Abstract
The antiapoptotic Bcl-2 family member Bfl-1 is up-regulated in many human tumors in which nuclear factor-κB (NF-κB) is implicated and contributes significantly to tumor cell survival and chemoresistance. We previously found that NF-κB induces transcription of bfl-1 and that the Bfl-1 protein is also regulated by ubiquitin-mediated proteasomal degradation. However, the role that dysregulation of Bfl-1 turnover plays in cancer is not known. Here we show that ubiquitination-resistant mutants of Bfl-1 display increased stability and greatly accelerated tumor formation in a mouse model of leukemia/lymphoma. We also show that tyrosine kinase Lck is up-regulated and activated in these tumors and leads to activation of the IkappaB kinase, Akt, and extracellular signal-regulated protein kinase signaling pathways, which are key mediators in cancer. Coexpression of Bfl-1 and constitutively active Lck promoted tumor formation, whereas Lck knockdown in tumor-derived cells suppressed leukemia/lymphomagenesis. These data demonstrate that ubiquitination is a critical tumor suppression mechanism regulating Bfl-1 function and suggest that mutations in bfl-1 or in the signaling pathways that control its ubiquitination may predispose one to cancer. Furthermore, because bfl-1 is up-regulated in many human hematopoietic tumors, this finding suggests that strategies to promote Bfl-1 ubiquitination may improve therapy.
Introduction
Defective apoptosis is a hallmark of many human tumors and is frequently responsible for resistance to therapy. It often results from up-regulation of antiapoptotic members of the Bcl-2 family that regulate the cellular response to death-inducing stimuli. Multidomain antiapoptotic Bcl-2 family proteins (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, Bfl-1) interact with BH3-only proapoptotic members of this family (Bid, Bad, Bim, Puma, Noxa, Hrk, Bmf, Nbk/Bik) to control activation of the multidomain proapoptotic Bcl-2 family proteins Bax and Bak, which trigger mitochondrial permeability and the release of cytochrome c and other proapoptotic factors that activate effector caspases, leading to cell death.1
Bfl-1 (Bcl2a1, A1) is an nuclear factor-κB (NF-κB)–inducible antiapoptotic Bcl-2 family protein that promotes the survival of immune and inflammatory cells and is highly up-regulated in therapy-resistant B-cell chronic lymphocytic leukemia (B-CLL), acute myelogenous leukemia with poor prognosis, large B-cell lymphomas, and other cancers.2-10 Bfl-1 plays a critical role in tumor cell survival and chemoresistance and is a key contributor to the arsenal of antiapoptotic proteins regulated by NF-κB because Bfl-1 knockdown with shRNA can restore apoptosis in drug-resistant B-CLL, large B-cell lymphoma, and several other leukemia/lymphoma cells in vitro.11-13 This points to the importance of Bfl-1 as a therapeutic target. It is therefore important to understand the mechanisms that control Bfl-1 levels and their impact on cancer.
Bfl-1 levels are tightly controlled and are regulated at multiple levels. Like Bcl-xL, bfl-1 is a transcriptional target of NF-κB, which is key in many cancers.2-6,14 Stimuli that activate the NF-κB signaling pathway rapidly up-regulate bfl-1 mRNA expression, enabling cells to quickly respond to NF-κB–mediated survival cues. bfl-1 is further controlled posttranscriptionally via alternative splicing to produce a shorter form, Bfl-1s, that is nuclear but remains protective.15 This action is reminiscent of the bcl-x and mcl-1 genes that encode the antiapoptotic Bcl-xL and Mcl-1 proteins and that can also produce shorter but proapoptotic forms Bcl-xS and Mcl-1S through alternative splicing.16-18
Several Bcl-2 family proteins also are regulated posttranslationally by phosphorylation or ubiquitination, which can critically affect their activities. For example, dephosphorylation of proapoptotic Bad triggers its release from 14-3-3, allowing it to inhibit Bcl-xL, and leads to cell death.19 Phosphorylation of proapoptotic Bim by the Ras/Raf-extracellular signal-regulated protein kinase 1/2 (ERK1/2) pathway causes its degradation by the ubiquitin-proteasome pathway and allows Bcl-xL and Mcl-1–mediated cell survival, which promotes tumorigenesis and chemoresistance.20 Phosphorylation of antiapoptotic Mcl-1 by GSK-3β promotes its degradation by the ubiquitin-proteasome pathway in a Mule- and β-TrCP–dependent manner,21-23 whereas Bcl-2 is further stabilized by phosphorylation by the mitogen-activated protein (MAP) kinase (ERK1/2) pathway.24 Because overexpression of antiapoptotic Mcl-1, Bcl-2, or Bcl-xL is commonly observed in human cancers and can lead to tumor formation in transgenic mice,25-27 it suggests that posttranslational modifications that either dictate rapid elimination of proapoptotic factors or that lead to sustained levels of antiapoptotic factors can significantly contribute to tumor development.
We previously reported that, in contrast to Bcl-2 and Bcl-xL, Bfl-1 is short-lived as the result of posttranslational control through ubiquitin-proteasome–mediated turnover (Bfl-1 T1/2: ∼ 2-3 hours).28 The mouse homologue of Bfl-1, A1, is similarly regulated.29 Although transcriptional up-regulation of Bfl-1 frequently is found in NF-κB–dependent tumors and clearly contributes to tumor cell survival and chemoresistance, little is known of the role that Bfl-1's posttranslational control plays in cancer. In this report, we show that ubiquitination of Bfl-1 is an important regulatory mechanism to control its oncogenic activity and that Bfl-1 functionally cooperates with tyrosine kinase Lck in tumorigenesis. Our results suggest that failure to posttranslationally down-regulate Bfl-1 can significantly predispose one to leukemia/lymphoma.
Methods
Plasmids and shRNA
Green fluorescent protein (GFP)–tagged wild-type Bfl-1 or mutants (Bfl-1ΔC, KKK/RRR, ST/DD) were previously described.28 Mouse FL5.12 pro-B cells stably expressing these proteins, or Bcl-xL, from the spleen focus-forming virus LTR promoter of pSFFV-neo also have been described.28 2xMyc-Bfl-1 in pSFFV-neo contained 2 Myc epitopes fused to the N-terminus of Bfl-1.30 Dominant-negative p53 (p53DD) was expressed from pcDNA3.1-IRES-puro (Invitrogen). Constitutively active Lck (Lck Y505F; a gift from H. Band, University of Nebraska Medical Center) was expressed in pcDNA3.1/Zeo (Invitrogen). pDsRed2-Mito vector was from Clontech. Murine Lck shRNAs and scrambled nonspecific shRNA control in vector pGeneClip hMGFP were from SuperArray Bioscience Corp.
Cells and transfection
Jurkat T-lymphocytic leukemia cells (ATCC), mouse B cells WEHI-3B and interleukin-3–dependent FL5.12 pro-B cells (both gifts from C. S. Duckett, University of Michigan), and tumor-derived mouse spleen cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 20mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 2mM l-glutamine, 50μM β-mercaptoethanol, penicillin (100 U/mL), and streptomycin (100 μg/mL). The culture medium for FL5.12 and tumor-derived cells was supplemented with 10% supernatant from confluent WEHI-3B cell cultures, as a source of interleukin-3. Cells were maintained at 37°C in an atmosphere of 5% CO2.
