In this issue of Blood, 2 groups—Thoennissen et al and Beer et al—using complementary approaches address the genetic basis for LT in MPDs, PV, ET, and PMF.1,2
Leukemic transformation (LT) is the most serious complication of myeloproliferative disorders (MPDs). For polycythemia vera (PV), a minimum spontaneous transformation rate of 1.5% has been established.3 Prospective studies of adequate duration are lacking for essential thrombocytosis (ET) but LT is unlikely to be more frequent. For primary myelofibrosis (PMF), however, an LT rate of 15% has been recorded. Treatment-induced acute leukemia (t-AL) occurs at a much higher rate in PV,3,4 but adequate prospective studies are lacking for ET and PMF. The mechanisms for t-AL are understood, but whether these apply to hydroxyurea as they do for other types of chemotherapy is still controversial. The etiology of spontaneous LT in the MPD is not understood, although it is more common in PMF and PV or ET after evolution to a PMF phenotype. Even more puzzling is JAK2V617F-negative AL arising in a JAK2V617F-positive MPD.
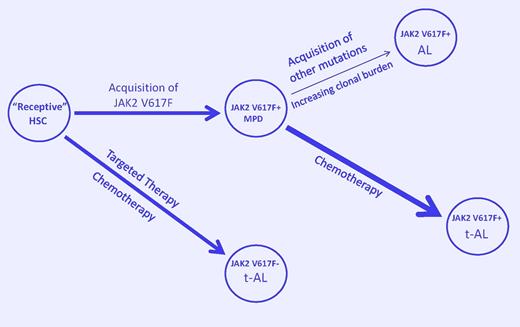
A model to explain JAK2V617F-positive and -negative leukemic transformation in the myeloproliferative disorders. Acquisition of JAK2V617F by a receptive hematopoietic stem cell (HSC) results in a myeloproliferative disorder (MPD). Acquisition of additional mutations due to genetic instability in the expanding clonal cell population favors spontaneous acute leukemia (AL). Exposure to chemotherapeutic agents that damage DNA but prevent its repair (such as HU) favors the emergence of a clonal constituent that is TP53-independent, can bypass normal replication checkpoints, and continues to acquire genetic damage until it transforms (t-AL). Alternatively, inhibition of more robust members of the dominant clone by chemotherapy may allow a less robust and more unstable clone to dominate. Finally, selective suppression of the JAKV617F-positive clone by targeted therapy or chemotherapy could lead to selection of a member of the “receptive” ancestral clone or transform it, leading to JAK2V617F-negative AL or t-AL. (Arrow width indicates event probability; arrow length, the time to transformation.)
A model to explain JAK2V617F-positive and -negative leukemic transformation in the myeloproliferative disorders. Acquisition of JAK2V617F by a receptive hematopoietic stem cell (HSC) results in a myeloproliferative disorder (MPD). Acquisition of additional mutations due to genetic instability in the expanding clonal cell population favors spontaneous acute leukemia (AL). Exposure to chemotherapeutic agents that damage DNA but prevent its repair (such as HU) favors the emergence of a clonal constituent that is TP53-independent, can bypass normal replication checkpoints, and continues to acquire genetic damage until it transforms (t-AL). Alternatively, inhibition of more robust members of the dominant clone by chemotherapy may allow a less robust and more unstable clone to dominate. Finally, selective suppression of the JAKV617F-positive clone by targeted therapy or chemotherapy could lead to selection of a member of the “receptive” ancestral clone or transform it, leading to JAK2V617F-negative AL or t-AL. (Arrow width indicates event probability; arrow length, the time to transformation.)
To examine these issues, Thoennissen et al used high-density single-nucleotide polymorphism (SNP) arrays to study genomic changes in 148 MPD patients, in chronic phase (CP) and LT. They found a 2- to 3-fold greater incidence of abnormalities with LT. ET patients had fewer abnormalities during CP but matched their MPD counterparts upon transformation. Commonly altered genes in LT included ETV6, TP53, and RUNX1, and MYC amplification in JAK2V617F-negative LT. SNP arrays recognize copy number–neutral loss of heterozygosity (CNN-LOH), and CNN-LOH on either 9p in association with JAK2V617F homozygosity, or 7q alone correlated with decreased LT survival. CNN-LOH was also found on 16q, 19p, and 21q, implicating additional LT candidate genes and also genetic instability, suggesting that MPD LT is a heterogenous process. Importantly, 17p deletions involving TP53 and chromosome 17 CNN-LOH were highly associated with hydroxyurea (HU) therapy and shortened survival. JAK2V617F did not appear to be associated with time to LT or overall survival. However, because the CP and LT patients were largely unmatched, cause-and-effect associations were not possible.
This is where Beer et al make a very important contribution. They studied 16 MPD patients before and after LT to understand the basis for JAK2V617F-negative AL arising in a JAK2V617F-positive MPD. They elegantly demonstrate that this was not caused by mitotic recombination, gene conversion, or gene deletion. They, too, identified some “usual suspects” associated with LT, RUNX1, TP53, WT1, CBL, NRAS, and TET2, and their expression was occasionally present during CP. Importantly, in this matched study, JAK2V617F-positive LT occurred mainly in PMF or PV and ET patients who had evolved to a PMF phenotype and was associated with RUNX1 mutations, whereas JAK2V617F-negative LT occurred in CP PV and ET patients and was associated with TP53 mutations or deletion. TP53 mutations and 17p deletions are, of course, a hallmark of HU exposure and, although we have only a numerator, 14 of the 16 patients received HU before LT, including all 9 JAK2V617F-negative AL patients, 6 of whom received HU alone.
What should we conclude from these studies? First, CNN-LOH occurring in a CP MPD indicates the presence of genomic instability, probably due to constitutive tyrosine kinase signaling, which promotes apoptosis resistance,5 augmented homologous recombination, a mutator phenotype,6 and loss of the tumor suppressor mechanism, heterochromatic gene silencing.7 These abnormalities may not only explain spontaneous AL in the MPD, particularly in PMF with its large clonal burden, they also are conducive to t-AL. Second, if the data are confirmed, SNP arrays may be a useful guide for MPD prognosis.
Third, with respect to MPD t-AL, HU can now be securely added to the list of agents that are leukemogenic in the MPD because HU is not different from them or even drugs like azathioprine that also impede DNA repair and cause t-AL. This is not to suggest that HU should not be used in the MPD but rather that its use be judicious. In all studies to date, HU has failed to prevent arterial or venous thrombosis or myelofibrosis and has prevented transient ischemic attacks only because it is a nitric oxide donor.4,8
Finally, what about JAK2V617F-negative AL arising in a JAK2V617F-positive MPD? It is apparent that JAK2V617F is not the initiating lesion in the MPD, and, because JAK2V617F-positive cells are more sensitive to HU than JAK2V617F-negative cells, we may only be transforming or selecting for a more resistant but less robust primitive ancestral clone by targeting the more sensitive one (see figure), suggesting that nonspecific therapy such as pegylated interferon9 may be more appropriate for MPD than targeted therapies.10
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health