Abstract
The relative contribution of founder effects and natural selection to the observed distribution of human blood groups has been debated since blood group frequencies were shown to differ between populations almost a century ago. Advances in our understanding of the migration patterns of early humans from Africa to populate the rest of the world obtained through the use of Y chromosome and mtDNA markers do much to inform this debate. There are clear examples of protection against infectious diseases from inheritance of polymorphisms in genes encoding and regulating the expression of ABH and Lewis antigens in bodily secretions particularly in respect of Helicobacter pylori, norovirus, and cholera infections. However, available evidence suggests surviving malaria is the most significant selective force affecting the expression of blood groups. Red cells lacking or having altered forms of blood group-active molecules are commonly found in regions of the world in which malaria is endemic, notably the Fy(a−b−) phenotype and the S-s− phenotype in Africa and the Ge− and SAO phenotypes in South East Asia. Founder effects provide a more convincing explanation for the distribution of the D− phenotype and the occurrence of hemolytic disease of the fetus and newborn in Europe and Central Asia.
Introduction
Hirszfeld and Hirszfeld1 showed the frequencies of blood groups A and B differ between populations. Their observations raised fundamental questions regarding the causes of these differences, which were eloquently summarized by Mourant et al2(p1) :
Were the differences the result of random genetic drift and founder effects, in small populations which later multiplied and stabilized the original, fortuitous, frequencies, or were they the result of natural selection, arising from differences in fitness between the various blood groups, fitnesses which themselves depended upon locally determined features of the external environment?
Mourant et al concluded that “most workers now agree that both processes are operative, but their relative importance remains in question.”2
We now have detailed information concerning almost all the genes giving rise to blood group polymorphisms, the structure of the gene products and the antigens themselves, and in many cases functional information sufficient to delineate mechanisms of interaction with external agents.3-5 In addition, studies on the tracking of Y chromosome and mtDNA haplotypes in human populations provide us with unprecedented information concerning the significance of genetic drift and founder effects in determining the genetic background of different world populations.6 Given this new information, it seems an appropriate time to revisit these questions and ask whether we are any nearer understanding the relative importance of natural selection and founder effects in determining the distribution of human blood groups.
Infectious diseases and selection for ABO blood group antigens
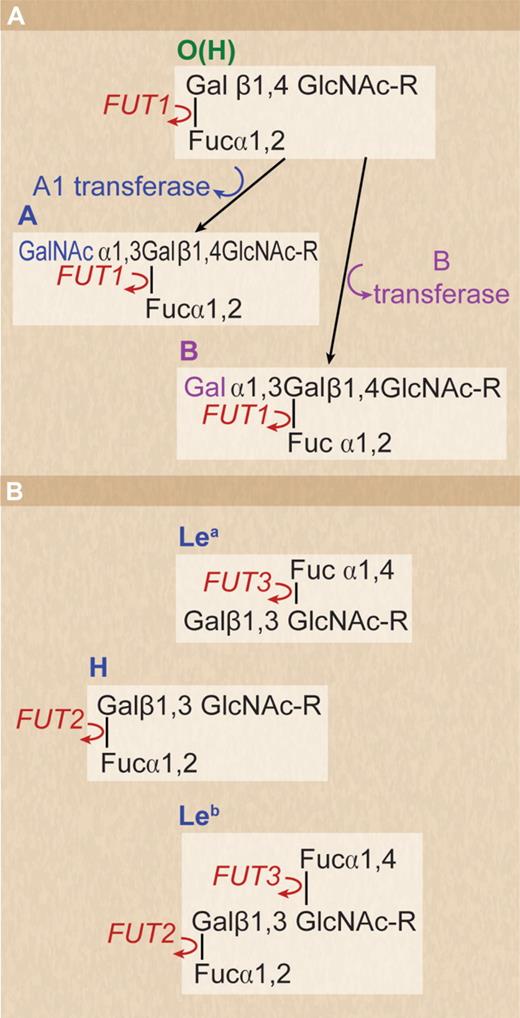
The molecular basis of the ABO blood group system was elucidated in 1990.7 The gene encodes a glycosyltransferase, which transfers N-acetyl D-galactosamine (group A) or D-galactose (group B) to the nonreducing ends of glycans on glycoproteins and glycolipids. The group O phenotype results from inactivation of the A1 glycosyltransferase gene, and the nonreducing ends of the corresponding glycans in group O subjects express the blood group H antigen (Figure 1A). The ABH antigens are not confined to red cells but are widely expressed in body fluids and tissues. The biologic significance of the A/B transferase has not been clearly demonstrated, but it would be expected that loss of this functional protein in group O patients would have some deleterious consequences for patients of this blood type.
Structure of ABO, H, and Lewis antigens. (A) Structure of ABO and H antigens on human red cells. H antigen formed by the action of FUT1 on oligosaccharide precursor chains in which the terminal D-galactose residue is linked to carbon 4 of the penultimate N-acetyl D-glucosamine residue (type II chain). (B) Structure of Le blood group antigens in bodily secretions. Secretor gene (FUT2) regulates the production of H antigen, which can be converted to A or B antigen if the corresponding active ABO glycosyltransferase is present. The ABH, Leb-active structures are formed on oligosaccharide precursor chains in which the terminal D-galactose residue is linked to carbon 3 of the penultimate N-acetyl D-glucosamine residue (type I chain) If FUT 2 is deficient the Lea active structure predominates.
