Abstract
Immunotherapy with allodepleted donor T cells (ADTs) improves immunity after T cell–depleted stem cell transplantation, but infection/relapse remain problematic. To refine this approach, we characterized the expression of surface markers/cytokines on proliferating alloreactive T cells (ATs). CD25 was expressed on 83% of carboxyfluorescein diacetate succinimidyl esterdim ATs, confirming this as an excellent target for allodepletion. Seventy percent of CD25− ATs expressed CD71 (transferrin receptor), identifying this as a novel marker to target ATs persisting after CD25 depletion. Comparison of residual alloreactivity after combined CD25/71 versus CD25 immunomagnetic depletion showed enhanced depletion of alloreactivity to host with CD25/71 depletion in both secondary (2°) mixed lymphocyte reactions (P < .01) and interferon-γ enzyme-linked immunospot assays (P < .05) with no effect on third-party responses. In pentamer/interferon-γ enzyme-linked immunospot assays, antiviral responses to cytomegalovirus, Epstein-Barr virus, and adenovirus were preserved after CD25/71 allodepletion. CD25/71 ADTs can be redirected to recognize leukemic targets through lentiviral transfer of a chimeric anti-CD19ζ T-cell receptor. Finally, we have established conditions for clinically applicable CD25/71 allodepletion under European Union Good Manufacturing Practice conditions, resulting in highly effective, reproducible, and selective depletion of ATs (median residual alloreactivity to host in 2° mixed lymphocyte reaction of 0.39% vs third-party response of 62%, n = 5). This strategy enables further clinical studies of adoptive immunotherapy with larger doses of ADTs to enhance immune reconstitution after T cell-depleted stem cell transplantation.
Introduction
The rigorous T-cell depletion required to avoid graft-versus-host disease (GVHD) after haplo-stem cell transplantation (SCT) delays immune reconstitution. The resultant high rate of leukemic relapse1 and mortality/morbidity from viral and fungal infections2 have limited the broader use of haplo-SCT. To overcome this, a variety of strategies have been developed to selectively deplete alloreactive T cells (ATs) after ex vivo stimulation of donor lymphocytes in a mixed lymphocyte reaction (MLR). These include negative selection of donor T cells expressing activation markers (eg, CD25, CD69, CD134, CD137, CD147, HLA-DR)3-10 using immunotoxins or immunomagnetic selection, fluorescence-activated cell sorting,11,12 killing activated T cells by photodynamic purging,13,14 chemotherapy agents,15 or inducing Fas-mediated apoptosis.16
We and others10,17 have demonstrated that allodepletion using a CD25 immunotoxin (IT) specifically deletes host-reactive T-cell responses with preservation of antiviral and antileukemic responses. Andre-Schmutz et al showed that adoptive transfer of allodepleted donor T cells (ADTs) generated using this IT after human histocompatibility leukocyte antigen (HLA)–mismatched SCT was safe and feasible.18 Solomon et al have adoptively transferred larger doses of ADTs generated using a similar approach in adults transplanted from HLA-matched sibling donors.19 In both of these studies, GVHD was inversely associated with the efficacy of allodepletion.
We have shown that adoptive transfer of CD25 IT generated ADTs at a dose of 3 × 105/kg accelerated T-cell reconstitution and recovery of cytomegalovirus (CMV)/Epstein-Barr virus (EBV)–specific immunity paediatric patients after haplo-SCT.20 However, the rate of leukemic relapse rate was high, reflecting the high-risk nature of this patient group and the low precursor frequency of leukemia-reactive T cells. In addition, 2 patients died of adenovirus-associated complications, and no patient had detectable T-cell responses to adenovirus within 9 months after SCT. Interestingly, one of these patients cleared adenoviremia after a single infusion of ADTs at a higher dose of 2.5 × 106/kg. These data suggest that larger doses of ADTs may be necessary to confer protective responses to pathogens, such as adenovirus, which evoke low-frequency T-cell responses in the donor21 and for a graft-versus-leukemia effect. Although the incidence of significant acute and chronic GVHD was low in our clinical study, in vitro data indicating that significant residual alloreactivity persists after CD25 IT–mediated allodepletion22 raise concerns about the safety of administering larger doses of ADTs in the haploidentical setting. There is thus a pressing need to enhance the degree of depletion of ATs.
Activated T cells express a variety of surface markers, including CD25, CD69, CD71, CD95, CD137, CD147, OX40, ICOS, HLA-DR, and they secrete Th1 cytokines interleukin-2 (IL-2) and interferon-γ (IFN-γ). As such, a plethora of potential targets now exist for allodepletion,3-8 but there are no data on the relative expression of these targets on ATs to enable identification of the optimal targets. The data above imply that some ATs are retained after CD25-based allodepletion, suggesting that CD25 may be expressed only in a subset of ATs and raising the possibility that targeting other molecules could enhance allodepletion. To rationally design strategies for enhanced allodepletion, we have systematically characterized the phenotype of ATs. While ATs have multiple phenotypes, proliferation in response to alloantigens is their most basic hallmark. We have identified proliferating ATs using carboxyfluorescein diacetate succinmidyl ester (CFSE) dye dilution. Godfrey et al11 have shown that flow cytometric depletion of CFSEdim T cells markedly reduces alloreactivity in a major histocompatibility complex class II disparate murine model. We have used this approach to characterize the expression of cytokines, effector molecules, and activation markers on proliferating ATs. Based on these studies, we have designed a clinically applicable approach to enhance allodepletion.
Methods
Generation of DCs and EBV-transformed LCLs
Ethical approval was obtained through the nonclinical Institutional Review Board at University College London. CD3+ T cells and CD14+ cells were isolated by positive selection of peripheral blood mononuclear cells (PBMCs) from normal donors with CD3 or CD14 microbeads (Miltenyi Biotec). Lymphoblastoid cell lines (LCLs) were generated as described.23 Dendritic cells (DCs) were generated from PBMCs/CD14+ PBMCs in serum-free CellGenix DC media as described.24 DCs and LCLs were irradiated (30 and 70 Gy, respectively) before use as stimulators for MLRs.
CFSE staining and flow cytometric characterization of alloreactive T cells and Tregs
CD3+ T cells were labeled with 2.5μM CFSE (Invitrogen) as described11 and cocultured with or without HLA-mismatched allogeneic irradiated DCs at a responder-to-stimulator (R:S) ratio of 5:1 at 2 × 106/mL in AIM V medium (Invitrogen). Samples from cocultures at days 0, 1, 3, 5, and 7 were stained with phycoerythrin/ peridinin chlorophyll protein/allophycocyanin-conjugated monoclonal antibodies for human CD3, CD25, CD69, CD71, HLA-DR, OX40, ICOS, CD95, CD45RA, CCR7, IFN-γ, tumor necrosis factor α (TNFα), and IL-2. Antibodies were from BD Biosciences, except for CCR7 (R&D Systems). Intracellular cytokine staining was performed as published12 using 1 μg/mL brefeldin A (BD Biosciences). Costaining with CD3 and CD25 allowed gating on the CFSEdim, CD3+, CD25− population. Samples were acquired on a FACS LSR (BD Biosciences) or CyAn flow cytometer (Dako). Analysis was done using WinList/Summit Version 4.1 software, and the proliferative index/precursor frequencies were obtained using ModFit LT software (Verity Software House). Regulatory T cells (Tregs) were stained for CD4/CD25 and then fixed and permeabilized for intracellular FoxP3 staining, as per the manufacturer's protocol (eBioscience).
Generation of allodepleted donor T cells and comparison of allodepletion methods
Normal donor PBMCs at 2 × 106/mL were cocultured with or without irradiated HLA-mismatched LCLs at a R:S ratio of 40:1 in AIM V in T-175 flasks for 3 days. For comparison of CD25 immunomagnetic bead and CD25 IT depletion, on day 3 of the MLR, cocultures were split and anti-CD25 microbeads (20 μL microbeads/107 PBMCs; Miltenyi Biotec) added to half the coculture and 4 μg/mL CD25 IT added to the other half. CD25 immunomagnetic negative selection was performed according to the manufacturer's instructions using LD columns and depletion using CD25 IT was performed overnight as described.17 Unmanipulated donor PBMC/LCL cocultures and donor PBMCs alone were used as controls. Unmanipulated cells or day 3 ADTs (both 2 × 105 cells) were sampled for fluorescence-activated cell sorter (FACS) analysis and in triplicate for primary (1°) proliferation assays. The remaining ADTs were rested for 2 days as below before restimulation in secondary (2°) MLRs and enzyme-linked immunospot (ELISPOT) assays.
