Abstract
This is a phase 2 study to assess the role of tumor histogenesis (subtype), fluorodeoxyglucose positron emission tomography (FDG-PET), and short-course etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin with dose-dense rituximab (SC-EPOCH-RR) in newly diagnosed HIV-associated CD20+ diffuse large B-cell lymphoma. Patients received a minimum of 3 and a maximum of 6 cycles with 1 cycle beyond stable radiographic and FDG-PET scans. Overall, 79% of patients received 3 cycles. Combination antiretroviral therapy was suspended before and resumed after therapy. Thirty-three enrolled patients had a median age of 42 years (range, 9-61 years), and 76% had a high-intermediate or high age-adjusted international prognostic index. At 5 years median follow-up, progression-free and overall survival were 84% and 68%, respectively. There were no treatment-related deaths or new opportunistic infections during treatment, and patients had sustained CD4 cell count recovery and HIV viral control after treatment. FDG-PET after 2 cycles had an excellent negative but poor positive predictive value. Tumor histogenesis was the only characteristic associated with lymphoma-specific outcome with 95% of germinal center B-cell (GCB) versus 44% of non-GCB diffuse large B-cell lymphoma (DLBCL) progression-free at 5 years. SC-EPOCH-RR is highly effective and less immunosuppressive with shorter duration therapy compared with standard strategies. However, new therapeutic advances are needed for non-GCB DLBCL, which remains the important cause of lymphoma-specific death. This trial was registered at www.clinicaltrials.gov as NCT000019253.
Introduction
The survival of acquired immunodeficiency syndrome–related lymphoma (ARL) has significantly improved over the past decade, but it has been mostly attributed to HIV control and not to advances in lymphoma treatment.1-6 We tested a strategy based on the dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (da-EPOCH) regimen that balanced the competing needs of lymphoma treatment and HIV management.7 This regimen used dose adjustment, based on the degree of immune suppression, and temporarily suspended combination antiretroviral therapy (cART) to obviate untoward drug interactions.8 da-EPOCH proved to be highly effective with progression-free (PFS) and overall survival (OS) of 73% and 60%, respectively, at 53 months in ARL, most of which were diffuse large B-cell lymphoma (DLBCL).7 Baseline CD4+ cells less than or equal to 100/μL was the only biomarker of decreased survival in a multivariate analysis, and patients in remission had significant recovery of immune function and HIV control. On the basis of these results, da-EPOCH has been identified as a treatment of choice for ARL.5,9
Herein, we report results on a second-generation regimen that aimed to improve efficacy and to decrease toxicity through the addition of dose-dense rituximab to EPOCH. The design was based on the hypothesis that rituximab would significantly enhance the efficacy of chemotherapy, thereby allowing a major reduction in the number of treatment cycles.10 Interestingly, years after our study commenced, a phase 3 study of cyclophosphamide. doxorubicin, vincristine, and prednisone (CHOP) with or without rituximab concluded that rituximab did not improve the outcome of ARL and was potentially unsafe in immune-compromised patients.4 As we show below, however, our present study does not support those conclusions.
A novel component of the present study was the use of sequential fluorodeoxyglucose positron emission tomography (FDG-PET) to assess early and late responses in HIV-associated DLBCL. Furthermore, this study actively used interim FDG-PET in the decision to reduce the number of treatment cycles. Our goal was to study for the first time whether DLBCL could be effectively treated with up to 50% fewer cycles than a standard course and to assess the role and specificity and sensitivity of FDG-PET in HIV-associated DLBCL.
We also wanted to examine the role of tumor biology in the outcome of HIV-associated DLBCL. Although studies have assessed histology and CD4 cell count, none have prospectively assessed molecular histogenesis of DLBCL that derive from a germinal center or an activated B-cell (GCB or ABC) and are independently prognostic in HIV-negative DLBCL.11-13 Importantly, insight into the molecular basis of treatment failure is critical to the development of more effective treatments in HIV-associated DLBCL. Thus, we wanted to assess whether tumor histogenesis is a main factor in lymphoma-specific survival and whether one or both molecular subtypes might benefit from additional novel interventions.
Methods
Patients
Forty-five patients with untreated CD20+ ARL entered on a study of short-course EPOCH and dose-dense rituximab (SC-EPOCH-RR) at the National Cancer Institute. Thirty-five patients had DLBCL, and 10 patients with Burkitt lymphoma will be reported separately. Two patients with DLBCL were excluded; 1 received treatment elsewhere, and 1 had primary mediastinal B-cell lymphoma (PMBL), putatively of thymic B-cell origin.14 Eligible patients were HIV seropositive by Western blot and had adequate organ function unless because of tumor. Patients with serious infections, pregnancy, breast-feeding, or primary central nervous system lymphoma were ineligible. Patients were consecutively enrolled between June 2001 and April 2009. The study was approved by the institutional review board and complied with the Declaration of Helsinki, and patients gave written informed consent.
