniae; concentration, 1.5 U; Sigma), again under nondenaturing conditions, was performed to remove N-linked and O-linked glycans, respectively. To remove terminal A antigenic carbohydrate structures, pd-VWF was incubated with 2U of α-N-acetylgalactosaminidase (A-Zyme; from chicken liver; Sigma-Aldrich) overnight at 37°C.
Quantitative analysis of VWF glycan expression
After each glycosidase digestion, quantitative changes in VWF carbohydrate expression were assessed with the use of modified sandwich enzyme-linked immunosorbent assays (ELISAs) as previously described.20,24 In brief, purified VWF diluted in phosphate-buffered saline (PBS) was coated onto MaxiSorb plates (Nalge Nunc International) and incubated for 2 hours. Biotinylated conjugated lectins Sambucus nigra, Maackia amurensis, and Ricinus communis agglutinin (Vector Laboratories) diluted 1/1000 in PBS were then added to the plates and incubated for 1 hour. The plates were washed with PBS containing 0.05% (vol/vol) Tween and then incubated with streptavidin–horseradish peroxidase (R&D Systems), diluted 1/200 in PBS containing 0.05% (vol/vol) Tween, for 1 hour. After a further 3 washes, the plates were incubated with Ortho-phenylenediamine-dihydrochloride (OPD). The reaction was stopped with 1M H2SO4, and the optical density was measured at a wavelength of 490 nm. Dilutions of purified pooled pd-VWF were used to construct standard curves for calibration, and levels of specific glycan expression before and after glycosidase treatment were expressed as a percentage of normal control.
HPLC-based determination of sialic acid expression on pd-VWF
For the preparation of samples selected for high-performance liquid chromatography (HPLC)–based quantitation of sialic acid, pd-VWF was incubated with or without α2-3,6,8,9 neuraminidase, PNGase F, or O-glycosidase as previously described. After glycosidase treatment, liberated glycan chains (ie, N-linked glycans after PNGase F; O-linked glycans after O-glycosidase; and sialic acid after neuraminidase digestion) were separated from VWF by centrifugation (2 minutes at 15 000g) through 50-kDa molecular weight cutoff columns (Vivaspin 500 columns; GE Healthcare Life Sciences). Liberated glycans were collected in the flow-through (FT), whereas undigested oligosaccharides remained attached to VWF (Bound). Sialic acid moieties were liberated from the terminal ends of FT and Bound oligosaccharide chains, respectively, by mild acid hydrolysis (0.1N HCl; 80°C for 60 minutes), and then desiccated in a speed-vacuum drier (Particular Sciences) at room temperature. After reconstitution in 100 μL of HPLC-grade water, this process was repeated to ensure that all traces of HCl were completely removed. Liberated sialic acid (Toronto Chemicals) was derivatized with 100 μL of OPD solution (20 mg/mL prepared in 0.25M sodium bisulfate) at 80°C for 40 minutes. OPD, sodium bisulphate, fetuin, asialofetuin, and all mobile-phase constituents were of analytical grade and purchased from Sigma-Aldrich. All HPLC-based analysis was performed with the use of a Shimadzu Prominence HPLC system (Mason Technology) and a TSK-C18 stationary phase (Supelco), whose temperature was maintained at 10°C always by a column oven. OPD derivatives of sialic acid were eluted with 2 mobile phases; the primary mobile phase (A) consisted of butylamine (0.15%, vol/vol), ortho-phosphoric acid (0.5%, vol/vol), and tetrahydrofuran (1.0%, vol/vol). The secondary mobile phase (B) consisted of a mixture of mobile phase A and acetonitrile (50:50). All assays were performed at a flow rate of 1.0 mL/min with isocratic conditions (A:B = 87:13) as previously described.25 Eluants (10 μL) were monitored for fluorescence at wavelengths of 340 nm and 420 nm for excitation and emission, respectively. All assays were performed in triplicate, and analytical samples were interspersed with water samples.
Proteolysis of glycan-modified VWF by ADAMTS13
Recombinant human ADAMTS13 (cDNA kind gift of Dr R. Montgomery, Medical College of Wisconsin) was stably expressed in HEK293 cells and concentrated by anion-exchange chromatography with the use of a MonoQ 5/50 column (GE Healthcare Life Sciences). Recombinant ADAMTS13 activity was determined by FRETS-VWF73 assay (Peptides International) as previously described.26 To assess the effect of glycan modification on VWF susceptibility to ADAMTS13 cleavage, 3nM rADAMTS13 was preincubated with 10mM BaCl2 for 10 minutes at 37°C. The activated ADAMTS13 was then incubated at 37°C with 6 μg/mL VWF in a reaction mix containing urea (1-2M), 10mM BaCl2, 5mM NaCl, and 15mM Tris-HCl (pH 7.8). At specific time points (0, 30, 60, 90, and 120 minutes), subsamples were removed, and 1mM EDTA was added to stop the cleavage reaction. VWF proteolysis was analyzed with VWF:CB or VWF multimer analysis as previously described.20 Neu-VWF preparations were also treated with chymotrypsin (60 U/mg VWF) and cathepsin B (4 U/mg VWF; both Sigma-Aldrich) at 37°C for 90 minutes, and the extent of cleavage was assessed by residual VWF:CB as before.
Data analysis and statistics
Data were analyzed with the use of the GraphPad Prism program (GraphPad Prism Version 5.0 for Windows; GraphPad Software). Experiments were performed in triplicate. All data are expressed as mean (± SEM). To assess statistical differences, the data were analyzed with the Student unpaired 2-tailed t test. P values less than .05 were considered significant.