For combined CD25/71 allodepletion, day 3 PBMC/LCLs cocultures were washed and resuspended in 60 μL of phosphate-buffered saline with 1% FCS/107 PBMCs. Biotinylated anti-CD71 antibody (BD Biosciences) was added (20 μL of antibody/107 PBMCs) for 15 minutes at 4°C. Cells were washed and labeled with anti-CD25 beads and anti-biotin beads (Miltenyi Biotec) using 20 μL of beads/107 PBMCs for 15 minutes at 4°C. Depletion of CD25/71 labeled cells was then performed on LD columns according to the manufacturer's instructions. For combined CD25/45RA depletions, PBMCs were labeled with CD25 and CD45RA beads (Miltenyi Biotec). Aliquots from the allodepleted fractions were analyzed flow cytometrically. The remainder of the negative fraction was resuspended in AIM V at 2 × 106/m: for 2 days and then analyzed for residual alloreactivity in 2° MLRs or IFN-γ ELISPOT assays.
Scale-up and final allodepletion protocol for clinical application
Normal donor PBMCs from the buffy coat of a 450-mL blood donation (median 6.58 × 108 PBMCs total) were cocultured at 2 × 106/mL with or without irradiated fully HLA-mismatched in vitro matured DCs at an R:S ratio of 10:1 in AIM V serum-free medium in T-175 flasks for 4 days under Good Manufacturing Practice (GMP) conditions in the Cell Therapy laboratory. Cells were harvested and stained with 2 μg of biotinylated clinical-grade anti-CD25 (daclizumab; Roche; biotinylated by Miltenyi Biotec) and 1.25 μg of biotinylated anti-CD71 (BD Biosciences) antibodies per 108 cells for 15 minutes at 4°, washed and stained with CliniMACS anti-biotin reagent (Miltenyi Biotec) for 30 minutes at room temperature according to the manufacturer's protocol. Cells were washed and resuspended in CliniMACS phosphate-buffered saline/ethylenediaminetetraacetic acid buffer (plus 0.5% human serum albumin; Bio Products Laboratory). Depletion was performed on the CliniMACS Plus using a CliniMACS tubing set (Miltenyi Biotec). The program used was depletion 2.1 with the frequency of positive cells set to 20%. Depleted cells were washed, resuspended at 2 × 106/mL, sampled for flow cytometry, and the remainder was plated in 6-well plates to be rested for 2 days before assessment of residual alloreactivity in 2° MLR.
Proliferation assays
[3H]thymidine incorporation from unmanipulated/allodepleted 1° MLRs was measured as described.25 To assess residual alloreactivity in 2° MLRs, on day 5 after primary stimulation, 2 × 105 ADTs were restimulated with 5000 irradiated LCLs (where LCLs were used as the original stimulator) or 2 × 105 irradiated PBMCs (where DCs were used as original stimulators) from either the original stimulator or HLA-mismatched third party. Controls consisted of unmanipulated PBMCs frozen on day 0 and thawed on the day of plating, ADTs alone, or LCLs alone.
IFN-γ ELISPOT assay
To further assess residual alloreactivity, on day 5 after 1° stimulation, 2 × 105 ADTs or thawed, unmanipulated donor PBMCs were plated per well in the presence of 2 × 105 irradiated host or third-party LCL stimulators in triplicate for 18 to 24 hours at 37°C. Controls consisting of 2 × 105 responders or stimulators alone were also plated. The assay was performed as published,17 plates were counted on a Bioreader 3000 (Bio-Sys), and the means of triplicate wells were calculated. The mean number of specific spot-forming cells was calculated by subtracting the mean number of spots of responder alone and stimulator alone wells from the mean number of spots in test wells.
Assessment of antiviral immunity
To detect virus-specific CD8+ T cells, 106 PBMCs or ADTs were costained with CD8 fluorescein isothiocyanate, CD3 peridinin chlorophyll protein and either isotype phycoerythrin control antibody or phycoerythrin-conjugated pentamers appropriate to the donors' HLA restriction (CMV pp65, HLA-A*0201-NLVPMVATV (A2-NLV) and HLA-B*0702-TPRYTGGGAM (B7-TPR); EBV-LMP-2, HLA-A*0201-CLGGLLTMV (A2-CLG); ProImmune). Staining/analysis was done as reported.20 The percentage of pentamer+ cells in the CD3+/CD8+ lymphocyte gate was expressed as a proportion of the CD8+ cells. Functional responses to CMV, EBV, and adenovirus were analyzed in IFN-γ ELISPOT assay as published.25
Construction of chimeric TCR and production of lentiviral supernatant
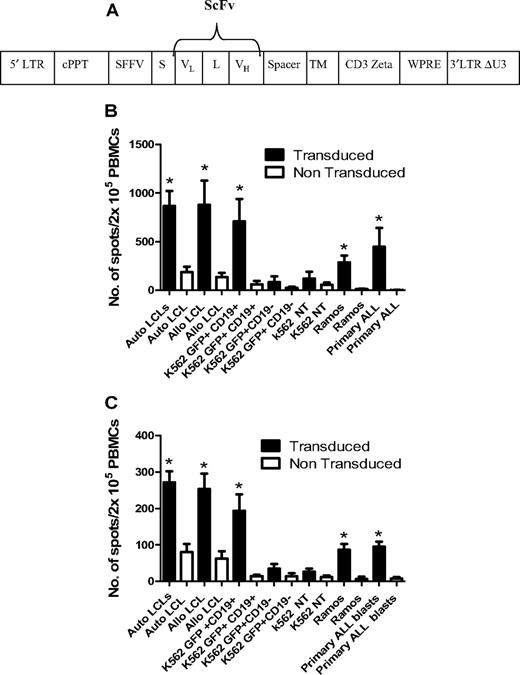
The CD19 chimeric T-cell receptor (TCR; CD19R) transgene consists of the variable domains of the CD19-specific murine monoclonal antibody FMC-63 assembled as a single-chain variable fragment (ScFv), in frame with a sequence encoding the human IgG1 hinge CH2-CH3, CD28 transmembrane domain and the cytoplasmic signaling domain of the CD3ζ. The CD19R transgene was subcloned as a Not1-BamH1 fragment in to the pHR–SIN-SE lentiviral vector26 to create the construct CD19R/pHR–SIN-SE (see Figure 6A). For lentiviral production, 293T cells were cotransfected with 40 μg of CD19R/pHR–SIN-SE and the packaging plasmids pMDG.2 (VSV-G envelope; 10 μg) and pCMVΔ8.74 (gag-pol; 30 μg). Viral supernatant was harvested at 48 and 72 hours after transfection and concentrated by ultracentrifugation.
Transduction of allodepleted T cells and functional assays of antileukemic responses
CD25/71 ADTs were resuspended at 106/mL in cytotoxic T lymphocyte medium25 with IL-2 (100 U/mL; R&D Systems). PBMCs were transduced at a multiplicity of infection (MOI) of 150 on day 4 and analyzed on day 8. Expression of the CD19R transgene was determined flow cytometrically using a goat anti-human IgG FcγCy5 antibody (Jackson ImmunoResearch Laboratories). Triplicate samples of anti-CD19ζ TCR/mock transduced ADTs were cocultured with CD19+/− tumor cell lines (K562, K562 stably transduced with green fluorescent protein (GFP) or a CD19-GFP transgene; Ramos), LCLs, or 1° ALL blasts at an R:S ratio of 1:1. After 18 hours, IFN-γ or granzyme B production was assessed in ELISPOT assays as described above and previously.27
Statistical analysis
A Wilcoxon matched pairs test was used to determine statistical differences between samples (GraphPad Software Version 5.0). FACS data are represented as mean plus or minus SD.
Results
Kinetics of expression of activation markers and cytokines during allo-MLR
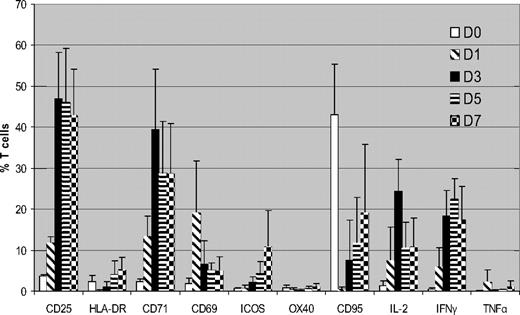
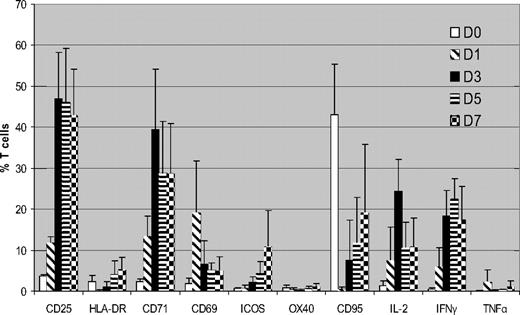
We initially determined expression of surface markers/cytokines flow cytometrically in CD3+ T cells cultured with HLA-mismatched DCs over a 7-day MLR using unmanipulated PBMCs from the same donors cultured under identical conditions as controls. Figure 1 summarizes the cumulative data from 5 donor-recipient (D-R) pairs. CD25 was up-regulated within 24 hours of MLR, peaking on day 3 (mean, 47% of total T cells) and then it plateaued. CD71 showed similar kinetics (mean, 39.3% T cells on day 3). This high level of expression was consistent cross all 5 pairs. There was little expression of CD25 or CD71 in the unstimulated T cells (mean, 1.4% and 2.6%, respectively, on day 3). CD69 showed rapid up-regulation on day 1 but expression subsequently declined. HLA-DR, ICOS, and CD95 all showed similar patterns, with progressively increasing expression, but on lower proportions of T cells. There was significant up-regulation of IL-2 and IFN-γ by days 3 to 5 of coculture but little increase in TNFα expression.