Structure of ABO, H, and Lewis antigens. (A) Structure of ABO and H antigens on human red cells. H antigen formed by the action of FUT1 on oligosaccharide precursor chains in which the terminal D-galactose residue is linked to carbon 4 of the penultimate N-acetyl D-glucosamine residue (type II chain). (B) Structure of Le blood group antigens in bodily secretions. Secretor gene (FUT2) regulates the production of H antigen, which can be converted to A or B antigen if the corresponding active ABO glycosyltransferase is present. The ABH, Leb-active structures are formed on oligosaccharide precursor chains in which the terminal D-galactose residue is linked to carbon 3 of the penultimate N-acetyl D-glucosamine residue (type I chain) If FUT 2 is deficient the Lea active structure predominates.
One of the most significant disease associations described for non-O (subjects of group A, B, or AB) versus O subjects is susceptibility to arterial and venous thromboembolism (VTE).8,9 Non–group O patients have a greater risk of VTE than patients of group O and have greater levels of von Willebrand factor (vWF) and factor VIII.8,10 The risk of VTE is probably related to the level of vWF and factor VIII because patients of group A2 have lower levels of these proteins than A1, B, and AB and have a lower risk of VTE.9 A, B, and H blood group antigens are expressed on N-glycans of vWF and influence the half-life of the protein (10 hours for group O and 25 hours for non-O subjects), providing an explanation for the greater levels in non-O patients.11 These observations raise the possibility that a greater propensity for blood clot formation in non-O patients conferred a survival advantage to early humans. Such an argument has been made for the occurrence of the prothrombotic mutations factor V Leiden and prothrombin 20210G>A, which are commonly found in white humans dated as occurring 20 000 to 24 000 years ago toward the end of the last ice age.12 It is proposed that mutations like factor V Leiden lower the risk of hemorrhage and/or severe infections and thereby the risk of death during pregnancy.13 A similar hypothesis could explain the function of A and B antigens on vWF.
What then was the stimulus that caused the inactivation of this gene and the creation of the group O phenotype, which is so prevalent throughout the world? Evidence supporting the view that blood group O provides a selective advantage against severe malaria has been recently reviewed.14-16 The argument is persuasive. Group O is presumed to have arisen in Africa before the migration of early humans. Severe malaria results in the death of millions each year before they reach child-bearing age, and therefore selects survival genes.17 Experimental support for the hypothesis is provided by Fry et al18 and by Rowe et al.19 Rowe et al19 report reduced rosetting of Plasmodium falciparum isolates from group O Malian children compared with non-O blood groups. Parasitized red cells form rosettes with uninfected red cells and adhere to vascular endothelium, causing vasocclusion and severe disease.
There are other examples of infectious diseases in which the severity of infection can be directly linked to ABO phenotype. The authors of numerous studies have shown that once a person is infected with cholera (Vibrio cholerae strains O1 El Tor and O139) the phenotype group O confers a greater likelihood of severe infections than non-O blood group phenotypes.20 Glass et al21 suggest that the low prevalence of group O and high prevalence of group B in the Ganges Delta in Bangladesh is directly related to selective pressure from cholera. Almost all recent cholera pandemics have emanated from this region of the world.22 Patients of group O were more susceptible in an outbreak of gastrointestinal infections caused by Escherichia coli O157 in Scotland in 1996. A total of 87.5% of patients who died were group O.23
However, suggestions that smallpox selects against A, thereby explaining the high frequency of group A in Europe, and that the low frequency of O in ancient plague centers in Mongolia and the Middle East is also a reflection of selection are not supported by adequate data (Vogel et al [1960], cited in Mourant et al2(p18) ; Kreiger and Morton24 ). More recent studies have linked the high frequency of the HIV-1 resistance mutation CCR5Δ32 in Europe with protection against smallpox and the Black Death.25 This proposal has also been questioned.26 The mutations A→O and CCRΔ32 occurred much earlier in human evolution than the plague and smallpox epidemics of medieval times. As discussed previously, the A→O mutation was likely driven by malaria in Africa before the migration of early humans to Europe, and CCR Δ32 has been described in skeletons from the Bronze Age.25 A combination of selection against infectious diseases, such as plague and smallpox, and genetic drift and founder effects in small populations (resulting from migration patterns of early humans) may ultimately explain the allele frequencies observed today.