Lck Y505F (2 μg of pcDNA3.1/Zeo-Lck Y505F) or Lck shRNAs (2 μg of murine Lck shRNA in vector pGeneClip hMGFP) were introduced into FL5.12 cells or GFP-Bfl-1ΔC/p53DD tumor #3–derived cells (2 × 106), respectively, by Amaxa nucleofection with solution V (100 μL) and Program G-016 (Amaxa Biosystems), followed by selection with Zeocin (600 μg/mL; Invitrogen) at 36 to 48 hours (LckY505F) or by sorting for GFP intensity (Beckman Coulter; EPICS Elite) at 4 days after nucleofection (mLck shRNA). Interaction of Bfl-1 mutants with Bak, Bax, or Noxa was assayed in human HeLa cells transfected with Lipofectamine 2000 (15 μg of DNA; Invitrogen), as was their subcellular localization.
Immunoblotting, coimmunoprecipitation, and immunofluorescence
The half-life of Bfl-1 mutants was analyzed in FL5.12 cells (5 × 105/mL) treated with protein synthesis inhibitor cycloheximide (10 μg/mL). Cell extracts were prepared in CHAPS lysis buffer30 and analyzed by immunoblotting. Antibodies used in this study were against GFP (Torrey Pines Biolabs), p53 (Oncogene Research), Lck, DAP 12, p38, JNK, Akt, ERK, IkappaB kinaseα/β (IKKα/β; Santa Cruz Biotechnology), phospho-Y505-Lck, phospho-p38, phospho-c-Jun N-terminal kinase (JNK), phospho-Akt, phospho-ERK, phospho-IKKα/β, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 (MEK1/2), phospho-MEK1/2 (Cell Signaling), macrophage inflammatory protein-1γ (MIP-1γ; PeproTech), Gab-1 (Upstate Biotechnologies), or actin (Sigma-Aldrich), followed by enhanced chemiluminescence (Amersham Life Sciences). Monoclonal anti-Rab27a was a gift from G. M. Griffiths (Imperial College). Precleared extracts from HeLa cells transfected with wild-type or mutant Bfl-1 or Mcl-1 as control (1 mg, Figure 4A; 2.5 mg, supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) were immunoprecipitated with monoclonal anti-Myc (Invitrogen) or anti-FLAG resin (Sigma-Aldrich) and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotting with antibodies to Bak (Upstate, a Millipore company), Bax (Santa Cruz Biotechnology), Noxa (Calbiochem), Myc (Invitrogen), FLAG (Sigma-Aldrich), or actin as control. Bfl-1 mutant subcellular localization was analyzed in transfected HeLa cells and photographed under ultraviolet illumination at 100× magnification.
In vivo tumorigenicity assays
FL5.12-derived cell lines (8 × 107 cells/0.5 mL phosphate-buffered saline), or splenocytes derived from Bfl-1ΔC/p53DD or KKK/RRR/p53DD tumor-bearing mice, were injected intravenously via the tail vein into female Balb/c nu+/nu+ (The Jackson Laboratory; Figure 2) or homozygous NCR nude mice (Taconic; Figures 4, 5, and 7). Mice were monitored every 2 to 3 days for splenomegaly and killed when they displayed manifestation of disease or loss of body condition. Before euthanasia, blood smears were stained with Wright-Giemsa (Fisher Scientific). Mouse spleens and livers were fixed in FormaFresh (Fisher Scientific), embedded, sectioned, and stained with hematoxylin and eosin by the Tissue Analytic Services of the Cancer Institute of New Jersey. Animals were used according to the National Cancer Institute Animal Care and Use Committee guidelines under an approved animal study protocol.
Affymetrix gene chip analysis
RNA (5 μg) was prepared from FL5.12 cells expressing Bfl-1ΔC/p53DD and from 2 different cell lines each (eg, T1 vs T1A) derived from 3 independent Bfl-1ΔC/p53DD tumors by the use of the RNeasy Mini Kit (QIAGEN) and hybridized to the Affymetrix Mouse Genome 430A_2.0 chip with 14 000 characterized mouse genes (Transcriptional Profiling Shared Resource of the Cancer Institute of New Jersey). Microarray data were analyzed by use of the Affymetrix Netaffex software (www.affymetrix.com/analysis/index.affx), the analysis suite from Ingenuity.com (www.ingenuity.com), and GeneSpring 7.2 to identify transcripts increased 2-fold or greater in tumor-derived cells versus parental cells as control. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE18204 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE18204).
Results
C-terminal mutations significantly alter Bfl-1 protein stability
We previously showed that deletion of 24 C-terminal residues of Bfl-1 (Bfl-1ΔC) or point mutations targeting serine 152 and threonine 156 or lysines 151, 163, and 172 in this region can markedly alter Bfl-1 ubiquitination and its antiapoptotic activity (Figure 1A).28 To better understand the biologic implications of Bfl-1 regulation at the posttranslational level, we analyzed the effects of these mutations on the half-life of Bfl-1 and on tumor development. Wild-type Bfl-1 fused to a green fluorescent protein tag had a half-life of approximately 3.2 hours when stably expressed in mouse FL5.12 pro-B cells, as observed upon treatment with the protein synthesis inhibitor cycloheximide (Figure 1B). Deletion or mutation of C-terminal lysines 151,163, and 172 to arginines markedly prolonged its half-life (11-14 hours), as did mutation of serine 152 and threonine 156 to aspartic acids (GFP-Bfl-1ΔC, KKK/RRR, ST/DD; Figure 1B). In contrast, mutation of serine 152 and threonine 156 to alanines accelerated Bfl-1 turnover, decreasing its half-life to only 1.2 hours (ST/AA; Figure 1B). This finding is consistent with our previous studies showing that these mutations significantly alter ubiquitination of Bfl-1 and highlights an important role for the C-terminus of Bfl-1 in controlling its degradation and cell death inhibition.
Mutations within Bfl-1's C-terminal region dramatically alter its half-life. (A) Schematic representation of GFP-Bfl-1 and of C-terminal deletion and point mutants. The BH1-3 domains and putative BH4 domain are shown. The C-terminal domain deleted in Bfl-1ΔC is underlined. Enlarged amino acids were substituted by mutagenesis with arginine (KKK/RRR), aspartic acid (ST/DD), or alanine (ST/AA). (B) Half-life of Bfl-1 mutants in FL5.12 cells treated with cycloheximide and collected at time intervals followed by Western blot with anti-GFP or actin as control. Protein amounts were quantified with the Alphaimager 2200 program.
Mutations within Bfl-1's C-terminal region dramatically alter its half-life. (A) Schematic representation of GFP-Bfl-1 and of C-terminal deletion and point mutants. The BH1-3 domains and putative BH4 domain are shown. The C-terminal domain deleted in Bfl-1ΔC is underlined. Enlarged amino acids were substituted by mutagenesis with arginine (KKK/RRR), aspartic acid (ST/DD), or alanine (ST/AA). (B) Half-life of Bfl-1 mutants in FL5.12 cells treated with cycloheximide and collected at time intervals followed by Western blot with anti-GFP or actin as control. Protein amounts were quantified with the Alphaimager 2200 program.
