Abstract
Natural killer (NK) cells play an important role in the immunosurveillance of leukemia. Their reactivity is governed by a balance of activating and inhibitory receptors including various members of the tumor necrosis factor receptor (TNFR) family. Here we report that human NK cells acquire expression of the TNFR family member CD137 upon activation, and NK cells of acute myeloid leukemia (AML) patients display an activated phenotype with substantial CD137 expression. CD137 ligand (CD137L) was detectable on leukemic cells in 35% of 65 investigated AML patients, but not on healthy CD34+ cells, and expression was associated with monocytic differentiation. Bidirectional signaling following CD137-CD137L interaction induced the release of the immunomodulatory cytokines interleukin-10 and TNF by AML cells and directly diminished granule mobilization, cytotoxicity, and interferon-γ production of human NK cells, which was restored by blocking CD137. Cocultures of NK cells with CD137L transfectants confirmed that human CD137 inhibits NK-cell reactivity, while activating signals were transduced by its counterpart on NK cells in mice. Our data underline the necessity to study the function of seemingly analog immunoregulatory molecules in mice compared with men and demonstrate that CD137-CD137L interaction enables immune evasion of AML cells by impairing NK-cell tumor surveillance in humans.
Introduction
Natural killer (NK) cells were initially identified as lymphocytes capable of lysing cells without prior sensitization; later, the involvement of low or absent expression of self major histocompatibility complex (MHC) class I molecules was discovered. Subsequently, multiple in vitro and in vivo studies demonstrated the important role of NK cells in the immunosurveillance of pathogens and tumor cells.1,2 The relevance of NK cells in antitumor immunity, particularly in leukemia, was recently highlighted by clinical evidence: studies of haploidentical stem cell transplantation (SCT), where the recipient's leukemia cells fail to inhibit donor NK cells via killer inhibitory receptors (KIRs), revealed that KIR disparity is associated with powerful graft-versus-leukemia effects and better clinical outcome.3-5 The observation that leukemia cells may down-regulate HLA class I molecules,6,7 presumably to escape adaptive immunity, suggests that NK cells are also involved in controlling leukemia in an autologous setting. This is supported by the observation that NK-cell counts and activity are reduced in patients with leukemia, and that activity levels of autologous NK cells are associated with survival.8-10 In the patients presenting with leukemia, antitumor immunity has clearly failed to eradicate the malignant cells, which may have, at least in part, been induced by the disease. Since NK-cell reactivity results from an integrative response emerging from multiple activating, inhibitory, and costimulatory receptors, the outcome of NK cell–leukemia cell interaction is dependent on various immunoregulatory molecules far beyond HLA class I–specific inhibitory KIRs.11,12
Among the molecules that affect NK-cell reactivity are various members of the TNF/TNF receptor (TNFR) family.13,14 The role of the TNFR family member CD137 as an inducible costimulatory receptor on T cells in cancer and autoimmunity has been extensively studied (for review, see Watts15 ). Triggering of CD137 by agonistic antibodies and aptamers as well as tumor-expressed CD137 ligand (CD137L) potently stimulates T-cell antitumor immunity.16-25 In addition, CD137 has been shown to stimulate the reactivity of mouse NK cells.26,27 However, although CD137L is expressed on human carcinoma and lymphoma cells,28,29 the consequences of CD137-CD137L interaction for antitumor reactivity of NK cells in humans are yet unclear.
Since available data suggest that certain TNFR family members may mediate different effects in mice and men,30,31 we here studied the role of CD137 and its ligand in NK-cell antitumor reactivity in humans compared with mice and used the interaction of allogenic NK cells with primary acute myeloid leukemia (AML) cells to mimic the situation, for example, after haploidentical SCT in patients with leukemia.
Methods
Patients
Blood samples from patients were obtained at time of diagnosis before therapy. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation. All patients gave their written informed consent in accordance with the Helsinki protocol, and the study was performed with the approval of all participating Ethics Committees. Diagnosis was confirmed by study of bone marrow specimens and flow cytometric immunophenotyping. Clinical data were collected the day when blood samples were taken.
Antibodies, cytokines, and fusion protein
The blocking anti–human CD137 monoclonal antibody (mAb) BBK-232 and the agonistic anti–human CD137 mAb 4B4-1 were from Neo Markers and Ancell, respectively. Anti–human CD137L antibody was produced as previously described.33 Anti–mouse CD137 as well as mouse and human IgG1 isotype control antibodies were from R&D Systems.
Anti–mouse CD137L mAb, rat isotype control mAb, anti–mouse CD3-PerCP, anti–NK1.1-FITC and rat IgG2a-FITC, anti–mouse IFN-γ–PECy7, rat IgG1-PECy7, anti–human CD69-PE, anti–CD80-PE, anti–CD86-PE, anti–HLA-A, anti–HLA-B, anti–HLA-C–PE, anti–CD33-PECy5, anti–CD34-PECy5, and anti–rat PE conjugates were from BD Biosciences. The goat anti–mouse PE conjugate was from Jackson ImmunoResearch Laboratories. Human IL-2 and IL-15 were from ImmunoTools. The CD137 fusion protein (human CD137 with human IgG1 tail) was generated as previously described.29
Transfectants
P388D.1 and HEK cells were transfected using the vector pcDNA3 containing the open reading frame of mouse and human CD137L, respectively, or empty vector as control (mock). Stable transfectants (P388D.1-mock, P388D.1-CD137L, HEK-mock, and HEK-CD137L) were established by subcloning and selection with 1.8 mg/mL G418 (Biochrom AG) as described previously.34
Preparation of NK cells and CD34+ cells
Resting NK cells were isolated from the PBMCs of healthy volunteers using the NK Cell Isolation Kit and MACS columns (Miltenyi Biotec), yielding a cell population of more than 98% NK cells. Polyclonal NK cells were generated by incubation of non–plastic-adherent PBMCs with irradiated RPMI8866 feeder cells in the presence of IL-2 (50 U/mL) over 10 days as previously described.34
CD34+ progenitor cells from healthy donors were obtained after informed consent during G-CSF–induced stem cell mobilization for SCT. Mononuclear cells were separated by Ficoll density gradient centrifugation. Enrichment of CD34+ cells was performed using immunomagnetic microbeads (Miltenyi Biotec) according to the manufacturer's instructions (purity > 95%).
Preparation of mouse splenocytes
Mouse splenocytes were obtained by passing spleens of C57BL/6 wild-type mice through a cell strainer before red blood cell lysis and washing. Cells were then cultured in the presence or absence of activating cytokines as indicated and used in functional assays.