Evaluation and treatment
Evaluation included routine blood tests, imaging (computed tomography [CT] of the body, magnetic resonance imaging of the brain, and FDG-PET), bone marrow biopsy, and lumbar puncture with cytology and flow cytometry. Serial plasma HIV-1 viral loads (mRNA copies/mL plasma) were measured by the Roche-Amplicor method, and T-lymphocyte subsets were determined by flow cytometry at baseline, at the end of SC-EPOCH-RR, and every 3 to 6 months thereafter.
The study was designed to administer 1 cycle beyond no significant radiographic changes for a minimum of 3 and a maximum of 6 cycles. cART was suspended before SC-EPOCH-RR and was reinstated on day 6 of the last cycle. Response was based on serial CT body and FDG-PET scans, beginning at cycle 3 day 1 and performed after each cycle thereafter until treatment completion (Figure 1). Thus, the number of scans performed was the same as the number of cycles received for each patient. In clinical practice, however, the posttreatment scans could be omitted if the previous scans were negative. SC-EPOCH-RR was stopped when there was less than 25% reduction in bidimensional products compared with the previous interim CT scan and the standardized uptake values on FDG-PET decreased greater than 50% compared with the pretreatment FDG-PET. This definition was developed to take into account HIV-associated reactive changes that may confound FDG-PET interpretations. Response designation followed the International Workshop criteria.15
SC-EPOCH-RR was administered through a central line on days 1 to 5 as a 96-hour continuous infusion (CIV) of etoposide (50 mg/m2/d), doxorubicin (10 mg/m2/d), and vincristine (0.4 mg/m2/d; no cap) and oral prednisone (60 mg/m2/d) with cyclophosphamide (750 mg/m2) on day 5 as previously described.16 Rituximab (375 mg/m2) was administered on day 1 (before CIV began) and on day 5 (after CIV completed and before cyclophosphamide). All patients received filgrastim 300 μg subcutaneously (pediatric dose, 5 μg/kg/d; maximum dose, 300 μg/d) from day 6 until absolute neutrophil count (ANC) was at least 5.0 × 109/L (5000 cells/mm3) beyond the nadir. Cycles were repeated every 21 days. Cyclophosphamide was reduced 25% for a nadir ANC less than 0.5 × 109/L (500/mm3) or platelet count less than 25.0 ×109/L (25 000/mm3) for 2 to 4 days and 50% if the nadir ANC was less than 0.5 × 109/L (500/mm3) or platelet count was less than 25.0 ×109/L (25 000/mm3) for 5 or more days, based on twice weekly blood counts. Patients received 12 mg of methotrexate intrathecally on days 1 and 5 of cycle 3 and repeated every 3 weeks for a total of 6 doses (ie, cycles 3-5), irrespective of when SC-EPOCH-RR stopped. Patients with a positive flow cytometry or cytology of cerebrospinal fluid received induction intrathecal or intraventricular methotrexate twice weekly for 2 weeks beyond negative flow cytometry for a minimum of 4 weeks, consolidation weekly for 6 weeks, and maintenance monthly for 6 months. Patients also received prophylaxis for Pneumocystis jiroveci and Mycobacterium avium if CD4 cells were less than 100/μL (100/mm3).
Immunohistochemical analyses
Immunohistochemistry (IHC) was performed on paraffin-embedded tissue.17 Sections were stained with monoclonal antibodies to Bcl-6 (clone PG-B6p), MUM-1 (clone MUM1p), and CD10 (clone 56C6 from Novocastra). For Bcl-6 and MUM-1, cases were scored as positive if expression occurred in at least 30% of neoplastic cells. CD10 stained uniformly positive or negative in all cases. Classification into GCB or non-GCB (ABC) subtypes was determined (by S.P.) with the use of the validated method of Hans et al.18 In situ hybridization analysis for Epstein-Barr virus (EBV) RNA was done on 4-μm-thick formalin-fixed, paraffin-embedded tissue with the use of the INFORM EBV-encoded nontranslated RNA probe (Ventana Medical Systems). The signal was visualized with the use of the ISH iVIEW Blue Detection kit (Ventana Medical Systems) with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate and a Fast Red nuclear counterstain. All the procedures were done on a BenchMark XT autostainer (Ventana Medical Systems) according to the manufacturer's instructions.
FDG-PET imaging
Patients fasted for 6 hours before FDG-PET and were injected with a nominal amount of FDG according to weight. Patients were scanned on a GE Advance or a GE Discovery ST PET/CT. Images were acquired from mid skull to proximal thighs, performed at a nominal time of 60 minutes after injection, and blindly read by J.A.C. Images were interpreted visually as positive when focal areas of uptake were seen that did not correspond to physiologic sites of uptake.19
Statistical analysis
The primary end point was to test the hypothesis that the number of chemotherapy cycles could be reduced from a standard of 6 cycles.7,20 On the basis of a mean of 4 cycles, the study would have an 80% power at 0.05 two-sided significance to detect a difference between a mean of 4 and 5 cycles with 1.5 standard deviation in 28 patients. On completion of accrual for the primary end point, enrollment was increased to more fully address the secondary end points of FDG-PET and immune recovery. Assessment of tumor histogenesis and EBV was obtained from standard immunohistochemical panels.