Ubiquitination-resistant mutant Bfl-1ΔC significantly predisposes nude mice to leukemia/lymphoma when coexpressed with mutant p53
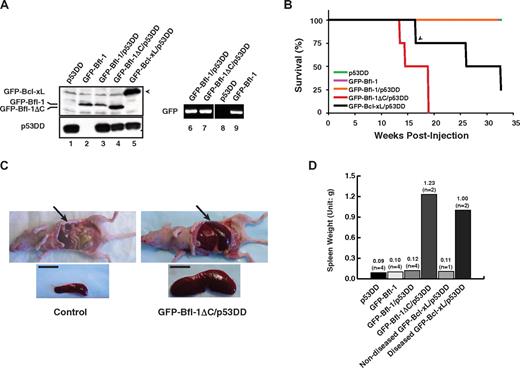
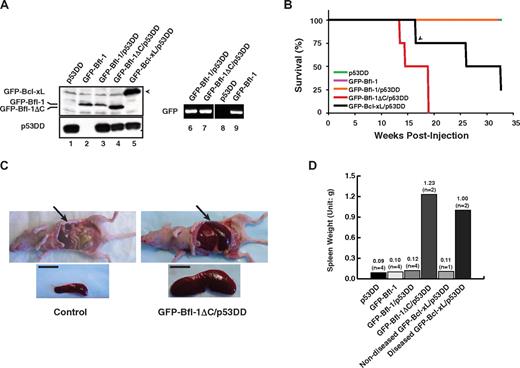
Given the increased levels of Bfl-1 in several human tumors, we investigated the role of its ubiquitin-mediated control in tumorigenesis using a previously described nude mouse model of leukemia/lymphomagenesis involving intravenous injection via the tail vein of nontransformed mouse FL5.12 pro-B cells coexpressing antiapoptotic Bcl-xL with dominant-negative mutant p53 (p53DD).31 Wild-type GFP-Bfl-1 or its degradation-resistant mutant GFP-Bfl-1ΔC was stably expressed, alone or together with p53DD, in FL5.12 cells (supplemental Table 1). Cells expressing p53DD alone or GFP-Bcl-xL plus p53DD served as controls. GFP-Bfl-1, GFP-Bfl-1ΔC, and p53DD protein expressions were verified by Western blot (lanes 1-5; Figure 2A). Because C-terminal deletion stabilizes Bfl-1, the use of complementary semiquantitative reverse transcriptase polymerase chain reaction allowed us to confirm equivalent mRNA levels in cells expressing wild-type GFP-Bfl-1 and GFP-Bfl-1ΔC (lanes 6 and 7; Figure 2A). Bfl-1 and Bfl-1ΔC transcripts were expressed at levels comparable with those of endogenous bfl-1 in chemoresistant ABC-DLBCL–derived tumor cells (data not shown).
Ubiquitination-resistant Bfl-1ΔC induces massive splenomegaly when coexpressed with p53DD in nude mice. (A left) Western blot of FL5.12 cells stably expressing p53DD or GFP-Bfl-1 alone or coexpressing p53DD with either GFP-Bfl-1, GFP-Bfl-1ΔC or GFP-Bcl-xL. (Right) Reverse transcriptase polymerase chain reaction shows equivalent amounts of GFP-Bfl-1 and GFP-Bfl-1ΔC mRNA in FL5.12 cell lines stably expressing GFP-Bfl-1/p53DD, GFP-Bfl-1ΔC/p53DD, or GFP-Bfl-1 alone. (B) Kaplan-Meier curves showing overt manifestation of disease in athymic nude mice (Balb/c nu+/nu+) injected intravenously with FL5.12 cells expressing p53DD alone (green), GFP-Bfl-1 alone (purple), GFP-Bfl-1/p53DD (orange), GFP-Bfl-1ΔC/p53DD (red), or GFP-Bcl-xL/p53DD-positive control (black). The arrowhead denotes a mouse that died of unknown cause. P values were determined by Student t test (GFP-Bfl-1ΔC/p53DD vs p53DD or GFP-Bfl-1 or GFP-Bfl-1/p53DD, P = .001; GFP-Bfl-1ΔC/p53DD vs GFP-Bcl-xL/p53DD, P = .042). (C) Splenomegaly in a representative GFP-Bfl-1ΔC/p53DD mouse compared with p53DD alone. Scale bar, 1cm. (D) Plot of average spleen weight at euthanasia.
Ubiquitination-resistant Bfl-1ΔC induces massive splenomegaly when coexpressed with p53DD in nude mice. (A left) Western blot of FL5.12 cells stably expressing p53DD or GFP-Bfl-1 alone or coexpressing p53DD with either GFP-Bfl-1, GFP-Bfl-1ΔC or GFP-Bcl-xL. (Right) Reverse transcriptase polymerase chain reaction shows equivalent amounts of GFP-Bfl-1 and GFP-Bfl-1ΔC mRNA in FL5.12 cell lines stably expressing GFP-Bfl-1/p53DD, GFP-Bfl-1ΔC/p53DD, or GFP-Bfl-1 alone. (B) Kaplan-Meier curves showing overt manifestation of disease in athymic nude mice (Balb/c nu+/nu+) injected intravenously with FL5.12 cells expressing p53DD alone (green), GFP-Bfl-1 alone (purple), GFP-Bfl-1/p53DD (orange), GFP-Bfl-1ΔC/p53DD (red), or GFP-Bcl-xL/p53DD-positive control (black). The arrowhead denotes a mouse that died of unknown cause. P values were determined by Student t test (GFP-Bfl-1ΔC/p53DD vs p53DD or GFP-Bfl-1 or GFP-Bfl-1/p53DD, P = .001; GFP-Bfl-1ΔC/p53DD vs GFP-Bcl-xL/p53DD, P = .042). (C) Splenomegaly in a representative GFP-Bfl-1ΔC/p53DD mouse compared with p53DD alone. Scale bar, 1cm. (D) Plot of average spleen weight at euthanasia.
Mice were monitored for evidence of disease for up to 8 months. All mice transplanted with cells expressing GFP-Bfl-1ΔC/p53DD developed massive splenomegaly and manifested disease between 13 and 19 weeks after inoculation (Figure 2B). In contrast, only a fraction of the mice injected with cells expressing GFP-Bcl-xL/p53DD manifested disease and did so with a significantly longer latency (26 and 32 weeks after injection; Figure 2B). At autopsy, mice injected with GFP-Bfl-1ΔC/p53DD cells had a 10- to 12-fold increase in spleen size compared with uninjected control mice or to the single GFP-Bcl-xL/p53DD mouse that died of unknown cause at week 17 with no evidence of disease (Figure 2C-D). In contrast to GFP-Bfl-1ΔC/p53DD, mice injected with cells expressing wild-type GFP-Bfl-1/p53DD, p53DD alone, or GFP-Bfl-1 alone failed to manifest disease for up to 8 months after inoculation (Figure 2B,D).
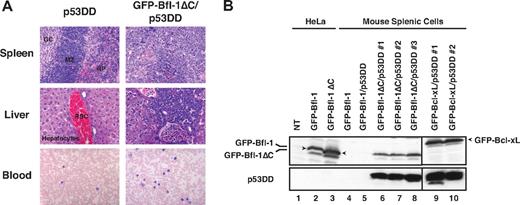
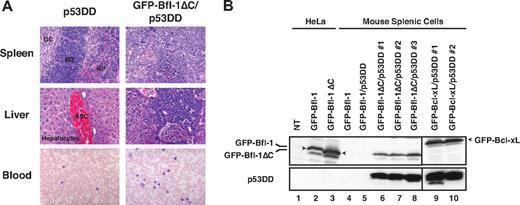
Histologic analysis revealed massive lymphoid infiltration in the spleen, liver, lymph nodes, and peripheral blood of diseased GFP-Bfl-1ΔC/p53DD mice. Spleen sections contained large aggregates of malignant cells in the white pulp and periarteriolar region and little evidence of internal splenic architecture remained (Figure 3A top). Liver sections showed malignant lymphoid infiltrates that percolated through the sinusoids (middle). Virtually all lymph nodes analyzed were replaced by sheets of malignant cells (data not shown). Peripheral blood smears showed evidence of atypical lymphoid proliferation with circulating malignant cells (bottom). The cytology of the lymphoid infiltrates was suggestive of mature diffuse large B-cell lymphoma. Spleen cells recovered from diseased GFP-Bfl-1ΔC/p53DD mice could be cultured in vitro as stable cell lines, as were those of GFP-Bcl-xL/p53DD mice. Western blots showed expression of GFP-Bfl-1ΔC or GFP-Bcl-xL along with p53DD (Figure 3B). In contrast, expression of GFP-Bfl-1 or p53DD was undetectable in spleen cells from mice transplanted with FL5.12 cells expressing GFP-Bfl-1 or GFP-Bfl-1/p53DD, and these spleen cells could not be maintained indefinitely in vitro (lanes 4-5; Figure 2B). Together, these results demonstrate that deletion of the Bfl-1 C-terminus and inactivation of p53 cooperate to promote leukemia/lymphomagenesis.
