Abstract
Although chronic lymphocytic leukemia (CLL) remains incurable, over the past decade there have been major advances in understanding the pathophysiology of CLL and in the treatment of this disease. This has led to greatly increased response rates and durations of response but not yet improved survival. Advances in the use of prognostic factors that identify patients at high risk for progression have led us to the question whether there is still a role for a “watch and wait” approach in asymptomatic high-risk patients or whether they should be treated earlier in their disease course. Questions remain, including, what is the optimal first-line treatment and its timing and is there any role of maintenance therapy or stem cell transplantation in this disease? CLL is a disease of the elderly and not all patients are eligible for aggressive up-front chemoimmunotherapy regimens, so what is the optimal treatment approach for more frail elderly patients? It is highly likely that our treatment approaches will continue to evolve as the results of ongoing clinical trials are released and that further improvements in the outcome of this disease will result from identification of therapies that target the underlying pathophysiology of CLL.
Introduction
It is estimated that 15 490 people (9200 men and 6290 women) will be diagnosed with chronic lymphocytic leukemia (CLL) in the United States in 2009.1 CLL is a disease of the elderly, with a median age at diagnosis of 72 years and median age at death from CLL of 79 years. Almost 70% of CLL patients are older than 65 years at the time of diagnosis; less than 2%, younger than 45; 9.1%, between 45 and 54; 19.3%, between 55 and 64; 26.5%, between 65 and 74; 30.0%, between 75 and 84; and 13.2%, 85+ years of age. The age-adjusted incidence rate is 4.1 per 100 000 men and women per year, with little evidence for any increase in the rate of CLL from 1975 to 2006. The disease is twice as common in males as females, more common in white than black Americans, rarer in Hispanics and Native Americans, and much rarer in the Asian population.
Among the strongest risk factors for the development of CLL is a family history of this or other lymphoid malignancies. Several familial clusters of CLL have been reported,2 and there is genetic anticipation, the process whereby the median age at onset in a child of a multigeneration family with malignancy is younger than that of the parent generations. In a report from the National Cancer Institute Familial Registry, the mean age at diagnosis among familial cases was 58 years, 14 years younger than that of sporadic cases.3 There is no difference in survival from diagnosis in familial compared with nonfamilial cases of CLL,4 and no increased risk of transformation to more aggressive non-Hodgkin lymphomas. Apart from the difference in age at presentation, familial CLL is essentially indistinguishable from sporadic CLL, favoring a genetic basis to disease development in general rather than a simple environmental etiology. It is highly likely that the study of families with multiple CLL cases will aid in delineating the genes and environmental factors that play a role in the development of CLL.
CLL is extremely heterogeneous in its clinical course; some patients live for decades with no need for treatment for their disease, whereas others have a rapidly aggressive clinical course. A major focus of research has been to try to identify those biologic factors that influence the clinical course. The goal of therapy has been to maintain the best quality of life and treat only when patients become symptomatic from their disease. For the majority of patients this means following a “watch and wait” approach to determine the rate of progression of the disease and assess for development of symptoms. Any alteration to this approach will require demonstration of improved survival with early institution of therapy, or identification of criteria that define patients as sufficiently “high risk” that they gain benefit by introduction of early therapy. There are many available therapies and, until recently, little consensus on an optimal first-line or relapse treatment. The following discussion presents my approach for the management of previously untreated CLL based upon 25 years of clinical practice in oncology, research, and review of the work of distinguished colleagues. Appropriate literature is cited to support treatment practice and recommendations.
How I diagnose CLL
I follow the guidelines that have been outlined by the International Workshop on CLL (iwCLL)5 and the diagnosis is made by the identification of cells bearing the unique phenotype of CLL using an immunophenotypic panel on peripheral blood (PB). The World Health Organization classification considers CLL and small lymphocytic lymphoma (SLL) to be simply different clinical manifestations of the same disease.6 The disease is called CLL when there is a leukemic component in PB and is called SLL when lymph nodes (LNs) or other tissues are infiltrated by cells with the identical morphologic and immunophenotypic features as CLL cells but in which there is no leukemic manifestations of the disease. Only 5% of patients present with clinical features of SLL without the leukemic component. CLL is always a B-cell neoplasm and the World Health Organization nomenclature reclassified the entity formerly known as T-CLL as T-prolymphocytic leukemia.
CLL cells are monomorphic small round B lymphocytes, with only rare prolymphocytes seen, and the diagnostic criteria are shown in Table 1. The diagnosis of CLL requires the presence of at least 5000 B cells/μL, and the presence of fewer than this number of B cells in the absence of lymphadenopathy is now defined as monoclonal B-lymphocytosis.7 The diagnosis is made by the detection of a clonal population of small B lymphocytes in PB or BM, or by LN biopsy showing cells expressing the characteristic morphology and immunophenotype. CLL cells express CD19, dim CD20, dim CD5, CD23, CD43, and CD79a and weakly express surface immunoglobulin M (IgM) and IgD, with cytoplasmic Ig detectable in 5% of cases. Expression of CD38 is variable and has prognostic significance in this disease,8,9 and for this reason CD38 should be included in the immunophenotypic panel in this disease. A scoring system has been proposed,10 and in difficult cases, particularly those in which there is an atypical immunophenotype, the detection of specific cytogenetic and molecular features can be helpful in making the definitive diagnosis. The immunophenotypic and genetic features of CLL compared with other small B-cell neoplasms are shown in Table 2. Dim expression of CD20 and surface immunoglobulin is highly characteristic of CLL and this can be useful in distinguishing from mantle cell lymphoma, especially in those rarer cases that lack expression of CD23.
