Abstract
One of the most puzzling observations in HIV research is the lack of pathogenicity in most nonhuman primate species that are natural hosts of simian immunodeficiency virus (SIV) infection. Despite this, natural hosts experience a level of viremia similar to humans infected with HIV or macaques infected with SIV. To determine the role of adaptive immune responses in viral containment and lack of disease, we delayed the generation of cellular and humoral immune responses by administering anti-CD8– and anti-CD20 lymphocyte–depleting antibodies to sabaeus African green monkeys (Chlorocebus sabaeus) before challenge with SIVsab9315BR. In vivo lymphocyte depletion during primary infection resulted in a brief elevation of viremia but not in disease. Based on the magnitude and timing of SIV-specific CD8+ T-cell responses in the lymphocyte-depleted animals, CD8+ T-cell responses appear to contribute to viral containment in natural hosts. We found no evidence for a contribution of humoral immune responses in viral containment. These studies indicate that natural hosts have developed mechanisms in addition to classic adaptive immune responses to cope with this lentiviral infection. Thus, adaptive immune responses in natural hosts appear to be less critical for viral containment than in HIV infection.
Introduction
AIDS virus infections in most non-natural hosts, including humans and Asian nonhuman primates, ultimately result in overt signs of disease and immune failure. However, there is strong evidence that adaptive immune responses contribute to partial containment of viremia in these species.1 Correlative investigations have shown that the early containment of viremia in humans and rhesus macaques coincides with the expansion of AIDS virus-specific CD8+ T-cell responses.2-4 More direct evidence for the role of CD8+ T cells in viral containment was obtained through in vivo depletion experiments in nonhuman primates, such as simian immunodeficiency virus (SIV) infection of rhesus macaques.5,6 Furthermore, SIV-challenged rhesus macaques vaccinated with vaccines that primarily elicit CD8+ T-cell responses experience more efficient viral containment and significantly longer survival than sham-vaccinated control animals.7
The role of humoral immune responses is more difficult to evaluate. HIV-specific B cells produce HIV envelope-specific antibodies as early as 2 weeks after infection.8 However, neutralizing antibodies (NAbs) develop only after months and are initially highly specific for the autologous transmitted/founder virus. NAbs also mostly fail to neutralize the contemporaneous virus during chronic infection because the virus rapidly evolves to escape autologous neutralization.9,10 Similarly, rhesus macaques infected with SIV are slow to develop NAbs and experience continued viral escape.11,12 However, the potential potency of humoral immune responses has been demonstrated by complete protection of rhesus macaques from pathogenic SHIV89.6P challenge after passive transfer of broadly NAbs.13,14 Recently, a limited number of studies have evaluated the role of B cell–mediated immune responses in SIV containment in rhesus macaques.15-17 Humoral immune responses did not contribute to viral containment in primary infection,15 but an accelerated disease progression was observed in some studies when B-cell function was impaired by anti-CD20 antibody administrations, suggesting that B cells are important during the chronic stages of infection.16
At least 40 different African apes and monkey species are natural hosts of lentivirus infections. Unlike humans and macaques, these animals only rarely show signs of disease.18,19 The general lack of pathogenicity in these species is still not completely understood. Recent studies in natural hosts of SIV demonstrate a similar dynamic of primary viremia, level of peak viremia, and eventual set point viremia compared with pathogenic models.20,21 Natural hosts, such as African green monkeys (AGMs) and sooty mangabeys, appear to have undergone evolutionary adaptations that enable these species to better cope with AIDS virus infections. These adaptations include the lack of chronic immune activation,22,23 a paucity of SIV target cells (CCR5+CD4+ T cells)24 and the apparent presence of a unique subset of T cells that provide helper function in the absence of CD4 expression.25,26
Prior studies suggest that adaptive immune responses seem to play a limited role in containing SIV in chronic infection in natural hosts.27,28 CD8+ T-cell responses are generally less robust than in pathogenic SIV infection and do not correlate with plasma viral load.28-30 Furthermore, CD8+ lymphocyte depletion in chronic infection in AGMs and sooty mangabeys resulted in a variable outcome with modest increase in viremia in some studies31 (R.C.Z., M.D.R., E. Hagan, R.W., A.C., V.M.H., M. C. Gauduin, J.S.A., and J.E.S., unpublished observations, September 2009) and a more significant increase in another study (A.K., Z. Wang, N. Kassis, R. M. Ribeiro, S.P., R. P. Johnson, K. Reimann, J.E.S., A. S. Perelson, H. M. McClure, and S. P. O'Neill, unpublished observations, October 2003). Humoral immune responses in chronic infection are mainly directed against Env, and only low titers of NAbs are elicited.27,32
Here, we used sabaeus AGMs (Chlorocebus sabaeus) to evaluate the role of the early development of adaptive immune responses in viral containment and lack of disease progression. We depleted CD8+ lymphocytes and B cells in vivo with monoclonal antibodies (mAb) to delay the generation of both CD8+ T-cell and humoral immune responses during primary infection.
Methods
Animals and viruses
A total of 12 sabaeus AGMs (C sabaeus) were recruited for this study. Three animals were imported from St Kitts in the Caribbean and 9 were purchased from New Iberia, LA. The animals were infected with an equivalent of 143 ng of p27 tissue-culture supernatant of Molt4(cl8) cells infected with SIVsab9315BR. This virus was originally isolated from cell-free homogenized brain fluid from a naturally infected sabaeus AGM.33 Lymph nodes were obtained by excisional biopsy under anesthesia with Telazol. For all other procedures, animals were sedated with ketamine hydrochloride. All animals were maintained in accordance with the guidelines of the Committee on the Care and Use of Laboratory Animals under a National Institute of Allergy and Infectious Diseases (NIAID)–approved animal study protocol,34 and all studies and procedures were reviewed and approved by the Institutional Animal Care and Use Committees of the National Institutes of Health (NIH) and Harvard University.