Flow cytometry
Analysis of CD137 and CD137L surface expression was performed using the specific mAb or isotype control (10 μg/mL) followed by anti–mouse or anti–rat PE conjugate (1:100) as secondary reagent. Intracellular analysis of IFN-γ was performed using the antimouse IFN-γ conjugate or isotype control (both 1:100). Within patient PBMCs, NK cells and AML cells were selected according to expression of CD56+CD3− and CD33 or CD34, respectively. NK cells among mouse splenocytes were selected according to expression of NK1.1+CD3−. Analysis was performed using a FACSCalibur (BD Biosciences) or FC500 (Beckman Coulter) flow cytometer. Specific fluorescence indices (SFIs) were calculated by dividing median fluorescences obtained with specific mAb by median fluorescences obtained with isotype control.
PCR analysis
Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed as described previously.29 CD137L primers were 5-GCGTCCATCTTCACACTGAG-3 and 5-GTAGGATTCGGACTCTGTCCA-3. Primers for CD137 were 5-TCTGTTGCTGGTCCTCAACTT-3 and 5-CTCCTTCCTGGTCCTGAAAAC-3. 18S rRNA primers were 5-CGGCTACCACATCCAAGGAA-3 and 5-GCTGGAATTACCGCGGCT-3.
Quantitative real-time PCR analysis was performed as described previously34 using 5-TCAGGACCAGAAGGGAGTGT-3 and 5-AACGGAGCGTGAGGAAGAAC-3 as primers for CD137. Samples were normalized to 18S rRNA.
Cytotoxicity assays
Cytotoxicity of NK cells against leukemia cells from patients with more than 80% blast count was analyzed by 2-hour BATDA Europium release assays as previously described.31,35 In assays with HEK transfectants, cytotoxicity was analyzed by 4-hour chromium release assays as previously described.34 Percentage of lysis was calculated as follows: 100 × (experimental release − spontaneous release) ÷ (maximum release − spontaneous release).
Determination of IFN-γ, IL-10, and TNF
Cytokine determination was performed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions using OptEIA sets from BD Pharmingen or DuoSet ELISA development systems from R&D Systems. Cytokine concentrations in supernatants are expressed as mean plus standard deviation of triplicates.
Results
CD137 is up-regulated on human NK cells upon activation and in patients with AML
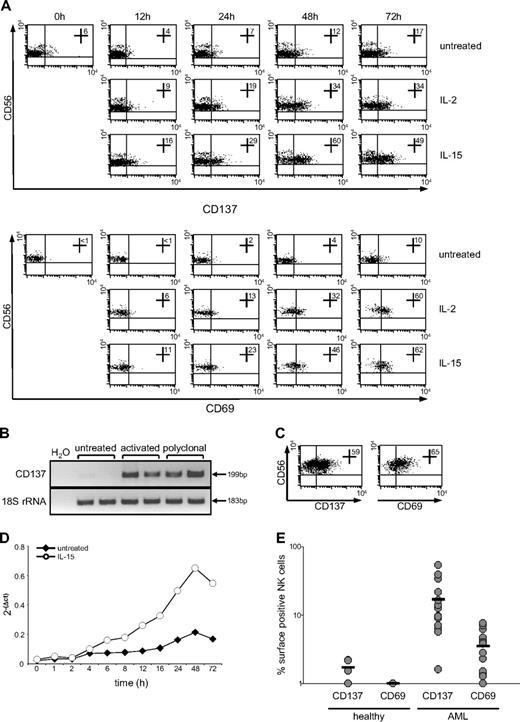
As a first step, we determined the expression and regulation of CD137 on human NK cells. While no marked expression of CD137 was detected in freshly isolated resting NK cells, activation with IL-2 or IL-15 clearly induced CD137 mRNA and surface expression (Figure 1A-B). Surface expression peaked after 48 to 72 hours, and treatment with IL-15 induced a more pronounced effect compared with IL-2. Up-regulation of CD137 on NK cells was associated with enhanced expression of the activation marker CD69. Of note, NK cells cultured in the absence of cytokines also acquired low levels of CD137 and CD69 expression, most likely due to unspecific activation by the isolation procedure and in vitro culture. Substantial CD137 surface and mRNA expression as well as CD69 expression was also detected in polyclonal NK cells which were predominantly used in subsequent functional studies (Figure 1B-C). Quantitative PCR analysis of CD137 mRNA induction in resting NK cells revealed that CD137 mRNA increased already after 4 hours of cytokine stimulation and peaked at approximately 48 hours, which is in line with the data obtained from fluorescence-activated cell sorter (FACS) analysis (Figure 1D).
CD137 expression on human NK cells. NK cells were incubated in the presence or absence of 50 U of IL-2 or 10 ng/mL IL-15. (A) Up-regulation of CD137 and CD69 after the indicated times was analyzed by FACS using specific mAb and mouse IgG1 as isotype control. Numbers in dot plots indicate the percentage of CD137+ or CD69+ CD56+CD3− NK cells. (B) CD137 mRNA expression in freshly isolated, IL-15–activated (48 hours) and polyclonal NK cells was investigated by RT-PCR analysis of equal mRNA levels; 18S rRNA served as control. (C) Expression of CD137 and CD69 on polyclonal NK cells was analyzed by FACS as described in panel A. (D) Total RNA of freshly isolated NK cells after culture for the indicated times in the absence or presence of IL-15 was isolated and reverse transcribed. Relative copy numbers of CD137 were determined by quantitative PCR and normalized with 18S rRNA expression. (E) The percentage of CD137- and CD69-expressing CD56+CD3− NK cells among the PBMCs of 10 healthy donors and 20 patients with AML as obtained by FACS is shown for each investigated donor/patient.
CD137 expression on human NK cells. NK cells were incubated in the presence or absence of 50 U of IL-2 or 10 ng/mL IL-15. (A) Up-regulation of CD137 and CD69 after the indicated times was analyzed by FACS using specific mAb and mouse IgG1 as isotype control. Numbers in dot plots indicate the percentage of CD137+ or CD69+ CD56+CD3− NK cells. (B) CD137 mRNA expression in freshly isolated, IL-15–activated (48 hours) and polyclonal NK cells was investigated by RT-PCR analysis of equal mRNA levels; 18S rRNA served as control. (C) Expression of CD137 and CD69 on polyclonal NK cells was analyzed by FACS as described in panel A. (D) Total RNA of freshly isolated NK cells after culture for the indicated times in the absence or presence of IL-15 was isolated and reverse transcribed. Relative copy numbers of CD137 were determined by quantitative PCR and normalized with 18S rRNA expression. (E) The percentage of CD137- and CD69-expressing CD56+CD3− NK cells among the PBMCs of 10 healthy donors and 20 patients with AML as obtained by FACS is shown for each investigated donor/patient.