A bone marrow (BM) aspirate or biopsy is not required at diagnosis in CLL. I perform BM biopsy at the time of requirement for treatment, and do this in the newly diagnosed patients only when they present with cytopenias, since this may be useful in evaluating whether cytopenias are immune mediated or caused by marrow replacement by disease. The BM infiltrate may be nodular, interstitial, or diffuse or may show a combination of these patterns. For the diagnosis of SLL a fine needle aspiration is not appropriate and an excisional biopsy of an accessible LN is required with review by an expert hematopathologist with expertise in lymphoma diagnosis. The LN infiltrate in SLL/CLL is composed of predominantly small lymphocytes with condensed chromatin, round nuclei, and occasionally a small nucleolus.11 Prolymphocytes and paraimmunoblasts with more prominent nucleoli and more dispersed chromatin are always present and are clustered in aggregates known as proliferation centers or pseudofollicles. I seek informed consent for use of excess PB and LN biopsies at the time of presentation and at each subsequent relapse of disease for research purposes to investigate the molecular biology of the disease.
Staging of disease
The clinical course of CLL is extremely heterogeneous and the value of the 2 widely used staging systems in CLL (Table 3) lies in their prognostic implications for survival.12,13 I discuss both staging systems with patients since they will likely come across both in their own reading about their disease and this can cause confusion. The Rai staging systems is based upon the premise that there is a progressive accumulation of neoplastic cells manifested by increasing lymphocytosis, progressive lymphadenopathy, splenomegaly, and hepatomegaly, followed by BM replacement with development of anemia and thrombocytopenia.12 At the time of initial diagnosis, 25% of patients are stage 0; 50%, stage I to II; and 25%, stage III or IV. The Binet system takes into consideration 5 potential sites of involvement: cervical, axillary and inguinal lymph nodes (either unilateral or bilateral counts as one site), spleen, and liver.13 Patients are staged according to the number of involved sites plus the presence of anemia with hemoglobin less than 100 g/L (10 g/dL) and/or thrombocytopenia with platelets less than 100 × 10 g/L. Advanced disease with anemia and thrombocytopenia are present at the time of initial presentation in 20% of cases, and up to 20% of cases present with B symptoms, defined as unintentional weight loss of 10% or more of body weight over the previous 6 months, fevers greater than 38°C for more than 2 weeks without evidence of infection, night sweats, or extreme fatigue (Eastern Cooperative Oncology Group performance status 2 or greater).14 An integrated system using both methods was recommended by iwCLL for uniformity in reporting clinical trials,15 but this has not been widely accepted by clinicians in their everyday practice who prefer to use the simpler Rai or Binet systems, and it is often difficult to extract iwCLL staging in multicenter studies.
Staging of CLL is performed by clinical examination and results of blood counts only, and for this reason the guidelines do not recommend computed tomography (CT) scan at diagnosis. Although care must be taken not to overuse CT scans in early-stage patients, in patients identified at higher risk of progression, CT scans provide a more accurate assessment of intra-abdominal disease than clinical examination, and upstaging a patient by CT scan criteria alone has prognostic significance.16 I therefore find it useful to perform CT scans to determine baseline adenopathy in patients who present with poor prognostic features, particularly del 11q, since this is often associated with an increased frequency of intra-abdominal lymphadenopathy. No other tests are required for the diagnosis of CLL, but additional tests provide important information on the pathogenesis of the disease and may be helpful to predict prognosis: for example, β-2 microglobulin to determine the possibility of Richter transformation or direct antiglobulin test (DAT) and bilirubin to assess underlying hemolysis. In patients in whom monoclonal antibody therapy is being considered, I also test for hepatitis B and C and for HIV.
Molecular profiling and how this is used in practice
The molecular profile of CLL provides insight into the underlying pathogenesis of the disease and provides predictors of time to progression, time to need for therapy, and overall survival. A molecular profile can be built from assessment of number of molecular biomarkers, the most important being cytogenetic analysis by fluorescence in situ hybridization (FISH), mutational status of the immunoglobulin heavy chain variable gene locus (IgVH), IgVH usage, the 70-kDa ζ-associated protein (ZAP70), lipoprotein lipase, and CD38 expression. High-risk features predictive of disease progression include the cytogenetic features deletion of the long arm of chromosome 11 (del 11q) and del 17p, IgVH unmutated status, use of the IGHV3-21 gene segment, and expression of ZAP70 or CD38 as discussed in more detail in “IgVH status and gene usage.” A current challenge is to understand how we should use this new information in clinical practice and whether we should alter treatment based upon the detection of high-risk features and assessment of the impact of these biomarkers is a vital component of research studies. Although it is tempting to speculate that these markers can now be useful in clinical practice, several questions remain. Indeed, it is by no means clear that the practicing clinician derives any benefit from obtaining these tests in routine clinical practice. There is not yet any evidence that patients presenting with high-risk disease features have any benefit with earlier treatment. This question is being addressed in ongoing and planned clinical trials and until the results of these studies are available, patients should not be offered treatment on the basis of any molecular marker until the standard criteria for treatment are reached.5
However useful the biomarkers may be in predicting how cohorts of patients will behave, they are less precise in predicting outcome in individual patients. It is often the patient who demands the results of the prognostic tests. Certainly, if I were to be diagnosed with CLL, I would want to have as much prognostic information as possible for planning purposes. Several of the factors, most notably IgVH mutational status and gene usage, cannot readily be obtained, and the current assays for expression of ZAP70 are inconsistent, with no clear guidelines on the established methodology or where the cutoff should be for designation as ZAP70-positive or -negative. There is considerable ongoing debate regarding the clinical utility of CD38 expression and its stability over time. The most robust and reproducible of these tests is cytogenetics assessed by FISH. However, cytogenetic abnormalities evolve over time, and it is generally recommended to perform this analysis at the time of institution of therapy. Clinicians may be better served using cheaper and more established markers of disease such as β-2 microglobulin, which can be incorporated into nomograms to assess risk of progression.17 My own feeling is that decision-making in clinical practice should be based on symptoms and clinical features of the disease, and the use of molecular profiles in the management of CLL remains a research question only. The only clear exception to this is in symptomatic patients with del 17p or p53 mutations, since this may change therapy; efforts should be made to treat these patients with agents that act independently of p53. Again it should be stressed that even the detection of this poorest of the prognostic markers is not an indication for earlier treatment in asymptomatic patients.