CD8+ and CD20+ lymphocyte depletion
Six AGMs were transiently depleted of CD8+ and CD20+ lymphocytes by the administration of the chimeric anti–human CD8α mAb, cM-T807 (NIH Nonhuman Primate Reagent Resource) and the anti–human CD20 mAb, Rituxan (rituximab), purchased from Genentech. cM-T807 was administered at 10 mg/kg body weight subcutaneously on day 0 (the day of SIV infection) followed by 5 mg/kg intravenous injections on days 3 and 7. Rituximab was administered intravenously at 50 mg/kg body weight on days −7, 14, and 35. One dose of human immunoglobulin (IgIV; NIH Nonhuman Primate Reagent Resource) was administered intravenously at 50 mg/kg on days 0 to 6 AGMs to serve as a control.
The effect of the lymphocyte-depleting antibody treatment and SIV infection on lymphocyte subset changes was determined by flow cytometric investigations using an LSR II instrument (BD Biosciences) and FlowJo software (TreeStar; supplemental Methods, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Plasma viral load assay
In brief, SIVsab9315BR plasma RNA levels were quantified by the Ultrasense One-Step Quantitative RT-PCR System (Invitrogen; also see supplemental Methods).
Amplification and cloning of the envelope and rev SIVsab9315BR DNA cassettes and serum-NAb assays
In brief, Env-rev cassettes were cloned from DNA obtained from cultured peripheral blood mononuclear cells (PBMCs) after infection with an animal challenge stock of molecularly cloned SIVsab9315BR. An assay stock of molecularly cloned SIVsab92315BR Env-pseudotyped viruses was prepared by transfection in 293T cells and was titrated in TZM-bl cells as described.35 Neutralization was measured as a function of reduction in luciferase reporter gene expression after a single round of infection in TZM-bl cells as described35 (also see supplemental Methods).
Cellular immune response
The interferon-γ (IFN-γ) ELISpot assay and the intracellular cytokine staining (ICS) assay was performed as previously described.29 The ICS was modified to accommodate a PBMC stimulation time of 9 hours that permits a greater sensitivity to detect cytokine responses compared with a 6-hour incubation period. The peptide pools for stimulation of AGM-derived PBMC in both assays consisted of overlapping 15-mer peptides spanning the SIVsab92018 Env protein or the Gag protein. A total of 2 μg/mL SIVsab92018 Gag pool or 2 μg/mL SIVsab92018 Env peptide pools (Mimotopes, and NIH/NIAID Reagent Resource Support Program for AIDS Vaccine Development, Quality Biological; R. L. Brown, principal investigator) was used for PBMC stimulations.
Statistical analyses
Statistical analyses and graphical presentations were computed with GraphPad Prism, Version 5.02 (GraphPad Prism software). P values of less than .05 were considered significant. Mann-Whitney tests were applied for comparison of 2 groups. A Spearman correlation test was performed to analyze the association between plasma viral RNA loads and various parameters (including absolute CD4+ T-cell counts and memory CD4+ T cells).
Results
Administration of cM-T807 and rituximab to sabaeus AGMs induces temporal depletion of CD8+ and CD20+ lymphocytes in peripheral blood and lymphatic tissues
To assess the role of adaptive immune responses in the control of SIV infection in sabaeus AGMs, we used CD8+ and CD20+ lymphocyte depletion to temporarily delay adaptive immune responses during primary SIVsab9315BR infection in 6 AGMs. A control group of 6 animals was also inoculated with SIVsab9315BR but received IgIV instead of the lymphocyte-depleting antibodies.
The CD8+ lymphocyte depletion resulted in an efficient elimination of CD8+ T cells in peripheral blood for 3 weeks in 5 of 6 animals (Figure 1B). A brief depletion of CD8+ T cells (1 week) was observed in 1 animal (no. 364). Similarly, we observed a near-total depletion of CD8+ T cells in lymph nodes at day 14 after infection (Figure 1D). As CD8+ T cells reappeared in peripheral blood, CD8+ T cells also reappeared in lymph nodes on weeks 5 and 10 after infection (Figure 1D). In contrast, significant changes in CD8+ T cells were not observed in the 6 IgIV-treated control AGMs (Figure 1A,C). Interestingly, all of the AGMs with efficient CD8+ lymphocyte depletion had a transient 2.5- to 5.0-fold (median, 4.2-fold) increase of CD8+ T-cell counts for 3 to 13 weeks after the reappearance of CD8+ T cells. The relatively high levels of CD8+ T cells slowly returned to pretreatment levels, with the exception of animal no. 366, which maintained high levels of CD8+ T cells until week 42 after infection. This massive expansion of CD8+ T cells on reappearance has not been observed in CD8+ lymphocyte depletion experiments in either noninfected or acutely SIV-infected rhesus monkeys.5,36,37
CD8+ T-cell and NK-cell depletion in SIV-infected AGMs. Absolute CD8+ T cells in peripheral blood (A-B) and lymph nodes (C-D) and peripheral blood NK-cell (E-F) counts in 12 sabaeus African green monkeys (AGMs) infected intravenously with SIVsab9315BR. Six AGMs received 1 subcutaneous dose of 10 mg/kg of the anti-CD8α mAb cM-T807 on day 0 before simian immunodeficiency virus (SIV) infection and 2 intravenous doses of 5 mg/kg cM-T807 on days 3 and 7 after infection to deplete CD8+ lymphocytes (B,D,F). These 6 animals also received 50 mg/kg anti-CD20 rituximab antibody intravenously at days −7, 14, and 35 after infection to deplete B cells. The other 6 animals received 1 intravenous injection of 50 mg/kg IgIV 7 days before infection to serve as control animals (A,C,E). The ↓ in panels B, D, and F indicate the injection of the CD8+ lymphocyte-depleting mAb. CD8+ T cells were gated as CD8+ CD3+ lymphocytes and NK cells as CD8+ CD3− lymphocytes. Absolute CD8+ T-cell and NK-cell numbers were calculated from white blood cell counts. p.i. indicates postinfection.