Since CD137 expression was found to be associated with NK-cell activation, and NK-cell activity may prevent disease progression in patients with leukemia,8,36 we next determined the expression of CD137 and CD69 on NK cells of patients with AML. Although virtually absent on the NK cells of 10 healthy donors, significantly (both P < .05; Mann-Whitney U test) enhanced CD137 and CD69 expression was detected on NK cells among the PBMCs of 20 patients with AML included in our study (Figure 1E). This indicated that signals via CD137 might affect NK-cell reactivity in patients with AML.
CD137L is expressed by AML cells
Since we had observed CD137 expression on NK cells of patients with AML, we next performed flow cytometry to determine whether CD137L was expressed on primary patient AML cells and thus might modulate NK-cell reactivity against leukemia by interacting with its cognate receptor. Malignant cells among the PBMCs of a total of 65 patients were selected by staining for CD33 or CD34 according to immunophenotyping. HLA class I surface expression was also determined. The clinical characteristics of each investigated patient with AML are given in Table 1. There were 25 women and 40 men (female-to-male ratio of 1:1.6), and the median age was 58 years (range, 18-84 years). The median peripheral blood blast count was 76% (range, 6%-98%). Bone marrow aspirates were available on all patients and were reviewed by an experienced hematologist. Adequate cytogenetic data were available for 53 (82%) of 65 patients, of which 27 (55%) had a normal karyotype.
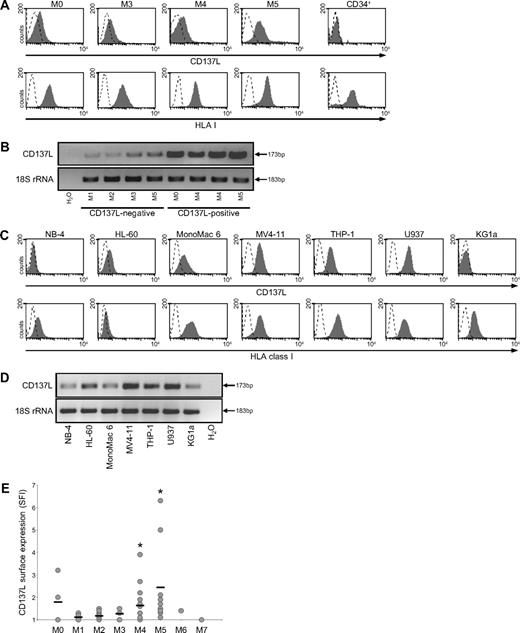
Patient AML cells expressed substantial levels of HLA class I. Intensities of CD137L cell-surface stainings were in general lower compared with HLA class I expression. We considered AML cells positive for CD137L in case of at least 1.5-fold increase of median fluorescence above background (SFI ≥ 1.5). Among the 65 investigated patients, 23 (35%) expressed substantial surface levels of CD137L (Table 1). No relevant CD137L expression was detected on CD34+ cells of 6 investigated healthy volunteers. In Figure 2A, CD137L surface expression on selected AML cells from patients and CD34+ cells of one exemplary healthy donor is shown. RT-PCR analyses using PBMCs from patients with AML with at least 80% blasts (uniform patient numbers [UPNs] 10, 12, 33, 56, 3, 43, 40, and 55) revealed CD137L amplicons in all investigated samples with substantial surface expression. Of note, low amplicons were also observed in patient samples that lacked CD137L surface expression on leukemia cells (Figure 2B). This could be due to CD137L expression on contaminating healthy cells, since, for example, healthy monocytes express substantial levels of CD137L (data not shown). To ascertain that in fact leukemic cells express CD137L, we used AML cell lines. We detected substantial CD137L surface expression in 5 of 7 investigated AML cell lines. Highly variable levels of HLA class I expression in AML cell lines were observed (Figure 2C). Of note, all AML cell lines, including the surface-negative NB-4 and KG1a cells, expressed CD137L mRNA, which may suggest that CD137L expression is regulated posttranscriptionally (Figure 2D).
CD137L expression in AML cells. (A) PBMCs from patients with AML and CD34+ cells of healthy donors were analyzed by FACS for CD137L and HLA class I expression using specific mAb (shaded peaks) and isotype control (open peaks). Malignant cells were selected as described in “Methods.” (B) RNA from the PBMCs of patients with AML was extracted, reverse transcribed, and investigated for CD137L expression by RT-PCR analysis of equal mRNA levels; 18S rRNA served as control. (C) CD137L and HLA class I surface expression on the indicated AML cell lines was investigated as described in panel A. (D) CD137L mRNA expression by the indicated AML cell lines was investigated as described in panel B. (E) SFI levels of CD137L surface expression on patient AML cells were determined by FACS. Patients were then subdivided according to FAB types. Association with specific subtypes was calculated by 1-way ANOVA. Horizontal bars indicate means within each group. *Statistically significant differences.
CD137L expression in AML cells. (A) PBMCs from patients with AML and CD34+ cells of healthy donors were analyzed by FACS for CD137L and HLA class I expression using specific mAb (shaded peaks) and isotype control (open peaks). Malignant cells were selected as described in “Methods.” (B) RNA from the PBMCs of patients with AML was extracted, reverse transcribed, and investigated for CD137L expression by RT-PCR analysis of equal mRNA levels; 18S rRNA served as control. (C) CD137L and HLA class I surface expression on the indicated AML cell lines was investigated as described in panel A. (D) CD137L mRNA expression by the indicated AML cell lines was investigated as described in panel B. (E) SFI levels of CD137L surface expression on patient AML cells were determined by FACS. Patients were then subdivided according to FAB types. Association with specific subtypes was calculated by 1-way ANOVA. Horizontal bars indicate means within each group. *Statistically significant differences.