Kinetics of surface markers and cytokine expression in alloreactive T cells (n = 5). FACS analysis of expression of surface markers and intracellular cytokines in CFSE labeled T cells cocultured with HLA-mismatched DCs. Results are means ± SD. Unstimulated control data has been subtracted from the stimulated data. Because CCR7 and CD45RA showed high expression in unstimulated PBMCs, they are not shown
Kinetics of surface markers and cytokine expression in alloreactive T cells (n = 5). FACS analysis of expression of surface markers and intracellular cytokines in CFSE labeled T cells cocultured with HLA-mismatched DCs. Results are means ± SD. Unstimulated control data has been subtracted from the stimulated data. Because CCR7 and CD45RA showed high expression in unstimulated PBMCs, they are not shown
Identification of proliferating alloreactive T cells using CFSE dye dilution
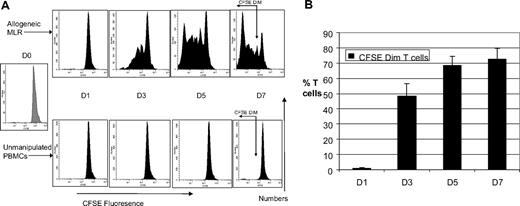
To characterize the phenotype of proliferating ATs, we cocultured CFSE-labeled T cells with or without HLA-mismatched DCs. Gating on the CFSEdim population enabled us to identify the proliferating alloreactive T-cell population. Figure 2A shows the progressive dilution of CFSE fluorescence with time in an allo-MLR and our gating strategy, taking into account the shift in CFSE fluorescence of unmanipulated PBMCs with time. By day 7 of the MLR, 70% of T cells in the MLR were CFSEdim. The mean alloreactive precursor frequency was 4.2% plus or minus 1.5% and there was a mean of 8 cell divisions. T cells cultured with autologous DCs showed minimal proliferation (mean, 1.4% CFSEdim T cells at day 7, data not shown). Thus, the CFSEdim population truly represents proliferating alloreactive T cells. Proliferation was greatest between days 1 and 3 of culture and plateaued between days 5 and 7 (Figure 2B).
Marked T-cell proliferation evident in allogeneic mixed lymphocyte reaction. (A) Kinetics of CFSE fluorescence in allogeneic MLR and unmanipulated T cells (n = 5). FACS analysis of a representative example of the kinetics of CFSE fluorescence in allo-MLR of CFSE-labeled T cells cultured with/without HLA-mismatched DCs. Proliferation of alloreactive T cells results in reduction in CFSE fluorescence intensity. Gating on the CFSEdim region, which selectively identifies the proliferating T-cell population, is shown on the day 7 FACS plots. (B) Time course of proliferation in allogeneic MLR (n = 5). FACS analysis demonstrating the percentage of CFSEdim proliferating alloreactive T cells in the MLR, assessed serially over a week. Results are the means ± SD. The percentage of CFSEdim populations in the unstimulated control has been subtracted from the results obtained in the MLR.
Marked T-cell proliferation evident in allogeneic mixed lymphocyte reaction. (A) Kinetics of CFSE fluorescence in allogeneic MLR and unmanipulated T cells (n = 5). FACS analysis of a representative example of the kinetics of CFSE fluorescence in allo-MLR of CFSE-labeled T cells cultured with/without HLA-mismatched DCs. Proliferation of alloreactive T cells results in reduction in CFSE fluorescence intensity. Gating on the CFSEdim region, which selectively identifies the proliferating T-cell population, is shown on the day 7 FACS plots. (B) Time course of proliferation in allogeneic MLR (n = 5). FACS analysis demonstrating the percentage of CFSEdim proliferating alloreactive T cells in the MLR, assessed serially over a week. Results are the means ± SD. The percentage of CFSEdim populations in the unstimulated control has been subtracted from the results obtained in the MLR.
Phenotypic characterization of proliferating alloreactive T cells
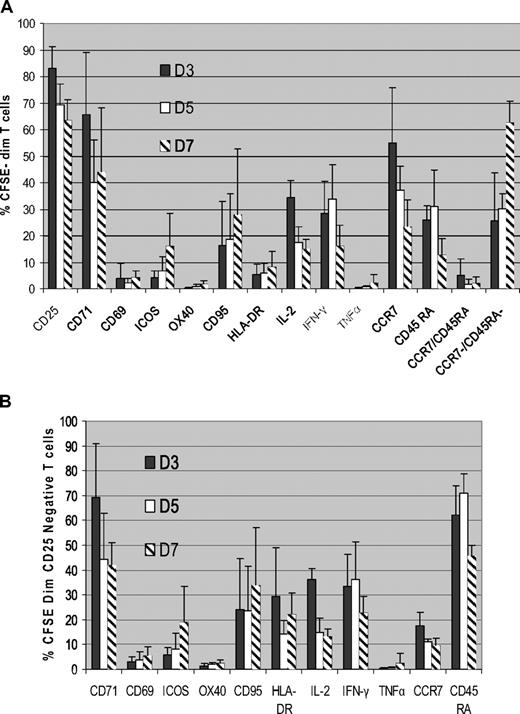
To determine the phenotype of ATs, we then analyzed surface marker and cytokine expression in the proliferating CFSEdim population. As shown in Figure 3A, CD25 was expressed in a mean of 83% plus or minus 8% of ATs by day 3 of coculture and showed little variability. CD71 expression had similar kinetics to CD25, being up-regulated on the majority of proliferating ATs by day 3 of MLR (mean 65% ± 23% of the CFSEdim T cells), with subsequent down-regulation at later time points. CD25 and CD71 were up-regulated in both CD4+ and CD8+ CFSEdim T cells.
CD25 and CD71 are highly expressed on alloreactive T cells. (A) Kinetics of surface marker and cytokine expression in proliferating alloreactive T cells (n = 5). Normal donor PBMCs were cocultured with HLA-mismatched DCs, and the expression of surface markers and intracellular cytokines in the proliferating alloreactive T-cell population was determined by gating on the CFSEdim population using FACS analysis. Data shown are means ± SD. (B) CD71 is highly expressed in proliferating CD25− alloreactive T cells (n = 5). CFSE labeled T cells were cocultured with HLA-mismatched DCs, and the kinetics of expression of surface markers and intracellular cytokines in the proliferating alloreactive CD25− population were determined flow cytometrically by gating on the CFSEdimCD25− cells. Results are means ± SD.
CD25 and CD71 are highly expressed on alloreactive T cells. (A) Kinetics of surface marker and cytokine expression in proliferating alloreactive T cells (n = 5). Normal donor PBMCs were cocultured with HLA-mismatched DCs, and the expression of surface markers and intracellular cytokines in the proliferating alloreactive T-cell population was determined by gating on the CFSEdim population using FACS analysis. Data shown are means ± SD. (B) CD71 is highly expressed in proliferating CD25− alloreactive T cells (n = 5). CFSE labeled T cells were cocultured with HLA-mismatched DCs, and the kinetics of expression of surface markers and intracellular cytokines in the proliferating alloreactive CD25− population were determined flow cytometrically by gating on the CFSEdimCD25− cells. Results are means ± SD.
The other activation markers analyzed were only expressed on a minority of proliferating CFSEdim T cells. CD69 was expressed on only 4% plus or minus 5% of CFSEdim cells at day 3 of MLR. Because a CFSEdim population was only discernible from day 3 onward, it was not possible to determine whether the expression of CD69 on proliferating ATs at earlier time points. ICOS and CD95 expression progressively increased over time, but on a minority of proliferating ATs (mean, 15% ± 12% and 27% ± 24%, respectively, at day 7). OX40 and HLA-DR were only expressed in small subpopulations of proliferating ATs (mean, 1.6% and 8% of CFSEdim T cells at day 7). We observed strong expression of CCR7 (mean, 54% ± 20% on day 3) and CD45RA (mean, 25.9% on day 3) in the CFSEdim population, but also high expression on the unstimulated PBMCs at the same time points (mean, 52.7% and 12.3% of total T cells, respectively). As time progressed, the percentage of CFSEdim T cells expressing CCR7 and CD45RA declined, consistent with a progressive increase in effector memory T cells (mean, 62% ± 8% by day 7).