Results
Characterization of neuraminidase-treated VWF
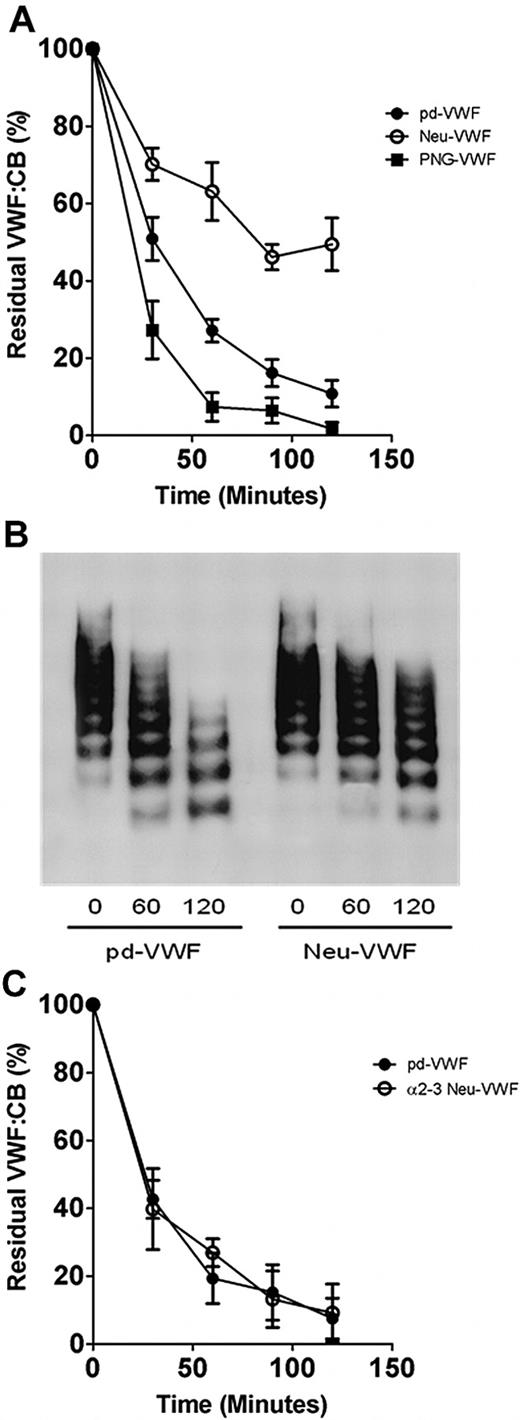
To assess the role of sialic acid in modulating VWF proteolysis by ADAMTS13, VWF was purified from either human blood group–specific plasma or from a commercial VWF/FVIII concentrate (Haemate P). The glycan structures of purified VWF were then modified by using different exoglycosidases. Quantitative sialic acid expression before and after neuraminidase treatment was studied by using lectin plate-binding ELISA (in keeping with previous reports) and subsequently by using HPLC analysis. Digestion with α2-3,6,8,9 neuraminidase resulted in a dose-dependent reduction in VWF sialic acid expression detected by S nigra (specific affinity for α2-6–linked, and to a lesser extent α2-3–linked sialic acid; Figure 1A). On the basis of initial lectin plate–binding ELISA, incubation with 8 mU or 1250 mU of α2-3,6,8,9 neuraminidase resulted in approximately 50% (50% Neu-VWF) and 95% (Neu-VWF) reductions in total S. nigra detectable sialic acid. Digestion with PNGase F (PNG-VWF) resulted in loss of 85% (± 2%) of total Sambucus binding, suggesting that most sialic acid on VWF is expressed on its N-linked carbohydrate chains. Similarly, VWF digestion with α2-3 neuraminidase resulted in a reduction in α2-3–linked sialic acid expression (predominantly O-linked) detected by M amurensis lectin (Figure 1B). Again, with the use of lectin plate-binding ELISA, incubation with 95 mU or 1560 mU of α2-3 neuraminidase resulted in dose-dependent removal of detectable α2-3–linked sialic acid. Interestingly, treatment with PNGase F to remove complex N-linked glycan structures significantly enhanced M amurensis lectin binding (148% ± 10%; P < .05). This unexpected increase in Maackia binding is presumably due to improved lectin accessibility to O-linked sialic acid and highlights the inherent limitations of lectin plate-binding ELISAs for studying VWF. Previous studies reported variable effects of glycosidase digestions on VWF multimer composition, although this variability was subsequently attributed to increased susceptibility to contaminating proteases. In keeping with this conclusion, we found that neither the removal of sialic acid nor the subsequent removal of penultimate galactose influenced VWF multimer composition (Figure 1C) or collagen binding activity (Figure 1D), respectively.
Characterization of neuraminidase-treated VWF. (A) Purified human VWF was incubated overnight with either α2-3,6,8,9 neuraminidase or PNGase F, and residual expression of α2-3,6–linked sialic acid on VWF was analyzed by modified S nigra lectin binding ELISA as described in “Methods.” All experiments were performed in triplicate, and results described represent the means ± SEM (**P < .001 and ***P < .001 in comparison to WT-VWF, respectively). (B) VWF was treated with either α2-3 neuraminidase or PNGase F, and residual expression of α2-3–linked sialic acid was analyzed by modified Maackia amurensis lectin ELISA. After treatment with either PNGase F, α2-3,6,8,9 neuraminidase, β1-3,4 galactosidase, or both neuraminidase and β-galactosidase, the multimeric structure of VWF was assessed by agarose gel electrophoresis in 1.8% gels under nonreducing conditions (C) or collagen binding activity (D).
