Abstract
Minimal residual disease (MRD) at the end of remission-induction therapy predicts relapse in acute lymphoblastic leukemia (ALL). We examined the clinical significance of levels below the usual threshold value for MRD positivity (0.01%) in 455 children with B-lineage ALL, using polymerase chain reaction amplification of antigen-receptor genes capable of detecting at least 1 leukemic cell per 100 000 normal mononucleated cells (0.001%). Of the 455 clinical samples studied on day 46 of therapy, 139 (30.5%) had MRD 0.001% or more with 63 of these (45.3%) showing levels of 0.001% to less than 0.01%, whereas 316 (69.5%) had levels that were either less than 0.001% or undetectable. MRD measurements of 0.001% to less than 0.01% were not significantly related to presenting characteristics but were associated with a poorer leukemia cell clearance on day 19 of remission induction therapy. Patients with this low level of MRD had a 12.7% (± 5.1%; SE) cumulative risk of relapse at 5 years, compared with 5.0% (± 1.5%) for those with lower or undetectable MRD (P < .047). Thus, low levels of MRD (0.001%-< 0.01%) at the end of remission induction therapy have prognostic significance in childhood ALL, suggesting that patients with this finding should be monitored closely for adverse events.
Introduction
The clinical outcome in children with acute lymphoblastic leukemia (ALL) has improved steadily over the past 4 decades through a progressive refinement of treatment strategies.1,2 Central to more recent improvements has been the increased precision of risk-assignment systems.3,4 This advance owes much to the development of robust methods for monitoring minimal residual disease (MRD). The enhanced ability to determine the degree and rapidity of leukemia cell clearance affords a powerful predictor of subsequent relapse.5-12 Hence, MRD measurements have been incorporated into many contemporary treatment protocols,1,11 in which residual leukemia is typically assessed at serial time points. Measurements at the end of remission induction or shortly thereafter appear to be the most informative.13
The methods most commonly used for MRD measurement are flow cytometric detection of aberrant immunophenotypes and polymerase chain reaction (PCR) amplification of immunoglobulin (IG) and T-cell receptor (TCR) genes.13,14 The cutoff level used by most investigators to define MRD positivity is 0.01% of bone marrow mononuclear cells because this is typically the limit of detection for routine flow cytometric and molecular assays and has been shown to discriminate patients with different risks of relapse.13 Thus, patients with an MRD level of 0.01% or higher in bone marrow by flow cytometry at any treatment interval had a significantly higher risk of relapse in our earlier studies.6,8,15 Likewise, MRD more than or equal to 0.01% on day 29 by flow cytometry was the strongest prognostic indicator in studies of the Children's Oncology Group.12 In the AIEOP BFM ALL 2000 study, MRD negativity was defined by the lack of PCR signals in assays with at least 0.01% sensitivity.11
The clinical significance of MRD levels less than the 0.01% threshold in children with ALL is unclear. We used PCR amplification of IG and TCR genes to monitor MRD in 455 children with newly diagnosed ALL. A criterion for inclusion in the study was that the PCR assay be sufficiently sensitive to detect 1 leukemic cell among 100 000 normal mononucleated cells (0.001%). Here we report the prevalence of low levels of MRD at the end of remission induction therapy in children with B-lineage ALL, and their relation to presenting features, early treatment response, and leukemic relapse.
Methods
Patients
From September 1993 to October 2007, 539 patients with newly diagnosed B-lineage ALL were enrolled in 4 Total Therapy studies (13A, 13B, 14, and 15) at our institution and had initial PCR studies performed to develop a patient-specific MRD test. Diagnostic immunophenotyping, karyotyping, and genetic analysis were performed by standard techniques.16-18 Deletions of the IKZF1 (IKAROS) gene were identified by single nucleotide polymorphism arrays.19 All studies were approved by the St Jude Institutional Review Board, with informed consent obtained from the parents or guardians of each child, and assent from the patients as appropriate in accordance with the Declaration of Helsinki.