PFS and OS were determined from on study until death, progression, or last follow-up by the Kaplan-Meier method, and statistical significance was determined by a log-rank test. For PFS, deaths unrelated to acute treatment or lymphoma were censored. Death within 30 days of chemotherapy was defined as treatment related. For PFS, Kaplan-Meier curves were developed for all prognostic factor analyses. An exact log-rank test was used to determine the effect of tumor histogenesis (GCB vs non-GCB) on PFS because there were so few failures in one group. For univariate prognostic factors analyses, if patients were grouped into 2 categories after preliminary evaluation of 3 categories, the subsequent P value was adjusted by multiplying the unadjusted P value by 2. This would account for the implicit testing that resulted in a decision to place patients into the 2 categories with a larger prognostic difference between groups. A Cox proportional hazards model analysis was performed to determine the joint association of factors initially found to have potential association with outcome in the univariate analyses (those with unadjusted P < .10 from a log-rank test). For PFS, because lactate dehydrogenase level divided at less than 226 versus greater than 266 (above normal) resulted in no failures in one group, the hazard ratio (HR) would be infinite; thus, for PFS, only cell type was available for use in a Cox model. The association between cell type and CD4 cell count was determined with the use of an exact Wilcoxon rank sum test. Other exploratory evaluations between cell type and other dichotomized factors were performed with the Fisher exact test. All P values are 2-tailed, and except as noted earlier, are reported without adjustment for multiple comparisons.
Results
Patient characteristics
Thirty-three patients with untreated DLBCL were enrolled. They had a median age of 42 years, and 76% had a high age-adjusted international prognostic index (IPI; Table 1). Specific adverse prognostic features included poor performance status in 39%, elevated lactate dehydrogenase level in 61%, and advanced stage disease in 82% of patients. Central nervous system involvement was found in 4 (12%) patients before treatment. Patients had a median CD4 cell count of 208 cells/μL (208 cells/mm3) with 42% having a CD4 cell count less than 100 cells/μL (100 cells/mm3), and 27% were cART naive.
Treatment and outcome
Patients received a median of 3 cycles (range, 3-5 cycles) of SC-EPOCH-RR; 79% received 3 cycles, 6% received 4 cycles, and 12% received 5 cycles (Figure 1). Complete response (CR) was observed in 30 (91%; 95% confidence interval [CI], 76%-98%) patients, and 1 patient achieved a partial response. Two patients progressed on therapy, and 3 patients relapsed. Four patients with leptomeningeal involvement by lymphoma achieved durable remissions, although 1 died of a non–lymphoma-related event. At the median potential follow-up of 5 years, the PFS and OS are 84% and 68%, respectively (Figure 2A-B). Of 10 deaths on study, 5 were due to lymphoma and 5 were in remission. Of these latter deaths, 3 were from complications of AIDS because of preexistent Mycobacterium avium complex infection and occurred at 5 weeks, 3 months, and 30 months after treatment. All 3 patients received treatment for Mycobacterium avium complex and were cART resistant with pretreatment and posttreatment (last available) CD4 cell counts of 0 and 1 (0 and 1), 3 and 4 (3 and 4), and 12 and 9/μL (12 and 9 cells/mm3) respectively. Two additional patients died at 31 months after treatment; 1 with progressive motor neuropathy after successful treatment of a secondary and clonally distinct Burkitt lymphoma, and 1 of exposure-associated hypothermia.
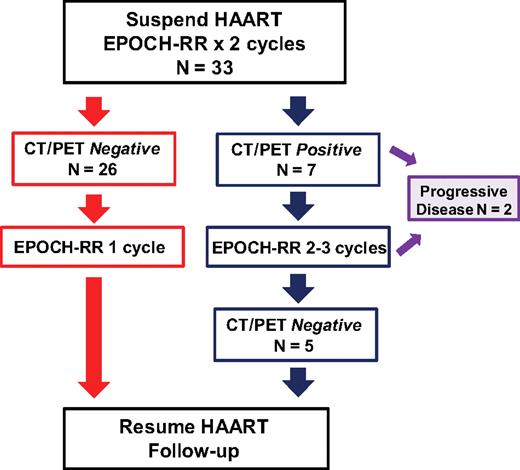
Treatment paradigm for SC-EPOCH-RR. Thirty-three patients with DLBCL received 2 cycles of EPOCH-RR after which time CT and FDG-PET scans were performed. Response was based on serial CT body and FDG-PET scans, beginning at cycle 3 day 1 and performed after each cycle thereafter until treatment completion. Patients who had “negative” studies, as defined in “Evaluation and treatment,” received 1 additional cycle (minimum of 3) of therapy. Patients who had a “positive” CT and/or FDG-PET study received additional cycles until they were negative, for a maximum of 6 cycles. Two patients with progressive disease on treatment did not complete their initial therapy.