Characterization of neuraminidase-treated VWF. (A) Purified human VWF was incubated overnight with either α2-3,6,8,9 neuraminidase or PNGase F, and residual expression of α2-3,6–linked sialic acid on VWF was analyzed by modified S nigra lectin binding ELISA as described in “Methods.” All experiments were performed in triplicate, and results described represent the means ± SEM (**P < .001 and ***P < .001 in comparison to WT-VWF, respectively). (B) VWF was treated with either α2-3 neuraminidase or PNGase F, and residual expression of α2-3–linked sialic acid was analyzed by modified Maackia amurensis lectin ELISA. After treatment with either PNGase F, α2-3,6,8,9 neuraminidase, β1-3,4 galactosidase, or both neuraminidase and β-galactosidase, the multimeric structure of VWF was assessed by agarose gel electrophoresis in 1.8% gels under nonreducing conditions (C) or collagen binding activity (D).
Neuraminidase treatment protects VWF from proteolysis by ADAMTS13
To elucidate the significance of N- and O-linked sialic acid expression in modulating VWF proteolysis, glycosidase-treated VWF preparations were incubated with rADAMTS13, and the rate of cleavage was determined by VWF:CB (Figure 2). In keeping with recent reports,19 removal of N-linked glycans (PNG-VWF) significantly enhanced the rate of VWF proteolysis by ADAMTS13 (Figure 2A). Paradoxically, in contrast to the enhanced rate of cleavage observed for PNG-VWF, ADAMTS13 proteolysis of Neu-VWF was significantly inhibited compared with both untreated pd-VWF and PNG-VWF, respectively (Figure 2A). At all time points after 60 minutes, Neu-VWF showed significant increased resistance to ADAMTS13 proteolysis (P < .05). After incubation with ADAMTS13 (3nM for 120 minutes), Neu-VWF collagen binding activity was reduced to 50% (± 14%), compared with 11% (± 7%) for pd-VWF (P < .01). Furthermore, 50% Neu-VWF also showed partial resistance to ADAMTS13 cleavage. The partial resistance was most apparent at 60 minutes, when residual VWF:CB values for pd-VWF, 50% Neu-VWF, and Neu-VWF were 27% (± 6%), 36% (± 7%), and 63% (± 15%), respectively (P = .08; data not shown). This attenuated susceptibility of Neu-VWF to ADAMTS13 cleavage was also confirmed by multimer analysis (Figure 2B). Although recent studies have shown that FVIII enhances the rate of VWF proteolysis by ADAMTS13 under shear stress,27 Neu-VWF resistance to ADAMTS13 cleavage was evident in both the presence (≤ 20nM FVIII) or absence of FVIII:C (data not shown). Despite the ADAMTS13-resistant phenotype of Neu-VWF compared with pd-VWF, digestion of VWF with α2-3 neuraminidase to specifically remove only α2-3– linked sialic acid expression (predominantly O-linked) did not influence ADAMTS13 proteolysis (Figure 2C). Thus, our data suggest a novel role for terminal α2-6–linked sialic acid expression on VWF in modulating proteolysis by ADAMTS13.
Variation in sialic acid expression on VWF and susceptibility to ADAMTS13 proteolysis. (A) To investigate whether sialic acid expression on VWF influences susceptibility to ADAMTS13 proteolysis, wild-type plasma–derived VWF (pd-VWF) α2-3,6,8,9 neuraminidase–treated VWF (Neu-VWF), and PNGase F–treated VWF (PNG-VWF) preparations were incubated with 3nM human rADAMTS13 in the presence of 1.5M urea. Rate of VWF cleavage was assessed by determining the rate of VWF:CB decrease over time. Results are expressed as residual VWF:CB (mean ± SEM). (B) VWF proteolysis was also studied by nonreducing 1% agarose gel electrophoresis. (C) To determine whether α2-3–linked sialic acid on VWF influenced ADAMTS13 proteolysis, pd-VWF and α2-3 neuraminidase–treated VWF (α2-3 Neu-VWF) were incubated with recombinant ADAMTS13 in the presence of 1.5M urea as before. Rate of proteolysis for α2-3Neu-VWF was compared with pd-VWF by determining residual collagen binding activity.
Variation in sialic acid expression on VWF and susceptibility to ADAMTS13 proteolysis. (A) To investigate whether sialic acid expression on VWF influences susceptibility to ADAMTS13 proteolysis, wild-type plasma–derived VWF (pd-VWF) α2-3,6,8,9 neuraminidase–treated VWF (Neu-VWF), and PNGase F–treated VWF (PNG-VWF) preparations were incubated with 3nM human rADAMTS13 in the presence of 1.5M urea. Rate of VWF cleavage was assessed by determining the rate of VWF:CB decrease over time. Results are expressed as residual VWF:CB (mean ± SEM). (B) VWF proteolysis was also studied by nonreducing 1% agarose gel electrophoresis. (C) To determine whether α2-3–linked sialic acid on VWF influenced ADAMTS13 proteolysis, pd-VWF and α2-3 neuraminidase–treated VWF (α2-3 Neu-VWF) were incubated with recombinant ADAMTS13 in the presence of 1.5M urea as before. Rate of proteolysis for α2-3Neu-VWF was compared with pd-VWF by determining residual collagen binding activity.