Since CD137L expression patterns differed between individual patients, we studied whether CD137L was associated with certain AML French-American-British (FAB) types. Among the investigated patients with FAB M4 and M5, leukemia cells were found to be positive for CD137L in 9 of 17 and 8 of 11 patients, respectively. Within the groups of patients with FAB types M0, M2, and M3, CD137L expression was detectable in 2 of 4, 1 of 19, and 2 of 4 patients, respectively. No substantial CD137L expression was found within the 7 patients with FAB M1 and in the single investigated patients with FAB M6 and FAB M7 (Table 1; Figure 2E). Statistical analysis using 1-way analysis of variance (ANOVA) revealed that CD137L expression was in fact significantly (P < .01) associated with monocytic (FAB M4 and M5) differentiation (Figure 2E). No significant association of CD137L expression patterns with HLA class I expression, cytogenetic risk, a particular cytogenetic abnormalitiy, blast count, or white blood cell count was observed (data not shown). Taken together, our data demonstrate that CD137L is expressed on leukemic blasts in a substantial percentage of patients with AML, preferentially within cases with monocytic differentiation.
Signaling via CD137L induces cytokine release of AML cells
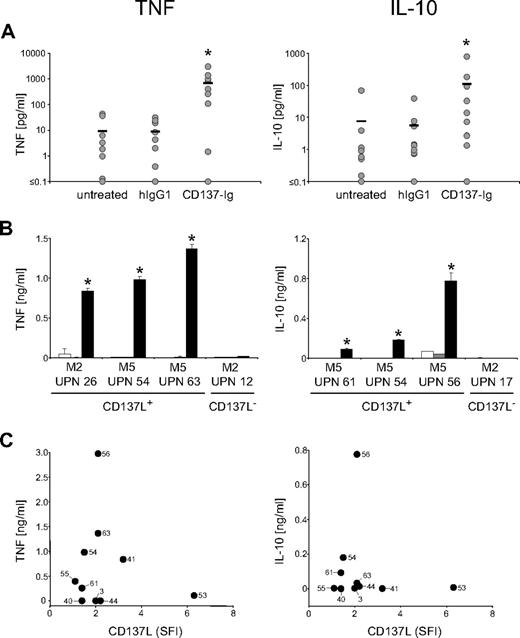
To study whether AML-expressed CD137L was functional, we stimulated CD137L on primary AML cells by incubation on immobilized CD137-Ig (which enables multimerization of CD137L) with human IgG1 as control and subsequently analyzed the culture supernatants by ELISA. CD137L signaling led to a substantial and significant (both P < .05; Mann-Whitney U test) induction of IL-10 and TNF production by AML cells after 12 hours and 6 hours, respectively, while isotype control had no effect (Figure 3A). It is noteworthy that enhanced cytokine production after CD137L stimulation was not only observed with AML cells of monocytic differentiation, but also with cells of undifferentiated FAB types. No effect of the CD137-Ig was observed when cells of patients with CD137L− AML were used, confirming that the effects of the CD137-Ig fusion protein observed with CD137L+ cells were specifically due to signaling via the CD137L (Figure 3B). Correlation analysis (Figure 3C) revealed that CD137L expression levels were not associated with the levels of IL-10 and TNF produced by the AML cells upon CD137-Ig stimulation (P = .62 and .95, respectively). Whereas the lacking correlation may be due to alterations in CD137L or its subsequent signaling pathways caused by the process of malignant transformation, it may also reflect distinct functional properties of the leukemic blasts from patients with varying FAB types of AML.
CD137L induces the release of immunosuppressive cytokines by AML cells. AML cells of patients with more than 80% blasts were cultured alone, on immobilized CD137-Ig or on human IgG1 as control. Levels of the indicated cytokines in culture supernatants were determined by ELISA. (A) Results of 10 investigated patients are shown. Horizontal bars represent the mean of the results obtained with each indicated culture condition. (B) Exemplary results (means of triplicates with SDs) with AML cells of single patients (□ indicates untreated;  , human IgG1; ■, CD137-Ig). (C) Correlation between CD137L SFI levels and cytokine production upon CD137-Ig stimulation. Numbers in diagrams represent the UPNs of the patients used. *Statistically significant differences.
, human IgG1; ■, CD137-Ig). (C) Correlation between CD137L SFI levels and cytokine production upon CD137-Ig stimulation. Numbers in diagrams represent the UPNs of the patients used. *Statistically significant differences.
CD137L induces the release of immunosuppressive cytokines by AML cells. AML cells of patients with more than 80% blasts were cultured alone, on immobilized CD137-Ig or on human IgG1 as control. Levels of the indicated cytokines in culture supernatants were determined by ELISA. (A) Results of 10 investigated patients are shown. Horizontal bars represent the mean of the results obtained with each indicated culture condition. (B) Exemplary results (means of triplicates with SDs) with AML cells of single patients (□ indicates untreated;  , human IgG1; ■, CD137-Ig). (C) Correlation between CD137L SFI levels and cytokine production upon CD137-Ig stimulation. Numbers in diagrams represent the UPNs of the patients used. *Statistically significant differences.
, human IgG1; ■, CD137-Ig). (C) Correlation between CD137L SFI levels and cytokine production upon CD137-Ig stimulation. Numbers in diagrams represent the UPNs of the patients used. *Statistically significant differences.
Together, our data demonstrate that CD137L can be functionally expressed by AML cells and can transduce signals resulting in the production of cytokines that may influence immunoediting by AML cells.
CD137-CD137L interaction impairs antileukemia reactivity of human NK cells
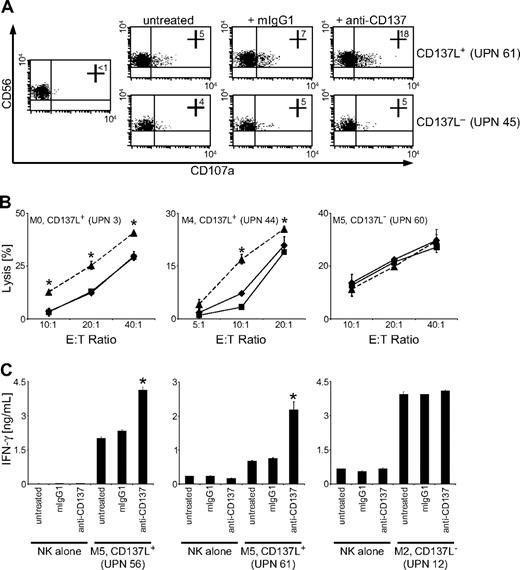
To determine how the interaction of AML-expressed CD137L with CD137 influenced antileukemia reactivity of NK cells in humans, we cultured CD137L+ primary AML cells with CD137-expressing allogenic polyclonal NK cells and determined expression of CD107a as a surrogate marker for NK-cell granule mobilization. Surprisingly, CD107a expression on NK cells in cultures with CD137L-expressing AML cells was substantially enhanced in the presence of an anti-CD137 mAb with defined capacity to block CD137-CD137L interaction (Figure 4A).32 This inhibitory effect of AML-expressed CD137L on NK-cell reactivity was also observed in Europium cytotoxicity assays: a substantial and statistically significant (P < .05, Student t test) increase of lysis of CD137L-expressing primary AML cells was observed by blocking CD137 on polyclonal NK cells (Figure 4B). Furthermore, we found that CD137L on AML cells also impaired NK-cell IFN-γ production. NK cells alone produced low levels of IFN-γ, which were not altered by the blocking CD137 mAb. Presence of AML cells markedly increased the levels of IFN-γ, and the production of IFN-γ was significantly (P < .05, Student t test) enhanced in the presence of the blocking CD137 mAb (Figure 4C). No effect of CD137 blockade on NK-cell granule mobilization, cytotoxicity, or cytokine production was observed when CD137L− AML cells were used as targets, which excluded that unspecific effects of the mAb were responsible for enhanced NK-cell reactivity (Figure 4A-C). Together, these data indicate that triggering of CD137 by AML-expressed CD137L impairs NK cell–mediated immunosurveillance of leukemia in humans.