Patients received remission-induction and consolidation therapy, followed by risk-directed continuation treatment including reinduction, as previously described.1,20 The differences between Total 13A and Total 13B treatment included 1 additional dose of intravenous mercaptopurine in the upfront window therapy with methotrexate before the beginning of remission-induction therapy, and the use of dexamethasone instead of prednisone during continuation treatment in Total 13B. The overall treatments for patients enrolled in Total 13B and Total 14 were essentially identical, with the exception of the use of different dosages of methotrexate as consolidation therapy. Remission-induction therapy in Total 15 was identical to that of the preceding protocols during the first 3 weeks. However, patients with MRD 1% or more on day 19 received 3 extra doses of asparaginase, and the cyclophosphamide, cytarabine, and mercaptopurine combination replaced the 3 doses of etoposide and cytarabine given in the previous protocols. In the postremission phase, no epipodophyllotoxins were administered in Total 15. In all protocols, patients deemed to have very high-risk leukemia underwent allogeneic hematopoietic stem cell transplantation.
MRD studies
PCR studies of MRD were performed with IG and TCR gene rearrangements as targets, as previously described.21 DNA collected at diagnosis was screened using the BIOMED-2 primers for IGK@, deleting element (Kde) gene rearrangements (Vk-Kde, intron-Kde), complete and incomplete TRD@ [Vd-(Dd)-Jd1, Dd2-Jd1, Vd2-Dd3, Dd2-Dd3], TRG@ (Vg-Jg1.3/2.3, Vg-Jg1.1/2.1), and TRB@ [Vb-(Db)-Jb, Db-Jb] rearrangements.22 IGH@ rearrangements [VH-(DH)-JH, DH-JH] were identified with the consensus V-region primers FR-1C23 or FR-2B24 and 7 DH family primers in combination with 1 JH consensus primer.25 PCR products were analyzed on 3% agarose gels, and potential rearrangements were further examined by heteroduplex analysis on 6% polyacrylamide–1× Tris-boric acid–ethylenediaminetetraacetic acid gels to discriminate between products derived from monoclonal or polyclonal cell populations.26 Biclonal or biallelic PCR products were separated by DNA cloning or by cutting amplicons from the polyacrylamide gel. The junctional regions of monoclonal PCR products were sequenced in the St Jude Hartwell Center for Bioinformatics and Biotechnology using Big Dye Terminator (Version 3.1) Chemistry on an Applied Biosystems 3730XL DNA Analyzer (Applied Biosystems). Patient-specific junctional region sequences were identified with the IMGT (http://imgt.cines.fr) or IgBlast (www.ncbi.nlm.nih.gov/igblast) databases, and sequences commonly available. We then designed at least 1 allele-specific oligonucleotide primer complementary to the junctional region sequence of each target gene, manually or using the Primer Express software (Applied Biosystems). Primers were synthesized on an ABI 3900 DNA Synthesizer (Applied Biosystems) and purified by reverse-phase chromatography (OPC cartridges; Applied Biosystems).
Real-time PCR analysis was performed by a single PCR amplification with sequence-specific TaqMan hydrolysis probes on ABI PRISM Sequence Detection System 7700 (Applied Biosystems).21,27 We made 10-fold serial dilutions of diagnostic DNA in pooled peripheral blood DNA extracted from mononuclear cells of 4 to 6 healthy donors, analyzed each dilution in duplicate, with the exception of the single copy dilution, for which 4 aliquots were analyzed. The DNA extracted from samples obtained on day 46 of treatment was analyzed in triplicate at the 100 000 genome copy level (equivalent to 600 ng of DNA), and 3 to 10 aliquots were analyzed at 10 000 copies diluted into normal blood DNA to estimate levels of MRD. For the limiting-dilution method, we used either a 2-round (either seminested or with the same patient-specific and consensus primer in both rounds) or a 1-round PCR assay with an initial touchdown phase.21
Flow cytometric studies of MRD, using 4-color analysis and multiple marker combinations individualized for each patient, were performed on days 19 and 46 (the end of remission induction treatment), as previously described.6,8,15,28 For each case, marker combinations allowing the identification of 1 leukemic cell per 104 normal nucleated bone marrow cells or greater were selected at diagnosis and then applied during clinical remission.