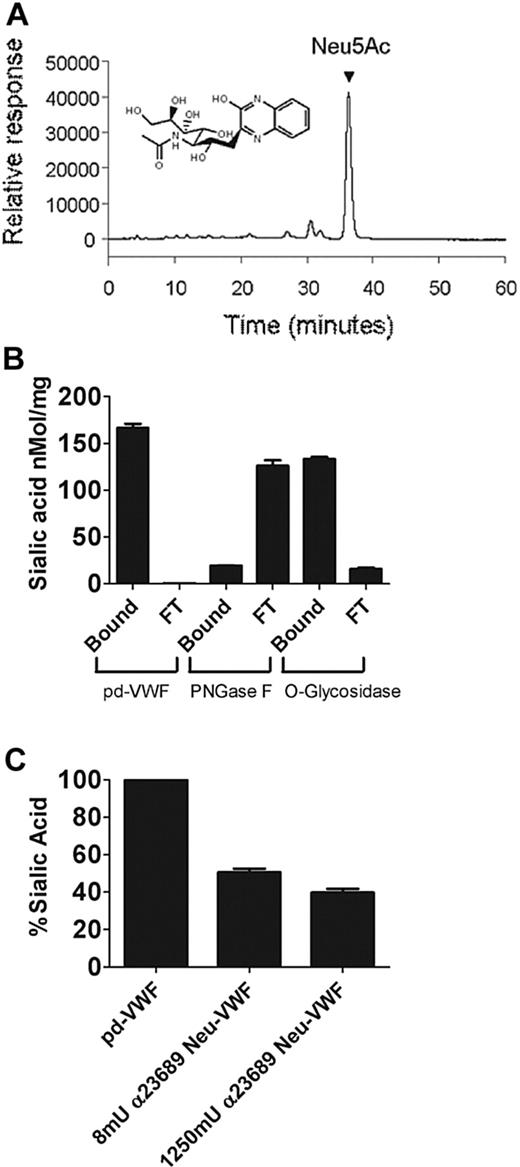
Quantification of VWF sialic acid expression with the use of HPLC-based analysis
Reverse-phase HPLC was used to define quantitative sialic acid expression on plasma VWF. Free sialic acid (Neu5Ac) was labeled with OPD to form a quinoxaline derivative that presented a baseline-resolved peak with retention time of 36.3 minutes (Figure 3A). Analysis of acid-hydrolyzed VWF (referenced with a calibration curve of free sialic acid; R2 = 0.99) showed that total sialic acid expression was 167 nmol/mg VWF (equivalent to 5.2% of total VWF mass), which is in keeping with previous reports.28 To determine the relative amount of sialic acid expressed on the N-linked glycans of VWF, pd-VWF was digested with O-glycosidase, and liberated oligosaccharide chains were separated from the intact protein as described. Sialic acid moieties from the retained protein (eg, N-linked) were subsequently liberated by mild acid hydrolysis, derivatized with OPD and quantified (Figure 3B). This experiment was also performed in reverse by digesting a separate aliquot of protein at the same concentration with PNGase F and, subsequently, separating intact (O-linked) and FT (N-linked) fractions. (Figure 3B lanes 1 and 2). N-linked sialic acid expression accounted for 133.4 nmol/mg VWF (80.1% total VWF sialic acid), whereas O-linked sialic acid represented 19.4 nmol/mg (11.6% total VWF sialic acid expression; Figure 3B). After combined PNGase F and O-glycosidase digestion, 8% of total sialic acid (removed by acid hydrolysis) remained undigested.
Quantification of VWF sialic acid expression with HPLC. (A) Reverse-phase HPLC analysis was used to quantify sialic acid expression on purified pd-VWF. A sample chromatograph of liberated sialic acid from bovine fetuin is represented. Hydrolyzed sialic acid (Neu5Ac) was labeled with OPD, and a baseline-resolved peak representing Neu5Ac, with a retention time of 36.3 minutes, was observed. A structural representation of Neu5Ac derivatized with OPD is shown. (B) To determine relative quantitative sialic acid expression on the N- and O-linked carbohydrate structures of VWF, HPLC analysis of residual VWF-bound sialic acid and free (flow-through, FT) was performed on digestion with either PNGase F or O-glycosidase, respectively. Bound sialic acid refers to sialic acid still intact on the glycans of VWF after digestion with a specific glycosidase; FT refers to liberated sialic acid that has been removed from VWF after glycosidase treatment. (C) Purified human VWF was incubated overnight with various concentrations of α2-3,6,8,9 neuraminidase as before, and residual α2-3,6–linked sialic acid expression on VWF was analyzed by HPLC.
Quantification of VWF sialic acid expression with HPLC. (A) Reverse-phase HPLC analysis was used to quantify sialic acid expression on purified pd-VWF. A sample chromatograph of liberated sialic acid from bovine fetuin is represented. Hydrolyzed sialic acid (Neu5Ac) was labeled with OPD, and a baseline-resolved peak representing Neu5Ac, with a retention time of 36.3 minutes, was observed. A structural representation of Neu5Ac derivatized with OPD is shown. (B) To determine relative quantitative sialic acid expression on the N- and O-linked carbohydrate structures of VWF, HPLC analysis of residual VWF-bound sialic acid and free (flow-through, FT) was performed on digestion with either PNGase F or O-glycosidase, respectively. Bound sialic acid refers to sialic acid still intact on the glycans of VWF after digestion with a specific glycosidase; FT refers to liberated sialic acid that has been removed from VWF after glycosidase treatment. (C) Purified human VWF was incubated overnight with various concentrations of α2-3,6,8,9 neuraminidase as before, and residual α2-3,6–linked sialic acid expression on VWF was analyzed by HPLC.
Lectin plate-binding ELISAs are strongly influenced by steric hindrance and preferentially detect glycans structures exposed on the surface of glycoproteins. These limitations are particularly relevant for VWF, given its multimeric composition and complex N-linked glycan structures. Consequently, quantitative sialic acid expression on VWF was also determined by HPLC after α2-3,6,8,9 neuraminidase digestion. After treatment with 8 mU of neuraminidase, HPLC analysis showed removal of 50% of total sialic acid expression (Figure 3C), which is consistent with results of lectin binding ELISA (Figure 1A). However, after digestion with 1250 mU of α2-3,6,8,9 neuraminidase, HPLC analysis showed that 29.2% of total sialic acid remained, although the lectin ELISA suggested that greater than 90% total sialic acid had been removed. Cumulatively, these HPLC data confirm that plasma VWF is heavily sialylated and show for the first time that 80% of this sialic acid expression is present on the N-linked glycan chains, where it is predominantly α2-6–linked to penultimate galactose residues. Moreover, approximately 30% of total sialic acid is not readily accessible to digestion with neuraminidase and cannot be detected by lectin binding plate assays.