Histologic analysis reveals evidence of leukemia/lymphoma in GFP-Bfl-1ΔC/p53DD mice. (A top and middle) Spleen and liver sections from representative GFP-Bfl-1ΔC/p53DD or p53DD mice stained with hematoxylin and eosin. (Bottom) Peripheral blood smears from GFP-Bfl-1ΔC/p53DD or p53DD control mice stained with Wright-Giemsa. (B) Splenic cells recovered from diseased mice express GFP-Bfl-1ΔC or GFP-Bcl-xL along with p53DD as seen by Western blot, whereas those from healthy mice do not. The blot was probed with anti-GFP (top) or anti-p53 (bottom). HeLa cells transiently transfected with GFP-Bfl-1 or GFP-Bfl-1ΔC were used as controls.
Histologic analysis reveals evidence of leukemia/lymphoma in GFP-Bfl-1ΔC/p53DD mice. (A top and middle) Spleen and liver sections from representative GFP-Bfl-1ΔC/p53DD or p53DD mice stained with hematoxylin and eosin. (Bottom) Peripheral blood smears from GFP-Bfl-1ΔC/p53DD or p53DD control mice stained with Wright-Giemsa. (B) Splenic cells recovered from diseased mice express GFP-Bfl-1ΔC or GFP-Bcl-xL along with p53DD as seen by Western blot, whereas those from healthy mice do not. The blot was probed with anti-GFP (top) or anti-p53 (bottom). HeLa cells transiently transfected with GFP-Bfl-1 or GFP-Bfl-1ΔC were used as controls.
Bfl-1 mutants ST/DD and KKK/RRR promote leukemia/lymphomagenesis attributable to compromised ubiquitination and degradation
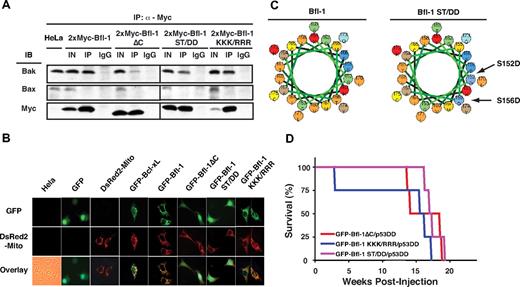
To determine whether Bfl-1ΔC predisposes mice to tumor formation as the result of its resistance to degradation or from other characteristics of this mutant, we analyzed the properties of ubiquitination-resistant point mutants KKK/RRR and ST/DD (Figure 1), which display increased antiapoptotic activity compared with wild type.28 We previously showed that part of Bfl-1's antiapoptotic activity is mediated via preferential interaction with endogenous proapoptotic Bak rather than Bax.30 Bfl-1ΔC retained the ability to interact with endogenous Bak but not Bax in coimmunoprecipitation assays but did so less efficiently than wild-type Bfl-1 or mutants KKK/RRR and ST/DD (Figure 4A). This finding is consistent with the fact that Bfl-1ΔC retains the ability to suppress Bak-mediated cell death both in wild-type and in Bax-deficient cells, albeit with reduced efficiency compared with Bfl-1.30
Ubiquitination-resistant Bfl-1 point mutants KKK/RRR and ST/DD coexpressed with p53DD induce lymphomagenesis with kinetics and efficiencies similar to that of Bfl-1ΔC. (A) Similar to wild-type Bfl-1 and Bfl-1ΔC, Bfl-1 point mutants KKK/ RRR and ST/DD selectively interact with endogenous Bak but not Bax, although Bfl-1ΔC and ST/DD did so with reduced efficiency. Coimmunoprecipitation of endogenous Bak or Bax with transiently transfected 2xMyc-tagged Bfl-1, Bfl-1ΔC, ST/DD, or KKK/RRR in HeLa cells. IN indicates 1/10 input; IP, immunoprecipitation with anti-Myc; IgG, immunoprecipitation with control mouse immunoglobulin. (B) Bfl-1, KKK/RRR, and Bcl-xL localize to mitochondria as seen by colocalization with DS-Red2-Mito tracker, whereas Bfl-1ΔC and ST/DD show diffuse localization by GFP fluorescence. (C) Helical wheel diagram showing that the ST/DD substitution adds significant negative charge on one side of the helix predicted in the C-terminus of Bfl-1. Basic (red), acidic (blue), nonpolar (orange), polar (green), and aromatic (brown) amino acids are indicated. (D) Kaplan-Meier curves showing similar kinetics of tumorigenesis in NCR nude mice injected intravenously with FL5.12 cells coexpressing Bfl-1 mutants GFP-Bfl-1ΔC (red, n = 4), KKK/RRR (blue, n = 4), or ST/DD (purple, n = 4) along with p53DD (GFP-Bfl-1ΔC/p53DD vs GFP-Bfl-1 KKK/RRR/p53DD, P = .401; GFP-Bfl-1ΔC/p53DD vs GFP-Bfl-1 ST/DD/p53DD, P = .469; GFP-Bfl-1KKK/RRR/p53DD vs GFP-Bfl-1 ST/DD/p53DD, P = .240).
Ubiquitination-resistant Bfl-1 point mutants KKK/RRR and ST/DD coexpressed with p53DD induce lymphomagenesis with kinetics and efficiencies similar to that of Bfl-1ΔC. (A) Similar to wild-type Bfl-1 and Bfl-1ΔC, Bfl-1 point mutants KKK/ RRR and ST/DD selectively interact with endogenous Bak but not Bax, although Bfl-1ΔC and ST/DD did so with reduced efficiency. Coimmunoprecipitation of endogenous Bak or Bax with transiently transfected 2xMyc-tagged Bfl-1, Bfl-1ΔC, ST/DD, or KKK/RRR in HeLa cells. IN indicates 1/10 input; IP, immunoprecipitation with anti-Myc; IgG, immunoprecipitation with control mouse immunoglobulin. (B) Bfl-1, KKK/RRR, and Bcl-xL localize to mitochondria as seen by colocalization with DS-Red2-Mito tracker, whereas Bfl-1ΔC and ST/DD show diffuse localization by GFP fluorescence. (C) Helical wheel diagram showing that the ST/DD substitution adds significant negative charge on one side of the helix predicted in the C-terminus of Bfl-1. Basic (red), acidic (blue), nonpolar (orange), polar (green), and aromatic (brown) amino acids are indicated. (D) Kaplan-Meier curves showing similar kinetics of tumorigenesis in NCR nude mice injected intravenously with FL5.12 cells coexpressing Bfl-1 mutants GFP-Bfl-1ΔC (red, n = 4), KKK/RRR (blue, n = 4), or ST/DD (purple, n = 4) along with p53DD (GFP-Bfl-1ΔC/p53DD vs GFP-Bfl-1 KKK/RRR/p53DD, P = .401; GFP-Bfl-1ΔC/p53DD vs GFP-Bfl-1 ST/DD/p53DD, P = .469; GFP-Bfl-1KKK/RRR/p53DD vs GFP-Bfl-1 ST/DD/p53DD, P = .240).