Treatment paradigm for SC-EPOCH-RR. Thirty-three patients with DLBCL received 2 cycles of EPOCH-RR after which time CT and FDG-PET scans were performed. Response was based on serial CT body and FDG-PET scans, beginning at cycle 3 day 1 and performed after each cycle thereafter until treatment completion. Patients who had “negative” studies, as defined in “Evaluation and treatment,” received 1 additional cycle (minimum of 3) of therapy. Patients who had a “positive” CT and/or FDG-PET study received additional cycles until they were negative, for a maximum of 6 cycles. Two patients with progressive disease on treatment did not complete their initial therapy.
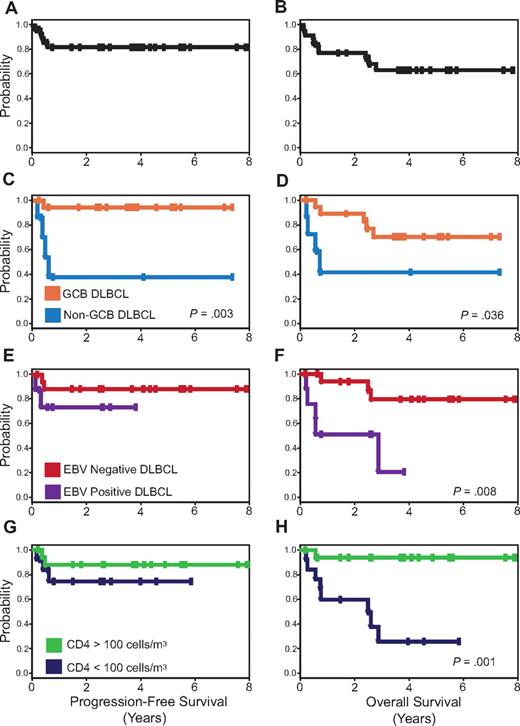
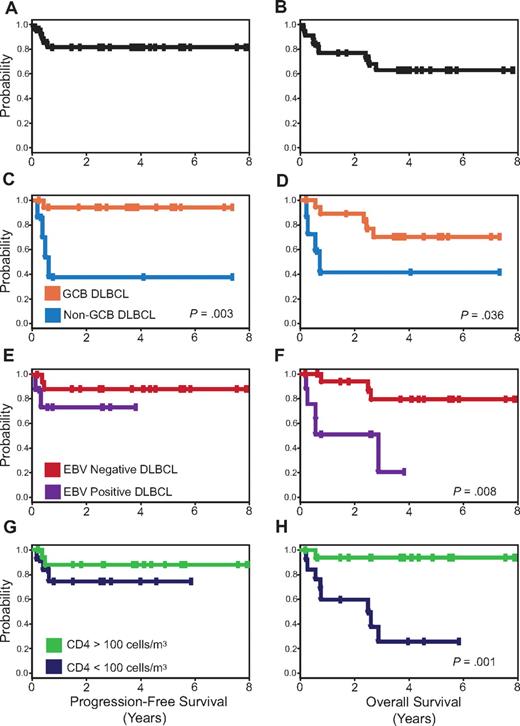
PFS and OS Kaplan-Meier curves. PFS (A) is 84% and OS (B) is 68% at the median follow-up of 5 years. PFS (C) and OS (D) for patients with GCB versus non-GCB DLBCL. PFS (E) and OS (F) for EBV-negative versus EBV-positive DLBCL, and PFS (G) and OS (H) for CD4 cell count greater than 100 cells/μL (100 cells/mm3) versus less than 100 cells/μL (100 cells/mm3) at diagnosis.
PFS and OS Kaplan-Meier curves. PFS (A) is 84% and OS (B) is 68% at the median follow-up of 5 years. PFS (C) and OS (D) for patients with GCB versus non-GCB DLBCL. PFS (E) and OS (F) for EBV-negative versus EBV-positive DLBCL, and PFS (G) and OS (H) for CD4 cell count greater than 100 cells/μL (100 cells/mm3) versus less than 100 cells/μL (100 cells/mm3) at diagnosis.