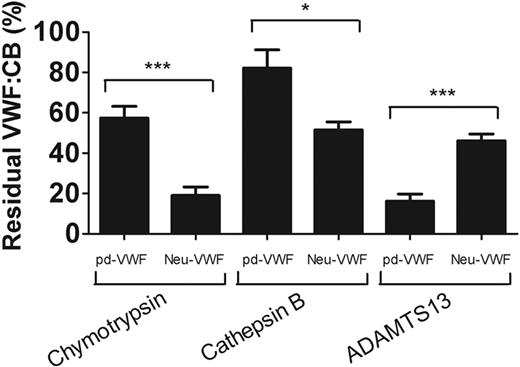
Sialic acid specifically enhances VWF proteolysis by ADAMTS13
Removal of sialic acid from many glycoproteins results in increased proteolysis.22 In view of the inhibitory effect of α2-3,6,8,9 neuraminidase treatment on VWF cleavage by ADAMTS13, we further investigated susceptibility of Neu-VWF to a series of other serine and cysteine proteases. In contrast to its resistance to ADAMTS13, Neu-VWF showed significantly enhanced cleavage by both chymotrypsin (final concentration, 60 IU/mg VWF) and cathepsin B (final concentration, 4 U/mg VWF) after 90 minutes compared with untreated wild-type (WT) VWF (P < .001 and P < .05, respectively; Figure 4). Consequently, although loss of sialic acid expression specifically protects VWF against ADAMTS13 proteolysis, it renders VWF more susceptible to cleavage by other proteases.
Expression of sialic acid specifically enhances VWF proteolysis by ADAMTS13. To establish the effect of desialylation of VWF on susceptibility to nonspecific proteolysis, VWF was treated with α2-3,6,8,9 neuraminidase and subsequently subjected to cleavage by chymotrypsin (60 U/mg VWF), cathepsin B (4 U/mg VWF), and ADAMTS13 (500nM/mg VWF) at 37°C for 90 minutes. Results are shown as residual collagen binding activity at 90 minutes ± SEM for each of the samples. *P < .05. ***P < .001.
Expression of sialic acid specifically enhances VWF proteolysis by ADAMTS13. To establish the effect of desialylation of VWF on susceptibility to nonspecific proteolysis, VWF was treated with α2-3,6,8,9 neuraminidase and subsequently subjected to cleavage by chymotrypsin (60 U/mg VWF), cathepsin B (4 U/mg VWF), and ADAMTS13 (500nM/mg VWF) at 37°C for 90 minutes. Results are shown as residual collagen binding activity at 90 minutes ± SEM for each of the samples. *P < .05. ***P < .001.
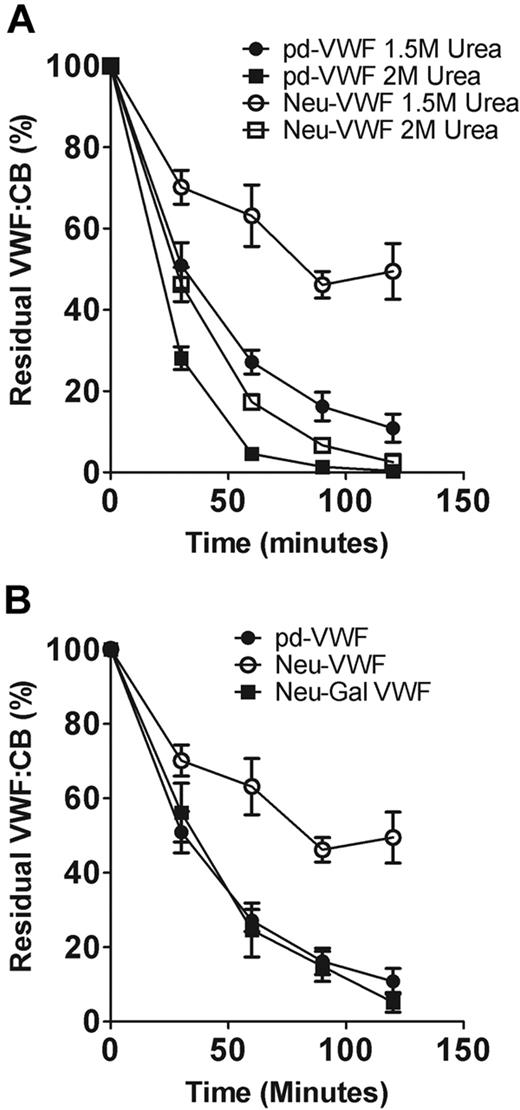
α2-6–linked sialic acid increases VWF proteolysis by ADAMTS13 through a conformational mechanism
Previous studies have shown that changes in glycan structures can influence glycoprotein conformation.29-32 To investigate whether α2-6–linked sialic acid expression enhances VWF proteolysis by altering its tertiary conformation, we studied the effects of α2-3,6,8,9 neuraminidase treatment on ADAMTS13 cleavage at differing urea concentrations. In keeping with previous reports,20 the rate of VWF proteolysis increased significantly as urea concentration was increased from 1M to 2M to progressively denature VWF (data not shown). However, the ability of α2-6–linked sialic acid to enhance VWF cleavage by ADAMTS13 was less evident at higher urea levels (Figure 5A). These data suggest that sialic acid may directly influence the conformation of pd-VWF. Alternatively, sialic acid may rely on normal VWF conformation to be able to affect specific interactions between VWF and ADAMTS13.