Bfl-1ΔC showed little to no significant interaction with endogenous Noxa compared with Bfl-1 or mutant KKK/RRR (supplemental Figure 1). Furthermore, Bfl-1ΔC was diffuse throughout the cells as seen by GFP fluorescence, as was mutant ST/DD (Figure 4B). In contrast, wild-type Bfl-1 and mutant KKK/RRR colocalized with the mitochondrial marker DS-Red2-Mito. A helical wheel diagram of the C-terminus of Bfl-1 demonstrated that substitution with 2 negatively charged aspartic acids in mutant ST/DD imposed a significant negative charge on one side of its predicted amphipathic helix that was proposed to function as an anchoring tail (Figure 4C).32 In other antiapoptotic Bcl-2 family members, this region is hydrophobic and anchors them to mitochondrial membranes. This may explain the altered distribution of Bfl-1ΔC and ST/DD compared with wild-type and KKK/RRR.
Because these mutants shared similar resistance to ubiquitination but showed different subcellular localization and association with Bak and Noxa, we compared the in vivo phenotype of FL5.12 cells stably expressing mutants Bfl-1ΔC, KKK/RRR, or ST/DD, along with p53DD, by intravenous injection in nude mice (supplemental Table 1). Consistent with the idea that sustained Bfl-1 levels are conducive to tumorigenesis, point mutants KKK/RRR and ST/DD induced massive splenomegaly with kinetics, efficiencies, and pathologies remarkably similar to those of Bfl-1ΔC (Figure 4D and data not shown). Hence, tumorigenesis was closely correlated with increased Bfl-1 stability rather than with changes in association with Bak or Noxa or altered subcellular localization compared with wild-type Bfl-1. These results underscore the importance of Bfl-1 ubiquitination as a mechanism to suppress oncogenesis.
Tyrosine kinase Lck and genes in the RANK signaling pathway are up-regulated in Bfl-1ΔC/p53DD-induced tumors
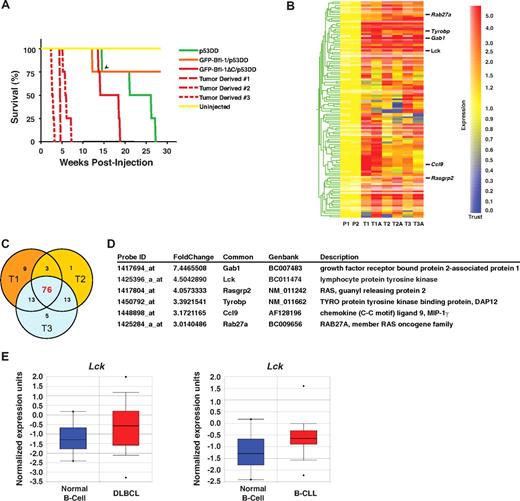
Spleen cells recovered at autopsy from 3 independent GFP-Bfl-1ΔC/p53DD mice were transformed and tumorigenic because intravenous transplantation in nude mice markedly accelerated disease manifestation by 3- to 5-fold compared with the parental FL5.12 GFP-Bfl-1ΔC/p53DD cells (2.5-7 weeks vs 13-19 weeks; P < .001; Figure 5A). Their spleens weighed 10-fold more than those of control animals. The significantly faster manifestation of disease suggested that FL5.12 GFP-Bfl-1ΔC/p53DD cells had acquired intrinsic changes during tumor development that perhaps contributed to the increased tumorigenicity of tumor-derived spleen cells. Importantly splenocytes recovered from independent KKK/RRR/p53DD mice also exhibited greatly accelerated tumor formation when reinjected into mice, with kinetics very similar to those of GFP-Bfl-1ΔC/p53DD tumor-derived cells, indicating that they were also transformed and tumorigenic (supplemental Figure 2).
Splenocytes recovered from diseased Bfl-1ΔC/p53DD mice are tumorigenic and display up-regulation of Lck and RANK signaling pathway. (A) Kaplan-Meier curves show accelerated tumorigenesis in nude mice transplanted with splenocytes derived from 3 independent Bfl-1ΔC/p53DD mice (GFP-Bfl-1ΔC/p53DD vs tumor derived 1, P = .001; GFP-Bfl-1ΔC/p53DD vs tumor-derived 2, P = .001; GFP-Bfl-1ΔC/p53DD vs tumor derived 3, P = .001). Uninjected mice and mice injected with FL5.12 cells expressing p53DD alone, GFP-Bfl-1/p53DD, or parental FL5.12 cells expressing Bfl-1ΔC/p53DD served as controls. The arrowhead denotes a mouse that died of unknown cause. (B) Hierarchical clustering of genes up- or down-regulated 2-fold or greater in microarrays from 3 independent GFP-Bfl-1ΔC/p53DD tumors analyzed in duplicate experiments with the use of 2 different cell lines isolated from each tumor (eg, T1 vs T1A) versus parental FL5.12 cells expressing GFP-Bfl-1ΔC/p53DD. Data were normalized with the use of GeneSpring 7.2 analysis software. (C) Venn diagram representing genes up-regulated by 2-fold or greater in GFP-Bfl-1ΔC/p53DD tumors. (D) Selected genes commonly up-regulated in 3 independent GFP-Bfl-1ΔC/p53DD tumors versus parental cells. (E) Box plots showing significant up-regulation of Lck mRNA in primary specimens from human diffuse large B-cell lymphoma (DLBCL) and B-cell chronic lymphocytic leukemia (B-CLL) compared with normal B cells (Rosenwald dataset), as extracted from the Oncomine database.
Splenocytes recovered from diseased Bfl-1ΔC/p53DD mice are tumorigenic and display up-regulation of Lck and RANK signaling pathway. (A) Kaplan-Meier curves show accelerated tumorigenesis in nude mice transplanted with splenocytes derived from 3 independent Bfl-1ΔC/p53DD mice (GFP-Bfl-1ΔC/p53DD vs tumor derived 1, P = .001; GFP-Bfl-1ΔC/p53DD vs tumor-derived 2, P = .001; GFP-Bfl-1ΔC/p53DD vs tumor derived 3, P = .001). Uninjected mice and mice injected with FL5.12 cells expressing p53DD alone, GFP-Bfl-1/p53DD, or parental FL5.12 cells expressing Bfl-1ΔC/p53DD served as controls. The arrowhead denotes a mouse that died of unknown cause. (B) Hierarchical clustering of genes up- or down-regulated 2-fold or greater in microarrays from 3 independent GFP-Bfl-1ΔC/p53DD tumors analyzed in duplicate experiments with the use of 2 different cell lines isolated from each tumor (eg, T1 vs T1A) versus parental FL5.12 cells expressing GFP-Bfl-1ΔC/p53DD. Data were normalized with the use of GeneSpring 7.2 analysis software. (C) Venn diagram representing genes up-regulated by 2-fold or greater in GFP-Bfl-1ΔC/p53DD tumors. (D) Selected genes commonly up-regulated in 3 independent GFP-Bfl-1ΔC/p53DD tumors versus parental cells. (E) Box plots showing significant up-regulation of Lck mRNA in primary specimens from human diffuse large B-cell lymphoma (DLBCL) and B-cell chronic lymphocytic leukemia (B-CLL) compared with normal B cells (Rosenwald dataset), as extracted from the Oncomine database.