CD8+ T-cell and NK-cell depletion in SIV-infected AGMs. Absolute CD8+ T cells in peripheral blood (A-B) and lymph nodes (C-D) and peripheral blood NK-cell (E-F) counts in 12 sabaeus African green monkeys (AGMs) infected intravenously with SIVsab9315BR. Six AGMs received 1 subcutaneous dose of 10 mg/kg of the anti-CD8α mAb cM-T807 on day 0 before simian immunodeficiency virus (SIV) infection and 2 intravenous doses of 5 mg/kg cM-T807 on days 3 and 7 after infection to deplete CD8+ lymphocytes (B,D,F). These 6 animals also received 50 mg/kg anti-CD20 rituximab antibody intravenously at days −7, 14, and 35 after infection to deplete B cells. The other 6 animals received 1 intravenous injection of 50 mg/kg IgIV 7 days before infection to serve as control animals (A,C,E). The ↓ in panels B, D, and F indicate the injection of the CD8+ lymphocyte-depleting mAb. CD8+ T cells were gated as CD8+ CD3+ lymphocytes and NK cells as CD8+ CD3− lymphocytes. Absolute CD8+ T-cell and NK-cell numbers were calculated from white blood cell counts. p.i. indicates postinfection.
Similar to humans and rhesus monkeys, AGMs harbor 2 distinct subsets of CD8+ T cells: CD8αα homodimer and CD8αβ heterodimer expressing cells.29 The CD8+ T cells that first reappeared after CD8+ lymphocyte depletion in the 5 well–depleted AGMs were mainly CD8αα+ T cells (5.5- to 21.2-fold increase from levels before depletion; median, 11.3-fold). CD8αβ+ T cells reappeared much slower and did not reconstitute to predepletion levels in 5 of 6 CD8+ and CD20+ lymphocyte-depleted AGMs during the observation period of 42 weeks after SIV infection (data not shown).
Similar to rhesus monkeys,38 natural killer (NK) cells from AGMs show a uniformly high expression of the CD8 molecule (data not shown). Therefore, as expected, cM-T807 administrations also led to a depletion of NK cells. In general, the duration of NK-cell depletion was comparable with the CD8+ T-cell depletion (Figure 1F). In contrast, the 6 control AGMs did not show a significant change in the number of NK cells after SIV infection and control Ab administration (Figure 1E).
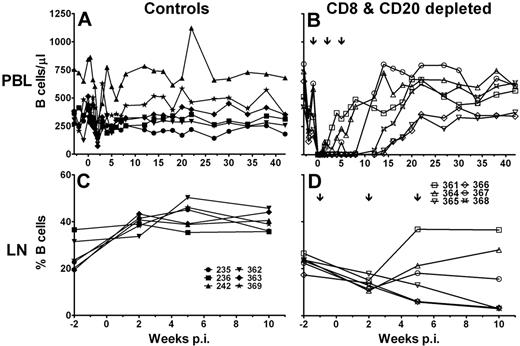
B-cell depletion in peripheral blood was efficient in 4 of 6 CD8+ and CD20+ lymphocyte-depleted AGMs and lasted for 8 to 14 weeks after infection (Figure 2B). Two AGMs (no. 361 and no. 364) responded inefficiently to rituximab administration and experienced a short-term B-cell depletion of only 1 week. In contrast to CD8+ lymphocyte counts, none of the animals showed an increase in B-cell counts above pre-CD20+ lymphocyte depletion values after reappearance of B cells in peripheral blood. The control animals experienced a transient decrease in B-cell counts coincident with the peak of viremia, 2 weeks after infection (Figure 2A). The pattern of B-cell depletion in lymph nodes (Figure 2D) was similar to what was observed in peripheral blood. The 3 animals with the longest B-cell depletion (no. 365, no. 366, and no. 368) had a continuous decline of B cells, and the percentage of B cells in lymphocytes obtained from these lymph nodes dropped to 3% at 10 weeks after infection. The other 3 CD8+ and CD20+ lymphocyte-depleted AGMs experienced only a transient B-cell depletion at week 2 after infection but did not show a long-lasting depletion in the lymph node. In contrast, the relative percentage of B cells in lymph nodes increased after SIV infection in control animals (median before SIV infection, 23.3%; range, 19.4%-36.6%; median week 10 after infection, 39.7%; range, 35.89%-45.7%; Figure 2C).
B-cell depletion in SIV-infected AGMs. Six sabaeus AGMs were depleted by mAb cM-T807 of CD8+ lymphocytes and by mAb rituximab of CD20+ lymphocytes (B,D), and 6 AGMs received purified control Ab IgIV (A,C). Absolute B-cell number in peripheral blood (A-B) and percentage of B cells in lymph node cell suspensions (C-D). The ↓ in panels B and D indicate the injection time points of the CD20+ lymphocyte-depleting mAb rituximab. p.i. indicates postinfection.
B-cell depletion in SIV-infected AGMs. Six sabaeus AGMs were depleted by mAb cM-T807 of CD8+ lymphocytes and by mAb rituximab of CD20+ lymphocytes (B,D), and 6 AGMs received purified control Ab IgIV (A,C). Absolute B-cell number in peripheral blood (A-B) and percentage of B cells in lymph node cell suspensions (C-D). The ↓ in panels B and D indicate the injection time points of the CD20+ lymphocyte-depleting mAb rituximab. p.i. indicates postinfection.
CD8+ and CD20+ lymphocyte depletion in SIV-infected AGMs results in a brief temporal delay of partial viral containment
The kinetics of plasma viral RNA levels in the control animals was comparable with that of pathogenic SIVmac infection of rhesus monkeys, although the magnitude of the viremia in AGM was generally lower. All control Ab-treated AGM experienced a peak of viremia at 2 weeks after infection followed by a nadir. Set-point viremia was established by 5 to 6 weeks after infection with a median of 2.5 × 104 SIV RNA copies per milliliter (range, 2 × 102 to 7.4 × 104 SIV RNA copies/mL; Figure 3A). Plasma viral RNA levels of the CD8+ and CD20+ lymphocyte-depleted AGMs were similar to the control AGMs at 2 weeks after infection and at set-point (median, 2.8 × 104 SIV RNA copies/mL; range, 3 × 102 to 9.3 × 104 SIV RNA copies/mL; Figure 3B). However, SIV RNA copies remained high until 3 weeks after infection and subsequently decreased until week 6 after infection. Thus, SIV RNA in plasma was significantly higher in CD8+ and CD20+ lymphocyte-depleted AGMs at weeks 3 and 4 compared with the control AGMs (Figure 3C). Despite this prolonged peak of viremia, the levels of set-point viremia did not differ between the 2 groups. AGM no. 364, which showed inefficient depletion, did not experience prolonged peak viremia and was more comparable with the control sabaeus AGMs (Figure 3B).