Statistical analysis
We analyzed associations between MRD, presenting features, and early treatment response with the χ2 test or Fisher exact test. The relation between levels of MRD by PCR and by flow cytometry was analyzed with the Spearman correlation test. In computations of the cumulative incidence of leukemic relapse, any type of leukemic relapse was the event of interest, whereas second malignancies, lineage switches, myelodysplastic syndrome, and death of any cause were considered competing events. Leukemic relapse-free time was calculated from the initial remission date to the date of leukemic relapse for those who experienced such an event, and from the initial remission date to the date of the competing event for patients experiencing a competing event. Patients who achieved remission and were still alive without any types of events were censored at the time of last follow-up. The cumulative incidence of leukemic relapse was compared between groups using the method of Gray,29 with estimation determined by the method of Kalbfleisch and Prentice.30
Results
Prevalence of MRD by PCR at the end of remission induction therapy and its relation to flow cytometric findings
Of 539 bone marrow samples from patients with newly diagnosed B-lineage ALL, 475 (88.1%) had clonal IG and/or TCR targets that were suitable for PCR monitoring of MRD and had an assays with a sensitivity of at least 0.001%, as demonstrated by tests with mixed leukemic and normal DNA. Bone marrow samples for MRD studies by PCR on day 46 of treatment (end of remission induction therapy) were available for 455 (95.8%) of these patients.
On day 46 of treatment, 76 patients (16.7%) had MRD 0.01% or more and 63 (13.8%) had MRD 0.001% to less than 0.01%. In 49 additional patients (10.7%), PCR signals were detected at a level less than 0.001%, whereas in the remaining 267 patients (58.4%) leukemia-associated PCR signals were not detected. The presence or absence of MRD was not significantly related to the PCR method used for analysis. Real-time PCR was used in 59 (42.4%) of the 139 patients with MRD 0.001% or more and in 135 (42.7%) of the 316 with lower levels or undetectable MRD; limiting dilution analysis was used in 80 (57.6%) and 181 (57.3%) of the patients, respectively.
Of the 455 samples studied by PCR on day 46, 412 (90.5%) were also studied by flow cytometry, with a routine sensitivity of 0.01%. As we previously reported,21 the results of the 2 assays correlated well above the 0.01% threshold (r = 0.8672; P < .001 by Spearman correlation analysis). Among the 68 cases with MRD 0.01% or more by PCR, 65 had MRD 0.01% or more by flow cytometry; 2 additional cases had MRD 0.008% each, whereas 1 case was MRD negative by flow cytometry but had PCR signals indicating a level of 0.051%. Among the 60 patients with MRD 0.001% to less than 0.01% by PCR who were also studied by flow cytometry, 10 had MRD 0.01% or more by flow cytometry, with levels close to the 0.01% threshold in most cases (median, 0.015%; range, 0.01%-0.1%). In an additional 12 cases of this group, flow cytometric signals less than 0.01% were detected (median, 0.005%; range, 0.001%-0.008%). Finally, among the 284 patients with MRD less than 0.001% or undetectable signals by PCR who were also studied by flow cytometry, only 2 had positive flow cytometry findings at a level of 0.01% or more (0.039% and 0.013%); an additional 2 had flow cytometry signals detected at the 0.003% level.
