Abstract
B-cell chronic lymphocytic leukemia (CLL) expresses CD160, a glycosylphosphatidylinositol-linked receptor found on normal natural killer (NK) and T cells, but not B cells. CD160 is a multifunctional molecule in normal lymphocytes, but its role in CLL biology is unknown. In vitro, CLL cells undergo rapid spontaneous apoptosis, which CD160 activation protected against—mean cell viability increased from 67% to 79% (P < .001). This was associated with up-regulation of Bcl-2, Bcl-xL, and Mcl-1, but not Bax. As expected from these changes in Bcl-2/Bax and Bcl-xL/Bax ratios, CD160 triggering reduced mitochondrial membrane potential collapse and cytochrome c release. CD160 stimulation also induced DNA synthesis, cell cycle progression, and proliferation. B-cell antigen receptor (BCR)–induced CLL proliferation was generally greater than with CD160, but marked variation was seen. Both BCR and CD160 signaling led to CLL secretion of interleukin-6 (IL-6) and IL-8, although CD160 induced greater increases of IL-6 (51-fold) and IL-8 (15-fold). Survival and activation signals mediated by CD160 showed dose-dependent suppression by phosphoinositide-3 kinase (PI3K) inhibitors. Thus, in vitro, CLL cells can use the CD160 pathway for survival and activation, mimicking CD160 signaling in normal NK and CD8+ T cells. Establishing the pathophysiologic relevance of these findings may reveal new therapeutic targets.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is characterized by the expansion of monoclonal CD5+ B lymphocytes that accumulate in peripheral blood (PB), secondary lymphoid organs, and bone marrow (BM).1 CLL remains incurable and this is partly attributable to cells being in the G0/G1 phase of the cell cycle and having high levels of antiapoptotic Bcl-2 family proteins.2,3 These malignant cells exhibit genetic abnormalities that can modify their resistance to apoptosis and response to selected microenvironmental signals giving both a growth and survival advantage. Despite their prolonged survival in vivo, CLL cells rapidly undergo spontaneous apoptosis once removed from their microenvironment,4 suggesting that survival signals available in vivo have been lost in culture conditions. This spontaneous in vitro apoptosis can be prevented by some cytokines,5,6 albumin,7 and stromal cells.8
CD160, a glycosylphosphatidylinositol (GPI)–linked membrane protein is recognized by 2 distinct monoclonal antibodies (mAbs)—the referenced BY55 and CL1-R2.9,10 CD160 is expressed by CLL cells, whereas in normal B cells there is no CD160 protein or RNA (our unpublished data). The GPI-linked protein CD160 was initially found on functional cytotoxic PB natural killer (NK) lymphocytes,11 triggering cytotoxicity as well as a unique profile of cytokine production: tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and interleukin-6 (IL-6).12,13 It is also expressed on CD8bright CD28− T lymphocytes14 and a subpopulation of CD4+ T cells in skin inflammation.15 CD160 recruits phosphoinositide-3 kinase (PI3K) and induces phosphorylation of Akt and extracellular signal-related kinase downstream signaling elements in NK cells.16 In CD8+ T cells, CD160 enhances CD3-induced proliferation and improves CD3-induced cell cytotoxicity14,17 and was found to coprecipitate with the tyrosine kinase, p56lck, and tyrosine-phosphorylated zeta chains upon T-cell receptor (TCR)–CD3 cell activation.17 Furthermore, it was demonstrated that antibody and ligand binding of CD160 on neoangiogenic blood vessels induced apoptosis of endothelial cells.18 This activating receptor has a broad specificity for the major histocompatibility complex class Ia and Ib molecules, including HLA-G.9 In contrast, a recent report indicated that CD160 mediated a profound inhibitory effect on the activation of normal human CD4+ T cells, although the underlying molecular mechanisms were not elucidated; furthermore, CD160 and herpes virus entry mediator were reported to be a new receptor/counterreceptor pair, with the potential for bidirectional signaling.19
IL-6 and IL-8 production has been described during CLL activation.20,21 IL-8 is known to be secreted by CLL cells, and to be a survival factor inducing Bcl-2 expression, but not to induce proliferation.22,23 However, the data on the role of IL-6 are contradictory. Although the IL-6Rα chain (CD126) is found on normal B cells and CLL cells,24 IL-6 has been shown to inhibit CLL cell proliferation induced by TNF-α.25 There are contradictory data with respect to serum levels of IL-6 and stage of CLL.25,26 Although triggering of CD160 on NK cells leads to cytotoxicity and IL-6 production,13 the role of CD160 in cytokine production in CLL is unknown.
Phosphoinositide-3 kinases are a family of enzymes that can be subdivided into 3 classes—class I, class II, and class III.27 The class I PI3K is involved in signaling by antigen and costimulatory receptors.28 A key function of class I PI3K is to phosphorylate phosphatidylinositol-4,5-bisphosphate (PIP2) to become phosphatidylinositol-3,4,5-triphosphate (PIP3).29 PI3K and PIP3 cooperate to regulate Akt activity through the direct binding of phosphoinositides to the Akt pleckstrin homology domain and the binding of PI3K-generated phospholipids to Akt. Phosphorylation of Akt has been shown to be critical to its activation.30 Activation of the PI3K/Akt pathway can occur in response to a variety of extracellular growth factors or engagement of surface receptors, mediating cellular proliferation, differentiation, and survival.28 The PI3K pathway has a key role in the antiapoptotic mechanisms in CLL as shown by transfection of primary cells with constitutively active Akt.31 Furthermore, specific inhibition of the p110α subunit of the PI3K class I isoform appears to be a therapeutic target in CLL.32 Although PI3K signaling, as well as other pathways, is important in CD160 activity in NK and T cells,16 the function of CD160 and the pathways involved are yet to be determined in CLL.
Here, we sought to determine the functional activity of membrane GPI-anchored CD160 in CLL. Using 2 different mAbs, we show that activation through CD160 leads to PI3K-dependent CLL protection from spontaneous in vitro apoptosis, as well as cellular activation with cell cycle progression and cytokine production.
Methods
Patients, cell preparations, and cell lines
The protocol was approved by the National Research Ethics Service, East London, and the City Health Authority Local Research Ethics Committee. The diagnosis of CLL was made by standard criteria: cellular morphology and immunophenotyping (CD5+, CD19+, CD23+, weak surface immunoglobulin [Ig] staining, and negative/weak CD79b with FMC7 negativity). All cases were either untreated or at least 6 months from the last treatment. Peripheral blood was obtained after written informed consent from patients with CLL in accordance with the Declaration of Helsinki.