We next determined Th1 cytokine expression on proliferating CFSEdim T cells using intracellular cytokine staining. As shown in Figure 3A, IL-2 expression peaked on day 3 of coculture (mean, 34% ± 6% of CFSEdim T cells) and progressively decreased thereafter. IFN-γ expression in the CFSEdim population peaked on day 5 (mean, 33% ± 13%) and decreased by day 7. In contrast, there was little up-regulation of TNFα in ATs.
Thus, the majority of phenotypic markers analyzed appear to be expressed only in small subpopulations of ATs, limiting their usefulness as potential targets for allodepletion strategies. Only CD25 and CD71 were expressed in the majority of T cells proliferating in response to alloantigens. These data provide strong support for targeting CD25 in allodepletion strategies and identify CD71 as a promising novel target for similar approaches.
Characterization of CD25− alloreactive T cells
Significant populations of proliferating ATs (17% ± 8% at day 3 of coculture; Figure 3A) do not express CD25 and would be retained by strategies targeting this molecule alone. We therefore gated on the CFSEdim, CD25− T-cell population to determine phenotypic markers that could be used to target this population. Data on the expression of surface markers and cytokines by this population are shown in Figure 3B. Our studies identified CD71 and CD45RA as the markers expressed on the majority of CD25− proliferating ATs (mean, 69% ± 21% and 62% ± 12%, respectively, at day 3 of MLR). In 5 D-R pairs, a mean of 94%/93% of proliferating ATs could potentially be deleted by effective depletion of cells expressing either CD71/CD25 or CD45RA/CD25 (Table 1). Other surface markers including CD69, ICOS, OX40, CD95, HLA-DR, CCR7, and the cytokines IL-2, IFN-γ, and TNF-α were only expressed on a minority of cells in this population and thus would be of limited value in enhancing allodepletion achieved with CD25-based approaches.
Comparison of CD25 immunomagnetic beads and CD25 immunotoxin
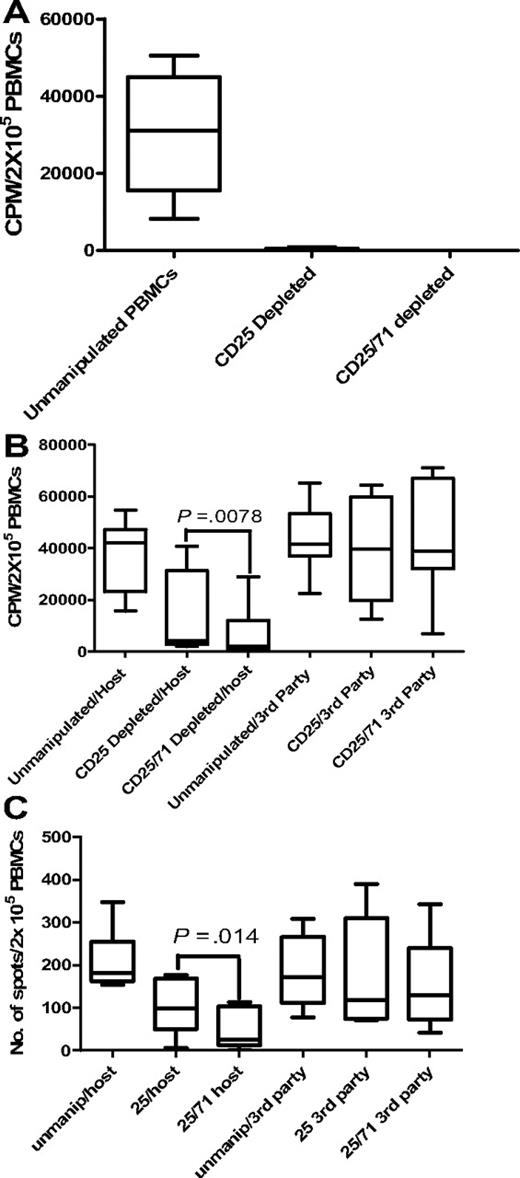
As a baseline, we initially compared residual alloreactivity after allodepletion using CD25 immunomagnetic beads or CD25 IT in 6 D-R pairs. Normal donor PBMCs were cocultured with HLA-mismatched LCLs and at day 3 of MLR, cultures were split and allodepletion was performed using anti-CD25 beads or IT. LCLs were used as stimulators in these and subsequent preclinical experiments based on our previous work.17 As shown in supplemental Figure 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article), there was no significant difference in residual alloreactivity to hosts assessed by 1° and 2° MLR and IFN-γ ELISPOT between the 2 methods. In view of this, we used anti-CD25 immunomagnetic bead depletion in further experiments to minimize cell manipulation in combined depletion methods.
Comparison of CD25 versus CD25/71 immunomagnetic depletion
Based on our phenotypic characterization of CD25 proliferating ATs, we then compared residual alloreactivity and third-party responses after CD25 versus combined CD25/71 immunomagnetic depletion to determine whether this enhanced the degree of selective allodepletion achieved with CD25-based methods. Normal donor PBMCs were cocultured with HLA-mismatched allogeneic LCLs in 8 D-R pairs, and at day 3 of MLR, cocultures were split into 2 arms and immunomagnetic negative selection for CD25+ or CD25+ and CD71+ cells was performed. As shown in Table 2, both CD25 and CD25/71 immunomagnetic depletions effectively deplete CD3+CD25+ T cells (mean, < 0.2% after both). However, there was a small CD3+CD71+ population remaining after CD25 allodepletion (mean, 0.62%), which was effectively removed with combined CD25/CD71 immunomagnetic depletion.
Aliquots of the 1° MLR cocultures were sampled to determine residual proliferative responses. Results were standardized by calculating the residual proliferation as: [cpm (donor PBMC + host LCL + allodepletion) − cpm (donor PBMC + allodepletion)]/[cpm (donor PBMC alone + LCL; no allodepletion) − cpm (donor PBMCs alone; no allodepletion)].
In 1° MLRs, no residual proliferation to host was detectable after CD25/71 depletion in any D-R pair tested (Figure 4A).
Allodepletion targeting CD25/71 is significantly better than CD25 alone. (A) Proliferative responses to host in primary MLR are undetectable after both CD25 beads and CD25/71 immunomagnetic allodepletion (n = 5). Residual proliferation after allodepletion with anti-CD25 beads or CD25/71 beads after stimulation of donor PBMCs with LCLs. Residual proliferation was calculated using the formula in “Comparison of CD25 versus CD25/71 immunomagnetic depletion.” The median residual proliferation for both CD25 beads and CD25/71 beads was 0%. Line = median, box = 25th-75th percentile, error bars = minimum, maximum values. (B) Enhanced depletion of secondary proliferative responses to host after CD25/71 allodepletion compared with CD25 depletion (n = 8). Rested allodepleted CD25 or CD25/71 PBMCs were restimulated with host or third-party LCLs in a 2° proliferation assay. CD25/71 allodepletion led to significantly reduced residual proliferation to host compared with CD25 alone (P < .01) without affecting third-party responses. (C) Residual alloreactivity to host is lower after CD25/71 allodepletion than CD25 in IFN-γ ELISPOT (n = 8). This figure shows the frequency of cells secreting IFN-γ as determined by ELISPOT assays. Rested allodepleted CD25 or CD25/71 PBMCs were restimulated with host or third-party LCLs in a 2° IFN-γ ELISPOT assay. CD25/71 allodepletion led to significantly reduced residual response to host compared with CD25 beads alone (P < .05) without affecting third-party responses.
Allodepletion targeting CD25/71 is significantly better than CD25 alone. (A) Proliferative responses to host in primary MLR are undetectable after both CD25 beads and CD25/71 immunomagnetic allodepletion (n = 5). Residual proliferation after allodepletion with anti-CD25 beads or CD25/71 beads after stimulation of donor PBMCs with LCLs. Residual proliferation was calculated using the formula in “Comparison of CD25 versus CD25/71 immunomagnetic depletion.” The median residual proliferation for both CD25 beads and CD25/71 beads was 0%. Line = median, box = 25th-75th percentile, error bars = minimum, maximum values. (B) Enhanced depletion of secondary proliferative responses to host after CD25/71 allodepletion compared with CD25 depletion (n = 8). Rested allodepleted CD25 or CD25/71 PBMCs were restimulated with host or third-party LCLs in a 2° proliferation assay. CD25/71 allodepletion led to significantly reduced residual proliferation to host compared with CD25 alone (P < .01) without affecting third-party responses. (C) Residual alloreactivity to host is lower after CD25/71 allodepletion than CD25 in IFN-γ ELISPOT (n = 8). This figure shows the frequency of cells secreting IFN-γ as determined by ELISPOT assays. Rested allodepleted CD25 or CD25/71 PBMCs were restimulated with host or third-party LCLs in a 2° IFN-γ ELISPOT assay. CD25/71 allodepletion led to significantly reduced residual response to host compared with CD25 beads alone (P < .05) without affecting third-party responses.