Toxicity was evaluable on all 109 cycles of treatment. Hematologic toxicity included neutropenia less than 0.5 × 109/L (500 cells/mm3) on 46% and thrombocytopenia less than 50.0 × 109/L (50 000/mm3) on 33% of cycles. Febrile neutropenia occurred on 31% of cycles, no new opportunistic infections were observed on treatment, and there were no treatment-related deaths. Nonhematologic toxicity was similar to our report of da-EPOCH.7
FDG-PET scans
FDG-PET scan after 2 cycles had an excellent negative predictive value and was not significantly different from the posttherapy scan (Table 2). The positive predictive value of FDG-PET scans was quite poor, however, presumably because of a high rate of HIV-associated reactive changes. Hence, FDG-PET had poor sensitivity and specificity for HIV-associated DLBCL when interpreted with the commonly recommended criteria.19
HIV and CD4 T-cell dynamics
A unique aspect of our strategy is suspension of cART during treatment to obviate adverse effects on the lymphoma treatment.2,7 We previously demonstrated this approach led to reversible viral load increase and CD4 cell decrement and excellent disease control with da-EPOCH.7 The current study aimed to reduce the adverse effects of chemotherapy on immune function through a reduction in cycle number that was achieved in all patients. The viral load increased a median of 0.47 log10 (range, −1.82 to 4.87 log10), and the CD4 count decreased a median of 64 cells/μL (64 cells/mm3; range, 271 to −541 cells/μL [271 to −541 cells/mm3]) among 28 patients without early deaths. Expectedly, cART-naive patients presented with higher viral loads and lower CD4 cell counts than patients on prior cART (Figure 3A-B). The median viral load declined below baseline in both groups by 3 months after treatment and was undetectable in most patients at 12 to 18 months, and the CD4 cells recovered to baseline by 6 to 12 months. We compared our current results to those with da-EPOCH and observed a significantly smaller decrement in median CD4 cell count of 63/μL (64/mm3; range, 271 to −541/μL [271 to −541/mm3]) versus 183/μL (183/mm3; range, 135 to −928/μL [135 to −928/mm3]; P = .03), respectively.7
HIV viral load and T-cell dynamics. (A) Median change in plasma mRNA HIV viral loads in 28 patients without early deaths. Viral loads increased with the peak shown at the end of therapy and declined below baseline at 3 months after completion of therapy and reinstitution of cART. cART-naive patients (♦) compared with patients with prior exposure (■) had slightly higher viral loads at presentation. (B) Median changes in CD4 cells in 28 patients without early deaths. CD4 cells declined to a nadir at end of therapy but recovered to baseline 6 to 12 months later. cART-naive patients (♦) compared with patients with prior exposure (■) had lower CD4 cells at baseline but equivalent CD4 cells 6 to 12 months after therapy. Medians with 95% CIs calculated by bootstrapping are shown.
HIV viral load and T-cell dynamics. (A) Median change in plasma mRNA HIV viral loads in 28 patients without early deaths. Viral loads increased with the peak shown at the end of therapy and declined below baseline at 3 months after completion of therapy and reinstitution of cART. cART-naive patients (♦) compared with patients with prior exposure (■) had slightly higher viral loads at presentation. (B) Median changes in CD4 cells in 28 patients without early deaths. CD4 cells declined to a nadir at end of therapy but recovered to baseline 6 to 12 months later. cART-naive patients (♦) compared with patients with prior exposure (■) had lower CD4 cells at baseline but equivalent CD4 cells 6 to 12 months after therapy. Medians with 95% CIs calculated by bootstrapping are shown.
Tumor biology and prognostic models
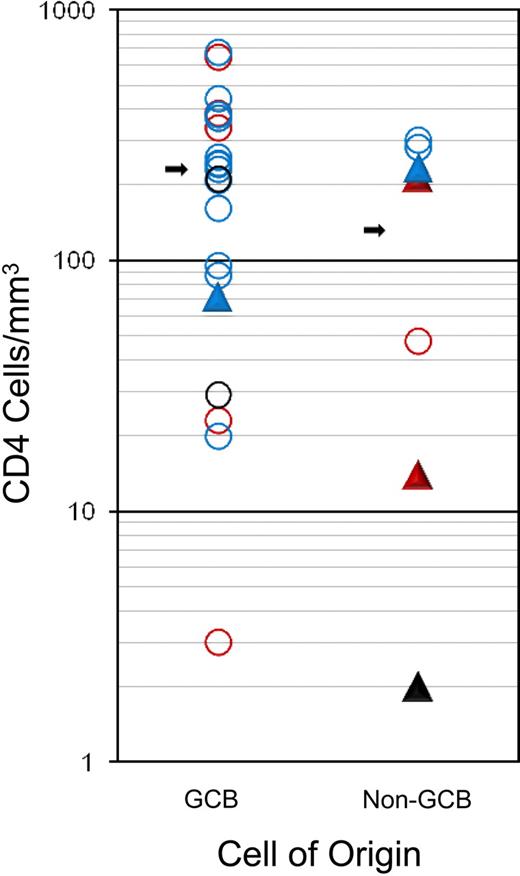
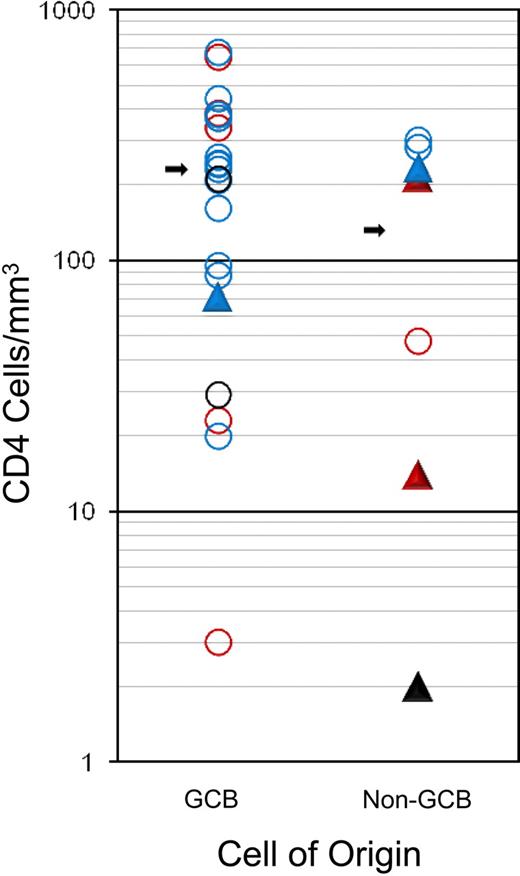