CD137 blockade enhances NK-cell reactivity against CD137L-expressing AML cells. Allogenic polyclonal NK cells were cultured with or without CD137L-expressing or CD137L− AML cells of patients with more than 80% blast count in the presence or absence of 10 μg/mL mouse IgG1 or blocking CD137 mAb. (A) Granule mobilization after 3 hours was analyzed by FACS for CD107a expression. Numbers in top right quadrants indicate the percentage of CD107a+ NK cells. (B) Cytotoxicity of NK cells alone (♦) or in the presence of mouse IgG1 (■) or CD137 mAb (▲) was evaluated by 2-hour Europium assays. (C) IFN-γ levels in culture supernatants were determined after 24 hours by ELISA. Means of triplicates with SDs of 1 representative experiment each of a total of at least 3 with similar results are shown. *Statistically significant differences.
CD137 blockade enhances NK-cell reactivity against CD137L-expressing AML cells. Allogenic polyclonal NK cells were cultured with or without CD137L-expressing or CD137L− AML cells of patients with more than 80% blast count in the presence or absence of 10 μg/mL mouse IgG1 or blocking CD137 mAb. (A) Granule mobilization after 3 hours was analyzed by FACS for CD107a expression. Numbers in top right quadrants indicate the percentage of CD107a+ NK cells. (B) Cytotoxicity of NK cells alone (♦) or in the presence of mouse IgG1 (■) or CD137 mAb (▲) was evaluated by 2-hour Europium assays. (C) IFN-γ levels in culture supernatants were determined after 24 hours by ELISA. Means of triplicates with SDs of 1 representative experiment each of a total of at least 3 with similar results are shown. *Statistically significant differences.
CD137 differentially affects reactivity of NK cells in mice and men
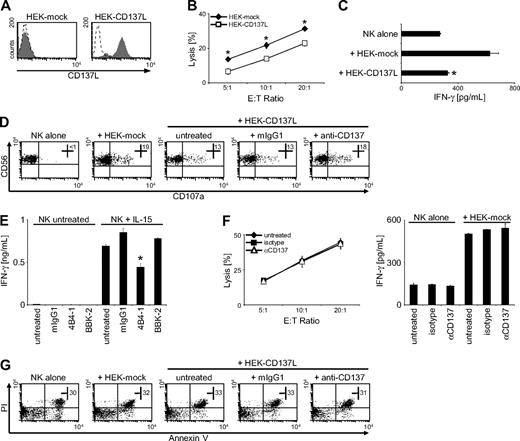
In light of available data reporting on a stimulatory role of CD137 in T cells and mouse NK cells, we aimed to ascertain that CD137 in fact inhibits effector functions of human NK cells. Thus, we used human HEK cells that do not constitutively express CD137L (or CD137; data not shown) to generate transfectants expressing human CD137L. As shown in Figure 5A, the HEK-CD137L transfectants expressed high levels of CD137L, whereas the respective mock-transfected HEK cells did not display CD137L expression. Chromium release assays with these HEK transfectants revealed that lysis by CD137-expressing polyclonal NK cells was substantially lower when CD137L was expressed on the target cells, confirming that CD137-CD137L interaction significantly (P < .01, Student t test) reduces cytotoxicity of human NK cells (Figure 5B). Similarly, ELISA of supernatants after 24 hours of coculture revealed that IFN-γ production of NK cells in cultures with HEK-CD137L cells was significantly (P < .01, Student t test) lower compared with cultures with the mock-transfectants (Figure 5C). Moreover, NK-cell degranulation was reduced by CD137L expression on HEK transfectants compared with mock controls and could be restored by addition of the blocking CD137 mAb (Figure 5D). Next, we wanted to ascertain that the observed inhibitory effect of CD137L on NK-cell reactivity was in fact due to transduction of inhibitory signals via CD137. We cultured human NK cells for 24 hours alone or with IL-15, which induces CD137 expression and IFN-γ production and induced CD137 signaling by incubation on immobilized agonistic anti-CD137 mAb (4B4-1) in the absence of target cells. Mouse IgG1 and the nonagonistic blocking mAb served as controls. In the absence of IL-15, NK cells did not produce substantial levels of IFN-γ, and no effect of the different antibodies was observed. Presence of IL-15 substantially induced IFN-γ release, which was significantly (P < .05, Student t test) reduced in the presence of agonistic anti-CD137 mAb, whereas isotype control or the blocking CD137 antibody (BBK-2) had no inhibitory effect (Figure 5E). This excluded that the reduced reactivity of CD137-expressing NK cells after interaction with tumor-expressed CD137L was solely caused by the potential effects of CD137L signaling into the tumor cells.