α2-6–linked sialic acid enhances ADAMTS13 proteolysis by reducing exposure of subterminal D-galactose. (A) The effects of α2-3,6,8,9 neuraminidase treatment in regulating VWF susceptibility to ADAMTS13 proteolysis were investigated at different urea concentrations. At 2M urea, the rate of VWF proteolysis was significantly increased for both pd-VWF and Neu-VWF compared with 1.5M urea. However, the resistant phenotype of Neu-VWF became less apparent at the higher concentration of urea. (B) Removal of capping α2-6–linked sialic acid from the N-linked glycans of VWF results in exposure of penultimate D-galactose residues. To determine whether the protective effect of α2-3,6,8,9 neuraminidase treatment was mediated through loss of sialic acid or was attributable to the subsequent increased galactose exposure, we studied the effects of combined α2-3,6,8,9 neuraminidase and β-galactosidase digestion. After combined glycosidase digestion, the protective effect of loss of α2-6–linked sialic acid only was no longer observed.
α2-6–linked sialic acid enhances ADAMTS13 proteolysis by reducing exposure of subterminal D-galactose. (A) The effects of α2-3,6,8,9 neuraminidase treatment in regulating VWF susceptibility to ADAMTS13 proteolysis were investigated at different urea concentrations. At 2M urea, the rate of VWF proteolysis was significantly increased for both pd-VWF and Neu-VWF compared with 1.5M urea. However, the resistant phenotype of Neu-VWF became less apparent at the higher concentration of urea. (B) Removal of capping α2-6–linked sialic acid from the N-linked glycans of VWF results in exposure of penultimate D-galactose residues. To determine whether the protective effect of α2-3,6,8,9 neuraminidase treatment was mediated through loss of sialic acid or was attributable to the subsequent increased galactose exposure, we studied the effects of combined α2-3,6,8,9 neuraminidase and β-galactosidase digestion. After combined glycosidase digestion, the protective effect of loss of α2-6–linked sialic acid only was no longer observed.
β-Galactosidase treatment of pd-VWF does not influence susceptibility to ADAMTS13 cleavage (data not shown). However, removal of α2-6–linked sialic acid from the N-linked glycans of VWF results in exposure of additional subterminal D-galactose residues.15,33 To determine whether these exposed galactose residues may play a role in mediating the ADAMTS13-resistant phenotype of Neu-VWF, we further treated Neu-VWF with β-galactosidase. Galactose expression before and after galactosidase treatment were determined with the use of another lectin binding assay by using R communis agglutinin, which specifically binds to β-D-galactosyl residues.34 Galactosidase treatment of Neu-VWF resulted in the removal of 92% (± 3%) exposed galactose (data not shown). Interestingly, on galactose removal, Neu-Gal-VWF no longer showed significant resistance to ADAMTS13 proteolysis and was cleaved at a rate identical to WT-VWF (Figure 5B). Consequently, our findings suggest that the balance of sialic acid to galactose expression at the termini of VWF glycans significantly influences susceptibility of pd-VWF to ADAMTS13 proteolysis.
N-linked sialic acid expression regulates the effect of ABO blood group on VWF proteolysis by ADAMTS13
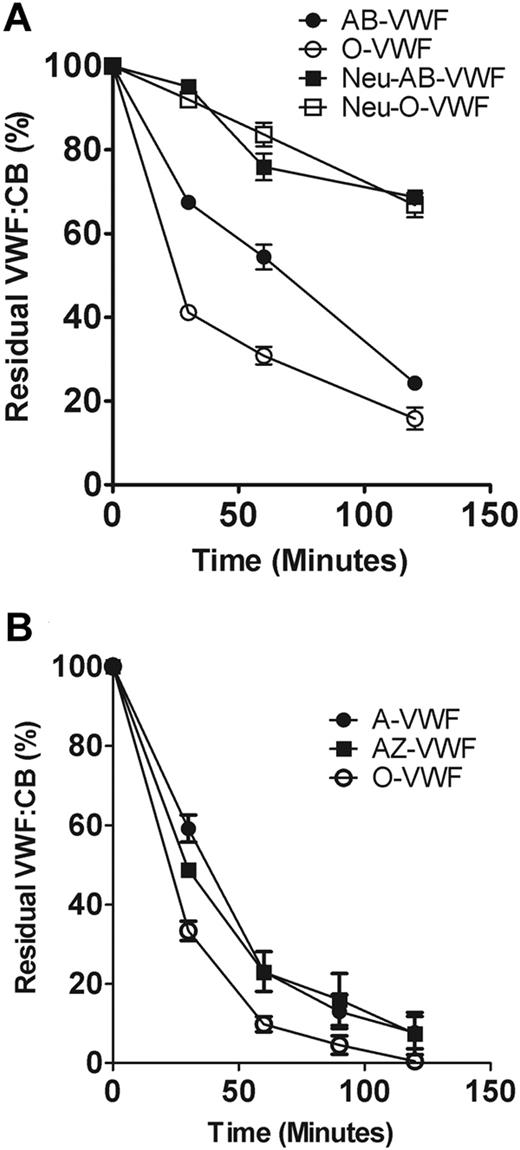
We and others have previously shown that VWF of different ABO blood groups exhibits differential susceptibility to cleavage by ADAMTS13 (O ≥ B > A ≥ AB).18,20 Although this effect was attributed to ABH determinant expression on VWF glycan structures, recent evidence suggests that the presence of ABH antigens can also modulate interactions involving α2-6– linked sialic acid expressed on adjacent glycans.35 Because α2-6–linked sialic acid and ABO(H) determinants are both expressed as terminal antigens on the N-linked glycans of VWF, we investigated the effects of neuraminidase digestion on ADAMTS13 proteolysis of blood group–specific VWF. As expected, group O VWF was cleaved significantly more quickly than group AB (P < .05; Figure 6A). However, after digestion with α2-3,6,8,9 neuraminidase, the ability of ABO blood group to regulate VWF susceptibility to ADAMTS13 proteolysis was no longer apparent, with both Neu-AB-VWF and Neu-O-VWF being cleaved at identical rates. To further define the role of ABH determinants in mediating the effect of ABO blood group, we studied purified group A VWF proteolysis by ADAMTS13 before and after digestion with N-acetyl-galactosaminidase treatment (A-Zyme). Although A-Zyme removed all detectable A antigen expression on VWF (data not shown), the rate of cleavage by ADAMTS13 was not significantly different from untreated group A VWF (Figure 6B). Furthermore, the A-Zyme–treated group A VWF was still cleaved significantly more slowly than group O VWF (P < .05; Figure 6B).