However, our previous data indicated that this assay may significantly underestimate residual alloreactivity. To skew our experimental system to detect this, ADTs were rested for 2 days and then restimulated with original host/third-party LCLs in IFN-γ ELISPOT and 2° MLR assays. Results were compared with the response observed with thawed unmanipulated PBMCs from the same donor. In 2° MLRs (Figure 4B), the median residual proliferation in response to host LCLs after combined CD25/71 depletion was significantly lower (4.8% of response of unmanipulated PBMCs; range, 0.98%-61.3%) than with anti-CD25 beads alone (9.9%; range, 5.5%-76.5%; P < .01). Responses to third-party LCLs were equivalent to unmanipulated PBMCs with both methods (median, 92.8% response of unmanipulated PBMCs for CD25/71 vs 95.01% for CD25 beads alone). Likewise, in the IFN-γ ELISPOT assay (Figure 4C), the median response of ADTs to host LCLs was >3-fold lower with combined CD25/71 allodepletion (14.1% of response of unmanipulated PBMCs; range, 0%-51.1%) than with anti-CD25 beads alone (54.6%; range, 0.04%-111.3%; P < .05). Again, third-party responses were maintained compared with unmanipulated PBMCs for both methods (median third-party response for CD25 beads, 69%; for CD25/71 beads, 76% of response of unmanipulated PBMCs). Thus, in 2 assays measuring distinct phenotypes, combined CD25/71 depletion led to significantly enhanced and more consistent allodepletion than did CD25 alone.
We also compared combined CD25/71 with CD25/45RA allodepletion, using negative selection for either CD25+ and CD71+ cells or CD25+ and CD45RA+ cells with immunomagnetic beads (n = 5). CD25/71 depletion gave nonsignificantly lower residual alloreactivity in 2° MLRs and IFN-γ ELISPOT assays compared with CD25/54RA (supplemental Table S1). Given that CD45RA is also expressed in a higher proportion of unstimulated T cells than CD71 (so that CD45RA depletion would result in a greater loss of bystander T cells), the CD25/71 combination was selected for further studies.
Antiviral responses are preserved but Tregs are depleted after CD25/71 allodepletion
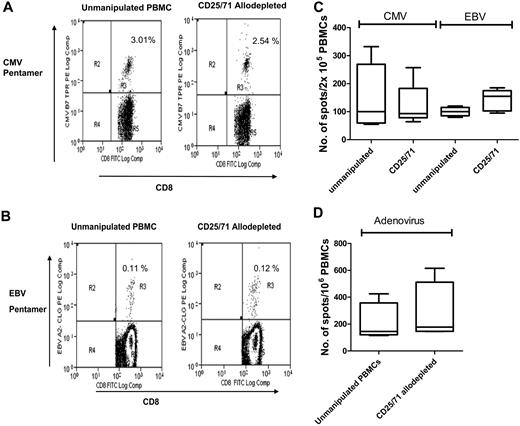
To determine the specificity of CD25/71 allodepletion, we then studied whether antiviral T-cell responses were retained. PBMCs from CMV+ or EBV+ donors known to have significant populations of virus-specific CD8+ cells detectable by pentamers were cocultured with HLA-mismatched LCLs for 3 days and then negatively selected for CD25/71 as above. As shown in Table 3 and the representative FACS plot in Figure 5A, in 4 donors there was no significant difference in the frequency of CMV-pp65–specific CD8+ T cells in ADT cultures compared with unmanipulated PBMCs from the same donors (median, 2.5% vs 3.3%, respectively). Similarly (Table 4 and representative FACS plot in Figure 5B), the frequency of EBV-specific pentamer-positive T cells in CD25/71 ADTs was equivalent to unmanipulated PBMCs (median, 0.34% vs 0.37%, respectively). These results suggest that virus-specific T cells are retained after allodepletion. To study the functionality of antiviral T cells, we then performed IFN-γ ELISPOT analyses to determine the frequency of CMV, EBV, and adenovirus-specific T cells before and after allodepletion. Unmanipulated PBMCs or CD25/71 ADTs from the same seropositive donors were stimulated with irradiated autologous PBMCs pulsed with a CMV pp65 peptide mix or transduced with an adenoviral vector (Ad5f35GFP) or autologous EBV LCLs. As shown in Figure 5C and D, the frequency of cells secreting IFN-γ in response to CMV, EBV, or adenoviral antigens in ADT cocultures and unmanipulated PBMCs was similar, implying that CD25/71 allodepletion does not affect antiviral responses.
Antiviral responses are preserved following CD25/71 allodepletion. (A) CMV-specific CD8+ T cells are preserved after CD25/71 allodepletion. The figure shows a representative FACS analysis from 1 of 4 D-R pairs demonstrating staining of either unmanipulated PBMCs (left) or CD25/71 allodepleted cells (right) in a HLA-A2–positive, CMV-seropositive donor with a HLA-A2–CMV pp65 pentamer (top right quadrants). The percentages of pentamer-positive cells as a proportion of CD8+ cells with isotype subtracted are shown. (B) EBV-specific CD8+ T cells are retained after CD25/71 allodepletion. Representative FACS analysis from 1 of 4 D-R pairs demonstrating staining of either unmanipulated PBMCs (left) or CD25/71 allodepleted cells (right) in a HLA-A2–positive, EBV-seropositive donor with a HLA-A2–CLG pentamer (top right quadrants). The percentages of pentamer-positive cells as a proportion of CD8+ cells with isotype subtracted are shown. (C) Functional T-cell responses to CMV and EBV are preserved after CD25/71 allodepletion (n = 5). The frequency of cells secreting IFN-γ in response to stimulation with irradiated autologous PBMCs pulsed with a peptide mix from CMV pp65 or autologous EBV LCL was determined by ELISPOT assays. Unmanipulated PBMCs or CD25/71 allodepleted T cells from the same seropositive donors were compared. Line = median, box = 25th-75th centile, error bars = minimum, maximum. (D) T cells responses to adenovirus are preserved after CD25/71 allodepletion (n = 4). The frequency of cells secreting IFN-γ in response to stimulation with irradiated autologous PBMCs transduced with an adenoviral vector (Ad5f35-GFP) was determined by ELISPOT assay. Unmanipulated or CD25/71 allodepleted T cells from the same seropositive donors were compared.
Antiviral responses are preserved following CD25/71 allodepletion. (A) CMV-specific CD8+ T cells are preserved after CD25/71 allodepletion. The figure shows a representative FACS analysis from 1 of 4 D-R pairs demonstrating staining of either unmanipulated PBMCs (left) or CD25/71 allodepleted cells (right) in a HLA-A2–positive, CMV-seropositive donor with a HLA-A2–CMV pp65 pentamer (top right quadrants). The percentages of pentamer-positive cells as a proportion of CD8+ cells with isotype subtracted are shown. (B) EBV-specific CD8+ T cells are retained after CD25/71 allodepletion. Representative FACS analysis from 1 of 4 D-R pairs demonstrating staining of either unmanipulated PBMCs (left) or CD25/71 allodepleted cells (right) in a HLA-A2–positive, EBV-seropositive donor with a HLA-A2–CLG pentamer (top right quadrants). The percentages of pentamer-positive cells as a proportion of CD8+ cells with isotype subtracted are shown. (C) Functional T-cell responses to CMV and EBV are preserved after CD25/71 allodepletion (n = 5). The frequency of cells secreting IFN-γ in response to stimulation with irradiated autologous PBMCs pulsed with a peptide mix from CMV pp65 or autologous EBV LCL was determined by ELISPOT assays. Unmanipulated PBMCs or CD25/71 allodepleted T cells from the same seropositive donors were compared. Line = median, box = 25th-75th centile, error bars = minimum, maximum. (D) T cells responses to adenovirus are preserved after CD25/71 allodepletion (n = 4). The frequency of cells secreting IFN-γ in response to stimulation with irradiated autologous PBMCs transduced with an adenoviral vector (Ad5f35-GFP) was determined by ELISPOT assay. Unmanipulated or CD25/71 allodepleted T cells from the same seropositive donors were compared.