To begin to address the gain-of-function responsible for the increased tumorigenicity revealed by Bfl-1ΔC expression, we compared the gene expression profiles of 3 independent GFP-Bfl-1ΔC/p53DD-derived tumors analyzed in duplicate experiments using 2 different cell lines derived from each (eg, T1 vs T1A) versus parental FL5.12 GFP-Bfl-1ΔC/p53DD cells with Affymetrix Mouse 430A_2 gene chips. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE18204 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE18204). Although there was variation in gene expression between duplicate experiments, by the use of gene tree clustering we identified 76 transcripts that were consistently and significantly up-regulated 2-fold or greater in tumor-derived GFP-Bfl1ΔC/p53DD spleen cells relative to parental FL5.12 Bfl1ΔC/p53DD cells (Figure 5B-C; supplemental Table 2). Of particular interest, the analysis suite from Ingenuity.com revealed that many up-regulated transcripts are involved in B- and T-cell signaling and in the MAP kinase/JNK signaling pathways (data not shown). Some of these transcripts include tyrosine kinase Lck (up 4.5-fold), the RAS oncogene family member Rab27a, Ras guanyl releasing protein 2 Rasgrp2, the MAP kinase/JNK pathway mediator Gab1, the protein tyrosine phosphatase Ptprs, and the tyrosine kinase binding protein Tyrobp (DAP12; Figure 5D). Interestingly although Lck is best known as a T-cell tyrosine kinase, ONCOMINE database search revealed that it is highly up-regulated in human DLBCL, as well as in B-CLL (Figure 5E), and previous studies showed that bfl-1 is also up-regulated in some subsets of these 2 tumor types.33,34 These findings suggest that Bfl-1ΔC promotes tumorigenesis by potentially enabling activation of Lck and other oncogenic pathways to further accelerate tumor progression.
Lck, ERK, and IKK kinases are selectively activated in Bfl-1ΔC/p53DD tumor-derived cells
Western blots confirmed up-regulated expression of these genes in spleen cells from diseased GFP-Bfl-1ΔC/p53DD mice compared with the parental FL5.12 GFP-Bfl-1ΔC/p53DD cells. Lck protein levels were increased up to 20-fold in tumor-derived cells from mice 1, 2, 3, and 7 versus control (lanes 5-7, 9 vs lane 4; Figure 6A) but not in tumor-derived cell lines 6 and 8 (lanes 8 and 10; Figure 6A). Western blots with anti–phospho-Src/Lck suggested activation of Src/Lck in all tumor-derived cell lines (lanes 5-10 vs lane 4; Figure 6A). Specific activation of Lck in tumor derived cells from mice 1, 2, 3, and 7 was confirmed by immunoprecipitation with anti-Lck followed by Western blot with anti–phospho-Lck (lanes 6, 13-15 vs lanes 5, 8-9, 16-18; Figure 6B). Jurkat T cells that express very high levels of active Lck served as positive control. Altogether, these data show that Lck is up-regulated and activated in a majority of Bfl-1ΔC/p53DD tumors.
Lck and proteins in the RANK signaling pathway are significantly up-regulated in Bfl-1ΔC/p53DD tumor-derived cells. (A) Western blots show increased levels of Lck and phospho-Lck in splenocytes from mice injected with FL5.12 cells expressing GFP-Bfl-1ΔC/p53DD versus parental FL5.12 GFP-Bfl-1ΔC/p53DD cells. The blot was probed with anti–phospho-Src/Lck, anti-Lck, or anti–β-actin. FL5.12 cells and FL5.12 cells expressing p53DD alone were negative controls; Jurkat T-cells are a positive control for Lck (1/5 input). (B) Immunoprecipitation of GFP-Bfl-1ΔC/p53DD-tumor-derived cells from mice 1, 2, 3, and 7 with anti-Lck, followed by Western blot with anti–phospho-Src-Lck. (C) Western blot showing elevated levels of MIP-1γ, DAP12, Gab1, and Rab27a in splenocytes derived from the mice analyzed in panel A. (D) Western blots show activation of the Akt, ERK, and IKK signaling pathways in splenocytes derived from the mice analyzed in panel A, as seen with anti-phospho p38, JNK, Akt, ERK, or IKK.
Lck and proteins in the RANK signaling pathway are significantly up-regulated in Bfl-1ΔC/p53DD tumor-derived cells. (A) Western blots show increased levels of Lck and phospho-Lck in splenocytes from mice injected with FL5.12 cells expressing GFP-Bfl-1ΔC/p53DD versus parental FL5.12 GFP-Bfl-1ΔC/p53DD cells. The blot was probed with anti–phospho-Src/Lck, anti-Lck, or anti–β-actin. FL5.12 cells and FL5.12 cells expressing p53DD alone were negative controls; Jurkat T-cells are a positive control for Lck (1/5 input). (B) Immunoprecipitation of GFP-Bfl-1ΔC/p53DD-tumor-derived cells from mice 1, 2, 3, and 7 with anti-Lck, followed by Western blot with anti–phospho-Src-Lck. (C) Western blot showing elevated levels of MIP-1γ, DAP12, Gab1, and Rab27a in splenocytes derived from the mice analyzed in panel A. (D) Western blots show activation of the Akt, ERK, and IKK signaling pathways in splenocytes derived from the mice analyzed in panel A, as seen with anti-phospho p38, JNK, Akt, ERK, or IKK.
Up-regulation of other genes identified in the microarrays was confirmed by immunoblotting in Bfl-1ΔC/p53DD tumor-derived cells versus parental control, including MIP-1γ, DAP12, Gab1, and Rab27a (Figure 6C). Interestingly, many of these reside in the Receptor Activator of NF-κB (RANK) signaling pathway and, like Lck, were selectively up-regulated in tumor-derived cells. Consistent with this observation, Western blots with phosphospecific antibodies revealed activation of the downstream ERK and IKK kinases but not p38, JNK, and/or Akt in Bfl-1ΔC/p53DD tumor-derived cells (Figure 6D). Lck (Figure 6A-B) and ERK were coactivated in tumors 1, 2, 3, and more weakly in tumor 7, as was MIP-1γ (lanes 5-7 vs lane 4; Figure 6C-D), whereas IKK was more strongly activated in tumors in which DAP12 was up-regulated but Lck was not (tumors 6-8, lanes 8-10 vs lane 4; Figure 6C-D). These data suggest that the Lck, ERK, and/or IKK kinases signaling pathways might contribute to the enhanced tumorigenic phenotype of Bfl-1ΔC/p53DD tumor-derived cells.