Relative importance of N-linked sialic acid and ABO blood group antigens in determining VWF proteolysis by ADAMTS13. (A) To investigate the effect of sialic acid removal on the cleavage of blood group–specific VWF, purified O or AB VWF was incubated with or without neuraminidase and then subjected to ADAMTS13 proteolysis in the presence of 1.5M urea. Rate of VWF cleavage was assessed by determining the decrease in VWF:CB over time. Results are expressed as residual collagen-binding activity (mean ± SEM). (B) To determine whether loss of the A antigenic carbohydrate structure from blood group A VWF (A-VWF) affects susceptibility to ADAMTS13 proteolysis, A-VWF was digested with A-Zyme (N-acetyl-galactosaminidase), and ADAMTS13 cleavage assay was carried out as before. A-VWF– and A-Zyme–treated VWF (AZ-VWF) were cleaved at the same rate by ADAMTS13, and both were cleaved significantly more slowly than O-VWF (P < .05).
Relative importance of N-linked sialic acid and ABO blood group antigens in determining VWF proteolysis by ADAMTS13. (A) To investigate the effect of sialic acid removal on the cleavage of blood group–specific VWF, purified O or AB VWF was incubated with or without neuraminidase and then subjected to ADAMTS13 proteolysis in the presence of 1.5M urea. Rate of VWF cleavage was assessed by determining the decrease in VWF:CB over time. Results are expressed as residual collagen-binding activity (mean ± SEM). (B) To determine whether loss of the A antigenic carbohydrate structure from blood group A VWF (A-VWF) affects susceptibility to ADAMTS13 proteolysis, A-VWF was digested with A-Zyme (N-acetyl-galactosaminidase), and ADAMTS13 cleavage assay was carried out as before. A-VWF– and A-Zyme–treated VWF (AZ-VWF) were cleaved at the same rate by ADAMTS13, and both were cleaved significantly more slowly than O-VWF (P < .05).
Discussion
To maintain normal physiologic hemostasis, VWF proteolysis by ADAMTS13 requires tight regulation.11 In patients with type 2A von Willebrand disease (VWD), increased proteolysis results in loss of high molecular weight multimer and confers a significant bleeding phenotype. Conversely, in thrombotic thrombocytopenic purpura, inherited or acquired ADAMTS13 deficiency causes accumulation of ultralarge VWF multimers in plasma and thereby contributes to the development of pathologic platelet-rich thrombi in the microvasculature.36 In addition, multivariate analyses suggest that reduced plasma ADAMTS13 levels are associated with increased risk of myocardial infarction.37,38 In this context, understanding the molecular basis through which VWF proteolysis by ADAMTS13 is regulated in vivo is of direct translational importance.
Recent reports have shown that carbohydrate structures expressed on VWF play an important role in regulating ADAMTS13 proteolysis.18-20 In this study, we demonstrate a novel role for sialic acid expression in mediating this effect, such that removal of terminal sialic acid significantly inhibits VWF cleavage by ADAMTS13 in a dose-dependent manner. Although impaired ADAMTS13 proteolysis was apparent after α2-3,6,8,9 neuraminidase treatment, α2-3 neuraminidase had no significant effect. Previous studies that used different methods have consistently shown that most sialic acid present on the N-linked chains of VWF is α2-6–linked to subterminal galactose,14-16 whereas O-linked can be either α2-3– or α2-6–linked.17 Neither α2-8– nor α2-9–linked sialic acid have been identified on the N- or O-linked glycans of human pd-VWF. Consequently, it seems highly probable that loss of α2-6–linked sialic acid (on either N- or O-linked chains) is responsible for mediating the ADAMTS13-resistant phenotype observed after α2-3,6,8,9 neuraminidase treatment. It is well established that sialic acid expression on other circulating glycoproteins influences susceptibility to proteolysis.22 However, to our knowledge, removal of sialic acid has always previously been reported to result in significantly enhanced proteolysis rates. In contrast to this accepted paradigm, we demonstrate that α2-6–linked sialic acid on VWF exerts different effects on susceptibility to proteolysis, depending on the protease involved. Thus, α2-6–linked sialic acid protects VWF against proteolysis by a variety of different plasma serine and cysteine proteases, but it also serves to specifically enhance cleavage by ADAMTS13.