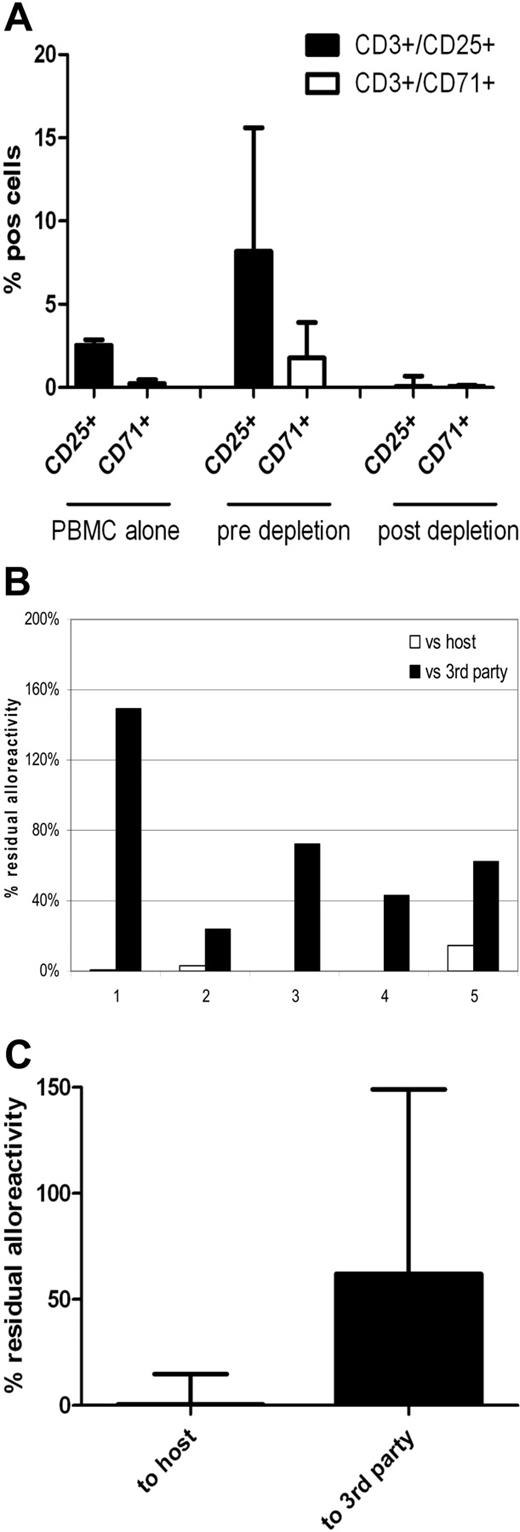
To determine the effect of CD25/71 allodepletion on Tregs, we performed flow cytometry after intracytoplasmic staining with FoxP3. As shown in supplemental Figure 2, Tregs expand during coculture with HLA-mismatched stimulators but are deleted by CD25/71 selection.
We next examined if CD25/71 allodepletion had differential effects on the naive and memory subsets. As shown in supplemental Figure 3, there was no change in the percentage of naive, central, and effector memory T-cell subsets during the period of coculture, whereas the frequency of terminally differentiated (CD45RA+CD62L−) cells increased slightly during coculture. This increase corresponded with a nonsignificant decrease in central memory (CD45RA−/CD62L+) T cells, consistent with maturation of alloreactive T cells in the central memory compartment. CD25/71 allodepletion resulted in a slight but significant reduction in the proportion of central memory T cells.
Enhancement of antileukemic activity of allodepleted PBMCs
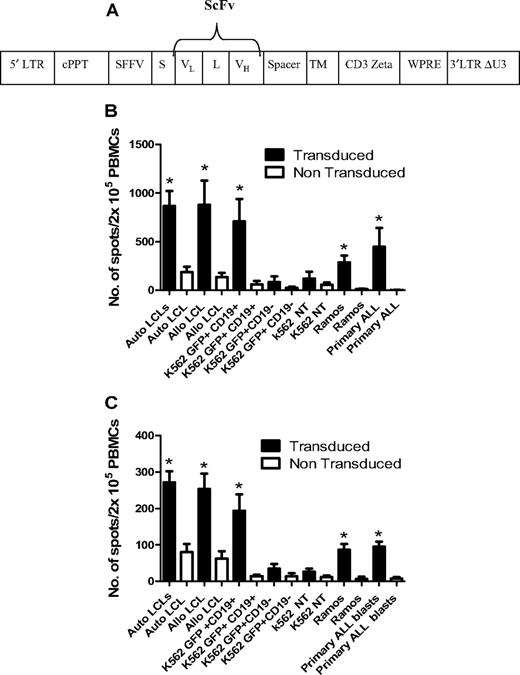
To enhance the antileukemic activity of ADTs against B-cell malignancies, CD25/71 ADTs from 6 D-R pairs were transduced with a lentiviral vector carrying a chimeric TCR recognizing CD19 (anti-CD19ζ TCR) using a method that preserves phenotype and antiviral responses.28,29 The mean transduction efficiency was 19.6 ± 6.4%. Transduced CD25/71 ADTs showed significantly enhanced IFN-γ (Figure 6B) and granzyme B (Figure 6C) secretion after stimulation with CD19+ tumor cell lines (K562-CD19 and Ramos, both P < .05), autologous and allogeneic LCLs (P < .05), and 1° blasts from patients with ALL (P < .05) compared with mock-transduced CD25/71 in ELISPOT assays. Transduced CD25/71 ADTs showed no significant IFN-γ/granzyme B secretion to CD19− targets (K562 or K562-GFP).
Transduction of CD25/71 allodepleted T cells with anti-CD19ζ TCR leads to efficient cytokine release in response to CD19 targets. (A) Schematic of the CD19R/pHR –SIN-SE. This consists of a self-inactivated (SIN) lentiviral construct, with a HIV central polypurine tract (cPPT), woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), and a spleen focus-forming virus (SFFV) promoter. The CD19R transgene consists of a human immunoglobulin leader sequence (S), the variable domains of the CD19-specific murine monoclonal antibody FMC-63 assembled as a single-chain variable fragment (ScFv; VL and VH), connected by a linker (L), in frame with a sequence encoding the human IgG1 hinge and CH2-CH3 domain (Spacer), the human CD28 transmembrane domain (TM), and the cytoplasmic signaling domain of the human CD3ζ. (B) Anti-CD19ζ TCR-transduced CD25/71 allodepleted PBMCs demonstrate significantly enhanced IFNγ secretion to CD19+ targets (n = 6). CD19R-transduced or mock-transduced CD25/71 allodepleted PBMCs were cultured with CD19+/− targets in an IFNγ ELISPOT assay. Transduced allodepleted PBMCs showed significantly enhanced IFNγ secretion to autologous and allogeneic LCLs, K562 cells stably transduced with a GFP-CD19+ transgene, Ramos, and 1° ALL blasts (*P < .05) compared with mock-transduced cells (mean ± SEM). (C) Anti-CD19ζ TCR-transduced CD25/71 allodepleted PBMCs demonstrate significantly enhanced granzyme B secretion to CD19+ targets (n = 6). CD19R-transduced or mock-transduced CD25/71 allodepleted PBMCs were cultured with CD19+/− targets in a granzyme B ELISPOT assay. Transduced allodepleted PBMCs showed significantly enhanced granzyme B secretion to autologous and allogeneic LCLs, K562 cells stably transduced with a GFP-CD19+ transgene, Ramos, and 1° ALL blasts (*P < .05) compared with mock-transduced cells (mean ± SEM).
Transduction of CD25/71 allodepleted T cells with anti-CD19ζ TCR leads to efficient cytokine release in response to CD19 targets. (A) Schematic of the CD19R/pHR –SIN-SE. This consists of a self-inactivated (SIN) lentiviral construct, with a HIV central polypurine tract (cPPT), woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), and a spleen focus-forming virus (SFFV) promoter. The CD19R transgene consists of a human immunoglobulin leader sequence (S), the variable domains of the CD19-specific murine monoclonal antibody FMC-63 assembled as a single-chain variable fragment (ScFv; VL and VH), connected by a linker (L), in frame with a sequence encoding the human IgG1 hinge and CH2-CH3 domain (Spacer), the human CD28 transmembrane domain (TM), and the cytoplasmic signaling domain of the human CD3ζ. (B) Anti-CD19ζ TCR-transduced CD25/71 allodepleted PBMCs demonstrate significantly enhanced IFNγ secretion to CD19+ targets (n = 6). CD19R-transduced or mock-transduced CD25/71 allodepleted PBMCs were cultured with CD19+/− targets in an IFNγ ELISPOT assay. Transduced allodepleted PBMCs showed significantly enhanced IFNγ secretion to autologous and allogeneic LCLs, K562 cells stably transduced with a GFP-CD19+ transgene, Ramos, and 1° ALL blasts (*P < .05) compared with mock-transduced cells (mean ± SEM). (C) Anti-CD19ζ TCR-transduced CD25/71 allodepleted PBMCs demonstrate significantly enhanced granzyme B secretion to CD19+ targets (n = 6). CD19R-transduced or mock-transduced CD25/71 allodepleted PBMCs were cultured with CD19+/− targets in a granzyme B ELISPOT assay. Transduced allodepleted PBMCs showed significantly enhanced granzyme B secretion to autologous and allogeneic LCLs, K562 cells stably transduced with a GFP-CD19+ transgene, Ramos, and 1° ALL blasts (*P < .05) compared with mock-transduced cells (mean ± SEM).