Relation among low levels of MRD, presenting features, and early treatment response
The presenting features commonly associated with treatment response in childhood ALL did not differ significantly between patients with MRD 0.001% to less than 0.01% at the end of remission induction and those with lower levels of MRD or no detectable MRD (Table 1). We also tested whether deletion of IKFZ1, a recently described abnormality in ALL associated with an inferior response to therapy,19,31 would predict the presence of low levels of residual disease after completion of induction therapy. Among 258 cases studied, IKFZ1 deletions were identified by single nucleotide polymorphism array in 28 (10.9%). As previously reported,19 patients with IKFZ1 deletions had a higher prevalence of MRD 0.01% or more at the end of remission induction than did those without this genetic abnormality: 15 of 43 (34.9%) versus 28 of the 230 (12.2%), respectively (P < .001). However, this relationship did not extend to MRD 0.001% to less than 0.01% or lower levels. Thus, among the 38 patients with MRD 0.001% to less than 0.01%, 4 (10.5%) had the deletion compared with 9 among the 176 (5.1%) with lower or undetectable levels of MRD (P < .254; Table 1). These findings clearly indicate that presenting prognostic features, as currently defined, do not reliably identify patients who will have low levels of MRD at the end of remission induction therapy.
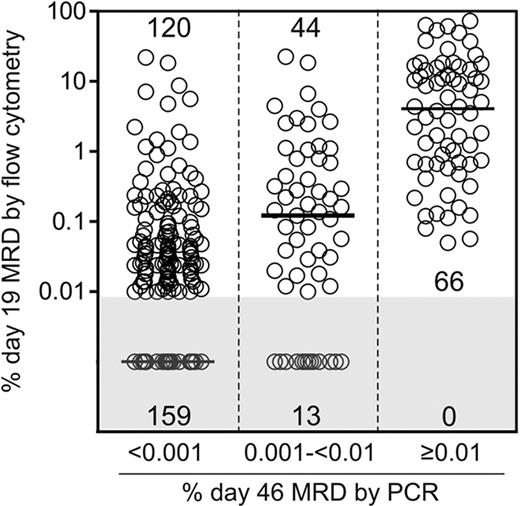
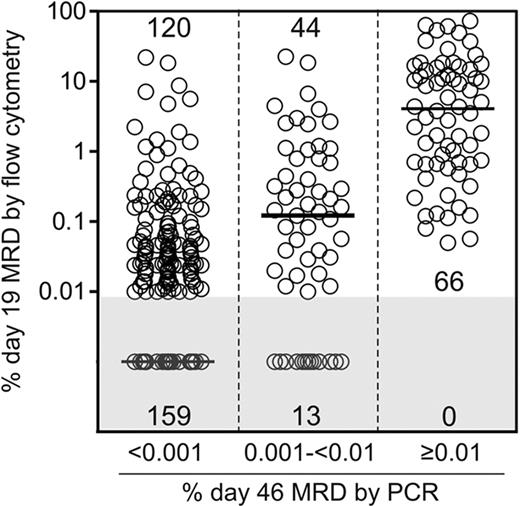
We next examined whether low levels of MRD at the end of remission induction therapy could be predicted by earlier treatment responses determined by flow cytometric testing of MRD on day 19 of therapy. Day 19 flow cytometric MRD data were available for 402 (88.4%) of the 455 of the patients who were also studied by PCR at the end of remission induction. As shown in Figure 1, all 66 patients with MRD 0.01% or more on day 43 by PCR in this group also had MRD 0.01% or more on day 19 by flow cytometry (median, 4.08%). Notably, 44 of the 57 (77.2%) patients with MRD 0.001% to less than 0.01% by PCR at the end of remission induction were also MRD positive by flow cytometry on day 19 (median, 0.12%), compared with 120 of the 279 (43.0%) with lower levels or undetectable disease on day 46 (median, < 0.01%; P < .001 by the Kruskal-Wallis 1-way analysis of variance test). Therefore, low levels of MRD at the end of remission induction therapy are strongly related to the degree of early clearance of leukemic cells.
Day 19 MRD levels by flow cytometry in 3 patient cohorts defined according to day 46 MRD levels by PCR. The size of each cohort is given within the diagram. Horizontal bars represent median values. Levels of minimal residual disease (MRD) on day 19 were significantly different in the 3 groups (P < .001 by Kruskal-Wallis 1-way analysis of variance test).
Day 19 MRD levels by flow cytometry in 3 patient cohorts defined according to day 46 MRD levels by PCR. The size of each cohort is given within the diagram. Horizontal bars represent median values. Levels of minimal residual disease (MRD) on day 19 were significantly different in the 3 groups (P < .001 by Kruskal-Wallis 1-way analysis of variance test).