My own experience is that patients usually request these tests, hoping that they will have good prognostic markers; the finding of poor-risk features can often lead to increased anxiety, while not changing management, and this requires considerable time in clinic explaining the potential significance of the findings. It is, therefore, important that those caring for CLL patients have a full understanding of the clinical significance of any investigation that has been ordered. A large number of parameters have been identified that are predictive of the clinical course; the most widely studied are shown in Table 4. A current challenge is to understand how we should use this new information in clinical practice and whether we should alter treatment based upon the detection of high-risk features.18
Cytogenetic abnormalities
Unlike many of the other low-grade B-cell malignancies, nonrandom reciprocal chromosomal translocations are rare, but their finding is associated with poor prognosis.19 Therefore conventional cytogenetic analysis in addition to a full CLL FISH panel is recommended. Using FISH, one or more cytogenetic abnormalities can be found in more than 80% of CLL patients and these have important prognostic significance.20 The most common recurrent chromosomal abnormalities observed include del 13q, del 11q, trisomy 12, del 17p, and del 6q.20,21 The most common abnormality is del 13q.14, which occurs in more than 50% of cases. The first report linking microRNAs to cancer was in CLL,22 where it was demonstrated that 2 microRNA clusters, mir-15a and mir16-1, were located within the deleted region at 13q14. The next most common cytogenetic abnormality is del 11q, seen in up to 20% of cases of CLL. This deletion is associated with a distinct clinical presentation, including younger age, male sex, bulky lymphadenopathy, and poor prognosis. The ATM gene is located within the minimal region of loss at 11q23, suggesting that alterations in this gene may be involved in the pathogenesis of the disease. This is further supported by the finding that mutations in the ATM gene are associated with poor prognosis.23 Trisomy 12 occurs in up to 20% of CLL cases but the molecular mechanism by which this genetic abnormality contributes to leukemogenesis is unknown. Although less common, occurring in less than 10% of patients at diagnosis, del 17p is associated with rapid progression of disease, poor response to therapy and short survival. The deletion involves the p53 locus at 17p13 and it is clear that both deletions and mutations in the p53 gene can contribute to disease progression and alter the sensitivity of CLL cells to chemotherapy agents. CLL cells often exhibit multiple cytogenetic abnormalities and there is a hierarchic structure.20 The detection of mutations of p53, del 17p, or del 11q is associated with poor risk, whereas del 13q as a sole abnormality is associated with good-risk disease. The cytogenetic changes that occur in CLL are not stable over time and it is important to use the whole panel of FISH markers on repeat testing. Ongoing studies are assessing the impact of specific cytogenetic abnormalities on response to particular therapeutic approaches.
IgVH status and gene usage
In addition to the heterogeneity of genetic abnormalities, CLL is also heterogeneous in its level of differentiation as evidenced by the status of the IgVH rearrangement. A major advance in the understanding of CLL was made with the demonstration that 50% of CLL cases have undergone somatic hypermutation in IgVH and that this has prognostic significance. Cases with somatic hypermutation have a more indolent clinical course and longer survival than those without somatic hypermutation.8,24 The degree of somatic hypermutation in any particular B cell is evaluated by comparison of the sequence of the rearranged variable region gene with germline sequences. Guidelines have been reported for analysis of IgVH rearrangements from the working group of European Research Initiative in CLL.25 Sequences with less than 98% homology to germline are considered to have undergone somatic hypermutation.
This finding led to the hypothesis of 2 subsets of B-cell CLL, based upon different cells of origin, with cases with unmutated IgV regions derived from naive, pre–germinal center cells whereas those that have mutated IgV regions arise from a post–germinal center cell that has encountered antigen. Gene expression profiling studies demonstrated that both subtypes of CLLs display a common and distinct gene expression profile, suggesting that both mutated and unmutated groups share a common cell of origin, and these findings are not supportive of the hypothesis of 2 distinct disease entities arising from different cells of origin. Analysis of variable region sequences demonstrated that CLL cells use a biased repertoire of V genes characterized by overrepresentation of selected Ig gene segments, in particular IGHV1-69, IGHV4-34, IGHV3-7, and IGHV3-21.26,27 Somatic hypermutation does not occur uniformly among IGHV genes: for example, IGHV1-69 consistently carries very few mutations as opposed to the typically mutated IGHV3-7, IGHV3-23, and IGHV4-34 genes. An apparent exemption to the generalization that mutated CLL cases have good prognosis is in the subgroup of patients with CLL cells that use IGHV3-21 since these patients have relatively aggressive disease even when the expressed IGHV3-21 is mutated.28 Not only is the Ig gene repertoire expressed by CLL cells biased, but it is also notable for the existence of subsets with near identical (stereotyped) B-cell receptors implying the recognition of structurally similar epitopes, likely selecting the leukemic clones.29 The nature of the antigens that these B-cell receptors might be recognizing and whether these are important in driving the pathogenesis of CLL remain unknown. The presence of such stereotypic rearrangements may also have prognostic significance.30,31 Sequencing and analysis of IgVH rearrangements is a research tool and is not required in routine management.