Lck functionally cooperates with Bfl-1 in tumorigenesis
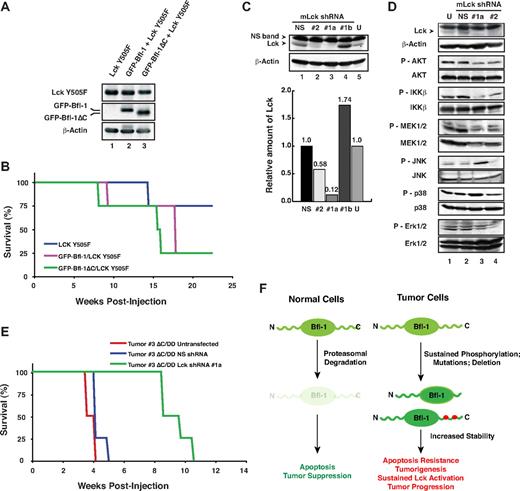
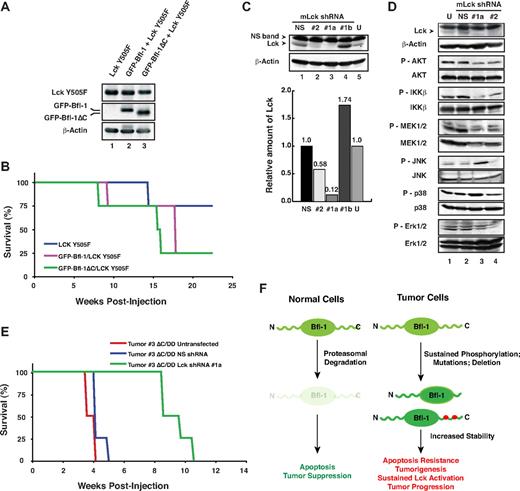
The significant up-regulation and activation of Lck in Bfl-1ΔC/p53DD tumor-derived cells led us to investigate its possible functional cooperation with Bfl-1 or Bfl-1ΔC in lymphomagenesis. Both wild-type Bfl-1 and Bfl-1ΔC accelerated lymphomagenesis induced by constitutively active Lck mutant Y505F, when coexpressed in FL5.12 cells injected intravenously in nude mice and did so even in absence of p53DD (Figure 7A-B). Conversely, we used specific mouse Lck (mLck) shRNAs to knockdown endogenous Lck in GFP-Bfl-1ΔC/p53DD tumor-derived cell line 3, which displayed the greatest level of mLck up-regulation and activation compared with parental cells (Figure 6A lane 7 vs lane 4). Nucleofection of mLck shRNA#1 coexpressed with GFP, followed by sorting for high GFP intensity achieved nearly 90% knockdown in tumor-derived cells (shRNA#1a, lane 3; Figure 7C), in contrast to mLck shRNA#1-nucleofected cells sorted for low GFP intensity (shRNA#1b, lane 4).
Lck synergizes with Bfl-1 in lymphomagenesis. (A) FL5.12 cells stably expressing constitutively active Lck Y505F alone or together with GFP-Bfl-1 or GFP-Bfl-1ΔC were analyzed by Western blot with anti-Lck, anti-GFP, or anti–β-actin. (B) Kaplan-Meier curves show accelerated tumorigenesis in NCR nude mice transplanted intravenously with FL5.12 cells expressing GFP-Bfl-1ΔC/Lck Y505F (green, n = 4) or GFP-Bfl-1/Lck Y505F (purple, n = 4) compared with those expressing Lck Y505F alone (blue, n = 4). (C top) Western blot showing that Lck shRNAs 1 and 2 reduce endogenous mLck levels in GFP-Bfl-1ΔC/p53DD tumor 3–derived cells at 4 days after nucleofection after sorting for high GFP expression (shRNAs 1a and 2, lanes 2-3) versus cells nucleofected with nonspecific scrambled shRNA control (NS) sorted for high GFP expression, cells nucleofected with shRNA1 sorted for low GFP expression (shRNA#1b) as control or untransfected cells (U; lanes 1, 4-5). (Bottom) Quantification of Lck knockdown efficiency relative to the NS shRNA control. (D) Stable knockdown of mLck in GFP-Bfl-1ΔC/p53DD tumor 3–derived cells selectively reduces phospho-Akt, -IKKβ, and -MEK activation and leads to activation of phospho-JNK versus controls as seen by Western blot with phospho-Akt, -IKKβ, -MEK1/2, -JNK, -p38, or -ERK1/2. (E) Kaplan-Meier curves demonstrate that stable knockdown of endogenous mLck with shRNA #1a (green, n = 4) significantly delays tumor formation in NCR nude mice injected intravenously with GFP-Bfl-1ΔC/p53DD-tumor 3–derived cells (ΔC/DD #3) versus those injected with tumor 3 cells expressing the nonspecific shRNA control (NS; blue, n = 4; P = .001) or untreated cells (red, n = 6; P = .001). (F) Model representing Bfl-1 ubiquitin-mediated regulation and its role in tumor suppression.
Lck synergizes with Bfl-1 in lymphomagenesis. (A) FL5.12 cells stably expressing constitutively active Lck Y505F alone or together with GFP-Bfl-1 or GFP-Bfl-1ΔC were analyzed by Western blot with anti-Lck, anti-GFP, or anti–β-actin. (B) Kaplan-Meier curves show accelerated tumorigenesis in NCR nude mice transplanted intravenously with FL5.12 cells expressing GFP-Bfl-1ΔC/Lck Y505F (green, n = 4) or GFP-Bfl-1/Lck Y505F (purple, n = 4) compared with those expressing Lck Y505F alone (blue, n = 4). (C top) Western blot showing that Lck shRNAs 1 and 2 reduce endogenous mLck levels in GFP-Bfl-1ΔC/p53DD tumor 3–derived cells at 4 days after nucleofection after sorting for high GFP expression (shRNAs 1a and 2, lanes 2-3) versus cells nucleofected with nonspecific scrambled shRNA control (NS) sorted for high GFP expression, cells nucleofected with shRNA1 sorted for low GFP expression (shRNA#1b) as control or untransfected cells (U; lanes 1, 4-5). (Bottom) Quantification of Lck knockdown efficiency relative to the NS shRNA control. (D) Stable knockdown of mLck in GFP-Bfl-1ΔC/p53DD tumor 3–derived cells selectively reduces phospho-Akt, -IKKβ, and -MEK activation and leads to activation of phospho-JNK versus controls as seen by Western blot with phospho-Akt, -IKKβ, -MEK1/2, -JNK, -p38, or -ERK1/2. (E) Kaplan-Meier curves demonstrate that stable knockdown of endogenous mLck with shRNA #1a (green, n = 4) significantly delays tumor formation in NCR nude mice injected intravenously with GFP-Bfl-1ΔC/p53DD-tumor 3–derived cells (ΔC/DD #3) versus those injected with tumor 3 cells expressing the nonspecific shRNA control (NS; blue, n = 4; P = .001) or untreated cells (red, n = 6; P = .001). (F) Model representing Bfl-1 ubiquitin-mediated regulation and its role in tumor suppression.
Consistent with silencing of mLck, mLck shRNA#1a cells showed significant down-regulation of downstream phospho-Akt, -IKKβ, and -MEK1/2 compared with cells nucleofected with the nonspecific shRNA control (NS, lane 3 vs lanes 1-2; Figure 7D). In contrast, JNK signaling was increased in tumor-derived cells in which Lck was silenced. Consistent with the roles of the Akt-IKK and MEK-ERK signaling pathways in tumorigenesis and the function of JNK signaling in cell death, silencing of mLck in Bfl-1ΔC/p53DD tumor 3-derived cells dramatically delayed lymphomagenesis in nude mice compared with those expressing the nontargeting shRNA control (NS) or untransfected tumor 3–derived cells (green curve vs red or blue curves, P < .001; Figure 7E). Together, these results provide compelling evidence that up-regulation of Lck contributes significantly to the increased tumorigenic properties of Bfl-1ΔC/p53DD tumor-derived cells and indicate that Bfl-1 and Lck can functionally synergize in lymphomagenesis. Future studies will help to determine the mechanism by which Bfl-1 cooperates with Lck in tumorigenesis.
Discussion
Several hematopoietic cancers are characterized by markedly elevated levels of bfl-1 transcripts, and accumulating evidence indicates that this contributes significantly to tumor cell survival and therapy resistance in B-cell chronic lymphocytic leukemia, large B-cell lymphoma, acute promyelocytic leukemia, and ALK-positive anaplastic large B-cell lymphoma.7-9,11-13 However, whether and how dysregulation of Bfl-1's posttranslational control contributes to tumorigenesis remained to be addressed. In this study we show that mutations that decrease Bfl-1's ubiquitination and increase its stability significantly accelerate leukemia/lymphomagenesis in a mouse model and that Bfl-1 functionally cooperates with Lck activation in tumorigenesis. These data demonstrate that ubiquitination is a critical mechanism to control Bfl-1's activity and that failure to appropriately down-regulate Bfl-1 can predispose one to cancer.