Optimization of CD25/71 allodepletion for clinical application
As a prelude to further clinical studies, we then optimized CD25/71 allodepletion for clinical scale use under GMP conditions using the CliniMACS system (Miltenyi Biotec). We initially compared irradiated LCLs (R:S ratio of 40:1) and DCs (R:S ratio of 10:1) from the same donors as stimulators in the coculture. While both stimulators gave similar up-regulation of CD25/CD71 and preclinical experiments showed equivalent depletion of antihost alloreactivity (data not shown), when GMP allodepletion was performed using the CliniMACS system, DCs gave reproducibly low levels of residual alloreactivity with preservation of third-party responses as assessed in delayed 2° MLRs, whereas LCLs gave inconsistent allodepletion. Because responses to EBV antigens presented by shared HLA molecules are preserved after allodepletion when DCs are used as stimulators, it was not possible to assess residual alloreactivity in IFN-γ ELISPOTs. In view of these data, in our subsequent protocol DCs were used as stimulators. We then optimized the coculture conditions for efficient allodepletion. As shown in supplemental Figure 4, the up-regulation of CD25 and CD71 in HLA-mismatched PBMC/DC cocultures from the same donor-recipient pairs was significantly higher when T-175 flasks were used than either cell differentiation bags (Miltenyi Biotec) or GP500 bioreactors (Wilson-Wolf). Because efficient up-regulation of activation markers on ATs is critical to effective allodepletion, we used T-175 flasks for coculture in our subsequent protocol.
Since both clinical grade anti-CD25 antibodies and anti-CD25 beads are available, we next compared immunomagnetic depletion using primary staining with anti-CD71 biotin followed by depletion with anti-CD25 beads and anti-biotin beads versus primary staining with anti-CD25 biotin plus anti-CD71 biotin together followed by negative selection with anti-biotin beads in 3 D-R pairs under GMP conditions using the CliniMACS system. For these experiments, a clinical grade anti-CD25, daclizumab (Roche) was biotinylated under GMP conditions using the Miltenyi Biotec CliniMACS biotinylation kit. As can be seen in supplemental Figure 5, both methods gave equivalent viability, yield, residual CD3+/25+ and CD3+/71+, and effective selective depletion of proliferative responses to host but not third-party PBMCs. To simplify the depletion procedure and reduce costs, primary staining with anti-CD25 biotin plus anti-CD71 biotin followed by negative selection with antibiotin beads was therefore used for our clinical-scale protocol. Finally, we compared CD25/71 immunomagnetic depletion using standard tubing set vs depletion tubing set for the CliniMACS system, which gave equivalent yields and median residual percentage CD3+/25+ (0.03% for both) and percentage CD3+/71+ (0.01% and 0.02%, respectively) in the allodepleted product (n = 3). In view of the higher cost of depletion tubing sets, standard tubing sets were used in our final protocol.
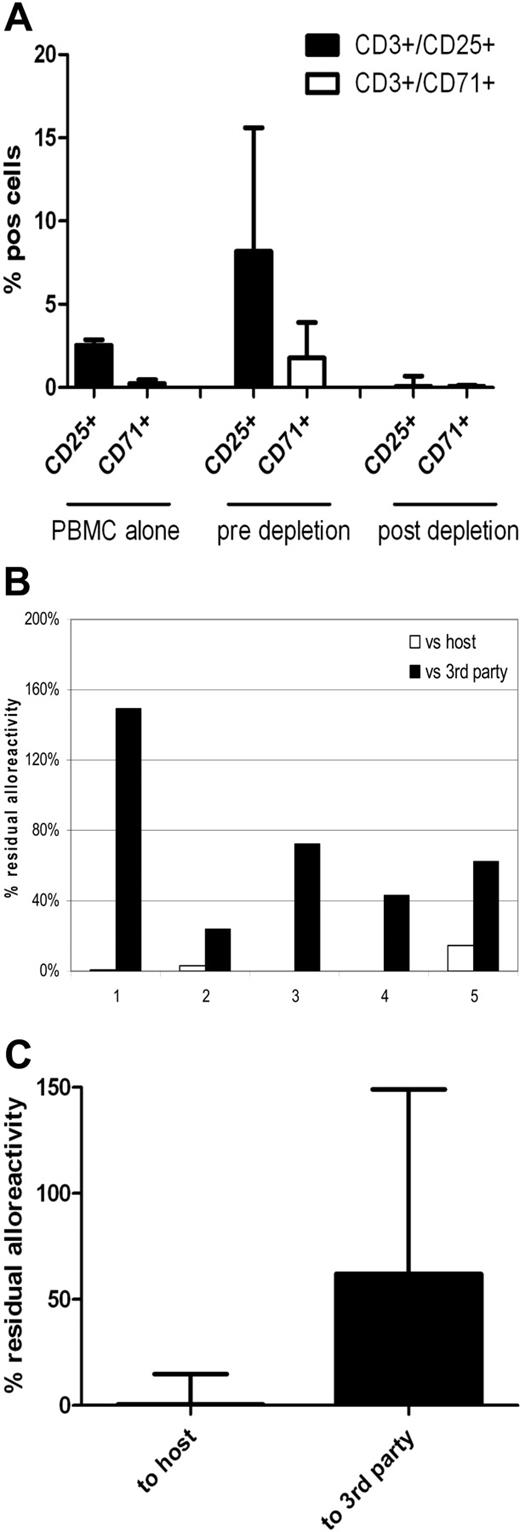
Based on these scale-up data, we designed a clinically applicable methodology for CD25/71 allodepletion using HLA-mismatched DCs to stimulate PBMCs from normal donor buffy coats, using T-175 flasks for coculture and depletion using staining with anti-CD25 biotin plus anti-CD71 biotin followed by negative selection with clinical grade anti-biotin beads on the CliniMACS system with TS tubing sets. As shown in Figure 7, allodepletion with this protocol performed under GMP conditions in 5 HLA-mismatched D-R pairs resulted in highly efficient and consistent depletion of CD3+/25+ and CD3+/71+ cells in the allodepleted fraction (median, 0.05%; range, 0.02-0.69% and 0.05%; range, 0-0.14%, respectively). Allodepleted T cells showed excellent viability (median, > 97% 7-aminoactinomycin D−) and the median yield from a single 500-mL blood draw was 7.6 × 106 allodepleted T cells. As can be seen in Figure 7B-C, in 2° MLRs this optimized protocol resulted in highly effective depletion of proliferative responses against host PBMCs (median residual alloreactivity, 0.39% of the response of unmanipulated donor PBMCs; range, 0.15-14.9%) with preservation of responses to third party (median, 62%; range, 24%-149%).
Clinical grade CD25/71 depletion of alloreactive T cells under GMP conditions using the CliniMACS system results in a 2-log reduction of alloreactivity (n = 5). PBMCs were cocultured with HLA-mismatched matured DCs (R:S ratio of 10:1) for 4 days in T-175 flasks. Cells were stained using biotinylated CD25 and CD71 antibodies followed by depletion using antibiotin immunomagnetic beads. Depletion was performed using standard CliniMACS tubing set and the CliniMACS system. (A) Flow cytometric analysis showing up-regulation of CD3+/25+ cells from a median of 2.55% to 8.17% and CD3+/71+ cells from 0.24% (0.07%-0.47%) to 1.77% (1.18%-3.9%) after 4 days of coculture and effective depletion of activated cells after allodepletion (median CD3+/25+, 0.05%; range, 0.02%-0.69%; median CD3+/71+, 0.05%; range, 0%-0.14%). (B) Residual alloreactivity of allodepleted cells in delayed 2° MLR. Individual data on all 5 depletions showing residual proliferative responses to host (□) and third-party (■) PBMCs are shown. (C) Cumulative data on residual alloreactivity in 2° MLR. Allodepleted T cells showed a median proliferative response to host PBMCs of 0.39% ± 0.063% compared with unmanipulated PBMCs with preservation of reactivity to third-party PBMCs (62% ± 0.478%).
Clinical grade CD25/71 depletion of alloreactive T cells under GMP conditions using the CliniMACS system results in a 2-log reduction of alloreactivity (n = 5). PBMCs were cocultured with HLA-mismatched matured DCs (R:S ratio of 10:1) for 4 days in T-175 flasks. Cells were stained using biotinylated CD25 and CD71 antibodies followed by depletion using antibiotin immunomagnetic beads. Depletion was performed using standard CliniMACS tubing set and the CliniMACS system. (A) Flow cytometric analysis showing up-regulation of CD3+/25+ cells from a median of 2.55% to 8.17% and CD3+/71+ cells from 0.24% (0.07%-0.47%) to 1.77% (1.18%-3.9%) after 4 days of coculture and effective depletion of activated cells after allodepletion (median CD3+/25+, 0.05%; range, 0.02%-0.69%; median CD3+/71+, 0.05%; range, 0%-0.14%). (B) Residual alloreactivity of allodepleted cells in delayed 2° MLR. Individual data on all 5 depletions showing residual proliferative responses to host (□) and third-party (■) PBMCs are shown. (C) Cumulative data on residual alloreactivity in 2° MLR. Allodepleted T cells showed a median proliferative response to host PBMCs of 0.39% ± 0.063% compared with unmanipulated PBMCs with preservation of reactivity to third-party PBMCs (62% ± 0.478%).