GCB and non-GCB DLBCL subtypes comprised 72% and 28% of cases, respectively, and EBV expression was detected in 31% of cases (Table 1). EBV expression was detected somewhat more frequently in the non-GCB (4 of 7; 57%) compared with GCB (5 of 19; 26%; P = .19) subtype, and in patients with low CD4 cell counts less than 100/μL (100/mm3; 5 of 11; 45%) compared with patients with higher counts (4 of 24; 17%; P = .10), although they did not reach statistical significance because of sample size. There was a trend toward a higher median CD4 cell number in patients with GCB (231 cell/μL [231 cells/mm3]) compared with non-GCB (131 cells/μL [131 cells/mm3)]) subtype (P = .15), but no difference was observed in the fraction who had CD4 cell counts less than compared with greater than 100 cells/μL (100 cells/mm3; P = .38; Figure 4).
Association of tumor histogenesis (cell of origin), EBV expression, immune status (CD4 cell count at diagnosis), and outcome. Outcome: progression/relapse (▲) or PFS (○). EBV status: positive (red), negative (blue), or unknown (black).
Association of tumor histogenesis (cell of origin), EBV expression, immune status (CD4 cell count at diagnosis), and outcome. Outcome: progression/relapse (▲) or PFS (○). EBV status: positive (red), negative (blue), or unknown (black).
To examine the role of GCB versus non-GCB histogenesis in the outcome of HIV-associated DLBCL, we developed prognostic models that were based on the characteristics in Table 1. In the univariate analysis, only tumor histogenesis was associated with PFS (Figure 2C), whereas tumor histogenesis, EBV, and CD4 cell count were all associated with OS (Figure 2D,F,H). Importantly, no clinical characteristics, including IPI, had prognostic value. The multivariate Cox model of PFS only showed tumor histogenesis (HR = 14.5; 95% CI, 1.6-131; P = .017), indicating tumor biology drove the lymphoma-specific survival. For OS, histogenesis and immune status were important in the Cox model; CD4 less than or greater than 100 cells/μL (100 cells/mm3; HR = 27; 95% CI, 2.9-251; P = .004) and non-GCB versus GCB (HR = 7.2; 95% CI, 1.2-42; P = .028). When HIV viral load was added to this model, it provided further evidence for the importance of HIV control in OS; CD4 cell count less than or greater than 100 cells/μL (100 cells/mm3; HR = 29.9; 95% CI, 2.6-351; P = .007), non-GCB versus GCB (HR = 64.3; 95% CI, 2.7-1509; P = .01), HIV-1 mRNA greater than versus less than 100 000 copies/mL plasma (HR = 15.9; 95% CI, 1.1-222; P = .04).
Discussion
Treatment of ARL presents the dual challenge of achieving tumor control while maintaining immune integrity. Although conventional wisdom argues for maintaining cART during lymphoma treatment to minimize the risks of uncontrolled HIV replication, we have hypothesized that the unpredictable effects of cART on chemotherapy pharmacokinetics and pharmacodynamics and lymphocyte apoptosis may reduce cure.7,8 Although it is established that structured cART suspension increases AIDS-associated events, a large randomized study showed it required a mean of 16.8 months (range, 5.7-42.3 months) of cART suspension for the CD4 count to decrease from greater than 400 cells/μL (400 cells/mm3) to less than 250 cells/μL (250 cells/mm3), far in excess of the 1.8-month mean suspension time in the present study.21 Furthermore, we previously showed recovery of immune function, HIV viral control, and reversion to wild-type virus after reinstitution of cART after da-EPOCH chemotherapy.7
We believe our current results provide further evidence for the safety of cART suspension. Indeed, only 3 patients died in remission of HIV-associated infections, and, in all cases, the infection(s) were preexistent and the patients were severely immune-compromised and cART-resistant. Furthermore, only 2 of these cases were immediately after completing chemotherapy treatment and occurred despite active treatment for their infections. Hence, there is no scientific basis to associate these deaths with suspension of cART. However, the results with SC-EPOCH-RR indicates that our treatment strategy provides excellent outcomes.