Surrogates for mutation status
Although I have access to IgVH sequence analysis in my research laboratory, it is not possible to obtain this routinely in clinical laboratories, and attempts have therefore been made to identify surrogate markers for mutational status. Expression of 2 proteins, ZAP70 and CD38, has been examined, both of which have prognostic significance. CLL cells demonstrate a continuum of expression of these proteins and it is necessary to determine a cutoff point at which a case is deemed to be positive or negative, leading to difficulties in standardization, since different laboratories use different criteria to define individual cases as being positive or negative for expression.
When gene expression profiles were analyzed comparing mutated and unmutated cases of CLL,32,33 only a small number of genes were found to be differentially expressed, the most specific being the gene encoding the ZAP70.34 Most mutated cases are ZAP70-negative and unmutated cases ZAP70-positive.34,35 ZAP70 expression can be measured by several methods including Western blotting, reverse transcriptase–polymerase chain reaction, immunohistochemistry, and flow cytometry.36-39 Levels of expression are higher in T cells and natural killer cells than in CLL cells and it is important to ensure that effective gating strategies are used to ensure that expression is being measured in the CLL cells. ZAP70 expression appears to be stable over time.38,40 Studies have demonstrated that there is not an absolute relationship between ZAP70 expression and IgVH mutational status, with discrepant cases occurring in up to 25% of cases.38,40 These discordant cases may have other biologic features with poor prognostic implications such as del 17p, del 11q, or use of IGHV3-21.41 Some studies have suggested that ZAP70 status is more useful as a predictor of time to progression than mutation status,38,42 but this remains controversial.
CD38 is a surface marker associated with CLL, and easily identified using standard immunophenotyping. It was initially found to correlate with IgVH mutation status,8 but the relationship is not absolute, and CD38 expression may vary over time.9,43 The field is somewhat confused by a variety of cutoffs ranging from 5% to 30% used in different series to define a case as being CD38+,43-45 and it has been suggested that CD38 should be evaluated by its modal expression by flow cytometry, or by antigen density. Other surrogates of mutation status have been investigated including expression of thymidine kinase, activation-induced cytidine deaminase, lipoprotein lipase A, and ADAM29.46-48 Analysis of microRNA arrays revealed a 13-gene signature correlated with ZAP70 status, unmutated IgVH expression,49 and disease progression,50 and recent work has suggested that altered microRNA expression regulates expression of genes regulating apoptosis and cell-cycle progression.51
With the finding that several molecular markers have prognostic significance, it is not surprising that many of these factors are correlated. However, there are discrepancies with many cases having some high-risk and other low-risk molecular features and more than 50% of IgVH unmutated cases have no unfavorable cytogenetics.41 There is an association between unfavorable cytogenetic aberrations (del 17p and del 11q) and unmutated CLL, although 13q− is more frequent in mutated CLL. Multivariate analysis identified IgVH mutational status, poor-risk cytogenetic abnormalities, white blood cell count, and lactate dehydrogenase as independent prognostic factors and when incorporated into models, clinical staging (using either the Rai or Binet staging systems) loses independent prognostic value.21 In a study involving more than 1000 CLL patients the relative value of ZAP70, CD38, and IgVH mutation status was examined and ZAP70 expression was found to be the strongest predictor of time from diagnosis to requirement for treatment.52 It may be that high-risk cytogenetics, IgVH mutational status, ZAP70, and CD38 provide complementary prognostic information, with expression of all markers conferring a poor prognosis; lack of expression of any, a good prognosis; and discordant expression, an intermediate prognosis.38,41,52
How I follow patients
My own practice is to follow patients who remain on an expectant course every 3 months for history, physical examination, and blood counts. This allows assessment of disease progression and measurement of the lymphocyte doubling time. Once it is established that patients are following a particularly stable clinical course, less frequent follow-up is sufficient. Special attention must be paid to any change in symptoms that might be suggestive of transformation, such as development of night sweats, increasing adenopathy at one site, or elevated lactate dehydrogenase. Repeat scanning is not indicated routinely but is reserved for patients presenting with specific symptoms or signs.
In all other leukemias early treatment is optimal, but this is not the case in CLL. Some patients have a smoldering clinical course and may have no difference in survival compared with age-matched controls. These patients do not merit therapy. Currently, the disease remains incurable using standard treatment approaches and previous trials have demonstrated no survival advantage of early treatment versus an initial watch and wait approach. More than 2000 patients with early disease have been enrolled in trials of immediate versus deferred chemotherapy and in a meta-analysis of these studies there was no statistically significant difference in survival between early versus deferred therapy,53 with in fact a trend toward a worse outcome for early treatment (10-year survival with immediate chemotherapy was 44% versus 47% for those whose therapy was deferred). It should be noted that all these studies were performed using alkylating agents.
When I institute therapy
I follow the iwCLL guidelines for when to initiate therapy and these recommend treatment for patients with active progressive disease.5 The most important treatment decision to be made in CLL is whether the patient merits therapy at any given time. In my own practice I almost never treat a patient based purely upon a high lymphocyte count alone and treat only for symptomatic disease, bulky progressive adenopathy, or marrow failure. The traditional goal of therapy of has been palliation and patients were usually treated until symptoms resolved. The availability of newer therapies has resulted in increased awareness of the importance of achieving a complete remission (CR) in CLL and updated criteria for response in CLL have been recently been established (Table 5).5 A major clinical trial question is whether identification of clinical or molecular risk factors can identify which patients are candidates for early therapy, and studies examining whether patients presenting with high-risk features have any benefit from more effective chemoimmunotherapy approaches are actively recruiting.