Temporally increased plasma SIV viremia in CD8+ and CD20+ lymphocyte-depleted SIV-infected AGMs. SIVsab9315BR RNA copy numbers were determined by quantitative PCR in control Ab-treated AGMs (A) and CD8+ and CD20+ lymphocyte-depleted AGMs (B). Median viral RNA copy number for both groups (C). *Statistically significant difference between the control (solid line, ●) and CD8+ and CD20+ lymphocyte-depleted group (dashed line, □) at the indicated time points (Mann-Whitney test, P ≤ .05). p.i. indicates postinfection.
Temporally increased plasma SIV viremia in CD8+ and CD20+ lymphocyte-depleted SIV-infected AGMs. SIVsab9315BR RNA copy numbers were determined by quantitative PCR in control Ab-treated AGMs (A) and CD8+ and CD20+ lymphocyte-depleted AGMs (B). Median viral RNA copy number for both groups (C). *Statistically significant difference between the control (solid line, ●) and CD8+ and CD20+ lymphocyte-depleted group (dashed line, □) at the indicated time points (Mann-Whitney test, P ≤ .05). p.i. indicates postinfection.
SIV-specific cellular immune responses are significantly increased in CD8+ and CD20+ lymphocyte-depleted AGMs after reappearance of CD8+ lymphocytes
The postpeak viremia decline of viral RNA in CD8+ and CD20+ lymphocyte-depleted AGMs coincided with the reappearance of CD8+ lymphocytes, suggesting that CD8+ lymphocytes partially contain virus replication in AGMs. Therefore, we examined the magnitude of SIV-specific adaptive immune responses in both groups of AGMs.
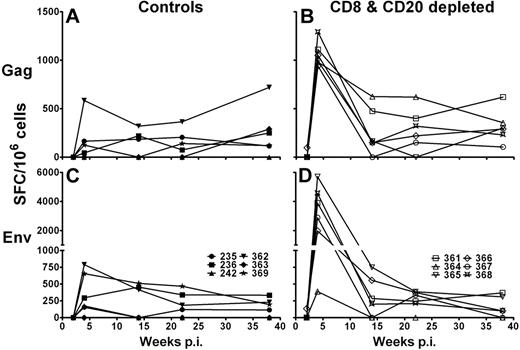
First, we investigated IFN-γ production of total PBMCs after stimulation with SIVsab Gag and Env 15-mer peptide pools in an ELISpot assay. Control Ab-treated AGMs had a range of Gag-specific IFN-γ responses between 0 and 720 spot-forming cells (SFC)/106 PBMCs (median, 228 SFC/106 PBMCs; Figure 4A) and Env-specific IFN-γ responses between 0 and 790 SFC/106 PBMCs (median, 315 SFC/106 PBMCs; Figure 4C). Gag and Env responses were first detectable in the control group at week 4 and were sustained throughout the study. CD8+ and CD20+ lymphocyte-depleted AGMs, however, had significantly higher IFN-γ responses at 4 weeks after infection after stimulation with SIVsab Gag (range, 940-1290 SFC/106 PBMCs; median, 1020 SFC/106 PBMCs; P = .005, Mann-Whitney test; Figure 4B) and SIVsab Env peptide pools (range, 390-5705 SFC/106 PBMCs; median, 3385 SFC/106 PBMCs; P = .009, Mann-Whitney test; Figure 4D). The high magnitude of IFN-γ production by CD8+ and CD20+ lymphocyte-depleted AGMs eventually declined and reached levels comparable with those observed in the control group. Interestingly, the inefficiently depleted AGM no. 364 did have a similar magnitude of IFN-γ response after Env peptide stimulation as the control AGMs.
Increased IFN-γ immune responses in CD8+ and CD20+ lymphocyte-depleted SIV-infected AGMs. SIVsab IFN-γ cytokine release of PBMCs obtained from 12 SIV-infected sabaeus AGMs. PBMCs from 6 control Ab-treated AGMs (A,C) and 6 CD8+ and CD20+ lymphocyte-depleted AGMs (B,D) were stimulated with SIVsab Gag and Env 15-mer peptide pools for 18 hours at 37°C. The values given represent the number of IFN-γ SFCs in 106 PBMCs in response to stimulation with the Gag (A-B) or Env peptide pool (C-D). Responses were considered positive if their value was greater than 2 times the SD of the mean of the media control wells. All values were background corrected using the media controls. p.i. indicates postinfection.
Increased IFN-γ immune responses in CD8+ and CD20+ lymphocyte-depleted SIV-infected AGMs. SIVsab IFN-γ cytokine release of PBMCs obtained from 12 SIV-infected sabaeus AGMs. PBMCs from 6 control Ab-treated AGMs (A,C) and 6 CD8+ and CD20+ lymphocyte-depleted AGMs (B,D) were stimulated with SIVsab Gag and Env 15-mer peptide pools for 18 hours at 37°C. The values given represent the number of IFN-γ SFCs in 106 PBMCs in response to stimulation with the Gag (A-B) or Env peptide pool (C-D). Responses were considered positive if their value was greater than 2 times the SD of the mean of the media control wells. All values were background corrected using the media controls. p.i. indicates postinfection.