The expression of ABH antigens in tissues and body fluids other than blood cells is regulated by the secretor gene (FUT2), which encodes an alpha 1,2-fucosyltransferase capable of transferring L-fucose to carbon 2 of galactose (beta, 1-3) N-acetyl D-glucosamine–containing glycans. In the absence of an active FUT2 gene (nonsecretor), the structure created is the Lea antigen.27 The product of the Le gene is an alpha 1,3/4 fucosyltransferase (FUT3), which transfers L-fucose to carbon 4 of the penultimate N-acetyl-D-glucosamine residue of the same glycans.28 The structure created in tissues by the combined action of FUT2 and FUT3 is the Leb antigen. A and B antigens can only be formed in the tissues of patients with an active FUT2 by the action of alpha-glycosyltransferases capable of transferring N-acetyl D-galactosamine or D-galactose to carbon 3 of the same glycans (Figure 1B). The secretions and tissues of a person with an active FUT2 (a secretor) can express A, B, H, and Leb antigens in those secretions according to the glycosyltransferase genes inherited. In European and African nonsecretors, the homozygous inheritance of a nonsense mutation (G428A) inactivating FUT2 denoted se428 is frequently found (20% of Europeans).29 In the Far East and Pacific regions, the commonest mutation in FUT2 (A385T, se385) causes a single amino acid change (Ile129Phe) in the stem region of the fucosyltransferase, resulting in a 5-fold reduction in active enzyme and a weak Le(a+b+) phenotype.30 Sequencing FUT2 in 732 patients from 39 populations confirmed the widespread occurrence of the se428 allele in Europe, Central Asia, and Africa and the se385 allele in the Far East and Pacific and mapped 2 further se alleles with a more restricted distribution (se302 and se571) to Central and South Asia and Cambodia, respectively.31 Possession of homozygosity for a nonsecretor phenotype has a demonstrable survival advantage for some infectious diseases.
One of the first proven associations of a blood group polymorphism with disease was that between group O and peptic ulceration.32,33 The gastric pathogen H pylori is now known to be a causative agent leading to peptic ulceration and gastric cancer. According to Björkholm et al,34 H pylori has established colonies in the stomachs of approximately one-half the world's population.34 Early studies demonstrated that a South American strain of H pylori P466 bound to blood group O Leb but not ALeb structures on the gastric epithelium, thereby providing a clear explanation for the greater susceptibility of group O secretors.35 More recent studies on strains of H pylori from different parts of the world have shown that not all strains are so specific for O Leb, with many strains from outside South America having binding capabilities for ALeb in addition to OLeb. Nevertheless, these strains have a greater binding affinity for OLeb compared with ALeb (approximately 5-fold [median] greater).36 Sequence analysis of the bacterial surface molecule responsible for binding to gastric epithelium BabA (blood group antigen binding adhesin) from different strains of H pylori showed that Peruvian strains were closely related to Spanish but not to Asian strains, raising the intriguing possibility that the OLeb-specific strains found in South American may have arisen after European colonization of South America in the 16th century and represent adaptation to a population that is almost entirely of the blood group O phenotype.36
Susceptibility to norovirus infection is also closely linked to the expression of ABH and Le antigens in the gastrointestinal tract. Noroviruses are the commonest cause of acute gastroenteritis in humans and are estimated to account for 60% to 85% of all gastroenteritis outbreaks in developing countries.37 They are transmitted by consumption of contaminated food, particularly oysters, which appear able to concentrate the virus, or exposure to contaminated water.37 The pivotal role of secretor status in determining susceptibility to norovirus has been clearly demonstrated by Thorven et al,38 who compared susceptibility to gastroenteritis in patients and medical staff involved in hospital outbreaks in Sweden. The results demonstrated that only those patients homozygous for nonsecretor were protected from infection. Larsson et al39 further demonstrated significantly lower antibody titers to norovirus GGII in nonsecretors compared with secretors. There are many different strains of norovirus, however, and some strains bind to nonsecretor Lea structures and cause symptomatic infection.40,41 The variable specificity of different strains for ABH and Leb structures reported reflects a similar diversity to that of the aforementioned H pylori. Evidence for greater susceptibility of secretors to influenza viruses, rhinoviruses, respiratory syncytial virus, and echoviruses has also been presented.42 Reduced risk of HIV type 1 infection was found in Senegalese commercial sex workers with the nonsecretor type.43 Slow disease progression of HIV-1 in nonsecretors was also reported by Kindberg et al.44