The present study extends our findings with da-EPOCH through reducing chemotherapy cycles by half with a reduction in cART suspension from 16 to 7 weeks. To achieve this, several modifications were made to da-EPOCH. First, we removed dose adjustment based on initial CD4 cell counts because of the improvement in HIV natural history; thus, patients received full-dose EPOCH on cycle 1 with subsequent reductions based on hematologic toxicity. We also incorporated dose-dense rituximab on days 1 and 5 of each cycle. When we developed SC-EPOCH-RR 9 years ago, we hypothesized that rituximab would be beneficial and that dose-dense administration would significantly increase drug exposure; it had been shown that rituximab T½ doubled and concentration maximum increased 50% between the first and fourth dose on a standard weekly schedule.22 We also restricted the present study to DLBCL to reduce the confounding effects associated with the biologic diversity of ARL.
With the use of a combination of CT and FDG-PET response criteria, most patients (79%) received 3 cycles of SC-EPOCH-RR. Overall, 91% of patients achieved CR, and 84% are progression free and 68% are alive at 5 years. SC-EPOCH-RR had significantly less immune toxicity than da-EPOCH, which was administered for 6 cycles, and achieved excellent immune reconstitution and HIV control after reinstitution of cART, similar to those findings when chemotherapy is administered with cART.23 The survival outcomes compare favorably with da-EPOCH, whereby PFS and OS were 73% and 60%, respectively, at 53 months, suggesting dose-dense rituximab may be beneficial, although the study was not designed to address this question.7 Our results appear to be significantly better than those achieved in a phase 3 study of CHOP with or without rituximab, which reported 50% time to progression (comparable with PFS in the present study) and OS with rituximab plus CHOP at 2.4 years.4 Importantly, our results do not support that trials' conclusion that rituximab may be risky because we observed no treatment-related deaths.20,24 Although SC-EPOCH-RR was associated with greater hematologic toxicity than da-EPOCH, probably because of higher chemotherapy dose intensity, we did not encounter any treatment-related deaths.7
The efficacy and safety of EPOCH and rituximab is further supported by preliminary results from the randomized phase 2 trial of concurrent versus sequential rituximab with da-EPOCH in ARL from the AIDS Malignancy Consortium (AMC) Trial 034.25 The AMC study, based on da-EPOCH and unpublished results from the present study, aimed to assess whether the CR rate of da-EPOCH and rituximab was probably superior to historical results with CHOP with or without rituximab and whether da-EPOCH with concurrent versus sequential rituximab was more toxic and/or more effective.4,7,25,26 Preliminary results found no increase in toxicity between the arms and rejected the null hypothesis of 50% (associated with CHOP with or without rituximab) in favor of 75% CR for da-EPOCH with concurrent rituximab (P = .005; power 0.89).4,25 These results provide additional evidence that rituximab does not increase chemotherapy toxicity and that da-EPOCH with concurrent rituximab is probably more effective than rituximab plus CHOP.
To our knowledge, this is the first study to use interim FDG-PET in a treatment paradigm to reduce chemotherapy cycles in DLBCL, and it provides a unique opportunity to study its utility in HIV-associated lymphomas. This is particularly important because HIV-associated nodal reactive hyperplasia and infections may confound FDG-PET interpretations.27,28 To assess the value of FDG-PET, they were blindly interpreted with the use of commonly accepted criteria.19 FDG-PET obtained after 2 cycles and at the end of therapy showed high negative predictive values of 91% and 87%, respectively, but poor positive predictive values of 15% and 7%, respectively. It was difficult to differentiate low-level inflammation from active lymphoma. Indeed, 65% of FDG-PET scans were positive after cycle 2, yet few of these patients relapsed. Although the role of FDG-PET needs further study in ARL, it will probably remain highly problematic.