How I treat CLL
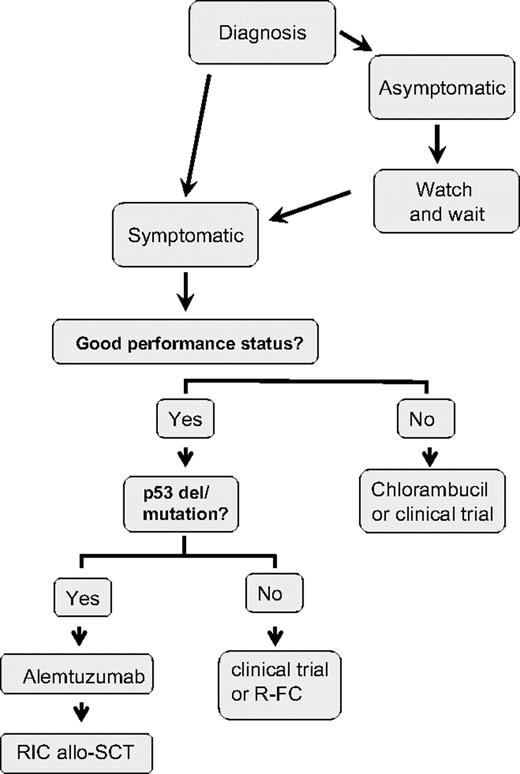
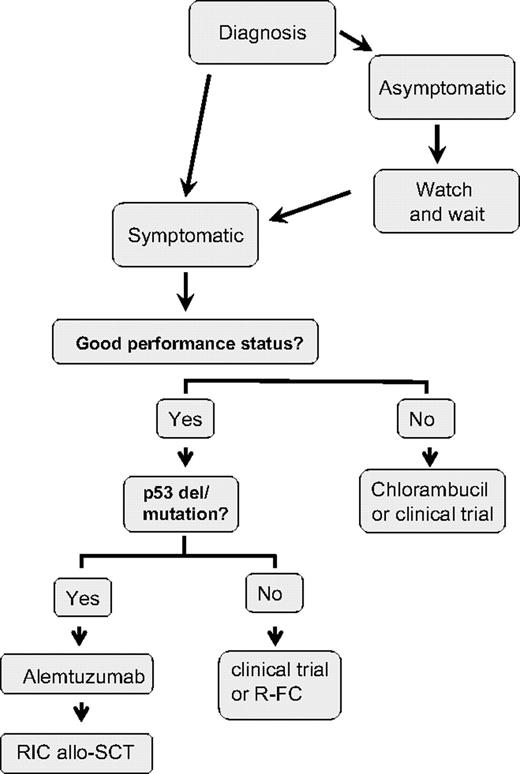
My treatment approach for the management of previously untreated CLL patients is shown in Figure 1. I initiate treatment in patients with symptomatic disease, bulky lymphadenopathy and/or splenomegaly, risk of local compressive disease, marrow compromise, or rapid disease progression. Once treatment is indicated, many treatment approaches are available. The concept that the approach can be either to continue to “do nothing” or to discuss an option with considerable morbidity and mortality such as stem cell transplantation (SCT) is a confusing one for patients (as well as for the physician), and considerable consultation time is required to review available treatment approaches.
The results of clinical trials in previously untreated CLL have demonstrated major advances over the last decade, as shown in Table 6. The most important advances have been the demonstration of improvement in outcome in CLL with combination chemotherapy and then further marked improvement with chemoimmunotherapy. It is by successful enrollment in clinical trials that we have been able to demonstrate improvement in outcome and wherever possible my preferred treatment for previously untreated CLL patients is enrollment in a clinical trial. For those patients who are ineligible or do not consent to be treated in clinical trials, my preferred treatment of choice (for patients with good performance status) is the combination of rituximab with fludarabine and cyclophosphamide (R-FC). I do this because it is clear from recent studies that patients who achieve CR have longer durations of response and that this is associated with improvement in performance status, and a primary goal of therapy now is for my patients to achieve CR. Phase 2 clinical studies demonstrated that R-FC is the most effective combination to date in terms of achieving CR in CLL in previously untreated,60,62 and treated63 patients. In a series of 300 previously untreated patients, overal response (OR) was 95%, with CR in 72%, nodular PR in 10%, PR due to cytopenia in 7%, and PR due to residual disease in 6%.60 At a median follow-up of 6 years OS was 77% and PFS, 51%. The German CLL Study Group (GCLLSG) CLL8 was the largest randomized clinical trial performed in CLL and demonstrated a significant improvement in response rates and duration of response with R-FC compared with FC alone.5 The use of R-FC was associated with a significantly higher CR rate, higher percentage of patients having eradication of minimal residual disease, and longer duration of response compared with FC. The study results highlight the importance of achieving CR and eradication of minimal residual disease in CLL, since these patients had longer duration of responses than those patients in whom residual disease was found. The study included analysis of cytogenetic abnormalities at study entry. The results of the CLL8 study led to approval of rituximab in combination with chemotherapy for CLL in both the United States and Europe.