Nonsecretors appear more susceptible to infections by Haemophilus influenzae,45 Neisseria meningitidis, and Streptococcus pneumoniae46 and urinary tract infection caused by E coli.47
A mutation (ΔF508) in the cystic fibrosis transmembrane regulator gene (CFTR) is common in European patients and was present in Europe during the Paleolithic period more than 10 000 years ago.48 The possibility that differences in A, B, and H antigen expression in the airway mucus might lead to differences in microbial binding and predispose to more severe lung disease was investigated in 808 patients homozygous for ΔF508. No association with ABO, Se, or Le genotype was observed.49
Rh blood groups and the origin of hemolytic disease of the fetus and newborn
The major clinical disease associated with the Rh blood group system is hemolytic disease of the fetus and newborn (HDFN). HDFN usually arises when a mother who is blood group D− carries a fetus who is blood group D+, and fetal red cells released into the maternal circulation immunize the mother to make antibody to D, which traverses the placenta and damages the fetus. Before the introduction of a successful prophylactic treatment in 1968, the frequency of the disease in England and North America was approximately 1 per 170 births.3 Recognition of the disease as a single entity was slow to emerge. In severe cases anti-D crosses the placenta and causes death of the fetus in utero, a condition known as hydrops fetalis. More commonly, disease occurs in the neonatal period, where severe and acute anemia and severe jaundice is fatal, a condition known as icterus gravis neonatorum. Roberts50 cites an account of Louyse Bourgeois, a midwife of Marie de Medici, who published in 1609 what is probably the earliest account of hydrops fetalis in one twin and neonatal jaundice in the other and credits Auden (1905) with several key observations relating to neonatal jaundice, in particular its appearance in successive children of the same parents. The recognition that hydrops fetalis and neonatal jaundice were manifestations of the same disease gradually emerged during the 1920s, and anti-D was shown to be the causative agent in 1939.51
There is now a formidable body of evidence to support the hypothesis that humans originated in Africa and to inform the timescale of various migrations from Africa, which have led to the world populations we have today.6 Simply by overlaying the known distribution of blood group frequencies on the world map of human migrations, the potential significance of genetic drift and founder effects is apparent. Wells6 argues, for example, that it is possible to account for all of the mtDNA and Y-chromosome types in Native Americans with a founding population of 10 to 20 people. Little wonder then that Native Americans are almost exclusively blood group O52 or that the Dia blood group polymorphism tracks the migration of humans from East Asia to the Americas.52 The occurrence of the Dia antigen in South East Poland also provides a measure of the extent to which the Mongol invasions penetrated Europe in more recent times.53,54
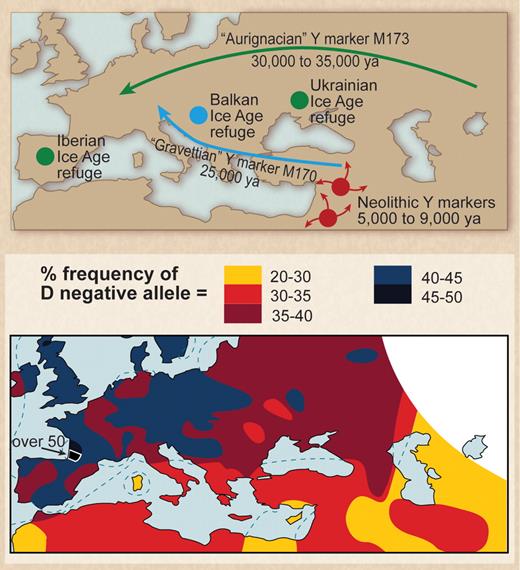
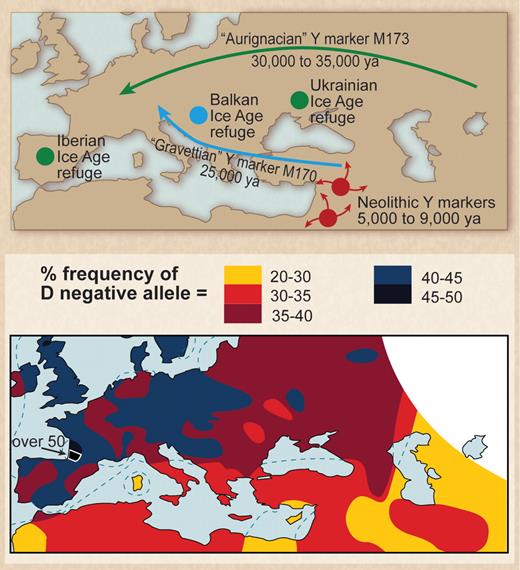
In Europe a similar founder effect can be invoked to explain the high frequency of the D− phenotype. The emergence of Paleolithic ancestors surviving the last ice age from refuges in the Basque region of Northern Spain and Southern France and the Ukraine 10 000 to 15 000 years ago and subsequent interbreeding of these survivors with Neolithic migrants from the Middle East provides an explanation for the occurrence of HDFN. To explain the high frequency of the D− allele in Europe, Mourant55 proposed a mixing of 2 populations, one essentially D− and the other D+. He noted that D− frequency was very high in the Basques and postulated the mixing of Paleolithic peoples from the Basque region with Neolithic migrants as the cause. This hypothesis has been largely ignored in the succeeding years, but recent observations made with the use of mtDNA and Y-chromosome markers have led to wide acceptance of the population-mixing hypothesis (Figure 2).52,56-58
Paleolithic settlers from the last glacial maximum may be the source of the high frequency of D− allele in Europeans. (Top) European location of Paleolithic refuges at the time of the last glacial maximum. Note migration of population containing marker M173 (from Gibbons58 ; reprinted with permission from American Association for the Advancement of Science). (Bottom) Distribution of the D− allele in Europe (from Mourant et al52 ; reprinted by permission of Oxford University Press).
Paleolithic settlers from the last glacial maximum may be the source of the high frequency of D− allele in Europeans. (Top) European location of Paleolithic refuges at the time of the last glacial maximum. Note migration of population containing marker M173 (from Gibbons58 ; reprinted with permission from American Association for the Advancement of Science). (Bottom) Distribution of the D− allele in Europe (from Mourant et al52 ; reprinted by permission of Oxford University Press).