CLL cells were isolated from PB by Ficoll-Hypaque density gradient centrifugation and used immediately or stored in liquid nitrogen prior to use. CLL cells were cultured in complete RPMI-1640 containing 10% fetal calf serum, 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). MEC-I B-cell line (German Collection of Microorganisms and Cell Cultures [DSMZ]), derived from a patient with prolymphocytic progression of CLL, and an Epstein-Barr virus (EBV)–transformed lymphoblastoid B-cell line (Sanchez) produced in our laboratory were cultured in complete RPMI medium.
Reagents
Purified anti-CD160 mAbs (CL1-R2, BY55) were produced in our laboratory. Anti-IgG,M,A F(ab)2 fragment (Pan-Ig) and the corresponding F(ab)2 control were purchased from Jackson ImmunoResearch Laboratories. IgG and IgM controls were obtained from Autogen Bioclear. Other reagents used were as follows: anti-Akt and anti–phosphorylated Akt from Cell Signaling; anti–Bcl-2 (100), anti-Bax (2D2), anti–Bcl-xL (s-18), and anti–cytochrome c–phycoerythrin (PE; 6H2) from Santa Cruz Biotechnology; anti–Ki67–fluorescein isothiocyanate (FITC; MIB-1) Kit from DAKO; anti–β-actin from Sigma-Aldrich; annexin V kit from BD Pharmingen; PI3K inhibitors, wortmannin and 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-1 (LY294002), and the fluorogenic substrates, Ac-DEVD-AFC for caspase-3, AC-IETD-AFC for caspase-8, and AC-LEHD-AFC for caspase-9, from Merck-Calbiochem. The tetramethylrhodamine methyl ester (TMRM) was from Invitrogen.
Flow cytometric and cytogenetic analysis
Immunophenotypic analysis.
The diagnosis of CLL was based on National Cancer Institute Working Group guidelines, which uses immunophenotyic analysis for hematologic neoplasms.33 Samples were prepared according to the standard operating procedure of the laboratories. All antibodies were directly conjugated and approved for diagnostic use. Diagnostic patient material was tested using a whole-blood postantibody ammonium chloride lyse/wash approach with the exception of analysis for light chain restriction. CD160 (BY55 monoclonal antibody; Beckman Coulter) was routinely incorporated into the diagnostic panel for all B-cell malignancies, with CD160 expressed in more than 98% of cases (CD160 positivity defined as > 20% surface expression; Table 1).
Cytogenetic analysis.
Fluorescence in situ hybridization was performed using the Vysis LSI p53/LSI ATM and LSI D13S319/LSI 13q34/CEP 12 Multi-color Probe set and LSI IGH/CCND1 Dual Color, Dual Fusion Translocation Probe according to the manufacturer's protocol. A total of 100 interphase nuclei were scored for each probe set. A clone/karyotype was defined according to the International System for Human Cytogenetic Nomenclature.
Measurement of ΔΨm by flow cytometry
For measuring mitochondrial membrane potential (ΔΨm), 4 × 106/mL CLL cells were treated with or without anti-CD160 mAb CL1-R2 (10 μg/mL) for 18 hours. Cells were stained with 20nM TMRM for 30 minutes at 37°C, and the ΔΨm collapse was measured using a FACSCanto flow cytometer (BD Biosciences). The TMRM was used to measure mitochondrial ΔΨm as described previously.34
Cytochrome c release assay
For mitochondrial cytochrome c, 4 × 106/mL CLL cells were treated with or without CL1-R2 for 18 hours and then permeabilized in 100 μL of mitochondria intact buffer (50 mg/mL digitonin, 100mM KCl in phosphate-buffered saline [PBS]) for 10 minutes on ice. Cells were fixed in fix buffer (4% paraformaldehyde in PBS) for 20 minutes at room temperature and washed 3 times in PBS.35 Cells were incubated with 20 μL of PE-conjugated anti–cytochrome c mAb for 1 hour at room temperature in the dark. Before analysis by flow cytometry, cells were washed once in PBS. Mitochondrial cytochrome c was measured using the FACSCanto flow cytometer in the PE channel.
Caspase activity assay
CLL cells were lysed after incubation with or without CL1-R2 for 18 hours and 50 μg of proteins was diluted in the fluorogenic assay buffer to 90 μL onto a 96-well plate. The reaction was initiated by addition of 10 μL of 200μM (final concentration was 20μM) fluorescent substrate, Ac-DEVD-AFC for caspase-3, AC-IETD-AFC for caspase-8, and AC-LEHD-AFC for caspase-9. The solution was incubated at 37°C for 15 minutes and the reaction stopped by adding 50 μL of stop solution.34 The caspase cleaved AFC was measured with a Bio-Tek Synergy HT Multi-Detection Microplate Reader (Bio-Tek Instruments).
Cell proliferation assay
In flat-bottom plates, 105 MEC-I cells were incubated in 200 μL of complete RPMI medium containing isotype control at 15 μg/mL (IgG or IgM) or anti-CD160 antibodies CL1-R2 (IgG1) or BY55 (IgM; 10 μg/mL). After 72 hours of culture in 5% CO2 incubator, cells were counted (3 times) using trypan blue exclusion. PI3K inhibitor LY294002 (10μM and 20μM) was cotreated with 10 μg/mL CL1-R2 for 72 hours; cells were counted by trypan blue exclusion.
CLL cells were cultured at 2 × 105 in 200 μL of complete RPMI medium with anti-CD160 antibodies, CL1-R2 (10 μg/mL) or BY55 (10 μg/mL), and anti-IgG,M,A F(ab)2 fragment (PAN-Ig; Jackson ImmunoResearch Laboratories). Negative controls were performed with appropriate antibodies mouse IgG or IgM and control F(ab)2 fragment (Jackson ImmunoResearch Laboratories) at 10 μg/mL. After 60 hours, cells were pulsed for 16 hours with 1 μCi (0.037 MBq) of 3H-thymidine (Amersham). All conditions were performed in triplicate. Cells were harvested in a Tomtec Harvester MKIII. Thymidine incorporation was quantified using a 1450 Trilux machine (PerkinElmer).
For Ki67 expression, 4 × 105 CLL cells were treated with anti-CD160 mAbs (10 μg/mL CL1-R2 or BY55) for 24 hours, and then washed once in PBS. Cells were fixed with 200 μL of buffer A for 20 minutes at room temperature and washed once in PBS. Then cells were permeabilized and stained with anti–Ki67-FITC mAb in 200 μL of buffer B for 30 minutes at room temperature in the dark. Cells were washed once in PBS and analyzed with a FACSCanto.