Discussion
Adoptive immunotherapy with CD25 IT ADTs improves T-cell immunity after HLA-mismatched SCT,18,20 but with transferred cell doses of 104/kg to 6 × 105/kg, leukemic relapse and infection by pathogens evoking low-frequency T-cell responses remain problematic. Given that some patients who received ADTs even at these doses developed GVHD, enhanced depletion of alloreactivity will be required to infuse larger doses of ADTs in this setting.
To refine our allodepletion strategy, we systematically characterized the activation and cytokine marker profile in proliferating ATs because proliferation in response to alloantigen is the most fundamental phenotype of ATs. Our data showed that CD25 and CD71 were expressed in the majority (> 80% and 65%) of proliferating ATs. In contrast, the other markers tested were expressed only in a minority of proliferating ATs, indicating that these are poor targets for allodepletion. CD69 is a well-established T-cell activation marker but was poorly expressed in the CFSEdim population. This may in part reflect down-regulation of CD69 by the time appreciable CFSE dye dilution has occurred, but CD69 showed more variable expression compared with CD25/CD71 and is up-regulated in bystander cells,5,30 limiting its usefulness as a target for allodepletion. Recent studies suggest that alloreactivity may reside predominantly in the CD45RA+ naive T-cell compartment.31,32 However, the expression of this marker on only on a minority of ATs and on bystander T cells, as well as the progressive maturation of ATs to a memory phenotype during MLR, suggests that targeting this molecule as a sole strategy for allodepletion is unlikely to be successful.
In contrast, our data provide strong support for targeting CD25 in allodepletion strategies. In 2 different assays we found no significant difference in residual alloreactivity to host between CD25 immunomagnetic and IT depletion. To reduce cell manipulation and in view of the regulatory constraints of using IT, we used CD25 beads in further studies. While CD25 depletion resulted in undetectable responses to hosts in 1° MLRs, significant residual alloreactivity was observed in 2° MLRs and ELISPOT assays. These residual responses are comparable with those seen by some investigators4,7,8,19 but are somewhat higher than those reported by others.3,9,10,14 However, comparing residual alloreactivity between studies is difficult because of differences in the methods used, including the wide confidence intervals for cytotoxic T-lymphocyte precursor and helper T-lymphocyte precursor assays9,10,14 and comparison of alloreactivity with donor PBMCs previously sensitized to the recipient rather than unmanipulated donor PBMCs.4,12,14 Furthermore, we have found that proliferative and cytokine responses to 2° stimulation with host antigen-presenting cells were highly dependent on the timing of 2° stimulation in relationhip to immunodepletion, with negligible responses if restimulation was done immediately after depletion.22 To skew our assays to optimize detection of residual antihost responses, in our assays cells were rested for 2 days after allodepletion before restimulation, allowing ATs that persist to proliferate and hence facilitating their detection.
While our data confirm CD25 as an excellent target for allodepletion strategies, a mean of 17% of proliferating ADTs do not express CD25. Therefore, we studied the phenotype of proliferating CD25−CFSEdim T cells. We identified CD71 as a novel target that is highly expressed on proliferating ATs. CD71 (transferrin receptor) is essential for iron transport into proliferating T cells but is not expressed on resting lymphocytes. We found that 70% of the CFSEdimCD25− population express CD71. Potentially, this enables us to target 2 independent phenotypes of ATs at the same time point within a single coculture. FACS analysis showed that immunomagnetic depletion deletes CD25+ and CD71+ cells to below background levels. In 1° MLRs, residual responses to the stimulator were undetectable after CD25/71 depletion. In 2 separate functional assays measuring distinct phenotypes, the combination of CD25/71 allodepletion led to significantly enhanced and more consistent allodepletion compared with CD25 alone without compromising third-party/antiviral responses. Our studies indicate that combined CD25/71 depletion results in a 1–2 log reduction of alloreactivity against host stimulators flow cytometrically, in 2° MLRs, and in IFN-γ ELISPOT assays. While extrapolation of these results to an in vivo setting requires an animal model,33,34 the fact that allodepletion is enhanced compared with CD25 depletion suggests that it may be possible to safely transfer larger doses of ADTs than hitherto possible using combined CD25/71 depletion. Potentially, this may confer protective immunity to pathogens that evoke low-frequency T-cell responses in the donor, such as adenovirus, which is the single most important cause of infectious death after haplo-SCT.35,36 Based on the precursor frequency of adenovirus-specific CTLs in normal donor PBMCs37 and limited clinical data from donor leukocyte infusion,20,38 we anticipate that cell doses of between 1 and 5 × 106/kg may be sufficient for such immunity.
The persistence of antileukemic responses after selective allodepletion is critical if the benefits of adoptive transfer are not to be offset by leukemic relapse. Our approach will deplete T-cell responses against the mismatched HLA alleles and minor histocompatibility antigens presented by the shared HLA alleles. While we17 have shown that T-cell responses to potential myeloid tumor antigens are preserved when LCLs are used as stimulators, this would not be predicted to be the case with our final methodology, which uses DCs as stimulators. To augment the antileukemic activity of ADTs, we investigated if lentiviral transfer of a chimeric TCR directed against CD19 could be used to redirect their specificity so that they recognize ALL and other B-lineage malignancies. Anti-CD19ζ TCR-transduced ADTs efficiently secreted IFN-γ and the cytolytic effector molecule granzyme B in response to CD19 tumor cell lines and primary ALL blasts. Potentially CD19-redirected ADTs could thus be used to augment both antiviral and antileukemic T-cell responses after haplo-SCT.
Chen et al39 have observed more rapid immune reconstitution after reduced intensity conditioning and CD3-negative selection of the graft compared with conventional intensity CD34-selected haplo-SCT. However, this was associated with a 36% incidence of significant acute GVHD, reflecting the increased residual T-cell dose infused. Furthermore, T-cell recovery was slower than that observed with our previous study of adoptive transfer of ADTs at 3 × 105/kg (median circulating T cells at 3 months post-SCT 350/μL vs 616 /μL) and there was no assessment of antiviral immunity. Thus, allodepletion offers a safer, more effective way of improving T-cell immunity after haplo-SCT.
As noted above, a plethora of potential methods for allodepletion now exist, but to date only CD25-based approaches and photodynamic purging13,14,40 have been used clinically, reflecting the technical challenges in translating such methodologies from bench to bedside. Our studies not only suggest that for strategies targeting surface markers/cytokines on ATs that CD25/71 is the optimal combination for effective allodepletion, but they also demonstrate that this methodology is robust and clinically applicable. We have established conditions for clinical-scale CD25/71 allodepletion under GMP conditions, resulting in a reproducible 2-log selective depletion of ATs with preservation of third-party responses. Our approach is serum-free, DC generation and negative selection are performed in a closed system, and the separation methodology used is already in widespread clinical use for CD34 selection in centers performing haplo-SCT. As such, this approach could be broadly applicable in many transplant centers. With the exception of anti-CD71biotin, all of the reagents used in the GMP runs (media, cytokines, anti-CD25 antibody, biotinylation reagent, and anti-biotin immunomagnetic beads) were approved for clinical use. We are currently generating a biotinylated anti-CD71 under GMP conditions for ex vivo use. The viability of the ADT product was consistently excellent and the median yield of ADTs in our GMP runs was 7.6 × 107 ADTs from a buffy coat derived from a 450-mL blood donation, so that this volume would be sufficient for addback of 106/kg CD25/71 ADTs in an adult recipient weighing 70 kg. While functional assays can provide useful comparisons of the efficiency of allodepletion, they cannot fully predict clinical outcome, and we therefore plan a further clinical study to determine whether immunotherapy with ADTs can safely augment immune reconstitution after T cell–depleted SCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
S.S. was a recipient of a training fellowship from the Medical Research Council of the United Kingdom, and C.M. was funded by the Leukemia Research Fund and Children with Leukemia. CD25 IT was a gift of Dr Ellen Vitetta (Cancer Immunobiology Center, Southwestern Medical Center, Dallas, TX).
Authorship
Contribution: S.S., C.M., M.P., N.N., H.K., J.B., and P.J.A. performed experiments and analyzed data; P.O., H.B.G., and P.V. helped define experimental strategy; P.J.A. defined the experimental strategy and supervised and interpreted the experiments; and S.S. wrote the manuscript with P.J.A.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Persis J. Amrolia, Department of Bone Marrow Transplantation, Great Ormond Street Hospital for Children NHS Trust, 70A Fellows Rd, London WC1N 3JH, United Kingdom; e-mail: AmrolP1@gosh.nhs.uk.
References
Author notes
S.S. and C.M. contributed equally to this work.