Previous studies have shown that glycan structures can directly mediate protein–protein interactions through either conformational and/or charge-mediated mechanisms.4,29-32,39,40 Subtle variations in glycan structures directly influence glycoprotein conformation, by altering conformational freedom of the local peptide backbone.29,41,42 We observed that the ability of sialic acid to enhance ADAMTS13 cleavage became less marked at progressively higher urea concentrations, suggesting that sialic acid may influence the conformation of VWF and that removal of N-linked sialic acid results in the A2 domain adopting a conformation that is less permissive for ADAMTS13 cleavage. A putative role for α2-6–linked sialic acid in determining VWF conformation is supported by previous studies showing that neuraminidase-treated VWF induced spontaneous platelet aggregation in the absence of ristocetin.43 Interestingly, Federici et al43 also reported that VWF treatment with combined neuraminidase and galactosidase (to remove terminal sialic acid and penultimate galactose residues) significantly attenuated the rate of spontaneous aggregation compared with that after VWF digestion with neuraminidase alone. These data are in keeping with our finding that treatment with galactosidase after α2-3,6,8,9 neuraminidase ablated the ADAMTS13 resistance observed on neuraminidase digestion. Cumulatively, these results support the hypothesis that the N-linked carbohydrate structures of VWF play a critical role in regulating the conformation of both the A1 and A2 domains of VWF. Further studies will be required to determine whether this effect is mediated by specific glycosylation site(s) within these domains.
ABO(H) blood group sugar determinants are expressed on the N-linked carbohydrate chains of human VWF and influence susceptibility to ADAMTS13.18-20 The significant effect of blood group on VWF proteolysis is surprising, given that ABO(H) determinants differ with respect to a single terminal sugar residue and are expressed on only 13% of N-linked glycan chains.15 In contrast, we have demonstrated by HPLC analysis that VWF is heavily sialylated and that the majority (80%) of this sialic acid is expressed on N-linked carbohydrate chains. In addition, although absence of terminal ABO(H) antigens is associated with increased ADAMTS13 cleavage, removal of α2-6–linked sialic acid significantly inhibits ADAMTS13 proteolysis. Consequently, the ability of VWF glycans to modulate ADAMTS13 cleavage is not simply determined by the length of the N-linked glycan chains as previously proposed.19,24 Rather, specific individual monosaccharide residues expressed at the terminal end of the N-linked glycans of VWF directly influence susceptibility to ADAMTS13 proteolysis. Moreover, we have also demonstrated that the ability of ABO blood group determinants to influence the rate of ADAMTS13 proteolysis is lost after α2-3,6,8,9 neuraminidase treatment, suggesting that capping sialic acid expression plays a more important role in regulating VWF susceptibility to ADAMTS13 proteolysis than terminal ABO(H) antigens. Intriguingly, we also observed that, even after removal of A antigenic determinants, blood group A VWF remained significantly more resistant to ADAMTS13 proteolysis than group O VWF. These findings are interesting, given that previous studies reported variation in quantitative sialic acid expression on erythrocyte membrane glycoproteins according to ABO blood group.44,45 Furthermore, an elegant recent study has clearly shown that ABO blood group can also modulate qualitative sialic acid presentation on erythrocyte surfaces.35 Further studies will be required to define whether quantitative and/or qualitative variations in sialic acid expression on VWF are important in mediating the effects of ABO blood group on VWF proteolysis and/or clearance.
On the basis of our data, we propose that VWF synthesized within endothelial cells is sialylated in the Golgi before constitutive or regulated secretion. This sialic acid expression specifically enhances ADAMTS13 cleavage at the endothelial surface after initial secretion, while also protecting VWF against nonspecific proteolysis by other plasma proteases. The sialic acid is progressively lost with VWF aging in the plasma, precipitating eventual clearance by the asialoglycoprotein receptor.
Quantitative sialic acid and ABO(H) expression on the N-linked glycans of plasma VWF has previously been reported to vary widely in the normal population.24,34 In addition, sialyltransferase expression levels vary between different tissues and in different pathologic states.22,46 The physiologic and pathologic significance of quantitative variation in VWF sialic acid expression remain unclear. Unsurprisingly, however, loss of sialic acid significantly reduced the half-life of VWF in animal studies, presumably because of enhanced clearance by the asialoglycoprotein receptor.47 Moreover, murine knockout of a specific sialyltransferase (ST3GalIV) also resulted in VWF deficiency because of increased clearance.34 Given its dual effects in regulating VWF proteolysis (both by ADAMTS13 and other plasma proteases) and VWF clearance, it is also significant that reduced VWF sialic acid expression has been observed in a cohort of patients with type I VWD.34,48 Furthermore, van Schooten et al49 reported an inverse correlation between O-linked sialylated T-antigen expression and plasma VWF levels. It is well established that up to 40% of families with type I VWD fail to show linkage to the VWF gene locus on chromosome 12.50 Further clinical studies will be required to determine the potential role of mutations and/or polymorphisms influencing quantitative α2-6–linked sialic acid expression in this group of patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Science Foundation Ireland President of Ireland Young Researcher Award (06/Y12/0925; J.S.O.), a Health Research Board Ireland Project Award (grant RP/2006/44; J.S.O.), and British Heart Foundation (project grant PG/06/111/21 534) and by the National Institute for Health Research (NIHR) Biomedical Research Center Funding Scheme.
Authorship
Contribution: R.T.M., T.A.J.M., B.B., E.M., V.T., and R.J.S.P. performed experiments; and R.T.M., T.A.J.M., B.B., R.J.S.P., R.O., M.A.L., and J.S.O. designed the research and analyzed the data. All authors were involved in writing and reviewing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James O'Donnell, Haemostasis Research Group, Institute of Molecular Medicine, St James's Hospital, Trinity College Dublin, Dublin 8, Ireland; e-mail jodonne@tcd.ie.
References
Author notes
R.T.M. and T.A.J.M. contributed equally to this study.