Cytokine quantification
Cells were incubated with the corresponding isotype control and either CL1-R2 or BY55 antibody. After 24 hours of culture, supernatants were collected and stored at −80°C. When proliferation and cytokine assays were performed for the same patient, the same cell aliquot was used. The human CBA Flex kit (BD Biosciences) was used for simultaneous measurement of IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, and vascular endothelial-derived growth factor (VEGF), according to the manufacturer's instructions. Analysis was performed using a FACSCanto flow cytometer and FACSDiva software (BD Biosciences). The mean fluorescence was compared with standard curves and cytokine concentrations (picograms per milliliter) calculated by the CBA software provided (FCAP array software; BD Biosciences).
CD160–mediated Akt activation
Primary CLL cells, the CLL-derived cell line, MEC-I, and the EBV-transformed B-cell line Sanchez were used in this study. Cells were starved in serum-free RPMI-1640 medium overnight. Cells, or starved cells, were then treated with or without 1μM wortmannin (Sigma-Aldrich) for 30 minutes at 37°C and washed with PBS 3 times. After resuspending in serum-free medium, cells were incubated with 10 μg/mL anti-CD160 antibody (BY55) up to 60 minutes at 37°C. Samples were collected at 0, 15, and 60 minutes for Western blotting.
Western blotting
Cells were lysed in the lysis buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1 sodium dodecyl sulfate, 1mM phenylmethylsulphonyl fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1mM sodium orthovanadate, 1mM dithiothreitol in PBS, pH 7.4) on ice for 30 minutes. Proteins were collected by centrifugation at 12 000g for 20 minutes and protein concentrations were normalized using the Bradford reagent (Bio-Rad). Proteins (50 μg) were subjected to standard 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis at 120 V for 40 minutes and transferred on to polyvinylidene fluoride membrane at 16 V for 1 hour. The polyvinylidene fluoride membrane was blocked with 5% nonfat milk in phosphate-buffered saline plus Tween-20 for 1 hour and probed for various proteins using the following mAbs: anti–phosphorylated AKT, anti-AKT, anti–Bcl-2, anti-Bax, anti–Bcl-xL, anti–Mcl-1, and anti–β-actin. Bound antibodies were detected using horseradish peroxidase–conjugated anti–mouse antibody (Santa Cruz Biotechnology) followed by detection using SuperSignal West Pico Chemiluminescent Substrate (Perbio Science UK Ltd).
Cell viability assay
Cell viability was determined using annexin V–FITC kit (BD Pharmingen). Fresh CLL cells (4 × 106/mL) were treated with 10 μg/mL anti-CD160 antibody (CL1-R2), 10μM LY294002, or 1 μg/mL wortmannin for the indicated times. Cells were then washed once with PBS, incubated with 100 μL of binding buffer, containing 5 μL of annexin V–FITC, and 10 μL of propidium iodide (PI) for 15 minutes at room temperature in the dark. The fluorescence of annexin V–FITC and PI was measured with a FACSCanto I. Annexin V–FITC and PI double-negative cells were defined as viable cells.
Statistical analysis
Data are presented as averages (± SEM) using a 2-tailed Student t test in the Microsoft Excel software package.
Results
Patient characteristics
This study involved 54 patients with B-cell chronic lymphocytic leukemia (36 males and 18 females; ratio = 1.9; median age at diagnosis, 58.5 years; range, 38-83 years), diagnosed according to current National Cancer Institute Working Group guidelines.33 All cases had a demonstrable light chain–restricted CD19+ lymphocytosis (> 4 × 109/L; range, 27.8-424.8 × 109/L) performed by flow cytometry. CD160 positivity was seen in 53 of 54 patients (1 was not tested) using a cutoff of surface antigen expression more than 20% (range, 26%-98%). All patient demographics and prognostic indicators (zeta-chain–associated protein kinase 70 [ZAP-70] and CD38 expression) are shown in Table 1.
CD160 inhibition of spontaneous cell death of CLL cells in vitro involves the PI3K pathway
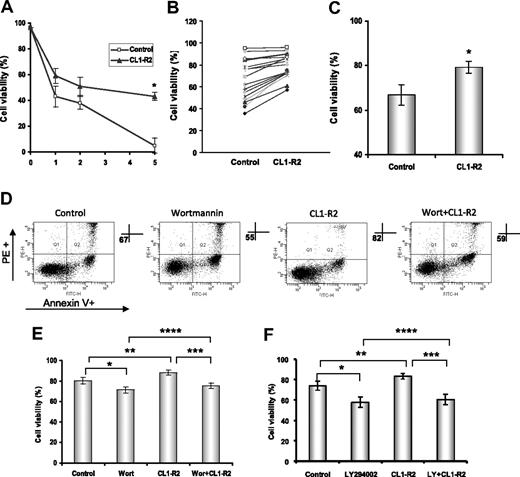
CLL cells are long-lived cells, largely arresting in the G0/G1 phase of cell cycle in vivo, but they have a marked propensity for rapid spontaneous apoptotic cell death when cultured in vitro.4 We investigated the direct effect of CD160 activation on CLL cell spontaneous cell death in vitro. CLL cells cultured with an anti-CD160 mAb (10 μg/mL CL1-R2) showed significantly improved survival compared with control conditions over a 5-day time course (P < .001, Figure 1A). We determined the effect of CL1-R2 on cell viability in 17 CLL cases after 18 hours of culture. CL1-R2 protected 15 of 17 CLL cases from spontaneous cell death (Figure 1B). The mean cell viability increased from 67% to 79% (P < .001) after incubation with CL1-R2 (Figure 1C).
Activation of CD160 protects against spontaneous in vitro apoptosis of CLL cells. Cell viability was assessed by annexin V/PI staining and measured with a FACSCanto. (A) CLL cells (4 × 106/mL) were treated with or without 10 μg/mL anti-CD160 (CL1-R2) for up to 5 days. Data shown are mean ± SD from 3 independent experiments (P < .001). (B) Cells were incubated with or without 10 μg/mL CL1-R2 for 18 hours and then viability was measured. Seventeen cases are presented. (C) Cell viability of all 17 cases is presented as the mean ± SEM (t test; *P < .001). (D-E) CLL cells were treated with CL1-R2, with or without a 1-hour preincubation by wortmannin (1μM) or (F) LY294002 (10μM), for 18 hours. (D) One representative experiment from 7 different patients. (E-F) Mean percentage of cell viability ± SEM (n = 7) of control versus wortmannin or LY294002 alone (E: *P = .036; F: *P < .001) and CL1-R2 alone (**P = .002; F: **P = .005); wortmannin/LY294002 plus CL1-R2 versus CL1-R2 alone (E: ***P < .001; F: ***P < .001) or wortmannin/LY294002 alone (E: ****P = .041; F: ****P = .013).