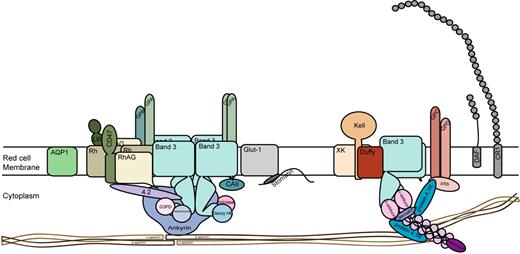
Tracking of haplotypes emerging from the Basque and Ukrainian refuges has shown that these populations migrated throughout Europe and Central Asia and into India and Pakistan.59 HDFN is found in all these regions. Mourant55 also suggested a link between the Basques and the Berbers of North Africa because of the high frequency of D− phenotypes among Berbers. This hypothesis is now supported by evidence from maternal DNA markers showing that ancestral Berbers occupied the Basque refuge area and migrated back into North Africa.60 In Western Europe, the D− phenotype results from a complete deletion of RHD.61 The molecular basis of D− phenotype has not been formally determined for Ukrainian D− people. Loss of RhD protein does not appear to be of significant detriment to red cell function. The best available structural models for RhD protein and its homologue RhCE protein indicate they do not function as transport proteins but rather serve to facilitate the assembly of the band 3 protein gas transport complex in the red cell membrane. These observations suggest there is considerable functional redundancy, with D and CE proteins effectively substituting for each other (Figure 3).62
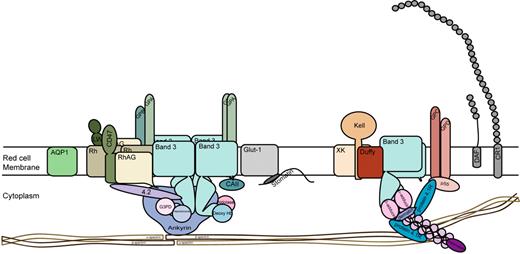
Structure of the human red cell membrane showing the major surface proteins and minor proteins Fy and CR1. Two major membrane complexes linked to the underlying red cell skeleton are depicted. The Band 3 complex containing glycophorins A (GPA) and B (GPB) and Rh proteins, Rh-associated protein (RhAG), CD47, LW glycoprotein (intercellular adhesion molecule–4), and the junctional complex comprising glycophorins C and D (GPC, GPD), Kell glycoprotein, XK glycoprotein, and Duffy (Fy) glycoprotein. Aquaporin 1 (AQP1), the glucose transporter (GLUT1), decay accelerating factor (DAF, CD55), and complement receptor 1 (CR1) are also shown. ABH active oligosaccharides known to be present on all major surface proteins except Rh proteins are not depicted.
Structure of the human red cell membrane showing the major surface proteins and minor proteins Fy and CR1. Two major membrane complexes linked to the underlying red cell skeleton are depicted. The Band 3 complex containing glycophorins A (GPA) and B (GPB) and Rh proteins, Rh-associated protein (RhAG), CD47, LW glycoprotein (intercellular adhesion molecule–4), and the junctional complex comprising glycophorins C and D (GPC, GPD), Kell glycoprotein, XK glycoprotein, and Duffy (Fy) glycoprotein. Aquaporin 1 (AQP1), the glucose transporter (GLUT1), decay accelerating factor (DAF, CD55), and complement receptor 1 (CR1) are also shown. ABH active oligosaccharides known to be present on all major surface proteins except Rh proteins are not depicted.
A counterargument to the population mixing hypothesis would be provided by a clear demonstration of selection for D− phenotype by environmental factors. In a thorough review of early studies seeking to identify associations between the D polymorphism and diseases, Mourant et al2 revealed no convincing associations. More recently, 2 studies have reported an association of the D polymorphism with disease. Busquets et al,63 in a study from Barcelona, reported an increased incidence of biliary complications in transplant recipients of livers mismatched for D. The presence of biliary complications in D-nonidentical graft-host cases (23 [30%] of 76) was greater than in D-identical grafts (47 [17%] of 269). Rh polypeptides are not expressed in liver,64 and therefore the mechanism of such an association is not clear. The fact that the study took place in the Basque region of Spain, where the D− phenotype is very common and may result from ancestral Paleolithic settlements, may be very relevant to the interpretation of these findings because it raises the possibility that other genes more relevant to transplantation and also occurring more commonly in Basques than in other populations may be influencing the results. In this context it is interesting to note evidence that donor human leukocyte antigen C (HLA-C) genotype has a profound impact on the outcome of liver transplants.65 HLA-C is the major inhibitory ligand for killer cell immunoglobulin-like receptors (KIRs). KIR genes are highly polymorphic and are expressed on natural killer cells and a subset of T lymphocytes.66,67 Several KIR genes (KIR2DS5, KIR3DS1, KIR2DL2) are significantly different in frequency in Basques, and 3 novel haplotypes were identified by Santin et al.68
Flegr et al69 in a study from the Czech Republic report an association of the polymorphism with Toxoplasma gondii infection whereby subjects (military conscripts) with the D− phenotype who were infected with T gondii (11 [6.08%] of 181) had slower reaction times and consequently were involved in more road traffic accidents than D+ patients infected with T gondii (17 [2.4%] of 709). Rh D protein is not reported to be expressed in brain; therefore, the likely mechanism of such an association is obscure, and given the small numbers of T gondii-infected patients involved in the study, a much larger cohort study will be required to prove the validity of this association.