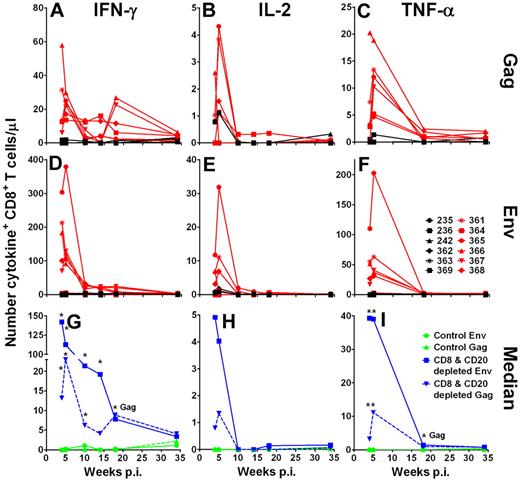
To further assess the cytokines produced after CD8+ T cells reappeared, we performed ICS after stimulation with SIVsab Gag and Env peptide pools. We found only very low responses (< 0.15% of CD4+ T cells) in the CD4+ T-cell subset without a significant difference between the groups of AGMs (data not shown). In CD8+ T cells, the strongest SIV-specific responses were observed by IFN-γ production (Figure 5A,D,G) followed by tumor necrose factor (TNF-α) production (Figure 5C,F,I). Relatively weak SIV-specific CD8+ T-cell responses were seen by interleukin-2 (IL-2) production (Figure 5B,E,H). The CD8+ T-cell responses showed a pattern of IFN-γ expression after peptide stimulation similar to the ELISpot assay, albeit at a lower sensitivity. Gag as well as Env peptide pool stimulation led to a 20- to 150-fold higher median number of IFN-γ–expressing CD8+ T cells/μL in the CD8+ and CD20+ lymphocyte-depleted AGMs than in the control AGMs (Figure 5G). Again, stimulation with Env (Figure 5D) resulted in a higher number of IFN-γ–producing cells than stimulation with Gag peptides (Figure 5A) in the CD8+ and CD20+ lymphocyte-depleted group (week 4, P > .082; week 5, P < .066; week 10, P > .093, week 14, P > .128; Mann-Whitney test). The number of IFN-γ+CD8+ T cells in the 2 groups of AGM was not significantly different after 18 weeks after infection.
Increased SIV-specific expression of IFN-γ, IL-2, and TNF-α cytokine responses in CD8+ T cells from CD8+ and CD20+ lymphocyte-depleted SIV-infected AGMs. Intracellular expression of IFN-γ (A,D,G), IL-2 (B,E,H), and TNF-α (C,F,I) in SIVsab Gag and Env peptide pool-stimulated CD8+ T cells. Freshly isolated PBMCs were stimulated for 9 hours at 37°C in the presence of either Env or Gag peptide pools. The intracellular staining in CD8+ T cells stimulated with Gag or Env peptide pools is shown for control Ab-treated AGMs in black and for CD8+ and CD20+ lymphocyte-depleted AGMs in red (A-F). (G-I) Median responses of the 6 control AGMs in green and of the 6 CD8+ and CD20+ lymphocyte-depleted AGMs in blue. *Statistically significant difference between the control and CD8+ and CD20+ lymphocyte-depleted group at the indicated time points (Mann-Whitney test, P ≤ .05). Responses were considered positive if their value was greater than 2 times the SD of the mean of the media control wells. All values were background corrected using the media controls. Responses and background were determined separately for the different cytokines. p.i. indicates postinfection.
Increased SIV-specific expression of IFN-γ, IL-2, and TNF-α cytokine responses in CD8+ T cells from CD8+ and CD20+ lymphocyte-depleted SIV-infected AGMs. Intracellular expression of IFN-γ (A,D,G), IL-2 (B,E,H), and TNF-α (C,F,I) in SIVsab Gag and Env peptide pool-stimulated CD8+ T cells. Freshly isolated PBMCs were stimulated for 9 hours at 37°C in the presence of either Env or Gag peptide pools. The intracellular staining in CD8+ T cells stimulated with Gag or Env peptide pools is shown for control Ab-treated AGMs in black and for CD8+ and CD20+ lymphocyte-depleted AGMs in red (A-F). (G-I) Median responses of the 6 control AGMs in green and of the 6 CD8+ and CD20+ lymphocyte-depleted AGMs in blue. *Statistically significant difference between the control and CD8+ and CD20+ lymphocyte-depleted group at the indicated time points (Mann-Whitney test, P ≤ .05). Responses were considered positive if their value was greater than 2 times the SD of the mean of the media control wells. All values were background corrected using the media controls. Responses and background were determined separately for the different cytokines. p.i. indicates postinfection.
As seen with the IFN-γ responses, TNF-α responses were significantly higher in the CD8+ and CD20+ lymphocyte-depleted animals than in the control group at weeks 4 and 5 after infection (Gag week 4, P < .045; Gag week 5, P < .007; Env week 4, P < .017; Env week 5, P > .004; Mann-Whitney test; Figure 5C,F,I). IL-2 responses also trended higher in CD8+ and CD20+ lymphocyte-depleted animals compared with the control animals (Gag week 4, P < .158; Gag week 5, P < .091; Env week 4, P > .104; Env week 5, P > .146; Mann-Whitney test; Figure 5B,E,H). As observed with the IFN-γ responses, the magnitude of Env peptide pool TNF-α and IL-2 responses was higher than the Gag peptide pool responses (TNF-α week 4, P = .09; TNF-α week 5, P = .06; IL-2 week 4, P < .225; IL-2 week 5, P > .463; Mann-Whitney test; Figure 5B,C,E-F,H-I). These results demonstrate that SIV-infected AGMs are capable of generating high-magnitude cellular immune responses. Furthermore, CD8+ T-cell immune responses are very probably involved in partial containment of SIV viremia.
Administration of rituximab delays the generation of NAbs in SIV-infected sabaeus AGMs
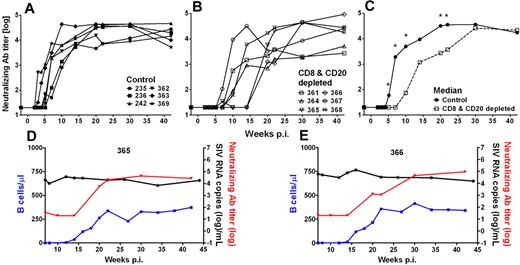
We and others have previously shown that AGMs and other natural hosts exhibit relatively low levels of SIV-specific NAbs.19,27,39 Using an SIVsab9315BR Env-pseudotyped HIV-1 virus to measure NAb activity, we were able to reproducibly detect SIVsab9315BR NAbs in all AGMs investigated (Figure 6). NAbs were first detected in control AGMs at weeks 3 to 7 and reached peak levels of NAb titers of 1.5 to 4.7 × 104 (median, 3.7 × 104, Figure 6A). In contrast, the CD8+ and CD20+ lymphocyte-depleted AGMs developed SIV NAb responses significantly later at weeks 7 to 20 (P = .02; Mann-Whitney test) but eventually reached levels similar to those observed in the control animals (range, 0.4-9.2 × 104; median, 3.8 × 104; Figure 6B). The 2 AGMs with the longest B-cell depletion (AGMs no. 365 and no. 366) also exhibited the most profound delay in SIV NAb responses. In weeks 5 to 22 after infection (except week 15 after infection), the SIV NAb responses in B cell–depleted AGMs were significantly lower compared with the control animals (Figure 6C). Additional screening for SIV antibodies by Western blot of AGMs serum against whole SIVsab9315BR lysate confirmed the results from the NAb assay (data not shown). Taken together, the data obtained by SIV-specific NAb assay demonstrated a solid reduction in humoral immune responses (NAb titer < 102) during the first 10 weeks in 4 AGMs (no. 364, no. 365, no. 366, and no. 368). However, the appearance of NAbs did not have a profound effect on the magnitude of viremia (Figure 3C). This is particularly evident in the 2 animals with the longest B-cell depletion (animals no. 365 and no. 366), which showed a relatively stable set-point viremia for more than 30 weeks, although a significant difference in the level of NAb was observed during this time period (Figure 6D-E).