CD137 impairs antitumor reactivity of human NK cells. (A) CD137L surface expression on HEK cells transfected with human CD137L (HEK-CD137L) and the respective mock controls (HEK-mock) was determined by FACS using CD137L mAb (shaded peaks) and isotype control (open peaks). (B) Cytotoxicity of polyclonal NK cells in cultures with the HEK transfectants was evaluated by 4-hour chromium release assays. (C) IFN-γ levels in cultures of polyclonal NK cells and HEK transfectants were determined after 24 hours by ELISA. (D) Granule mobilization of polyclonal NK cells after 3-hour cultures with HEK transfectants in the presence or absence of 10 μg/mL mouse IgG1 or blocking CD137 mAb was analyzed by FACS. Numbers in top right quadrants indicate the percentage of CD107a+ NK cells. (E) Freshly isolated NK cells were cultured for 24 hours with or without 10 ng/mL IL-15 followed by incubation for additional 24 hours with or without IL-15 in the presence or absence of immobilized agonistic (4B4-1) and blocking (BBK-2) CD137 mAb or isotype control. Subsequently, IFN-γ levels in culture supernatants were determined by ELISA. (F) Polyclonal NK cells were incubated for 24 hours in medium alone or on immobilized agonistic CD137 mAb or isotype control. The respective culture supernatants (untreated, IgG1, and αCD137, respectively) were then harvested, and fresh polyclonal NK cells of the same donor were incubated with HEK-mock cells using the different supernatants as culture medium. Cytotoxicity was analyzed after 4 hours by chromium release assays (left), and IFN-γ production was determined after 24 hours by ELISA (right). (G) Apoptosis of polyclonal NK cells was determined after culture with HEK transfectants for 48 hours by FACS for Annexin V/PI. Numbers in dot plots indicate the percentage of Annexin V/PI+ CD56+CD3− cells. One representative experiment each of a total of at least 3 with similar results is shown. Results shown in panels B, C, E, and F represent means of triplicates with SDs. *Statistically significant differences.
CD137 impairs antitumor reactivity of human NK cells. (A) CD137L surface expression on HEK cells transfected with human CD137L (HEK-CD137L) and the respective mock controls (HEK-mock) was determined by FACS using CD137L mAb (shaded peaks) and isotype control (open peaks). (B) Cytotoxicity of polyclonal NK cells in cultures with the HEK transfectants was evaluated by 4-hour chromium release assays. (C) IFN-γ levels in cultures of polyclonal NK cells and HEK transfectants were determined after 24 hours by ELISA. (D) Granule mobilization of polyclonal NK cells after 3-hour cultures with HEK transfectants in the presence or absence of 10 μg/mL mouse IgG1 or blocking CD137 mAb was analyzed by FACS. Numbers in top right quadrants indicate the percentage of CD107a+ NK cells. (E) Freshly isolated NK cells were cultured for 24 hours with or without 10 ng/mL IL-15 followed by incubation for additional 24 hours with or without IL-15 in the presence or absence of immobilized agonistic (4B4-1) and blocking (BBK-2) CD137 mAb or isotype control. Subsequently, IFN-γ levels in culture supernatants were determined by ELISA. (F) Polyclonal NK cells were incubated for 24 hours in medium alone or on immobilized agonistic CD137 mAb or isotype control. The respective culture supernatants (untreated, IgG1, and αCD137, respectively) were then harvested, and fresh polyclonal NK cells of the same donor were incubated with HEK-mock cells using the different supernatants as culture medium. Cytotoxicity was analyzed after 4 hours by chromium release assays (left), and IFN-γ production was determined after 24 hours by ELISA (right). (G) Apoptosis of polyclonal NK cells was determined after culture with HEK transfectants for 48 hours by FACS for Annexin V/PI. Numbers in dot plots indicate the percentage of Annexin V/PI+ CD56+CD3− cells. One representative experiment each of a total of at least 3 with similar results is shown. Results shown in panels B, C, E, and F represent means of triplicates with SDs. *Statistically significant differences.
Next, we aimed to exclude that the inhibitory effects of CD137 on NK-cell reactivity were due to induction of soluble factors causing paracrine inhibitory effects within the NK-cell population. Thus, we cultured polyclonal NK-cells alone, on immobilized agonistic CD137 mAb or isotype control, and harvested the culture supernatants after 24 hours. We then performed functional analyses within these different culture supernatants using fresh polyclonal NK cells from the same donor as effectors and the CD137L− HEK-mock cells as targets. As shown in Figure 5F, supernatants obtained with CD137-stimulated NK cells did not alter target cell lysis or IFN-γ production in this experimental setting. Together, these data excluded that the mAb used for blocking CD137-CD137L interaction in experiments with AML cells directly affected NK-cell reactivity and confirmed that the reduction of NK effector functions after interaction with CD137L was due to transduction of inhibitory signals via CD137 into NK cells and not, at least not in great part, due to signaling into tumor cells or paracrine effects.
Next, we performed cocultures of CD137-expressing polyclonal NK cells with the HEK transfectants and searched for alterations in the number of apoptotic NK cells. Annexin V/propidium iodide (PI) staining of NK cells after 24 and 48 hours of coculture did not reveal relevant differences in the numbers of apoptotic NK cells depending on CD137L expression (Figure 5G and data not shown). Thus, impaired NK-cell reactivity after CD137 triggering is not due to induction of apoptosis but rather to transduction of inhibitory signals.