Relation between low levels of MRD at the end of remission induction therapy and treatment outcome
As previously shown,6,8 patients with MRD 0.01% or more at the end of remission induction had a higher risk of relapse than those with lower levels. The 5-year cumulative incidence of relapse for the 76 patients with MRD 0.01% or more was 22.7% (± 5.8%; SE) compared with 6.3% (± 1.5%) for the 379 patients with lower levels or undetectable MRD (P < .001). Within this group of 76 patients, levels of MRD were also associated with outcome: 5-year cumulative incidence of relapse was 10.1% (± 5.8%) for patients with MRD 0.01% to less than 0.1% (n = 41), 32.5% (± 13.1%) for those with MRD 0.1% to less than 1% (n = 21), and 44.9% (± 16.5%) for those with MRD 1% or more (n = 14; P = .053). Thus, MRD 0.01% or more at the end of remission induction retained its strong prognostic significance even though 60 of the 76 patients were enrolled in the Total 14 and 15 study protocols, which featured intensified treatment for patients with MRD above this threshold level.
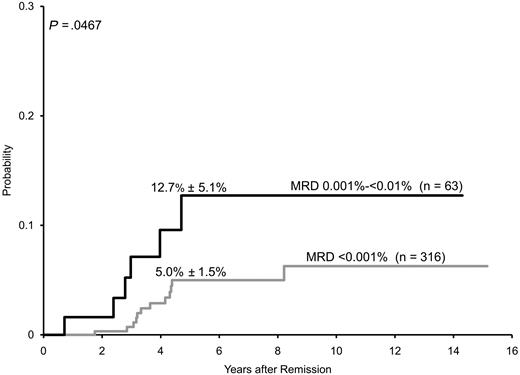
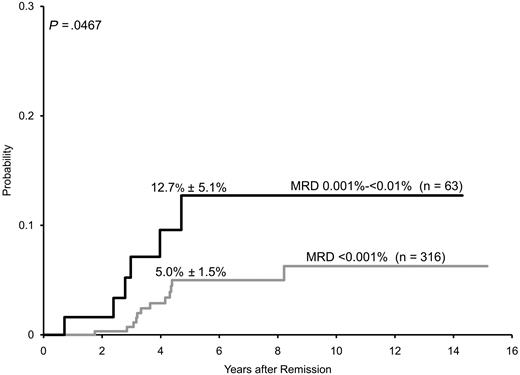
Because MRD less than 0.01% was regarded as “MRD-negative” by protocol criteria, risk assignment for the remaining 379 patients was solely based on presenting clinicobiologic features. In this group of patients were 18 with leukemic relapses (13 hematologic, 4 isolated central nervous system relapses and 1 testicular relapse; 16 of the 18 relapses occurred off-therapy). Analysis of these cases with PCR showed that 6 of the 63 patients with MRD 0.001% to less than 0.01% at the end of remission induction subsequently relapsed, compared with 12 of the 316 with lower levels or undetectable MRD. The estimated 5-year cumulative incidence of leukemic relapse for patients with MRD 0.001% to less than 0.01% was 12.7% (± 5.1%) versus 5.0% (± 1.5%) for patients with lower MRD levels or undetectable leukemia-associated PCR signals (Figure 2; P < .047). The risk of relapse for patients with PCR signals lower than 0.001% was not significantly different from that of patients with undetectable PCR signals: 7.7% (± 4.3%) versus 4.4% (± 1.6%) (P < .501). When estimates of relapse risk were limited to the 293 patients enrolled in Total 15, the 5-year rates were 13.3% (± 6.0%) for the 55 patients with MRD 0.001% to less than 0.01% and 4.8% (± 1.8%) for the 238 patients with lower levels or undetectable MRD (P = .065).