Considering the evidence thus far, it appears most likely that the frequency of D+ and D− negative phenotypes in Europe and Central Asia is a reflection of genetic drift and migration rather than natural selection, with the early colonists of Europe emerging from Africa with a deletion of RHD (Figure 2). There remains the possibility the original stimulus driving this deletion occurred in Africa as a result of selection. The concomitant occurrence of the D− phenotype in African populations resulting from a different molecular mechanism70,71 may be suggestive of some ancient selective pressure.
Malaria: evidence for selection of blood group phenotypes that are rare outside areas in which malaria is endemic
It appears likely that the most devastating effects of malaria on human populations coincided with a change of lifestyle from hunter gatherer to more sedentary agricultural practices circa 10 000 years ago.15 The clearance of trees from forest areas created the potential for pools of stagnant water and breeding grounds for the mosquitoes carrying parasites.
The clearest examples of selection in the face of malaria are reflected in the widespread distribution of inherited anemias, particularly sickle cell anemia and alpha thalassemia and the occurrence of hemoglobin C in regions of the world where malaria is endemic.72,73 The mutation giving rise to sickle cell disease (SCD; HbS) may have arisen at 3 different sites in Africa (Atlantic West Africa, Central West Africa, and Bantu-speaking Central and Southern Africa) with expansion of the mutation occurring 2000 to 2500 years ago.74 In this case, patients who inherit an HbS gene from both parents have SCD, whereas those who are heterozygous inheriting the HbS gene from 1 parent and the normal HbA gene from the other parent have substantial protection against malaria. A similar protective effect for the heterozygote seems likely in South East Asia, where HbE is very common and red cells from patients of genotype HbAE are markedly less susceptible to malaria parasite invasion in vitro.75
Plasmodium vivax and the blood group Fy(a−b−) phenotype
Complete absence from red cells of the molecule carrying the Duffy blood group antigens (aka DARC) is found in almost 100% of West Africans, and this absence is clearly and unambiguously demonstrated to provide protection from P vivax.77 The molecular basis of this Duffy deficiency is a point mutation in the binding site for the transcription factor GATA-1.78 GATA-1 is a DNA-binding protein essential for erythropoiesis, and its failure to bind to the Duffy gene promoter means that the Duffy protein is absent from the red cells of affected subjects. In Africans the mutation occurs on a Duffy allele that would otherwise generate a Fy(b+) phenotype. The same GATA-1 mutation appears to have occurred on a second occasion in South East Asia, where it occurs on a Duffy allele that would otherwise generate a Fy(a+) phenotype.79 Another mutation creating weak expression of Duffy (Fyx) may also be relevant to malaria, but relevant population studies have not been reported.80 Recently, evidence for the emergence of P vivax strains capable of invading Fy(a−b−) red cells has emerged in South America and East Africa.81,82
The protective effect of the Fy(a−b−) phenotype against P vivax is clear and unambiguously established. Not so clear are any deleterious consequences of this mutation for the subjects expressing the phenotype. Duffy protein is expressed on endothelial cells in these subjects but not on red cells,83 so any attempt to understand the consequences of red cell Duffy deficiency must take account of the functional role of endothelial Duffy. The Duffy protein is a member of the 7 membrane-spanning chemokine receptor family (Figure 3) but unlike most chemokine receptors does not effect intracellular signaling through G proteins. It binds several proinflammatory chemokines of both the CXC and CC subfamilies but does not bind homeostatic chemokines.84 Recent evidence suggests Duffy protein on endothelial cells binds chemokines and facilitates leukocyte extravasation contributing to disease pathogenesis through inflammation.85 Evidence for up-regulation of Duffy expression in the vascular endothelium during infection and transplant rejection supports this view.86,87
The lack of Duffy on red cells in Fy(a−b−) patients alters the balance of proinflammatory chemokines in the body because the very large capacity of red cell binding is absent but the consequences of this change are presently unclear. Lee et al88 provide evidence that red cell and endothelial Duffy regulate the kinetics of chemokine bioavailability between the circulation and extravascular sites during inflammation. Clearly this regulation would be altered in Fy(a−b−) subjects. In a mouse model, inflammation induced by polycytidylic acid significantly enhanced alloimmunization to red cells.89 In this context it is interesting to note that patients with SCD are predominantly of the Fy(a−b−) phenotype and that the production of multiple red cell alloantibodies upon transfusion (usually with blood from white donors) is a frequent and significant problem encountered by employees of blood banks seeking to provide compatible blood for the patients (reviewed in Anstee90 ). SCD patients in sickle cell crisis and mouse models of human SCD have many indicators of an inflammatory response.91 These data suggest that the enhanced propensity for alloimmunization in SCD patients is related to inflammation and also pose the question as to the significance of Fy(a−b−) in this process. Are Fy(a−b−) SCD patients more likely to make alloantibodies in response to transfusion than SCD patients of normal Fy phenotype? Is there a link between regulation of proinflammatory chemokine availability by red cell Fy and the adaptive immune response?