Activation of CD160 protects against spontaneous in vitro apoptosis of CLL cells. Cell viability was assessed by annexin V/PI staining and measured with a FACSCanto. (A) CLL cells (4 × 106/mL) were treated with or without 10 μg/mL anti-CD160 (CL1-R2) for up to 5 days. Data shown are mean ± SD from 3 independent experiments (P < .001). (B) Cells were incubated with or without 10 μg/mL CL1-R2 for 18 hours and then viability was measured. Seventeen cases are presented. (C) Cell viability of all 17 cases is presented as the mean ± SEM (t test; *P < .001). (D-E) CLL cells were treated with CL1-R2, with or without a 1-hour preincubation by wortmannin (1μM) or (F) LY294002 (10μM), for 18 hours. (D) One representative experiment from 7 different patients. (E-F) Mean percentage of cell viability ± SEM (n = 7) of control versus wortmannin or LY294002 alone (E: *P = .036; F: *P < .001) and CL1-R2 alone (**P = .002; F: **P = .005); wortmannin/LY294002 plus CL1-R2 versus CL1-R2 alone (E: ***P < .001; F: ***P < .001) or wortmannin/LY294002 alone (E: ****P = .041; F: ****P = .013).
PI3K/Akt pathway plays an important role in the survival of CLL cells30 and in CD160 activity in NK cell and T cells.14 The role of the PI3K/Akt pathway in CD160-mediated CLL cell survival was examined using the PI3K inhibitors, wortmannin and LY294002. Figure 1D shows the increase in annexin V/propidium iodide double-negative population (ie, living cells) with CL1-R2, whereas wortmannin decreased CLL survival in both the control and CL1-R2–treated groups. In a further set of experiments (n = 14), CD160 activation was shown to increase CLL cell survival compared with control after an 18-hour culture (P = .002 [Figure 1E]; P = .005 [Figure 1F]). Both wortmannin and LY294002 induced apoptosis in CLL cells (n = 7 for each inhibitor). However, the viability of cells treated with CL1-R2 in the presence of either inhibitor was still greater than that of cells incubated with wortmannin or LY294002 alone (****P = .041 [Figure 1E]; ****P = .013 [Figure 1F]): overall, the mean increased survival of CL1-R2 plus inhibitor versus inhibitor alone was 2.9% (P = .003).
Thus, the survival effect of CD160 activation appears to act largely through the antiapoptotic signaling PI3K/Akt pathway. The inability of wortmannin or LY294002 to completely block the CD160 signal suggests incomplete blockade of the PI3K/Akt pathway (as shown in Figure 5) and/or involvement of other pathways in CD160-mediated signaling.
CD160 regulates expression of Bcl-2 family proteins
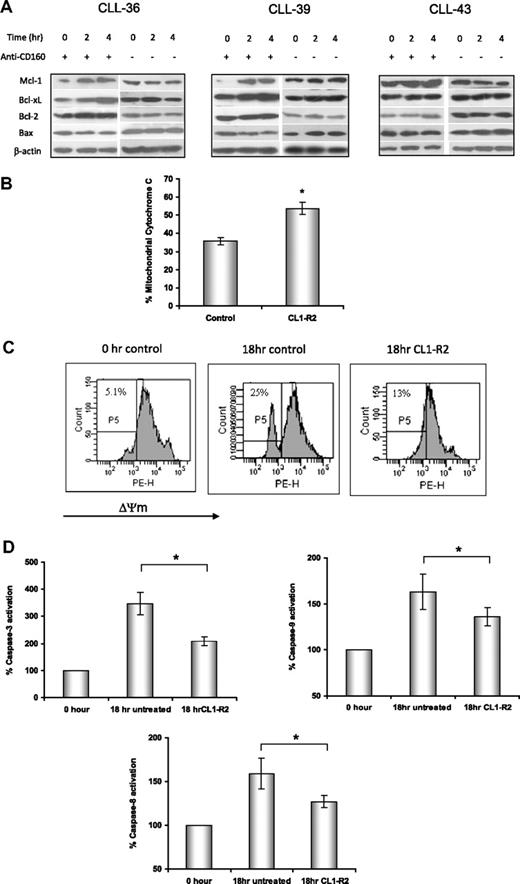
Mitochondria are critical regulators of the intrinsic apoptotic pathway in response to DNA damage, growth factor withdrawal, or oncogene deregulation. Proapoptotic and antiapoptotic Bcl-2 family proteins regulate mitochondrial outer membrane permeability and cytochrome c release from the mitochondrial intermembrane space. After treatment with CL1-R2 for up to 4 hours, expression of Bcl-2 family proteins was detected by Western blotting. CL1-R2 increased expression of antiapoptotic proteins Bcl-2, Bcl-xL, and Mcl-1, but not proapoptotic Bax (Figure 2A). This result indicates that CD160 activation markedly increased the ratios of Bcl-2/Bax and Bcl-xL/Bax. Increase in the ratio of Bcl-2/Bax proteins prevents cytochrome c release from mitochondria, caspase activation, and apoptotic cell death.
Activation of CD160 prevents mitochondrial dysfunction via an increase in the ratio of Bcl-2/Bax. (A) Expression of Bcl-2, Bcl-XL, Mcl-1, and Bax proteins in CLL cells was detected by Western blot after treatment with 10 μg/mL anti-CD160 mAb (BY55) for 2 and 4 hours. (B) Mitochondrial cytochrome c levels were detected by anti–cytochrome c–conjugated PE mAb (6H2) and measured with a FACSCanto. Data show the average percentage of mitochondrial cytochrome c ± SEM from 8 patients, *P < .001. (C) CLL cells were treated with or without 10 μg/mL CL1-R2 for 18 hours and then incubated with 20nM TMRM for 30 minutes at 37°C. Cells were analyzed with a FACSCanto for loss of mitochondrial membrane potential (ΔΨmLOW) and were gated as P5. Numbers indicated in each graph are the percentage of ΔΨmLOW cells. The figure shows 1 representative experiment from 7 patients. (D) Activation of caspase-3 and caspase-9 was tested after treatment with 10 μg/mL CL1-R2 for 18 hours, with data normalized to the basal caspase-3, -8, and -9 level (0 hour, expressed as 100%). Data represent the average percentage of 8 patients ± SEM; caspase-3: *P < .001; caspase-9: *P = .01; caspase-8: *P = .026.