Cumulative incidence of relapse (mean ± SE) among 379 children with B-lineage ALL whose MRD levels were less than 0.01% on day 46 (end of remission induction therapy). Patients with MRD 0.001% to less than 0.01% had a significantly higher incidence of relapse than those with lower levels or undetectable MRD by PCR.
Cumulative incidence of relapse (mean ± SE) among 379 children with B-lineage ALL whose MRD levels were less than 0.01% on day 46 (end of remission induction therapy). Patients with MRD 0.001% to less than 0.01% had a significantly higher incidence of relapse than those with lower levels or undetectable MRD by PCR.
To assess whether the association between MRD levels and relapse differed for patients treated in different protocols, we compared the results obtained in patients enrolled in Total 13 and in Total 15 (no separate analysis was performed was Total 14 patients as there were only 25 patients enrolled in this study, all with MRD < 0.001%). Results for Total 13 were similar to those estimated for Total 15. Thus, cumulative incidence of relapse was 12.5% (± 12.5%) for patients with MRD 0.001% to less than 0.01%, enrolled in Total 13 (n = 8) and 13.3% (± 6.0%) for the 55 enrolled in Total 15 (P > .992). For patients with MRD less than 0.001%, it was 7.5% (± 3.7%; n = 53) and 4.8% (± 1.8%; n = 238), respectively (P > .483).
The 6 patients with MRD 0.001% to less than 0.01% who relapsed had heterogeneous presenting features: 2 were younger than 10 years of age and had high hyperdiploid (> 50 chromosomes) karyotypes; the other 4 were 10 to 12 years old with leukocyte counts ranging from 3.3 to 65.9 × 109/L and the t(1;19), t(11;19), t(5;7), or t(8;14) translocation. After relapse, 4 of the 6 patients achieved a second remission and remain in remission 30 to 46 months later.
None of the conventional presenting prognostic features was significantly associated with relapse among patients with MRD less than 0.01% (Table 2). Nevertheless, we examined the prognostic impact of MRD 0.001% to less than 0.01% versus lower levels in a model, including presenting variables that had a P value of less than .10 in the univariate model, that is, ETV6-RUNX1, t(1;19) or TCF3-PBX1. The MRD level remained a significant prognostic factor after adjusting for these 2 factors (P < .042 and P > .041, respectively). Although t(9;22) or BCR-ABL1 also had a P value less than .10 in the univariate model (Table 2), there were only 5 patients with this abnormality in our cohort, precluding a meaningful analysis, but none of the patients with MRD 0.001% to less than 0.01% who relapsed had this cytogenetic abnormality.
To determine the degree of response to postremission induction therapy in patients with MRD 0.001% to less than 0.01%, we measured MRD on week 7 of continuation therapy (∼ 3 months from diagnosis) in 53 patients of this group and compared the results with those obtained in 47 patients with MRD 0.01% or more on day 46. The prevalence of MRD on week 7 by PCR was significantly different in the 2 groups: only 3 of the 53 patients (5.6%) with MRD 0.001% to less than 0.01% on day 46 had MRD 0.001% or more on week 7 of continuation therapy compared with 14 of the 47 (29.8%) with higher levels of MRD on day 46 (P < .003). These results indicate that patients with higher levels of MRD on day 46 continue to clear leukemia more slowly than those with more pronounced early clearance. Of note, 1 of the 3 patients with MRD 0.001% to less than 0.01% on day 46 and persistent MRD on week 7 of continuation therapy remained MRD-positive at subsequent time points and relapsed on therapy.
Discussion
The clinical significance of MRD in childhood ALL is well established.5-13 However, the predictive value of MRD below the 0.01% threshold typically used for risk assignment remains unclear. If the risk of relapse is directly proportional to the level of residual disease at the end of remission induction therapy, as shown by previous studies,5-12 patients with low levels of MRD (0.001%-< 0.01%) should have a measurably higher risk of relapse than those with lower levels. Yet, it is entirely possible that the prognostic significance of such low levels of disease (corresponding to an estimated total leukemic burden of 107 to 108 cells) might be abolished by effective reinduction/consolidation and continuation therapy. Using PCR methods that routinely detect at least 0.001% ALL cells in bone marrow, we specifically addressed this issue and found that patients with MRD levels between 0.001% and 0.01% have a significantly higher risk of relapse than do those with a lower level of MRD or no detectable disease. Within this group of patients, none of the presenting features we tested was prognostically informative.