The data of Afenyi-Annan et al92 provide evidence that SCD patients with the Fy(a−b−) phenotype are more susceptible to chronic organ damage and proteinuria than SCD patients of normal Fy phenotype and are consistent with such an hypothesis. Interpretation is likely also influenced by genetic differences of immune response and cytokine genes in African populations compared with other world populations,93,94 but the genetic backgrounds of SCD patients with normal and Fy(a−b−) phenotype may be sufficiently comparable to allow conclusions regarding alloimmunization and the role of Fy to be drawn. Should Fy(a−b−) subjects be more susceptible to alloimmunization, then the potential use anti-inflammatory therapies in the treatment of vaso-occlusion95,96 may have the added bonus of reducing rates of red cell alloimmunization and provide a much needed alternative approach to a major transfusion problem.
A further consequence of selection for the Fy(a−b−) phenotype in Africa may be to alter the kinetics of HIV-1 infection in those with this phenotype. Several HIV-1 strains bind to Duffy on normal red cells, facilitating the transfer of HIV-1 to its target cells (CD4+/CCR5+ T lymphocytes) with 5- to 12-fold greater efficiency than Fy(a−b−) red cells.97 He et al97 calculate that patients with the Fy(a−b−) phenotype have a 40% greater likelihood of acquiring HIV than those lacking the phenotype; however, the disease, once acquired, has a slower progression than in infected patients of normal Fy type. They conclude that these differences are related to loss of competition for binding HIV-1 between plasma chemokine CCR5 and Duffy on red cells in Fy(a−b−) subjects and consequent changes in the inflammatory state those infected. The findings of this study have been contested by Walley et al,98 who used different methodology to analyze a different cohort of HIV+ and HIV− African American subjects and found no association between Fy genotype and progression to AIDS or risk for HIV acquisition. They also point out that the number of HIV− patents used by Walley et al98 was much smaller (227 vs 814) and suggest this difference may be a major factor affecting the analysis.99
Different strains of P falciparum use different blood group proteins as receptors
The dual availability of in vitro culture systems to study the invasion of human red cells by P falciparum and well-characterized rare blood group phenotypes made it possible to identify red cell receptors used by different parasite strains. Early studies on cells lacking Glycophorin A (Ena− cells100 ) and glycophorin B (S-s− cells101 ) provided evidence that these sialic acid–rich red cell-surface glycoproteins were parasite receptors and these observations have been confirmed.102-105 Glycophorins C (GPC) and D (Ge− red cells) are also receptors for some strains of P falciparum.106-108 Glycophorins are major proteins at the red cell surface (Figure 3). Glycophorin A (GPA) and the major anion transport protein (AE1, Band 3) with approximately 106 copies/red cell are the most abundant red cell surface proteins with glycophorins B, C, and D together accounting for a further 450 000 copies per red cell.109,110
Perhaps surprisingly, there is little experimental evidence to suggest selection against the expression of GPA has occurred in response to P falciparum infection. Red cells from patients having the hybrid GPA-GPB protein Dantu, which is common in certain parts of Africa, are reported to resist invasion,111 and it has been suggested that elevated expression of Band 3 occurring in patients with the GPB-GPA-GPB MiIII protein common in South East Asia may be relevant to malaria survival.112 The importance of sialic acid on GPA in forming a receptor for P falciparum102 suggests that red cells expressing glycosylation variants of GPA found commonly in Africans in which N-acetyl D-glucosamine is present in some of the sialic acid–rich O-glycans at the N-terminus (patients with the M1 antigen113 ) may be relevant to one's susceptibility to malaria.
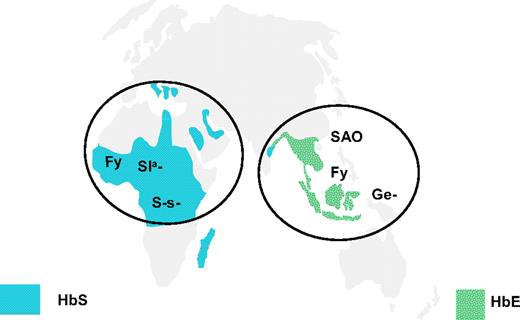
In contrast to the situation with GPA, subjects lacking glycophorin B are found in high frequency in central Africa.105 Patients with red cells lacking GPC and glycophorin D (Ge−, Leach phenotype) are very rare, but those with Ge− red cells having an altered GPC resulting from deletion of exon 3 of GYPC114,115 are common in Melanesians, most notably in Papua New Guinea, and the resulting phenotype provides protection against P falciparum (Figure 4).72,108 Clearly, different strains of P falciparum target glycophorins associated with one or other of the membrane complexes, providing key cytoskeletal linkages maintaining the stability of the red cell membrane (Figure 3) and selection, resulting in loss or alteration of glycophorins in either of these sites confers a survival advantage.