Activation of CD160 prevents mitochondrial dysfunction via an increase in the ratio of Bcl-2/Bax. (A) Expression of Bcl-2, Bcl-XL, Mcl-1, and Bax proteins in CLL cells was detected by Western blot after treatment with 10 μg/mL anti-CD160 mAb (BY55) for 2 and 4 hours. (B) Mitochondrial cytochrome c levels were detected by anti–cytochrome c–conjugated PE mAb (6H2) and measured with a FACSCanto. Data show the average percentage of mitochondrial cytochrome c ± SEM from 8 patients, *P < .001. (C) CLL cells were treated with or without 10 μg/mL CL1-R2 for 18 hours and then incubated with 20nM TMRM for 30 minutes at 37°C. Cells were analyzed with a FACSCanto for loss of mitochondrial membrane potential (ΔΨmLOW) and were gated as P5. Numbers indicated in each graph are the percentage of ΔΨmLOW cells. The figure shows 1 representative experiment from 7 patients. (D) Activation of caspase-3 and caspase-9 was tested after treatment with 10 μg/mL CL1-R2 for 18 hours, with data normalized to the basal caspase-3, -8, and -9 level (0 hour, expressed as 100%). Data represent the average percentage of 8 patients ± SEM; caspase-3: *P < .001; caspase-9: *P = .01; caspase-8: *P = .026.
CD160 protects mitochondrial membrane integrity and inhibits caspase activation in CLL
The effect of CD160-mediated increases in Bcl-2/Bax and Bcl-xL/Bax ratios on mitochondrial outer membrane permeability was investigated. The mitochondrial cytochrome c level was determined by flow cytometry after CLL cells were incubated with or without CL1-R2 for 18 hours. CL1-R2–treated cells retained significantly more cytochrome c compared with the control group (P = .001, Figure 2B), indicating that CD160 activation prevented mitochondrial outer membrane permeability and cytochrome c release.
The integrity of the mitochondrial inner membrane was also assessed by measuring the mitochondrial membrane potential (ΔΨm). A reduction of ΔΨm in CLL cells undergoing spontaneous in vitro cell death was detected at as early as 5 hours of culture, and this collapse was diminished by anti-CD160 (data not shown), with an even more marked prevention of the ΔΨm reduction after 18 hours (Figure 2C).
Caspase activation is crucial for mitochondrial-dependent or -independent apoptosis. The activation of caspases-3, -8, and -9 in CLL cells was assessed by a fluorogenic assay after treatment with or without CL1-R2 for 18 hours. The activation of caspases-3, -8, and -9 in CLL cells was significantly inhibited by CL1-R2 (Figure 2D: caspase-3, P = .001; caspase-9, P = .01; caspase-8, P = .026).
CD160 signaling induces proliferation and cell cycle progression of CLL cells
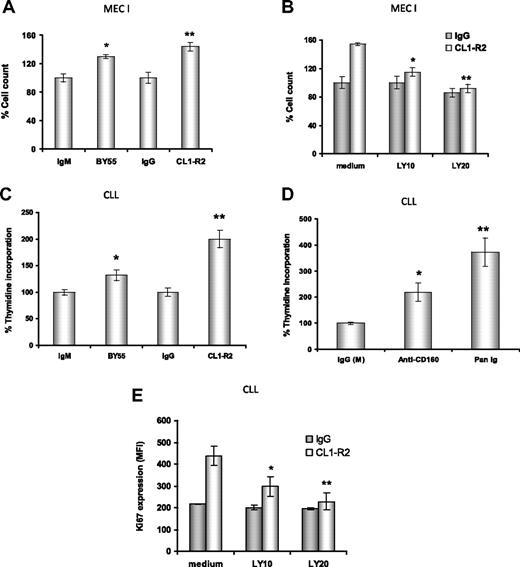
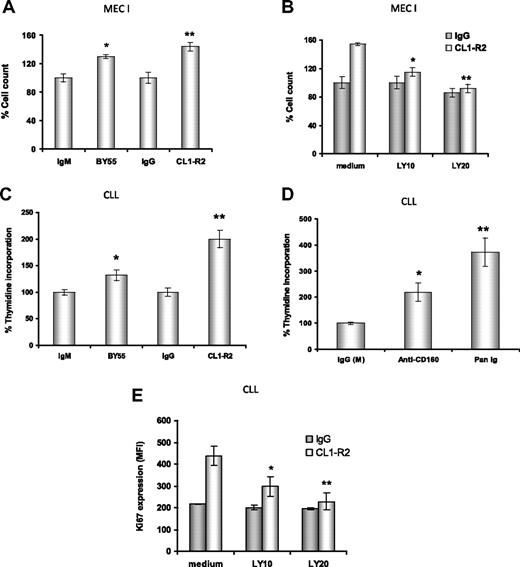
CD160 activation leads to effector function in NK cells12 and enhancement of TCR-mediated signaling, proliferation, and cytotoxicity in CD8+ T cells.14,17 Hence, we examined whether cross-linking of CD160 induced CLL cell proliferation. First, both anti-CD160 mAbs, CL1-R2 or BY55 (P = .02 and P = .01, respectively), led to enhanced proliferation of MEC-I cells, a cell line derived from a patient with prolymphocytic progression of CLL, with a 30% to 50% increase in cell numbers compared with isotype controls (Figure 3A). Both BY55 and CL1-R2 mAbs alone also induced proliferation in primary CLL samples in a 3H-thymidine assay (Figure 3C: P = .005 and P = .004, respectively, vs isotype control). Activation of surface receptors, such as CD4036 and BCR,37 also induced proliferation in CLL. Comparing anti-CD160 mAb (CL1-R2 or BY55) and BCR activation with Pan-Ig (anti-IgG,M,A), Pan-Ig induced more proliferation than anti-CD160 mAb in CLL cells (Figure 3D). However, there was marked heterogeneity, with this pattern reversed in individual cases (data not shown). In keeping with these observations, anti-CD160 mAb also induced cell-cycle progression in CLL cells, as indicated by increased levels of Ki67 after 24-hour culture (Figure 3E).