Delayed appearance of SIV-specific humoral immune responses has no effect on set-point viremia of SIV-infected AGMs. SIVsab9315BR NAb titers in control Ab-treated AGMs (A) and CD8+ and CD20+ lymphocyte-depleted AGMs (B). The median NAb titer was calculated for both groups (C). *Statistically significant difference between the control (solid line, ●) and CD8+ and CD20+ lymphocyte-depleted group (dashed line, □) at the indicated time points (Mann-Whitney test, P ≤ .05). The 2 animals with the most efficient/longest B-cell depletion, no. 365 (D) and no. 366 (E), in the group of antibody-treated and SIV-infected sabaeus AGMs are shown. The appearance of NAbs (red lines) coincided with the reappearance of peripheral blood B cells (blue lines) but had no effect on set-point viremia. p.i. indicates postinfection.
Delayed appearance of SIV-specific humoral immune responses has no effect on set-point viremia of SIV-infected AGMs. SIVsab9315BR NAb titers in control Ab-treated AGMs (A) and CD8+ and CD20+ lymphocyte-depleted AGMs (B). The median NAb titer was calculated for both groups (C). *Statistically significant difference between the control (solid line, ●) and CD8+ and CD20+ lymphocyte-depleted group (dashed line, □) at the indicated time points (Mann-Whitney test, P ≤ .05). The 2 animals with the most efficient/longest B-cell depletion, no. 365 (D) and no. 366 (E), in the group of antibody-treated and SIV-infected sabaeus AGMs are shown. The appearance of NAbs (red lines) coincided with the reappearance of peripheral blood B cells (blue lines) but had no effect on set-point viremia. p.i. indicates postinfection.
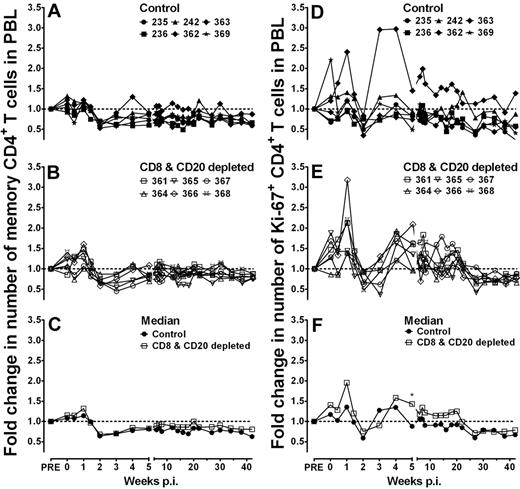
CD8+ and CD20+ lymphocyte depletion does not affect the number of memory and Ki-67+ CD4+ T cells or lead to the induction of an AIDS-like disease in SIV-infected AGMs
One feature of nonpathogenic AIDS virus infection is the maintenance of relatively stable CD4+ T-cell counts, whereas pathogenic AIDS virus infection is characterized by a progressive loss of CD4+ T cells. We observed an initial small gain of memory CD4+ T cells until 1 week after infection (Figure 7A) in 5 of the 6 control AGMs. After this transient increase, memory CD4+ T-cell numbers decreased by 36% at peak viremia (2 weeks after infection). The levels of memory CD4+ T cells did not recover to preinfection values but remained stable over weeks 6 to 42 after infection (median before infection, 140 cells/μL; median after infection, 107 cells/μL, P = .009, Mann-Whitney test). A similar phenomenon was observed in the lymphocyte-depleted AGMs (median before infection, 151 cells/μL, median after infection, 128 cells/μL, P = .013, Mann-Whitney test, Figure 7B). Memory CD4+ T-cell numbers did not differ significantly between the 2 groups at any time point (Figure 7C).
CD8+ and CD20+ lymphocyte depletion in SIV-infected AGMs did marginally affect the number of memory CD4+ T cells and Ki-67+ memory CD4+ T cells in peripheral blood. CD4+ T cells of control Ab-treated AGMs (A-D) and CD8+ and CD20+ lymphocyte-depleted AGMs (B-E) were stained with anti-CD28 and anti-CD95 to determine naive (CD95−) and memory (CD95+) CD4+ T-cell subsets. The fold change in memory CD4+ T-cell numbers (A-C) and the fold change in Ki-67+ memory CD4+ T-cell numbers (D-E) were calculated for each time point compared with the median of 3 preantibody injection time points. (C) The median fold change in memory CD4+ T cells from the 6 control Ab-treated AGMs (solid line, filled symbols) and the 6 CD8+ and CD20+ lymphocyte-depleted AGMs (solid line, open symbols) are shown. (F) The median fold change in Ki-67+ memory CD4+ T cells from the 6 control Ab-treated AGMs (solid line, filled symbols) and the 6 CD8+ and CD20+ lymphocyte-depleted AGMs (solid line, open symbols) are shown. *Single time point with a significant difference (Mann-Whitney test) between the control group and the antibody-treated group of animals. No significant differences were detected between the control and antibody-treated groups at any of the other time points. p.i. indicates postinfection.
