In this issue of Blood, the noncanonical NF-κB pathway and/or the canonical pathway, is aberrantly activated in 17% of patients with MM and 40% of MM cell lines through various gene abnormalities that result in NIK stabilization mostly.1
The NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathway involves dimers of transcription factors (TFs) of the Rel/NF-κB family, comprising RelA/P65, c-rel, RelB, NF-κB1 (p50 and its p105 precursor) and NK-κB2 (p52 and its precursor p100).2 NF-κB pathway plays a central role in infection and inflammation, and in lymphopoiesis, particularly in normal B-cell and plasma cell development. These TFs are normally kept inactive in the cytoplasm through binding with inhibitors called IκB (inhibitor of κB) or unprocessed NF-κB1 or NF-κB2. Cell activation may result in activation of IκB kinases (IKKs), mainly IKKβ in the canonical pathway and IKKα in the noncanonical pathway. Activation of IKKβ results in the phosphorylation, ubiquitination, and degradation of IκBα and other IκBs, releasing TF dimers (mainly P65/P50) to the nucleus that activate NF-κB target genes. In the noncanonical pathway, NF-κB–inducing kinase (NIK) induces phosphorylation and activation of IKKα dimers, resulting in phosphorylation and proteasome processing of NK-κB2 precursor into P52 subunit. P52 form active dimers with RelB that migrate to the nucleus and activate NK-κB target genes. The NK-κB canonical pathway is activated via numerous receptors, namely TNF receptor, T-cell receptor, Toll-like receptors activated by bacterial products, and BCMA and TACI receptors for BAFF or APRIL growth factors. The noncanonical NK-κB pathway is activated by a more restricted sets of stimuli activating, namely CD40, Rank, lymphotoxin-α receptor, or BAFF-receptor.
In this issue, Demchenko and colleagues have further documented the mechanisms of action of the gene abnormalities targeting the regulatory elements of canonical and noncanonical NK-κB pathway in multiple myeloma cell lines (MMCLs).1 These gene abnormalities were initially reported in 2007 in 40% of MMCLs and primary myeloma cells of 17% of patients with multiple myeloma (MM).3,4 These gene abnormalities target genes encoding either for products amplifying one of the 2 NK-κB pathways (TACI, CD40, LTB-R, NK-κB1, NIK) or for inhibitors of the canonical (CYLD) or noncanonical NK-κB pathway (cIAP1-2, TRAF2, TRAF3, NK-κB2). Indeed, in inactive cells, NIK is rapidly degraded through ubiquitination by cIAP1-2 ubiquitin ligases. NIK binds TRAF3 first and the NIK/TRAF3 complex is recruited to cIAP1-2/TRAF2 via TRAF3 binding to TRAF2. Activation of receptors by external stimuli induces recruitment of c-IAP1-2/TRAF2/TRAF3 complex to the activated receptors, increasing ubiquitination and degradation of TRAF3. TRAF3 decrease makes it possible to increase NIK and phosphorylation and activation of IKKα dimers. In MM, the most frequent mutations involve TRAF3 gene located on chromosome 14q32, but also include TRAF2 or cIAP1-2, resulting in increased NIK expression.
Demchenko et al have shown that nuclear p52 and RelB are detected in MMCLs with abnormalities targeting the noncanonical pathway, but also nuclear P65 and p50 that are indicators of canonical pathway activation. A deletion of cIAP or TRAF2 results in loss of NIK down-regulation, whereas TRAF3 mutations or deletions partly remove NIK control. Actually, Demchenko et al have shown that TRAF2 can directly interact with NIK and recruit cIAP to NIK, promoting NIK ubiquitination and degradation in the absence of TRAF3. In addition, Demchenko et al have shown that an overexpression or knockout of NIK in 2 MMCLs results in up-regulation or down-regulation of the noncanonical NK-κB pathway (increased nuclear P52 and RelB) but also of the canonical NK-κB pathway (increased nuclear P65 and P50). Finally, Demchenko et al show that the overexpression of IKKβ or NIK in one MMCL resulted in the same pattern of gene expression in one MMCL, but less overlapping in the second one.
The importance of NK-κB pathway deregulation in MM disease is a consequence of its pivotal role in the process of generation of normal plasma cells. Activation of CD40 B-cell molecules by the CD40 ligand on cognate T cells and of Toll-like receptor by bacterial products induce noncanonical and canonical NF-κB pathway activation resulting in IRF4 expression, down-regulation of Bcl6 B-cell TF, and up-regulation of Blimp1 and XBP1 plasma cell TFs.5 The generated plasmablasts exit the lymph node and have to home to bone marrow or mucosa and find an empty niche that will provide further differentiation and survival signal. Normal plasma cells may survive for many years in the bone marrow and mucosa niches provided the right survival factors are provided. Among numerous survival factors, BAFF and APRIL are important ones because treatment of mice with a BAFF/APRIL receptor TACI fused with Fc fragment abrogates plasma cell survival in mice and reduces survival in humans.6,7
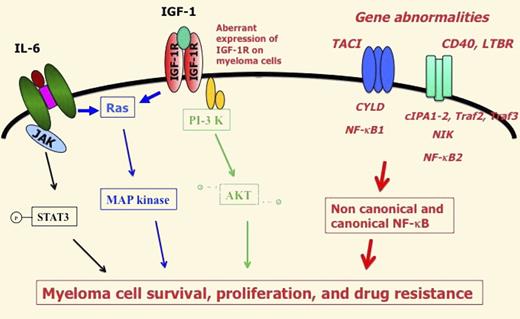
But it is important to emphasize that a cooperation of NK-κB pathway with other pathways should be critical to promote MMC and normal plasma cell survival (see figure). As an example, TNFα is a moderate growth factor for myeloma cell lines, whereas it induces a strong activation of canonical NF-κB pathway in MMCLs.8 BAFF and APRIL are frequently mentioned as activator of NF-κB pathways, but it is worthwhile to recall that they efficiently induce MAPK and AKT phosphorylation in myeloma cell lines.9 Primary MMCs express aberrantly the receptor for insulin growth factor type 1 in association with a poor prognosis. Insulin growth factor type 1 is the major growth factor for myeloma cell lines,10 but it actives MAPK and PI-3/AKT pathways, unlike NF-κB pathway. The same holds true for IL-6 that activates JAK/STAT3, MAPK, unlike NF-κB pathway or PI3/AKT pathway.
The main gene abnormalities targeting NF-κB pathway are displayed in red and italic font. They result in aberrant activation of noncanonical and canonical NF-κB pathway that together with other signaling pathway activation (JAK/STAT, MAP kinase, PI3K/AKT) trigger myeloma cell survival, proliferation and drug resistance.
The main gene abnormalities targeting NF-κB pathway are displayed in red and italic font. They result in aberrant activation of noncanonical and canonical NF-κB pathway that together with other signaling pathway activation (JAK/STAT, MAP kinase, PI3K/AKT) trigger myeloma cell survival, proliferation and drug resistance.
Strategies to block NF-κB pathway should consider resistance mechanisms linked to the activation of these other important pathways in MMCs and the association of drugs targeting these different pathways.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■