Anti-CD160 antibodies induce cell proliferation through PI3K activation. (A) MEC-I cells (105/mL) were cultured with isotype control (IgG or IgM) or anti-CD160 (CL1-R2 or BY55) mAb, (B) with or without the PI3K inhibitor LY294002, and the cells were counted after 72-hour incubation. Results are expressed as a percentage of basal control growth (ie, isotype control = 100%), and are representative of 3 independent experiments ± SEM. (A) *P = .02; **P = .01 (anti-CD160 vs isotype control). (B) *P = .036; **P = .029 (CL1-R2 plus LY294002 vs CL1-R2 alone). (C) CLL cells (2 × 105) were treated with isotype control (IgG or IgM) mAb and CL1-R2 or BY55. 3H-thymidine was added for the last 16 hours of a 72-hour incubation. Data are expressed as average percentage of basal growth (ie, isotype control = 100%) of 22 patients ± SEM, *P = .005, **P = .004 (anti-CD160 vs isotype control). (D) CLL cells were treated with isotype control mAb (IgG or IgM), CL1-R2, or Pan-Ig mAb (anti-IgG,M,A), all at a concentration of 10 μg/mL. 3H-thymidine was measured and analyzed as in panel C; the bars represent the mean values of 22 patients ± SEM; *P = .004, **P = .002 compared with control. (E) CLL cells were coincubated with control IgG or CL1-R2 and LY294002 for 24 hours and Ki67 expression was measured by flow cytometry. The data represent the mean ± SEM of 5 patients (*P = .002, **P = .001).
Anti-CD160 antibodies induce cell proliferation through PI3K activation. (A) MEC-I cells (105/mL) were cultured with isotype control (IgG or IgM) or anti-CD160 (CL1-R2 or BY55) mAb, (B) with or without the PI3K inhibitor LY294002, and the cells were counted after 72-hour incubation. Results are expressed as a percentage of basal control growth (ie, isotype control = 100%), and are representative of 3 independent experiments ± SEM. (A) *P = .02; **P = .01 (anti-CD160 vs isotype control). (B) *P = .036; **P = .029 (CL1-R2 plus LY294002 vs CL1-R2 alone). (C) CLL cells (2 × 105) were treated with isotype control (IgG or IgM) mAb and CL1-R2 or BY55. 3H-thymidine was added for the last 16 hours of a 72-hour incubation. Data are expressed as average percentage of basal growth (ie, isotype control = 100%) of 22 patients ± SEM, *P = .005, **P = .004 (anti-CD160 vs isotype control). (D) CLL cells were treated with isotype control mAb (IgG or IgM), CL1-R2, or Pan-Ig mAb (anti-IgG,M,A), all at a concentration of 10 μg/mL. 3H-thymidine was measured and analyzed as in panel C; the bars represent the mean values of 22 patients ± SEM; *P = .004, **P = .002 compared with control. (E) CLL cells were coincubated with control IgG or CL1-R2 and LY294002 for 24 hours and Ki67 expression was measured by flow cytometry. The data represent the mean ± SEM of 5 patients (*P = .002, **P = .001).
CD160 signaling enhances CLL cell proliferation in a PI3K-dependent manner
CD160 activation of NK-cell effector functions is dependent on the recruitment of PI3K.16 First, we investigated the MEC-I cell line. There was a dose-dependent reduction in CD160-mediated proliferation of MEC-I with the PI3K inhibitors, LY294002 at 10μM and 20μM (P = .036 and P = .029, respectively, Figure 3B) and wortmannin (data not shown). Similarly, in primary CLL cells, PI3K inhibition prevented the CD160-mediated up-regulation of Ki67 in a dose-dependent manner (P = .001 with 20μM LY294002, Figure 3E). Of note, at the doses used, these PI3K inhibitors did not diminish the basal level of proliferation of MEC-I or the Ki67 expression in CLL samples, suggesting that the PI3K/Akt pathway is not constitutively activated in these malignant B cells (which was confirmed in Figure 5).
Activation of CD160 triggers IL-6 and IL-8 secretion in CLL cells
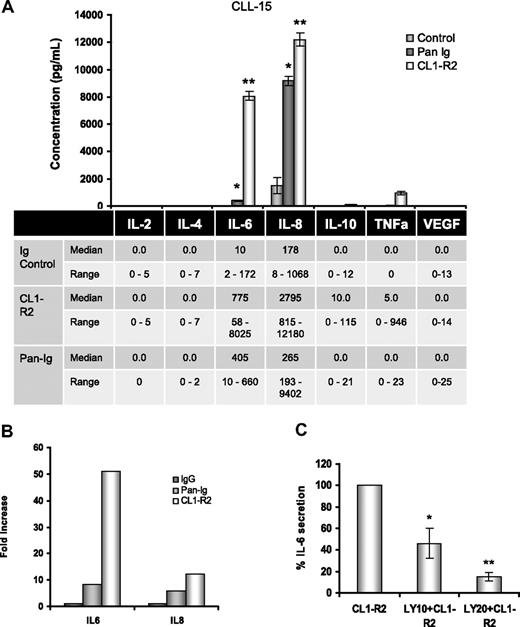
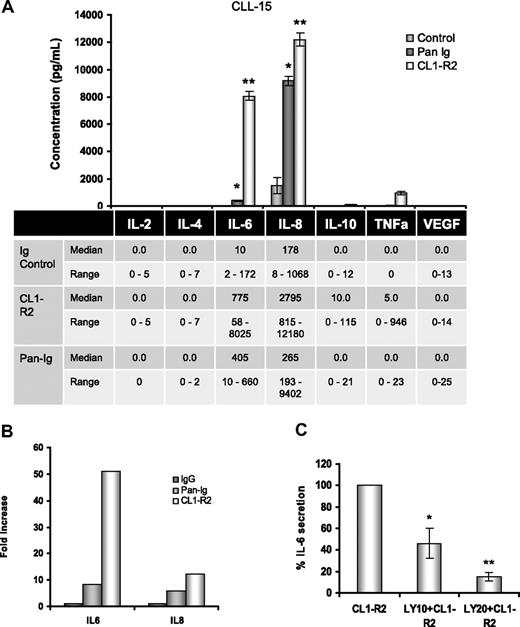
In vitro, CLL cell spontaneous apoptosis can be prevented by a variety of cytokines.22,38 CD160 activation of normal human NK cells is associated with a unique cytokine profile, including IL-6.13 The role of CD160 on CLL cytokine secretion was investigated for IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, and VEGF. The only cytokines consistently found at baseline were low levels of IL-6 and IL-8. We tested the cytokine expression of 10 CLL cases after CD160 or BCR activation. The results for 1 case are shown in the bar histogram (Figure 4A top panel), with a 800-fold increase in IL-6 (8025 pg/mL) and a 12-fold increase in IL-8 (12 480 pg/mL) after CD160 stimulation; TNF-α (946 pg/mL) and IL-10 (115 pg/mL) were also detected. BCR activation led to IL-8 (9402 pg/mL) production, but lower levels of IL-6 (405 pg/mL) and TNF-α (23 pg/mL). Data for all cases are shown in the bottom panel in Figure 4A: CD160 triggering was associated with marked up-regulation of IL-6 and IL-8, but only low levels of TNF-α and IL-10 (median, 5 and 10 pg/mL, respectively). Comparing the fold increase over baseline demonstrates that CD160 induced markedly greater IL-6 and IL-8 secretion than BCR activation (Figure 4B). The role of the PI3K/Akt pathway in CD160-mediated cytokine production was investigated. Figure 4C shows a dose-dependent decrease in IL-6 production from CLL cells treated with CL1-R2 in the presence of PI3K inhibition (P < .001 for 20μM LY294002).