Although low levels of MRD on day 46 could not be predicted by presenting characteristics, they were strongly associated with MRD levels on day 19. We would stress that this correlation was not absolute, as 21% of patients in the 0.001% to less than 0.01% MRD category still appeared to have excellent disease clearance according to flow cytometric measurements of MRD on day 19. We cannot be certain whether low levels of MRD on day 46 represent the reemergence of resistant disease or the persistence of disease not initially detectable by flow cytometry. Patients with MRD 0.001% to less than 0.01% on day 46 had a 5-year cumulative incidence of leukemic relapse that was similar to that of patients with MRD 0.01% to less than 0.1%: 12.7% (± 5.1%) versus 10.1% (± 5.8%). It should be noted, however, that the majority of patients were enrolled in studies Total 14 and Total 15, in which patients with MRD 0.01% or more received intensified therapy, which might have reduced their risk of subsequent relapse. Indeed, in a previous study in which MRD was not used for risk assignment, patients with MRD 0.01% to less than 0.1% had a 5-year cumulative incidence of relapse of 23% (± 11%).8
PCR-based MRD assays have advantages and disadvantages compared with flow cytometry–based assays. The most obvious advantage of PCR is its superior sensitivity, allowing the detection of 1 leukemic cell per 100 000 in the majority of cases. Indeed, we found that such level of sensitivity could be achieved in 475 (96.7%) of 491 cases with clonal IG and/or TCR targets. Interestingly, of the 60 cases with MRD 0.001% to less than 0.01 by PCR that were also studied by flow cytometry, 24 (40.0%) had detectable leukemic cells by this method. Therefore, with improvements in flow cytometric methods, it might be possible to identify an increased proportion of such patients. With current techniques, however, most cases with PCR signals less than 0.01% are negative by flow cytometry.
Because contemporary effective treatment regimens result in cure rates for children with ALL as high as 90%, it has become particularly important to identify patients with a higher risk of relapse, also considering that patients who relapse despite intensive therapy could be more difficult to salvage. It is widely accepted that MRD 0.01% or more at the end of remission-induction therapy mandates postremission treatment intensification. Our findings raise the question of whether therapy should also be intensified for patients in the MRD 0.001% to less than 0.01% group. Even though this group of patients had a higher rate of relapse than those with lower levels of MRD or undetectable disease in our study, the relapse rate was relatively low (12.7% at 5 years). This and the fact that 4 of the 6 patients who relapsed responded to salvage therapy and are still in remission indicate that further treatment intensification for all patients with this feature may not be justified. However, these patients should be carefully monitored for persistent or increasing MRD levels, which would be an indication for alternative treatment strategies, such as hematopoietic stem cell transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute (grants CA52259, CA60419, and CA21765) and the American Lebanese Syrian Associated Charities.
Authorship
Contribution: P.S., L.K., X.C., Q.P., and G.A.N. performed PCR studies of minimal residual disease; E.C.-S. performed flow cytometric studies of minimal residual disease; C.G.M. provided data on IKFZ1 deletions and mutations; Y.Z. performed statistical analysis; C.-H.P. led the clinical protocols; and D.C. initiated the studies, analyzed the data, and wrote the manuscript with the input of all other authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for X.C. is Division of Oncology, Children's Hospital of Philadelphia, Philadelphia, PA. The current affiliation for Q.P. is Department of Pathology, Montefiore Medical Center, Bronx, NY. The current affiliation for G.A.N. is Hartwell Center for Bioinformatics and Biotechnology, St Jude Children's Research Hospital, Memphis, TN.
Correspondence: Dario Campana, Department of Oncology, St Jude Children's Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105; e-mail: dario.campana@stjude.org.