Care has to be taken when administering rituximab to patients with CLL, because tumor lysis syndrome and deaths have been reported, particularly in patients with high circulating tumor load, likely because of high levels of cytokines released.64 For this reason, for patients with high white blood counts I usually use 100 mg of rituximab on day 1 of the R-FC regimen and administer the remainder of the dose on day 2 of treatment, particularly for the frailer patients, although this is not performed in all centers. This is required only in the first treatment cycle since by the time of starting the second cycle the white blood count has usually fallen considerably. Although there have been no published reports documenting improvement in outcome with the use of growth factors with R-FC, the R-FC regimen is associated with significant hematologic toxicities, and in my own practice I use growth factor support to ensure that patients can continue to be treated at full dosage and on time on the 28-day cycle. The dose of rituximab in CLL is somewhat controversial. The labeled dose of rituximab in CLL and the dose that has been used in all clinical trials performed with R-FC is 375 mg/m2 in cycle 1, and 500 mg/m2 in subsequent cycles. I continue to use this dosage until there is clinical trial data demonstrating that alternative dosing schedules do not result in inferior outcome.
The anti-CD52 monoclonal antibody, alemtuzumab, is approved for use in previously untreated CLL, having been approved initially for fludarabine refractory patients. A phase 3 randomized study evaluated first-line therapy with alemtuzumab compared with chlorambucil in 297 patients with progressive CLL.56 This study demonstrated significantly superior response rates for alemtuzumab compared with chlorambucil (OR 83% vs 56%; P < .001 and CR rates 24% vs 2%; P < .001). Further follow-up is awaited to determine survival outcomes from this study. I use alemtuzumab as preferred first-line therapy for CLL patients with del 17p or p53 mutations, because this agent has been shown to have efficacy in this patient population.65 If such patients have bulky adenopathy, I suggest enrollment in clinical trials or use alemtuzumab with corticosteroids.
Other chemoimmunotherapy regimens have been evaluated, notably pentostatin, cyclophosphamide, and rituximab (PC-R) and fludarabine and rituximab (F-R). These regimens may have less hematologic toxicity than R-FC. There have been no randomized clinical trials comparing R-FC with either PC-R or F-R. My preference for R-FC is based on considerable personal experience using the R-FC regimen, and the fact that this regimen has been evaluated in randomized clinical trials.
Maintenance or consolidation therapy in CLL
I do not administer maintenance therapy in CLL, because this has no established role at present. Alemtuzumab has been assessed in this setting with some intriguing results obtained,66 but enthusiasm has been tempered by the high toxicity observed. Ongoing clinical trials are examining maintenance therapy with rituximab, lenalidomide, and alternative schedules of administration of alemtuzumab.
Hematopoietic stem cell transplantation (SCT) has been evaluated in first remission in phase 2 clinical trials in high-risk patients.67 SCT is not a suitable option for the majority of CLL patients since most patients are too elderly and have an extremely indolent course. There have been no published studies in CLL that have compared the outcome after standard chemotherapy with either autologous or allogeneic SCT. The biggest challenges remain the decision of which patients are eligible for consideration of SCT and when in their disease course SCT should be offered. Because of the more elderly age of CLL patients, the approach of choice is usually reduced-intensity conditioning allogeneic SCT.68 The European Bone Marrow Transplant guidelines outline indications for SCT in CLL.69 These guidelines support the use of allogeneic SCT in patients requiring treatment who have p53 abnormalities. These patients have sufficiently poor prognosis to merit transplantation in first remission, since they continue to have poor survival. These patients should be referred early to transplant centers for discussion of this approach and identification of suitable donors. Allogeneic SCT is also recommended for younger patients with CLL who fail to respond to first-line combination chemotherapy.
Impact of prognostic markers on treatment outcome
Most of the modern prognostic markers were validated by retrospective analysis, often from single-center studies, but have now been applied to prospective randomized clinical trials. These studies suggest that the same molecular markers that identify patients with more aggressive disease also impact on outcome after treatment. This finding is not surprising since these same factors have been predictive of overall survival in retrospective studies, where it would have been expected that the same treatment options would have been offered to patients with and without risk factors. As shown in Table 7, 3 studies have been published examining the impact of these factors in response to prospective randomized trials in previously untreated patients with CLL,59,70,71 and these results have been confirmed in several other studies that have been reported in abstract format only. These findings suggest that poor-risk features for CLL are largely also predictive of poor response. There is not yet sufficient evidence to alter therapy based upon molecular features, but the one exclusion from this is the group of patients who present with del 17p. These patients have poor response to chemotherapy and impaired survival. Although this represents only a small group of previously untreated patients, these patients should ideally be treated in clinical trials examining agents that have efficacy in patients without functional p53 or with alemtuzumab-based therapy.
Treatment of the elderly patient with CLL
The problem with the R-FC regimen is that CLL is a disease of the elderly and the performance status of many patients is too impaired to consider this aggressive chemoimmunotherapy approach. It must be stressed that patient performance status is more important than chronologic age in determining suitability for chemoimmunotherapy, and adequate renal function is required for safe administration of fludarabine. The majority of clinical trials have enrolled younger patients who are not representative of the patients most often seen in practice. A notable exception is the GCLLSG CLL5 study.55 This multicenter phase 3 trial enrolled patients older than 65 years and compared first-line therapy with fludarabine or chlorambucil. One hundred ninety-three patients with a median age of 70 years were randomized to receive fludarabine (25 mg/m2 for 5 days intravenously, every 28 days, for 6 courses) or chlorambucil (0.4 mg/kg body weight with increase to 0.8 mg/kg, every 15 days, for 12 months). The results demonstrated that although fludarabine resulted in a significantly higher OR (72% versus 51%; P = .003) and CR (7% versus 0%; P = .011) rate, there was no difference in progression-free survival (19 months with fludarabine, 18 months with chlorambucil; P = .7) or overall survival (46 months in the fludarabine versus 64 months in the chlorambucil arm; P = .15).55 The results demonstrate no clinical benefit for fludarabine compared with chlorambucil as the first-line therapy of elderly CLL patients.