CD8+ and CD20+ lymphocyte depletion in SIV-infected AGMs did marginally affect the number of memory CD4+ T cells and Ki-67+ memory CD4+ T cells in peripheral blood. CD4+ T cells of control Ab-treated AGMs (A-D) and CD8+ and CD20+ lymphocyte-depleted AGMs (B-E) were stained with anti-CD28 and anti-CD95 to determine naive (CD95−) and memory (CD95+) CD4+ T-cell subsets. The fold change in memory CD4+ T-cell numbers (A-C) and the fold change in Ki-67+ memory CD4+ T-cell numbers (D-E) were calculated for each time point compared with the median of 3 preantibody injection time points. (C) The median fold change in memory CD4+ T cells from the 6 control Ab-treated AGMs (solid line, filled symbols) and the 6 CD8+ and CD20+ lymphocyte-depleted AGMs (solid line, open symbols) are shown. (F) The median fold change in Ki-67+ memory CD4+ T cells from the 6 control Ab-treated AGMs (solid line, filled symbols) and the 6 CD8+ and CD20+ lymphocyte-depleted AGMs (solid line, open symbols) are shown. *Single time point with a significant difference (Mann-Whitney test) between the control group and the antibody-treated group of animals. No significant differences were detected between the control and antibody-treated groups at any of the other time points. p.i. indicates postinfection.
Similarly, no statistically significant difference was detected in the fold change of the number of Ki-67+CD4+ T cells between both groups of animals (except on 1 time point at week 5 after infection; Figure 7D-F). In addition, no statistically significant differences were detected in the fold change of the absolute number of CD4+ T cells and number of naive CD4+ T cells (supplemental Figure 1A-F). Besides a typical lentiviral infection-induced lymphopenia around the peak viremia, which was observed in both groups of animals, the number of CD4+ T cells (total number and number of memory cells, naive cells, and Ki-67+ cells) showed little change compared to levels seen before infection.
Consistent with the similarity in CD4 T lymphocytes between the 2 groups, the disease-free survival was not affected during a follow-up period of 1 year in the CD8+ and CD20+ lymphocyte-depleted AGMs. Regardless of the treatment, none of the 12 animals investigated showed any signs of an AIDS-like disease. These observations furthermore underline the robustness of the AGM immune system toward SIV infection, even under harsh treatment such as simultaneous CD8+ and CD20+ lymphocyte depletion.
Discussion
This study demonstrates that transient inhibition of CD8+ T cell– and B cell–mediated adaptive immune responses during primary SIV infection of sabaeus AGMs did not lead to the induction of an AIDS-like disease. However, the transient absence of CD8+ lymphocytes did result in a delay of the initial viral containment. The reappearance of CD8+ lymphocytes and the generation of a high frequency of SIV-specific CD8+ T cells were associated with a reduction of viremia to set-point levels. In contrast, the delay in humoral immune responses in B cell–depleted animals did not correlate with viremia.
A number of investigations have shown that natural hosts are capable of eliciting SIV-specific adaptive immune responses and that these responses are of a similar or slightly lower magnitude compared with non-natural hosts infected with SIV.27-30,39-41 However, there is also accumulating evidence suggesting that natural hosts may use evolutionary adaptations to remain disease-free.42 To determine the role of adaptive immune responses in the maintenance of a disease-free course of infection, we used a combined treatment with both anti-CD8 and anti-CD20 antibodies to suppress the generation of adaptive immune responses in sabaeus AGMs. Even under these extreme conditions, the lymphocyte-depleted AGMs showed only a temporary elevation in viremia during primary infection. Similar to the control group, the lymphocyte-depleted AGMs showed only a brief decline in total CD4+ T cells in peripheral blood. As expected in AIDS virus infections, CCR5+ CD4+ T cells (data not shown) and memory CD4+ T cells showed some decline after infection, albeit we could not detect a difference between both groups of AGMs studied. None of these changes had any impact on the course of SIV infection; both groups of AGMs remained disease-free.
This observation is in sharp contrast to observations made in CD8+ or CD20+ lymphocyte depletion studies in pathogenic SIV infection models.5,15,16,43 CD8+ lymphocyte depletion during primary viremia in rhesus monkeys resulted in an extremely high viremia (in the range of 108 copies viral RNA/mL plasma). As a consequence of CD8+ lymphocyte depletion during primary infection, CD8+ T-cell responses, B-cell responses, and CD4+ T-cell responses were significantly impaired or did not develop, and survival was drastically decreased.36,44 Similarly, CD8+ and CD20+ lymphocyte depletion during primary SIVagm infection of pigtailed macaques resulted in increased viremia and accelerated disease.45
Studies of rhesus monkeys during primary SIV infection showed that humoral immune responses do not contribute to early viral containment. In these studies, the level of primary viremia was indistinguishable between B cell–depleted and control animals.15,16,45 In the present study, it is difficult to assess the role of humoral immune responses as we impaired the generation of both cellular and humoral immune responses. However, correlative evidence in the control group suggests that humoral immune responses do not contribute to viral containment in primary SIV infection of sabaeus AGMs and to a disease-free course of infection. Thus, a stable set-point viremia was established at week 5 after infection at a time when NAbs were still undetectable in most animals.
In contrast to primary infection, some investigators have recently suggested that humoral immune responses may contribute to viral control in chronic SIV viremia.16 In these studies, a significantly reduced survival was observed in long-term B cell–depleted SIV-infected rhesus monkeys. In the present study in sabaeus AGMs and in recent investigations in vervet AGMs,45 the appearance of NAbs did not affect the magnitude of plasma viral RNA in lymphocyte-depleted AGMs. In addition, B-cell depletion did not affect survival. Also, recent B-cell depletion experiments in SIV-infected sabaeus AGMs performed by other investigators confirmed our findings: inhibition of humoral immune responses in AGMs did not result in any adverse side effects or the induction of an AIDS-like disease.46 Still, we cannot formally rule out that more aggressive and longer-term inhibition of humoral immune responses may result in a different outcome. However, in general, there is little evidence in HIV infection of humans and SIV infection of nonhuman primates that NAbs significantly reduce the level of viremia. There is evidence that the decreased survival after long-term depletion of B cells in SIV-infected rhesus monkeys may be the result of an indirect effect. We observed a lower magnitude of CD8+ T-cell responses during chronic SIV infection in long-term B cell–depleted SIV-infected rhesus monkeys (M. de la Rosa, P. Chugh, V. Evans, S. Finstad, S. Westmoreland, A.C., Michael Piatak, J. D. Lifson, D. C. Montefiori, and J.E.S., unpublished observation, January 2008).