Numerous studies have identified IPI and CD4 cell counts as the important predictors of ARL outcome in the post-cART era.4,6,7 Indeed, there has been some concern that withholding cART during chemotherapy will promote CD4 cell loss and decrease both HIV and lymphoma-specific survival. Although there is ample evidence that ARL pathogenesis is influenced by immune status, there is no scientific evidence or rationale that treatment sensitivity of established lymphomas are dependent on the CD4 cell count.13 To help address this issue, we analyzed clinical and biologic markers of outcome in our study. Unlike other studies, no clinical prognostic characteristics, including IPI, were associated with PFS or OS. Only tumor histogenesis was significantly associated with lymphoma-specific outcome with 95% of GCB and 44% of non-GCB DLBCL being progression free at 5 years. Furthermore, both tumor histogenesis and CD4 cell count were independently associated with survival, reflecting both lymphoma and HIV-specific deaths. These results mirror the importance of tumor histogenesis in the outcome of HIV-negative DLBCL.11,12,29 We also examined the relationship between tumor histogenesis, immune status, and EBV expression and found an association, albeit a trend, between non-GCB tumor cell type and lower CD4 cell counts and EBV expression, suggesting a role for immune status in pathogenesis. It is important to recognize the potential limitations of IHC determination of tumor histogenesis. As validation for our methods, we previously showed full concordance between IHC and gene expression profiling, the gold standard, in 12 biopsies of DLBCL.18,30,31
Our results show for the first time that tumor biology is the main factor in lymphoma-specific survival in HIV-associated DLBCL. In contrast, a recent publication from the AMC found no relationship between tumor histogenesis or EBV infection and outcome in HIV-associated DLBCL, which is contrary to our own results and numerous publications in HIV-negative DLBCL.11,12,18,29,32 Several factors may explain these negative findings. First, the AMC study was retrospective and included biopsy specimens from 2 different studies and 4 different treatment regimens; CHOP with or without rituximab and EPOCH with concurrent or sequential rituximab. This probably confound the results because rituximab and the chemotherapy platform (CHOP vs EPOCH) appear to influence the effect of tumor histogenesis on outcome.11,12,29 These results are further limited by the relatively short and variable follow-up in the 2 studies. Second, the outcome measures did not distinguish lymphoma-specific survival from OS and thus may be confounded by a high rate of non–lymphoma-specific deaths. Third, the accuracy of immunohistochemical determination of tumor histogenesis can be highly variable, and validation of the laboratory technique against gene expression profiling is an important control.30,31
Understanding the relationship of tumor biology to outcome is important for the identification of molecular targets and for improvement of therapy. Our present finding that SC-EPOCH-RR is curative in nearly all cases of GCB DLBCL is similar to our results in HIV-negative cases (unpublished observations, September 2009).29,33 In vitro, sustained exposure of tumor cells to topoisomerase II inhibition by etoposide and low-dose doxorubicin promote the p53-p21 pathway and activates the check-point kinase (Chk2) independently of ATR, pathways that are associated with the GCB Bcl-6 transcription factor.34,35 Such studies indicate that prolonged exposure to topoisomerase II inhibition, as achieved with EPOCH, may be particularly effective in GCB DLBCL. In contrast, our results indicate that therapeutic advances are needed in non-GCB DLBCL, similar to findings in HIV-negative non-GCB DLBCL.11,29 In HIV-negative DLBCL, most cases identified as non-GCB by immunohistochemistry have an ABC DLBCL gene expression profile.18 The poor outcome of non-GCB (ABC) DLBCL may be related to the constitutive activation of the nuclear factor-κB (NF-κB) pathway, which has been ascribed to activity of a signaling cascade that involves CARD11, BCL-10, and MALT1, leading to activation of inhibitor of κB kinase.36-38 Furthermore, inhibition of NF-κB in ABC DLBCL cell lines is toxic, in keeping with the ability of this pathway to inhibit apoptosis.36,39 Clinically, it has been shown that the combination of EPOCH with the proteasome inhibitor bortezomib, which can inhibit NF-κB through blocking inhibitor of κBα degradation, had significantly greater benefit in recurrent ABC versus GCB DLBCL, a strategy that may improve the outcome of untreated ABC DLBCL.30
In conclusion, most HIV-associated DLBCLs are curable with 3 cycles of SC-EPOCH-RR, and tumor histogenesis is the most important determinant of lymphoma-specific survival. Although FDG-PET is useful when used alongside CT scans to determine when treatment is completed, they should be used with caution in HIV-associated DLBCL. These results suggest that SC-EPOCH-RR is an important advance for HIV-associated DLBCL, although AIDS-related deaths and non-GCB DLBCL remain important barriers to overall survival.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Millie Whatley, RN, MT, and the nuclear medicine technologist for their technical assistance with the PET scans.
National Institutes of Health
Authorship
Contribution: K.D. conducted and analyzed the study and wrote the paper; R.F.L. designed and conducted the study; S.P., J.A.C., and E.S.J. analyzed the study; N.G. analyzed the study and managed the data; A.S.W. conducted the study and analyzed the data; S.M.S. designed the study and analyzed the data; R.Y. performed study consultation and sample analysis; and W.H.W. designed and conducted the study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests. However, for full disclosure, rituximab was provided by Caner Therapy Evaluation Program (CTEP), NCI, which had a Cooperative Research and Development Agreement (CRADA) with Genentech. W.H.W. has participated on Genentech Advisory Board meetings on drug development. All participation was conducted by W.H.W. as official business of NCI, and he received no personal remuneration.
Correspondence: Wyndham H. Wilson, Metabolism Branch, Bldg 10, Rm 4-N-115, Bethesda, MD, 20892-1868; e-mail: wilsonw@mail.nih.gov.