Chlorambucil was the first effective agent used in the treatment CLL. Chlorambucil is rapidly absorbed from the gastrointestinal tract and peak plasma concentrations occur within one hour of ingestion. Metabolism is primarily hepatic and excretion of metabolites, via renal clearance. There has been great variability in dosage and schedule of administration, but the 2 commonly used approaches are low-dose continuous therapy using a continuous dose of 0.08 mg/kg (usual dose 4 to 8 mg by mouth) or pulsed intermittent dosage of 0.8 mg/kg given (usual dose 40 to 80 mg) in a single dose by mouth given every 3 to 4 weeks. The drug has fallen out of fashion in the United States, but continues to be widely used in Europe and the results of the CLL5 trial suggest it still has a role to play in patients with decreased performance status. Ongoing clinical trials are assessing the addition of monoclonal antibodies, including rituximab or ofatumomab, to chlorambucil compared with chlorambucil alone. Additional agents being assessed in clinical trials in this patient population include bendamustine alone and in combination with rituximab, lenalidomide, and ABT263.
Unique complications of CLL
CLL is frequently associated with autoimmune phenomena, the most common being autoimmune hemolytic anemia (AIHA) and immune thrombocytopenia (ITP).72 In practice, these complications may occur in patients with no other requirement for treatment, or in patients in whom chemotherapy treatment is imminent or already started.
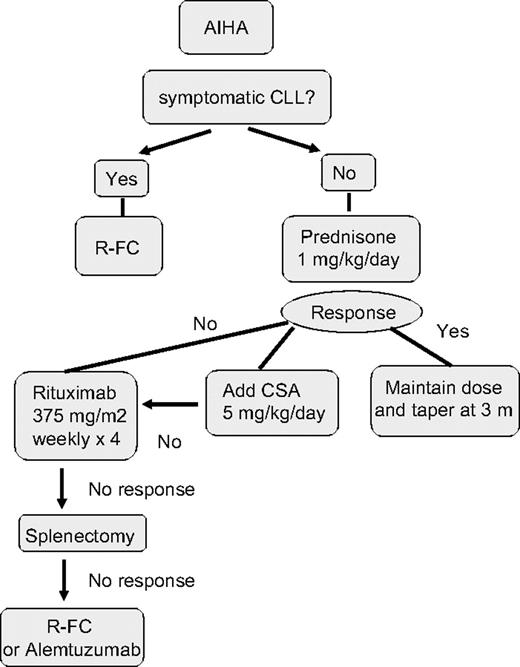
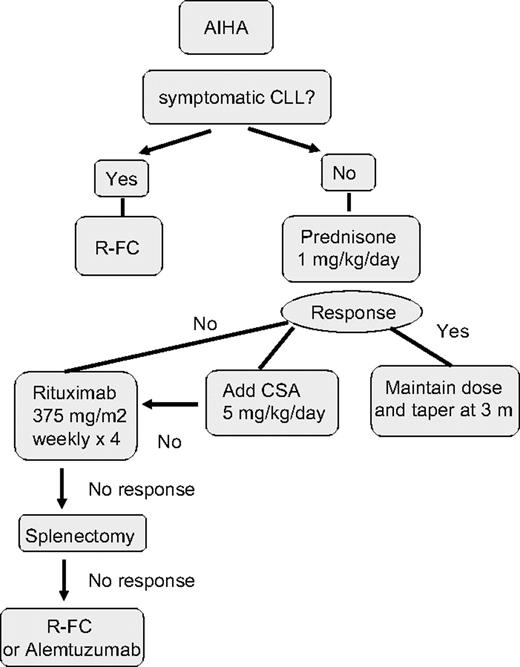
Up to 33% of CLL cases have a positive direct antiglobulin test (DAT) during the course of disease, but overt AIHA occurs much less frequently. In a report of 1203 patients with CLL consecutive cases reported from a single institution, 52 (4.3%) cases of AIHA were observed, 19 at the time of diagnosis.73 Factors associated with an increased risk of development of AIHA at diagnosis included a high white blood count, older age, and male sex. AIHA alone was not itself associated with poor prognosis. The diagnosis of AIHA is usually based on the presence of an isolated fall in hemoglobin associated with a positive DAT, increased reticulocytes, and serum bilirubin. The DAT may be negative despite overt hemolysis. A fall in serum haptoglobin may be a helpful measure of hemolysis. There have been no controlled trials of treatment for AIHA in CLL and the treatment approach is based on personal experience. I follow the algorithm shown in Figure 2. I treat autoimmune cytopenias with prednisone 1 mg/kg orally for 2 to 4 weeks, followed by a slow taper. In severe cases, a single high dose of intravenous methylprednisolone (1 g) or intravenous immunoglobulin (IVIg) (0.4 mg/kg per day for 5 days) can be given and is effective in 40% of cases. In nonresponders or those patients who relapse on steroid withdrawal, the use of cyclosporine (CSA; 5-8 mg/kg per day) or mycophenolate mofetil can be beneficial. More than 60% of patients respond to CSA, with median duration of response of 10 months. Splenectomy is still indicated in refractory patients with vigorous uncontrolled hemolysis and splenic irradiation may be an alternative for patients in whom surgery is contraindicated. I recommend that patients receive immunization against pneumococcus, Haemophilus influenzae B, and meningococcus, ideally 2 to 3 weeks before surgery, followed by lifelong penicillin (or equivalent) prophylaxis. Rituximab (375 mg/m2 per week for 4 weeks) has been used in the treatment of many autoimmune diseases, including AIHA74,75 and alemtuzumab has also been reported to have activity in this setting.76
There has been controversy whether some chemotherapy agents, particularly purine analogs, induce or worsen AIHA. In a trial comparing outcomes of treatments using chlorambucil, fludarabine, or fludarabine in combination with cyclophosphamide, a positive DAT was found in 14%, and AIHA occurred in 10% of patients.72 AIHA occurred more often in patients treated with chlorambucil than fludarabine, and occurred least frequently in patients receiving the combination of fludarabine and cyclophosphamide. For patients requiring therapy, a positive DAT test had poor prognostic significance, even in the absence of AIHA. The results suggest that the most successful treatment of AIHA in patients requiring chemotherapy treatment is the treatment associated with the best response rate.