CD8+ lymphocyte depletion experiments in natural hosts and non-natural hosts suggest that CD8+ T-cell responses participate in viral control. Interestingly, significant differences were seen between rhesus macaques and sabaeus AGMs when CD8+ lymphocytes reappeared. In rhesus monkeys, the recovery of CD8+ T cells and the generation of SIV-specific responses were highly suppressed.36,44 In contrast, SIV-infected sabaeus AGMs that usually exhibit only relatively low CD8+ T-cell responses showed a dramatic increase in SIV-specific responses when CD8+ T cells reappeared. The transient prolongation of primary viremia in CD8+ lymphocyte-depleted AGMs may have crossed a threshold necessary to induce a vigorous expansion of SIV-specific CD8+ T cells. Observations made in the CD8+ lymphocyte-depleted AGM no. 364 support this notion. CD8+ lymphocytes were only inefficiently and briefly depleted in this animal, and replenishment was not associated with a high number of Env-specific CD8+ T cells. The magnitude of SIV-specific CD8+ T-cell responses seen after the reappearance of CD8+ lymphocytes in most of the CD8+ lymphocyte-depleted AGMs also refutes the notion that natural hosts are only capable of relatively subdued immune responses to SIV. Our data clearly show that sabaeus AGMs are capable of generating robust SIV-specific CD8+ T-cell responses. Our findings also suggest that the presence of viremia does not abrogate the function of virus-specific CD8+ T-cell responses in AGMs. Thus, CD8+ lymphocyte recovery in AGMs (in contrast to rhesus monkeys36,44 ) may be associated with an appropriate response sufficient to reduce the viremia to an “asymptomatic” set-point level.
In addition to the effect of CD8+ lymphocyte depletion on SIV viremia, we measured a significantly increased level of cytomegalovirus (CMV) viremia during lymphocyte depletion (supplemental Figure 2). This indicates a critical role of these cells in controlling CMV infection in AGMs. This finding is in agreement with previous observations in other nonhuman primates, humans, and mice.31,47-49 Interestingly, we did not observe a generalized immune activation despite the reactivation of CMV or the massive expansion of CD8+ T cells after the reappearance of these cells, indicating an inherent stability and regulation of the immune system in these animals.
Our observations suggest that CD8+ T cells are at least partially involved in viral containment in AGMs as the vigorous expansion of SIV-specific CD8+ T cells coincided with the partial containment of viremia. We cannot rule out that NK cells participate in the partial control of the viremia as they also are depleted by the anti-CD8α antibody and reappear coincident with CD8+ T cells. In addition, the ultimate role and possible limitations of CD8+ T-cell responses in AGMs are still unknown. We currently have no data on the breadth of the SIV-specific immune responses beyond Env and Gag responses. However, it was remarkable that the primary CD8+ T-cell response was mainly directed against Env (similar to SIV-specific antibody responses). The eventual decline of these Env responses to levels similar to those seen for Gag responses suggests that SIVsab may also undergo sequence mutations resulting in immune escape. Limited data from another natural host, SIVsm-infected sooty mangabeys, have suggested that escape from cellular immune responses does occur.50 However, this area requires further investigation.
It is tempting to generalize the observations made here to other natural host species. However, the mechanisms of protection used by natural hosts to evade pathogenic consequences of SIV infection are probably the result of complex evolutionary adaptations and thus may differ considerably between the 40 different monkey and ape natural host species. Therefore, future efforts should include additional natural host species to determine the breadth of immune and nonimmune protection mechanisms used by natural hosts. The study of natural hosts gives researchers the unique opportunity to study AIDS virus-infected animals that do not develop disease despite relatively high viral loads. In contrast, less pathogenic AIDS virus infections of non-natural hosts (ie, HIV-2 in humans or SHIV89.6 in rhesus macaques) are always associated with a relatively low-level set-point viremia.
In conclusion, this study suggests that CD8+ lymphocytes contribute to partial viral containment during primary SIV infection in AGMs. However, the temporal inhibition of adaptive immune responses had no apparent effect on the SIV-infected AGMs. It would be reassuring for current AIDS vaccine efforts if adaptive immune responses also contribute to the disease-free course of infection observed in natural hosts. However, it is still unclear whether these responses are at all necessary for preventing outbreak of overt signs of SIV-mediated disease in natural hosts. Future in vivo manipulations aiming to achieve longer-lasting suppression of adaptive immune responses and/or induction of chronic immune activation will be required to address these questions. A more precise picture of the breadth of mechanisms used to prevent disease in natural hosts of lentivirus infection may provide important insights for the generation of a more successful AIDS vaccine and/or development of novel treatment modalities.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the NIH (grants RR000168, New England Primate Research Center; AI43890, A.K.; AI30034, D.C.M.; AI065335, J.E.S.), NIAID Center for HIV/AIDS Vaccine Immunology (grant AI067854, J.E.S., N.L.L.), and the Harvard Medical School Center for AIDS Research (grant AI060354). Reagents used in this work were provided by the NIH Nonhuman Primate Reagent Resource (grants AI040101 and RR016001).
National Institutes of Health
Authorship
Contribution: R.C.Z., V.M.H., J.S.A., and J.E.S. conceived and designed the experiment; M.D.R., M.L., H.T., B.K.-S., H.B., R.W., and S.P. performed the experiment; R.C.Z., N.L.L., A.K., D.C.M., V.M.H., J.S.A., and J.E.S. analyzed the data; R.C.Z. and J.E.S. wrote the paper; and A.C. performed the experimental work with nonhuman primates.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Correspondence: Jörn E. Schmitz, Division of Viral Pathogenesis, Beth Israel Deaconess Medical Center, Center for Life Science, E/CLS 1037, 330 Brookline Ave, Boston, MA 02215; e-mail: jschmitz@bidmc.harvard.edu.