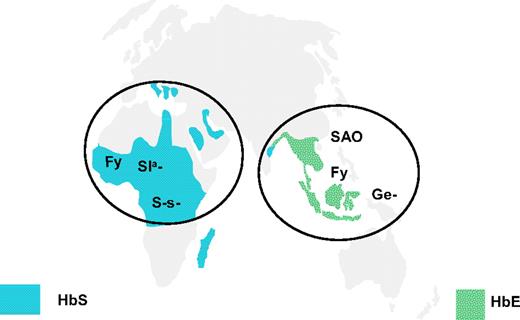
Distribution of rare blood group phenotypes selected by malaria in Africa and South East Asia. The location of rare blood group phenotypes lacking glycophorin B (S-s−), having altered glycophorin C (Ge−; Gerbich-negative), Fy (Duffy) blood group–null allele (Fy), Sl(a−) allele of complement receptor 1 (CR1), and the Band 3 mutation causing South East Asian ovalocytosis (SAO) in comparison with the distribution of HbS and HbE alleles.72
Distribution of rare blood group phenotypes selected by malaria in Africa and South East Asia. The location of rare blood group phenotypes lacking glycophorin B (S-s−), having altered glycophorin C (Ge−; Gerbich-negative), Fy (Duffy) blood group–null allele (Fy), Sl(a−) allele of complement receptor 1 (CR1), and the Band 3 mutation causing South East Asian ovalocytosis (SAO) in comparison with the distribution of HbS and HbE alleles.72
Melanesians also exhibit another example of selection, affording protection against cerebral malaria, a phenotype known as South East Asian ovalocytosis. South East Asian ovalocytosis cells, as the name implies, have an abnormal shape. They are also characterized by weakened expression of a large number of blood group antigens, including antigens found on Band 3, GPA, and the Rh blood group proteins.116 In this case selection favors the heterozygote. Heterozygotes inherit a normal Band 3 gene together with a mutant inactive Band 3 gene resulting from a deletion, causing a loss of 9 amino acids at the point at which the cytoplasmic N-terminal domain enters the cytosolic face of the lipid bilayer (reviewed in Bruce117 ). Homozygous inheritance of this mutation would result in total Band 3 deficiency. Because Band 3 is essential for respiration (Cl/HCO3 exchange) and for maintaining the integrity of the red cell membrane, it must be assumed in evolutionary terms that such an inheritance is incompatible with survival. Rare subjects with total Band 3 deficiency states have been described, but survival depends on extensive medical support, particularly in the neonatal period.118
Complement receptor 1 (CR1; Figure 3) carries antigens of the Knops blood group system. CR1 expression is very variable between patients and red cells expressing fewer than 100 copies CR1 per cell show reduced rosetting with P falciparum strain R29R, as do red cells expressing the Sla− blood group phenotype. The Sla− phenotype, which results from a single nucleotide polymorphism (R1601G) in long homologous repeat D, occurs in only 1% of the white population but reaches 70% in Malians.119-121
Conclusions
The significance of human blood groups can now be seen more clearly in the context of population movement, and the constant battle between humans and infectious disease. Evidence for selection by infectious diseases at the level of the ABO and secretor genes is persuasive but for other blood group antigens, founder effects appear more likely to account for the distribution of blood group polymorphisms except that is, in parts of the world in which malaria is endemic. Available data suggest survival from malaria has been the most significant selective force acting on the blood groups.
Rare blood group phenotypes revealed through compatibility testing in transfusion centers and blood banks throughout the world have provided powerful tools with which to investigate the mechanisms whereby malarial parasites invade human red cells. However, a comprehensive study of the distribution of known blood group polymorphisms in areas in which malaria is endemic has not been undertaken. Now that almost all the blood group genes have been cloned and the molecular bases of most antigens determined, it is feasible to conduct such a study with the use of high-throughput DNA-based methods.122-125 Furthermore, the availability of rapid DNA sequencing methodologies presages an era in which mass screening of genes encoding red cell membrane proteins could be used to identify new polymorphisms of relevance to malarial invasion. Studies of this type, focused in tropical Africa, South East Asia, and Latin America, would provide a valuable database of new information about blood group diversity in populations inhabiting these regions not only for malarial epidemiologists but also for those investigating human susceptibility to new emerging infectious diseases given that zoonoses from wildlife in these regions have been identified as the most significant growing threat to global health of all emerging infectious diseases.126
Acknowledgments
The author thanks Lesley Bruce of the Bristol Institute for Transfusion Science for preparing Figure 3, David Briggs of NHS Blood and Transplant for helpful discussions regarding HLA-C and KIR receptors, and Geoff Daniels of the Bristol Institute for Transfusion Science for critical reading of the manuscript.
The author's work is supported by Department of Health (England).
Authorship
Contribution: D.J.A. wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: David Anstee, Bristol Institute for Transfusion Sciences, NHS Blood and Transplant, Bristol, BS34 7QG United Kingdom; e-mail: david.anstee@nhsbt.nhs.uk.