Activation of CD160 triggers IL-6 and IL-8 secretion in CLL cells. (A) Cells (5 × 105) in 100 μL of medium were incubated with the isotype control and either CL1-R2 or Pan-Ig antibody (anti-IgG,M,A). The human CBA Flex kit was used for simultaneous measurement of IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, and VEGF expression by a FACSCanto after 24-hour incubation. (Top panel) Complete results for 1 case (CLL15) are shown. Pan-Ig and CL1-R2 significant production of IL-6 (*P = .023, **P = .019) and IL-8 (*P = .015, **P = .045) versus control conditions. (Bottom panel) The median and range of cytokine release for all cases are shown (IL-8, n = 5; other cytokines, n = 10). (B) Fold increase of IL-6 and IL-8 compared with control conditions for all cases (IL-8, n = 5; IL-6, n = 10). (C) Cells (5 × 105) in 100 μL of medium were pretreated with 10μM or 20μM LY249002 for 1 hour and then incubated with isotype control or CL1-R2 for 24 hours. IL-6 expression was detected with the CBA Flex kit. The data shown are the mean ± SEM from 3 patients; *P = .014, **P < .001 comparing CL1-R2 alone versus LY294002 followed by CL1-R2.
Activation of CD160 triggers IL-6 and IL-8 secretion in CLL cells. (A) Cells (5 × 105) in 100 μL of medium were incubated with the isotype control and either CL1-R2 or Pan-Ig antibody (anti-IgG,M,A). The human CBA Flex kit was used for simultaneous measurement of IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, and VEGF expression by a FACSCanto after 24-hour incubation. (Top panel) Complete results for 1 case (CLL15) are shown. Pan-Ig and CL1-R2 significant production of IL-6 (*P = .023, **P = .019) and IL-8 (*P = .015, **P = .045) versus control conditions. (Bottom panel) The median and range of cytokine release for all cases are shown (IL-8, n = 5; other cytokines, n = 10). (B) Fold increase of IL-6 and IL-8 compared with control conditions for all cases (IL-8, n = 5; IL-6, n = 10). (C) Cells (5 × 105) in 100 μL of medium were pretreated with 10μM or 20μM LY249002 for 1 hour and then incubated with isotype control or CL1-R2 for 24 hours. IL-6 expression was detected with the CBA Flex kit. The data shown are the mean ± SEM from 3 patients; *P = .014, **P < .001 comparing CL1-R2 alone versus LY294002 followed by CL1-R2.
CD160 activates the PI3K/Akt pathway in CLL
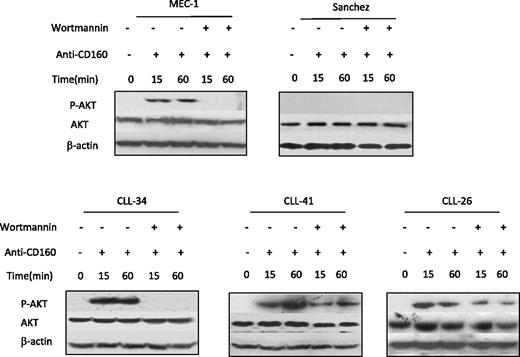
PI3K/Akt is an important pathway that regulates multiple cell processes, such as cell proliferation, differentiation, survival, growth, and angiogenesis in various cells. In NK cells, CD160 activation of cell effector functions was to found to be dependent on PI3K/Akt pathway.16 To understand CD160 signaling in CLL, we analyzed the phosphorylation status of Akt (pSer473), the best characterized target of PI3K. Western blot was preformed for Akt expression in the CD160-positive MEC-I and the CD160-negative Sanchez lymphoblastoid cell line (CD160 expression shown in supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article) and CLL. Phosphorylated Akt was not detected at baseline. CD160 triggering induced pAkt on Ser473, which was detectable at 15 and 60 minutes in CLL and MEC-I (Figure 5). The addition of wortmannin, a PI3K inhibitor, prevented Akt phosphorylation induced by anti-CD160 (Figure 5). The CD160-negative EBV cell line did not show pAkt at baseline or after addition of anti-CD160 mAb (Figure 5). These findings are in keeping with the hypothesis that CD160 signaling can act through the PI3K/Akt pathway and that this is associated with cellular activation in CLL.
CD160 activates the PI3K/AKT signaling pathway. CLL cells (5 × 106/mL), MEC-I cells (106/mL), and Sanchez cells (106/mL) were pretreated with or without 1μM wortmannin for 1 hour and then incubated with 10 μg/mL BY55 for the indicated times. Akt and phosphorylated Akt protein expression was detected by Western blot. The β-actin expression acted as a loading control.
CD160 activates the PI3K/AKT signaling pathway. CLL cells (5 × 106/mL), MEC-I cells (106/mL), and Sanchez cells (106/mL) were pretreated with or without 1μM wortmannin for 1 hour and then incubated with 10 μg/mL BY55 for the indicated times. Akt and phosphorylated Akt protein expression was detected by Western blot. The β-actin expression acted as a loading control.
Discussion
In healthy people, CD160 distribution is restricted to circulating and tissue cytotoxic NK cells and T cells, with no expression in normal B cells.11,38 In disease states, CD160 expression is up-regulated on CD3+CD8+ T cells during infection with human immunodeficiency virus39 and is expressed on the neoangiogenic vasculature of tumors18 as well as CD4+ T cells infiltrating inflammatory skin disease.15 In this study, all 53 cases tested for CD160 expression were positive (Table 1).