Transcription of antiapoptotic Bcl-2 family members is tightly controlled. Previous studies from our group and others showed that bfl-1 is a transcriptional target of NF-κB and that it physiologically regulates the survival of activated lymphocytes, macrophages, and neutrophils.2-6,35-38 NF-κB signaling allows for rapid and significant transcriptional induction of bfl-1/a1 to confer cell survival under physiologic conditions. Indeed, B cells from c-Rel/NF-κB knockout mice fail to express mouse bfl-1/a1 and undergo antigen receptor ligation-induced apoptosis, which is rescued by ectopic bfl-1/a1 expression.4
The recent discovery that Bfl-1 is also regulated by ubiquitination suggests that additional mechanisms to control Bfl-1 levels are likely to be vital to regulate its activity. In support of this idea, our in vivo studies showed that mutations that compromise Bfl-1 ubiquitination and increase its half-life (Bfl-1ΔC, KKK/RRR, ST/DD) appreciably hastened tumor formation compared with wild-type Bfl-1. This finding suggests that Bfl-1 degradation may be required for cells to undergo signal-induced apoptosis under physiologic conditions and that sustained Bfl-1 levels can promote oncogenesis. Hence, ubiquitination of Bfl-1 and/or its phosphorylation-dephosphorylation are likely to be critical determinants of its oncogenic activity. Further studies to identify the ubiquitin ligase and the kinases/phosphatases that regulate Bfl-1's turnover will help to better understand its posttranslational control.
The significantly shorter latency of disease presentation in animals transplanted with Bfl-1ΔC/p53DD tumor-derived spleen cells compared with parental cells suggested that genetic alterations acquired in vivo might contribute to Bfl-1's role in tumor development and maintenance. Consistent with this, gene expression profiling identified multiple genes up-regulated in tumor-derived cells, including genes involved in signal transduction pathways previously implicated in cancer. Among them, we demonstrated that Bfl-1 functionally cooperates with tyrosine kinase Lck in tumorigenesis and that this leads to activation of the kinases ERK and IKK in these tumors, which were previously implicated in cancer. This finding suggests that sustained levels of Bfl-1 enable acquisition of secondary oncogenic mutations that further accelerate tumor progression.
The Src-family tyrosine kinase Lck is preferentially expressed in T cells and plays an important role during lymphocyte maturation in the thymus and in T-cell receptor signaling during activation and proliferation of mature T cells.39,40 Although Lck is best known for its role in T cells, Lck expression also was reported in normal B-1, B-2, and chronic lymphocytic leukemia B cells, where it is involved in regulation of B-cell receptor signal transduction and oncogenic transformation.41,42 Our findings that Lck is induced during the development of Bfl-1ΔC/p53DD tumors and that it functionally cooperates with Bfl-1 in this context are consistent with its role as an oncogene. Indeed aberrant overexpression of Lck in thymocytes is sufficient for tumorigenesis in vivo.43 Lck also regulates apoptosis triggered by various death-inducing signals including radiation, ceramide and cytotoxic drugs, and activates the mitochondrial death pathway through up-regulation of proapoptotic Bak, in a manner independent of its role in T-cell receptor signaling.44,45
It is noteworthy that lck is sharply elevated in primary specimens from DLBCL and B-CLL compared with normal B cells (Figure 5E) and that some subsets of these 2 tumor types, including activated B cell–like (ABC)–DLBCL and fludarabine-resistant rB-CLL, are characterized by up-regulation of bfl-1 and depend on Bfl-1 for survival and drug resistance.7,10-13 Our results raise the possibility that sustained Bfl-1 levels conferred by increased transcription or reduced ubiquitination can contribute to leukemia/lymphomagenesis. However, the mechanism by which Bfl-1 contributes to oncogenesis remains to be clarified, and further studies will be needed to determine whether the mechanism by which it cooperates with Lck in tumorigenesis involves suppression of Lck-induced cell death.
Chemoresistance is a major challenge for successful cancer treatment. bfl-1 expression is strongly correlated with chemoresistance and poor prognosis and knockdown studies demonstrated that it is an important therapeutic target in drug-resistant B-CLL and other leukemia/lymphoma cells.11-13 Bfl-1 expression is also correlated with resistance of some tumors to the small molecule inhibitors ABT-737 and ABT-263 (BH3 mimetics), which selectively target antiapoptotic Bcl-2 family members Bcl-2, Bcl-xL, and Bcl-w but not Bfl-1 or Mcl-1 and are currently in clinical trials.46-48 An important implication of our findings is that ubiquitin-mediated turnover of Bfl-1 could perhaps be exploited to improve the response of Bfl-1–expressing tumor cells to anticancer treatment and pave the way for effective combination therapies for drug-resistant Bfl-1–positive tumors.
Recent data showing that combining ABT-737 with chemotherapeutic agents that lead to dysfunction or elimination of Mcl-1 improves the response of Mcl-1-positive tumors to ABT-737 support this hypothesis.49,50 Finally our data showing that ubiquitination-resistant Bfl-1 mutants significantly predisposed mice to tumor development raise the possibility that some human tumors may harbor naturally occurring mutations in bfl-1, or in the pathways that control its degradation, that may increase Bfl-1 stability and contribute to tumor maintenance and/or chemoresistance. Future studies will help to determine whether such alterations exist in tumors in which Bfl-1 is implicated and the extent to which failure to appropriately down-regulate Bfl-1 contributes to human tumors and therapy resistance.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank H. Band (University of Nebraska Medical Center) and G. M. Griffiths (Imperial College) for the gift of reagents; T. Choi, C. Krier, and J. Friedman from the shared resources for Analytical Cytometry/Image Analysis, Transcriptional Profiling, and Tissue Analytic Services of the Cancer Institute of New Jersey; J. Shearstone (University of Massachusetts Medical School) for assistance with microarray data submission to the GEO database; N. Gupta, Y. Fan, P. Molli, Y. Guo, and K. Madura for fruitful discussions during the course of this work; and P. Molli and Y. Guo for useful comments on the manuscript.
This work was supported by National Institutes of Health/NCI grants R01CA083937 (to C.G.) and R37CA53370 (to E.W.) and also partly by the Foundation of UMDNJ (10-07,64-08; to C.G.).
National Institutes of Health
Authorship
Contribution: G.F. and M.J.S. contributed to the experimental design, performed the research, and analyzed the data; S.G. performed the research and analyzed data; J.D.-S., Y.R., and C.-C.C. contributed to performing the research; J.K. contributed to experimental design and some of the research; C.M. analyzed data and performed statistical analysis; E.W. contributed to experimental design, analysis and interpretation of the data, and writing the paper; D.W. analyzed data; C.G. designed the research and analyzed and interpreted data; and C.G. and G.F. wrote the paper.
The current affiliation for G.F. is Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. The current affiliation for M.J.S. is Department of Cancer Biology, University of Massachusetts Medical School, Worcester, MA. The current affiliation for J.D.-S. is Agios Pharmaceuticals Inc, Cambridge, MA. The current affiliation for J.K. is Université de Lyon 1, Lyon, France. The current affiliation for C.M. is System Biosciences, Mountain View, CA.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Céline Gélinas, CABM, 679 Hoes Ln, Piscataway, NJ 08854;e-mail: gelinas@cabm.rutgers.edu.
References
Author notes
G.F. and M.J.S. contributed equally to this work.