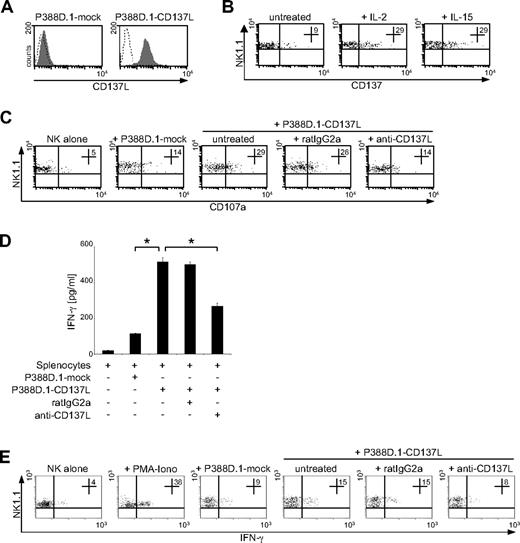
Now, we aimed to determine whether CD137 in fact mediates different effects in mice and men and set out to characterize the role of CD137 in antitumor immunity of mouse NK cells. To this end, we used a CD137/CD137L− mouse cell line (P388D.1) to generate CD137L and mock-transfectants. As shown in Figure 6A, our P388D.1-CD137L transfectants expressed substantial levels of mouse CD137L, while the respective mock-transfected P388D.1 cells did not reveal relevant CD137L expression. To study the expression and regulation of the receptor CD137 on mouse NK cells, we studied splenocytes of C57/BL6 mice using FACS analysis with anti-NK1.1 doublestaining and exclusion of CD3+ mouse T cells. In line with the findings in human NK cells, CD137 was virtually absent on freshly isolated resting mouse NK cells, and a weak up-regulation of expression, possibly due to the isolation procedure and in vitro culture, was observed after 48 hours. In contrast, substantial levels of expression were detectable on the mouse NK cells after treatment with IL-2 and IL-15 for 48 hours (Figure 6B). Next, we performed degranulation assays using mouse splenocytes pretreated with IL-15 for 48 hours to induce CD137 expression and our P388D.1 transfectants as targets. Substantially higher CD107a expression levels as surrogate marker for cytotoxicity were observed on mouse NK1.1+CD3− NK cells in cultures with P388D.1 cells expressing mouse CD137L compared with cultures with the mock-transfectants. Blocking interaction of mouse CD137L with its receptor in cultures with P388D.1-CD137L transfectants reduced degranulation of mouse NK cells, whereas isotype control had no marked effect (Figure 6C). Furthermore, we cultured activated mouse splenocytes with P388D.1-CD137L or P388D.1–mock-transfectants and analyzed the IFN-γ levels in culture supernatants after 24 hours by ELISA. Expression of mouse CD137L on transfectants resulted in significantly enhanced cytokine levels, and this stimulatory effect of mouse CD137L was significantly (both P < .01, Student t test) reduced by antibody blocking of CD137-CD137L interaction, while isotype control had no effect (Figure 6D). To ascertain that in fact IFN-γ production of NK cells among the activated splenocytes was enhanced by expression of the mouse CD137L on the transfectants, we analyzed IFN-γ in NK1.1+CD3− NK cells using intracellular FACS. A substantially higher percentage of IFN-γ–secreting NK cells was detected in cultures with the P388D.1-CD137L transfectants compared with cultures with the mock-transfected controls, which could again be abolished by blocking CD137-CD137L interaction.
CD137 enhances antitumor reactivity of mouse NK cells. (A) CD137L surface expression on P388D.1 cells transfected with mouse CD137L (P388D.1-CD137L) and mock controls (P388D.1-mock) was determined by FACS using CD137L mAb (shaded peaks) and isotype control (open peaks). (B) Mouse splenocytes were cultured for 48 hours in the presence or absence of 250 U/mL human IL-2 or 10 ng/mL human IL-15. Subsequently, CD137 expression was determined using CD137 mAb and rat IgG1 isotype control. Numbers in top right quadrants indicate the percentage of CD137+ NK1.1+CD3− NK cells. (C-E) Mouse splenocytes were cultured for 48 hours with 250 U/mL human IL-2 and then incubated with P388D.1 transfectants in the presence or absence of 10 μg/mL rat IgG2a or blocking CD137L mAb. (C) Granule mobilization after 3 hours was analyzed by FACS. Numbers in top right quadrants indicate the percentage of CD107a+ NK1.1+CD3− mouse NK cells. (D) IFN-γ levels in culture supernatants were determined after 24 hours by ELISA. Results represent means of triplicates with SDs. (E) Frequency of IFN-γ–producing mouse NK cells was determined after 8 hours by intracellular FACS. Stimulation with PMA/ionomycin served as positive control. Numbers in top right quadrants indicate percent of IFN-γ+ NK1.1+CD3− NK cells. One representative experiment each of a total of at least 3 with similar results is shown. *Statistically significant differences.
CD137 enhances antitumor reactivity of mouse NK cells. (A) CD137L surface expression on P388D.1 cells transfected with mouse CD137L (P388D.1-CD137L) and mock controls (P388D.1-mock) was determined by FACS using CD137L mAb (shaded peaks) and isotype control (open peaks). (B) Mouse splenocytes were cultured for 48 hours in the presence or absence of 250 U/mL human IL-2 or 10 ng/mL human IL-15. Subsequently, CD137 expression was determined using CD137 mAb and rat IgG1 isotype control. Numbers in top right quadrants indicate the percentage of CD137+ NK1.1+CD3− NK cells. (C-E) Mouse splenocytes were cultured for 48 hours with 250 U/mL human IL-2 and then incubated with P388D.1 transfectants in the presence or absence of 10 μg/mL rat IgG2a or blocking CD137L mAb. (C) Granule mobilization after 3 hours was analyzed by FACS. Numbers in top right quadrants indicate the percentage of CD107a+ NK1.1+CD3− mouse NK cells. (D) IFN-γ levels in culture supernatants were determined after 24 hours by ELISA. Results represent means of triplicates with SDs. (E) Frequency of IFN-γ–producing mouse NK cells was determined after 8 hours by intracellular FACS. Stimulation with PMA/ionomycin served as positive control. Numbers in top right quadrants indicate percent of IFN-γ+ NK1.1+CD3− NK cells. One representative experiment each of a total of at least 3 with similar results is shown. *Statistically significant differences.
Together, these data clearly demonstrate that CD137 inhibits cytotoxicity and cytokine production of human NK cells, while effector functions of mouse NK cells are activated by signals transduced by its mouse counterpart. Thus, CD137 in fact mediates opposite effects in human compared with mouse NK cells.
Discussion
The development of clinically apparent malignant disease is largely dependent on the interaction of tumor cells with the immune system, a reciprocal process that substantially influences whether transformed cells are eliminated or progress to a life-threatening malignancy.37 Among others, many members of the TNF/TNFR family have been shown to play an important role in host-tumor interaction.13,14 In this study, we addressed the yet unknown role of the TNF family member CD137L in human AML and the consequences of CD137-CD137L interaction for NK cell functions in humans compared with mice.
CD137 was originally identified as a molecule expressed on activated T cells and has subsequently also been shown to be functionally expressed on activated mouse NK cells.15,26,27 Surprisingly little is yet known regarding CD137 on NK cells in humans. We found that CD137 is up-regulated on human NK cells upon cytokine activation, while being virtually absent in resting state. In agreement with previously reported findings,26 a similar expression pattern was observed with mouse NK cells. Of note, Lin and coworkers reported that CD137 is expressed on human NK cells after combined stimulation with Fc-receptor triggering and IL-2, and only inconsistently with IL-2 stimulation alone.36 This discrepancy to our findings is most likely due to differences in experimental procedures.