Although CLL cells are characterized by being largely in G0/G1 phase of the cell cycle and long lived with high levels of antiapoptotic molecules in vivo,2,3 they rapidly die when cultured in vitro. The mechanism of CLL spontaneous apoptosis in vitro is unclear. However, some cytokines,5,6 chemokines,8 albumin,7 and stromal cells32 have been shown to protect from spontaneous apoptosis in vitro. Recently, constitutive activation of Akt was shown to increase expression of antiapoptotic proteins Mcl-1 and Bcl-xl and cell survival in CLL.31 However, findings in the literature concerning constitutive phosphorylation of Akt in CLL cells are variable and in many cases are negative for constitutive pAkt expression.31,40,41 In this study, we demonstrated that CD160 promotes CLL survival by up-regulating Bcl-2, Bcl-xL, and Mcl-1 expression and blocking cytochrome c release, ΔΨm collapse, and caspase activation. Activation of CLL cells by anti-CD160 mAb triggered Akt phosphorylation (Figure 5), and up-regulation of Mcl-1, Bcl-xL, and Bcl-2 (Figure 2A). Blockade of Akt phosphorylation by wortmannin or LY294002 led to increased spontaneous apoptosis and inhibition of CD160-induced survival (Figure 1E-F). Antiapoptotic Bcl-2, Bcl-xL, and Mcl-1 are localized in the inner and/or outer mitochondrial membranes and prevent apoptosis by blocking cytochrome c release from the outer mitochondrial membrane and inhibiting the ΔΨm collapse induced by Bax activation. Caspase activation is a crucial step for apoptosis in both the mitochondrial-dependent and -independent pathways. Extrinsic death factors, such as TNF-α and TNF-related apoptosis-inducing ligand, activate caspase-8, which then triggers the apoptotic process either by directly activating downstream caspase-3 or by cleaving Bid to induce cytochrome c release. Cytochrome c release initiates caspase-9 activation, which activates caspase-3, but not caspase-8. CD160 activation decreased not only caspase-3 and caspase-9 activity, but also caspase-8 activity, indicating that spontaneous apoptosis of CLL cells in vitro may involve both mitochondrial-dependent and -independent pathways.
A long-standing dogma in CLL biology is that CLL cells are quiescent in the G0/G1 phase of the cell cycle and are resistant to apoptosis, which is associated with overexpression of cell-cycle inhibitor P27kip142 and antiapoptotic molecules.8 However, recent work has shown that CLL may show substantial proliferation, with turnover of between 0.1% to more than 1% of the entire clone per day.43 In contrast, in vitro, the spontaneous apoptosis of CLL cells means assessing proliferation by counting cell numbers or by cell colony formation is unreliable; therefore, alternative techniques, such as 3H-thymidine and bromodeoxyuridine incorporation or Ki67 expression, have been used.44-46 In this study, we observed changes in the cell cycle using 3H-thymidine incorporation and Ki67 expression, which indicated that CD160 can regulate the shift of quiescent CLL cells into the cell cycle and an increase in S phase distribution. In the MEC-I cell line, it was possible to show that CD160 activation actually increased cell numbers (Figure 3A). The in vitro proliferation of B cells induced by cross-linking of the BCR, or the IL-4 receptor, is crucially dependent on PI3K/Akt activation.47,48 Blockade of Akt phosphorylation by the PI3K inhibitors LY294002 or AS-604850 led to significant reduction of thymidine incorporation.47 The mechanism of phosphorylated Akt-mediated proliferation is not fully understood, but Akt activation has been implicated in cell-cycle regulation via down-regulation of the cell-cycle inhibitor protein p27 and up-regulation of cell-cycle promoter proteins, cyclin A, D2, and E.47
In vitro, many cytokines have prosurvivial and antiapoptosis function in CLL and activation of CD40 induces IL-6, IL-8, IL-10, and TNFα secretion.36 In this study, CLL cell secretion of IL-2, IL-4, IL-10, TNFα, and VEGF was universally undetectable/low in control conditions; neither CD160 nor BCR stimulation significantly affected these cytokines (with the exception of IL-10 and TNFα in 2 cases only; Figure 4A top panel shows the strongest IL-10 and TNFα responses). On the other hand, activation of CD160 or the BCR triggered very high-level IL-6 and IL-8 secretion, with CD160 mediating a 50-fold increase in IL-6 and 15-fold in IL-8. IL-8 provides a survival signal in CLL leading to Bcl-2 up-regulation, but it has no effect on proliferation.22,23 IL-6 is a growth and differentiation factor for normal B cells and a growth factor for multiple myeloma and some B-cell malignancies,49,50 but its role in CLL biology is unclear. IL-6 gene expression and IL-6 secretion by CLL cells have been reported by several groups.20,21 Aderka et al showed that the low-level production of IL-6 by CLL cells was strongly up-regulated by in vitro activation using phorbol myristate acetate in Binet stage A but not in stage C CLL. Interestingly, IL-6 has been reported to inhibit TNF-α–induced CLL proliferation,25 whereas high serum IL-6 levels have been shown to be associated with both good25 and poor26 prognosis. Normal circulating human NK cells produce a unique profile of cytokines in response to CD160 triggering: IFN-γ, TNF-α, and IL-6,13 involving multiple signal pathways.16 In CLL, CD160-induced IL-6 secretion was at least partially dependent on the PI3K/Akt pathway. The Janus kinase 2 inhibitor, AG490, also blocked CD160-induced IL-6 secretion (data not shown), indicating that CD160 signaling also involves the Janus kinase 2/signal transducer and activator of transcription 3 pathway.
The functional data presented here show the potential for CLL cells to use the CD160 pathway to enhance their survival and cellular activation, mimicking the activity of CD160 in normal NK cells and CD8+ T cells.12-14,16,17 Triggering of CD160 on CLL cells protects against apoptosis, induces cytokine production, most notably IL-6, and leads to a subtle shift in the cell cycle as shown by increased Ki67 expression. The pathophysiologic relevance of these observations remains to be established, but a variety of CD160 ligands are expressed in the microenvironment of lymphoid tissue, both by CLL cells themselves and nonmalignant cells: major histocompatibility complex class I,9 CD1d,51 HLA-G,9,52 and herpes virus entry mediator.19 Understanding the biology of these multiple and complex interactions may reveal new therapeutic targets in CLL and other B-cell lymphoproliferative disorders, which remain incurable disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
J.G. was funded by a grant awarded by Barts and the London NHS Trust/Queen Mary University of London. T.F. was funded by a Cancer Research Committee grant awarded by Barts and the London NHS Trust/Queen Mary University of London. This study was supported by a generous donation from Relgate Investments Ltd (Charitable Foundation).
Authorship
Contribution: S.G.A., F.-T.L., and J.G. designed the research; F.-T.L., J.G., T.F., L.J., and S.G.A. performed the research and analyzed the data; J.G.G. performed the research and coauthored the paper; A.B. provided new reagents and coauthored the paper; and F.-T.L., T.F., L.J., and S.G.A. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: S. Agrawal, Stem Cell Laboratory, St Bartholomew's Hospital, West Smithfield, London, EC1A 7BE, United Kingdom; e-mail: s.g.agrawal@qmul.ac.uk.
References
Author notes
F.-T.L. and J.G. contributed equally to this work.