Pure red cell aplasia (PRCA) rarely occurs in CLL. The diagnosis is suspected by the finding of worsening anemia in the setting of an absence of a reticulocyte response and is confirmed by the finding of an absence of erythroid precursors in the BM. It is important to rule out viral infections including cytomegalovirus, Epstein-Barr virus, or parvovirus before assuming this is due to autoimmunity. The treatment approach is the same as for AIHA, and response to steroids or cyclosporin in PRCA can be assessed by following the reticulocyte count. Rituximab has been successful in the treatment of refractory PRCA.77
Approximately 2% of CLL patients develop clinically significant ITP. The diagnosis is made on the basis of an unexplained fall in platelet count in the absence of BM failure due to leukemic infiltration or hypersplenism, and platelet autoantibody tests lack sensitivity and specificity. Approximately one-third of cases also have AHA (Evans syndrome). I treat ITP with prednisone 1 mg/kg given orally, which is associated with a response in more than 50% of cases. Similar response rates have been seen with IVIg. In nonresponding or relapsed cases, splenectomy may be effective. Rituximab has been used with good results, but the optimal dosing schedule has not been defined. Autoimmune neutropenia occurs less frequently and can be diagnosed by assessment of antineutrophil antibodies and treatment is as with AIHA.
Infections are a major cause of morbidity and mortality in CLL patients, mediated through impairment in humoral and cellular immunity inherent in the primary disease and in the further immunosuppression related to the treatment. Hypogammaglobulinemia is the most important immune defect in terms of risk of severe bacterial infections, its frequency and severity progressing with the duration of the disease. Although there have been problems with supply of this agent, I use IVIg in patients with frequent severe bacterial infections. In a randomized crossover study among patients with severe hypogammaglobulinemia, the incidence and severity of infections were less when patients received IVIg replacement therapy.78 The frequency of infections may also be increased and altered after therapy.79 Although bacterial infections are most common, because of the resulting T-cell dysfunction after treatment, treated patients are at risk for a wide spectrum of opportunistic infections including Listeria monocytogenes, Pneumocystis carinii, cytomegalovirus, herpes simplex virus, and mycobacteria.80,81 Long-term follow-up suggests that although the purine analogues have an impact on opportunistic infections, complications of infection are more common in those patients with incomplete response to therapy or with progressive disease, suggesting that the disease itself has more impact than the therapy.82 Although opportunistic infections are also seen after therapy with alemtuzumab,83 patients treated with this agent were fludarabine refractory and serious infectious complications are high in this patient population. Serious infectious complications in patients with fludarabine refractory disease occurred in 89% of patients, with infections being bacterial in 78.5%, viral in 12.5%, fungal in 4.5%, and opportunistic in 4.5%.84 Many fewer infections were seen when alemtuzumab was used in previously untreated patients.56
Richter syndrome
Richter syndrome (RS) refers to the development of high-grade non-Hodgkin lymphoma and occurs in up to 10% of CLL patients.85 The large cells of RS either arise through a transformation of the original CLL clone by the acquisition of new genetic abnormalities or less frequently represent a new secondary neoplasm. The clinical outcome of the disease is generally poor, with median survival of months from transformation, but prognosis is better when transformation occurs in previously untreated patients. Treatment is usually with regimens that are effective in high-grade non-Hodgkin lymphoma and although numerous regimens have been proposed, there is no consensus on the best therapeutic approach for RS patients. RS can be suspected in patients who develop progressive adenopathy at some sites, development of B symptoms, or rising lactate dehydrogenase. The role of positron emission tomography scanning remains a research tool in early detection of RS.
Conclusions
The past decade has been very exciting in terms of advances in understanding CLL and in seeing huge improvements in outcome with treatment. Despite these improvements, the disease remains incurable, and much work still remains to ensure that we move toward a cure in this disease as quickly as possible. The ability to test new agents in this disease, move effective agents alone and in combination into phase 2 studies, and then provide proof of efficacy in randomized clinical trials remains important. This approach will enable us determine the optimal therapy, when treatment should be initiated, and whether treatment should be tailored by specific risk factors of the disease. Obtaining the answers to these questions will ensure that we can help our CLL patients achieve the longest duration of response and improve the quality of their lives.
Acknowledgments
This work was supported by program grant PO1 CA 81538 from the National Cancer Institute (J.G.G.) to the CLL Research Consortium.
Authorship
Contribution: J.G.G. conceived and wrote this article.
Conflict-of-interest disclosure: J.G.G. has received honoraria for advisory boards from Roche Pharmaceuticals, GSK, Celgene, and Mundipharma.
Correspondence: John G. Gribben, Institute of Cancer, Queen Mary University of London, Barts and The London School of Medicine and Dentistry, Charterhouse Square, London EC1M 6BQ, United Kingdom; e-mail: j.gribben@qmul.ac.uk.