The cognate ligand of CD137, CD137L, is mainly expressed on antigen-presenting cells like macrophages, dendritic cells (DCs), and B cells in mice and men, but has also been found in several other healthy tissues.15 We have previously shown that human carcinoma cells can express CD137L, and Palma and coworkers reported on CD137L expression in cell lines of lymphatic origin.28,29 Here, we demonstrate that a substantial proportion of AML cell lines and primary AML cells from patients display CD137L expression. Interestingly, the presence of CD137L mRNA in AML cells did not always translate into detectable CD137L surface levels. Although contamination with CD137L+ healthy cells may explain these findings within the PBMCs of patients with AML, the fact that similar results were obtained with AML cell lines may be indicative of a potential regulatory or mutational blockade of CD137L surface expression. Of note, surface expression of CD137L on malignant cells can be reduced by shedding, and elevated levels of soluble CD137L have been detected in sera of patients with leukemia.33 This may explain why some AML cells displayed no substantial surface expression despite expression of CD137L mRNA. Although CD137L surface expression varied between individual patients, it was significantly associated with monocytic differentiation (FAB types M4/5), which is in line with the observation that healthy cells of the myeloid lineage like monocytes, macrophages, and DCs express high levels of CD137L.15
Two recent studies demonstrated that CD137L is expressed on hematopoietic stem/progenitor cells in mice.38,39 Lee and coworkers reported that CD137L regulates myelopoiesis and inhibits differentiation of mouse hematopoietic progenitor cells.38 Thus, it is tempting to speculate on a potential role of CD137L in blocking differentiation of AML blasts and thus AML pathogenesis. However, the group of Schwarz demonstrated that CD137L stimulates proliferation and differentiation of mouse hematopoietic progenitors and reported similar effects in the very small subset of CD34+ cells found to express CD137L in humans.39,40 Thus, the definite role of CD137L in healthy and malignant myelopoiesis requires further elucidation, especially since we did not detect substantial levels of CD137L on healthy human CD34+ cells.
The ability to signal bidirectionally is a characteristic feature of many ligands of the TNF family, and CD137L signaling has been shown to alter cytokine production of healthy myeloid cells, but also of carcinoma cells.29,41 We report here that signaling via CD137L into AML patient blasts stimulates the release of IL-10 and TNF. Whereas IL-10 has been shown to mediate immunosuppression (eg, by inhibition of T-cell cytotoxicity and DC functions or by favoring the induction and differentiation of regulatory T cells),42 TNF is a molecule with potent immunomodulatory properties that largely depend on the cellular context and cell type. Although it mediates profound proinflammatory effects and is considered a prototypical proinflammatory cytokine, TNF can also cause immunosuppressive effects such as induction of lymphopenia or inhibition of T-cell signaling and DC functions.43 AML-expressed CD137L may thus substantially affect the reciprocal interaction of the leukemia cells with the immune system. Moreover, human CD137L also directly diminishes the antileukemia reactivity of NK cells: blocking CD137 in cocultures of CD137L-expressing AML cells with allogenic human NK cells significantly increased their granule mobilization, cytotoxicity, and IFN-γ production.
Because CD137 was reported to stimulate mouse NK cells, we aimed to ascertain that CD137 in fact caused opposite effects in NK cells in mice and men. To this end, we used human and mouse cell lines transfected to express human and mouse CD137L in cocultures with human NK cells and NK cells among mouse splenocytes, respectively. In the mouse system, target cell–expressed CD137L stimulated both NK-cell cytokine production and granule mobilization as a surrogate marker for cytotoxicity. This is partially in contrast to the findings of Melero and coworkers, who observed stimulation of cytokine production but not cytotoxicity of NK cells by mouse CD137,26 and may most likely be attributed to differing experimental conditions. Reactivity of human NK cells was, in line with our data obtained with AML blasts, reduced by expression of CD137L on transfectants. Moreover, the inhibitory effect of CD137 on human NK cells was confirmed in experiments using agonistic mAb for triggering of CD137 in the absence of CD137L-expressing tumor cells.
Available data indicate that CD137L also causes opposite effects on the antitumor immunity of human T cells compared with NK cells.29,44 Moreover, Houtenbos and coworkers demonstrated recently that addition of CD137L to cocultures of AML-derived DCs and T cells efficiently enhanced T-cell effector functions. T cells primed in the presence of CD137L efficiently recognized and lysed leukemic blasts.45 This indicates that CD137-CD137L interaction, in contrast to our findings with NK cells, enhances reactivity of T cells against AML cells. While our results clearly demonstrate that CD137 differentially affects human and mouse NK cells and inhibits reactivity of human NK cells, our study does not unravel the underlying intracellular pathways and molecular mechanisms, which require further elucidation. Of note, the same agonistic CD137 mAb that potently stimulated antitumor activity in mice has been shown to prevent or ameliorate various autoimmune conditions.46 CD137 can thus apparently mediate both inhibitory and stimulatory effects, which may depend on the cell type, the disease, and/or the levels of immune response.15,47 Our approach of using primary AML blasts and allogenic NK cells is well-suited to elucidate the role of the CD137-CD137L system in human NK-cell antitumor responses, because it mimics the situation in patients with leukemia after SCT. As NK cells may potently combat leukemia and prevent leukemic relapse,3-5,10 it is important to define whether a given leukemia is susceptible to NK-cell responses, and numerous attempts are presently made to engraft NK cells in the treatment of leukemia by approaches or interventions that prevent NK-cell suppression or stimulate NK-cell reactivity.48,49 Based on encouraging results obtained in mouse tumor models with systemic administration of agonistic anti-CD137 mAb,16-22 clinical phase 1/2 trials using humanized agonistic anti-CD137 mAb in patients with cancer have been initiated (www.clinicaltrials.gov; keyword CD137). Results are not yet available, but considering our data, it is questionable whether mouse models may in fact serve well as predictors of the effects that will be encountered in humans. The data presented in this study implicate that one should exercise caution in applying anti-CD137 therapeutically, and great care is still needed in the transition of CD137 agonists from preclinical work to the treatment of patients with cancer or autoimmune diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ingrid Kumbier and Carolin Fenner for excellent technical assistance.
This work was supported by grants from the German Research Foundation (SA 1360/4-1) and the Wilhelm Sander Stiftung (2007.115.1).
Authorship
Contribution: T.B. and J.E.C. performed the majority of the experiments; B.J.S., F.G., M.K., and A.W. contributed some of the experiments; H.-G.R. designed some of the experiments and contributed to the writing of the paper; and H.R.S. designed the project and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helmut Salih, Department of Hematology and Oncology, Eberhard Karls-University, Otfried-Mueller Str 10, 72076 Tuebingen, Germany; e-mail: helmut.salih@med.uni-tuebingen.de.
References
Author notes
T.B. and J.E.C. contributed equally to this work.