Abstract
In 2003, an international working group last reported on recommendations for diagnosis, response assessment, and treatment outcomes in acute myeloid leukemia (AML). Since that time, considerable progress has been made in elucidating the molecular pathogenesis of the disease that has resulted in the identification of new diagnostic and prognostic markers. Furthermore, therapies are now being developed that target disease-associated molecular defects. Recent developments prompted an international expert panel to provide updated evidence- and expert opinion–based recommendations for the diagnosis and management of AML, that contain both minimal requirements for general practice as well as standards for clinical trials. A new standardized reporting system for correlation of cytogenetic and molecular genetic data with clinical data is proposed.
1. Introduction
In 1990 and 2003, expert working groups published recommendations for diagnosis, standardization of response criteria and treatment outcomes, and reporting standards for clinical trials in acute myeloid leukemia (AML).1,2 These have been widely adopted in general practice, within clinical trials, and by regulatory agencies. During recent years, considerable progress has been made in deciphering the molecular genetic and epigenetic basis of AML and in defining new diagnostic and prognostic markers. A growing number of recurring genetic changes have been recognized in the new World Health Organization (WHO) classification of AML.3 Furthermore, novel therapies are now being developed that target some of the genetic lesions. All these developments prompted an international expert panel to provide updated recommendations for the diagnosis and management of adult patients with AML, excluding acute promyelocytic leukemia (APL) for which recommendations were published separately.4 These recommendations provide standard requirements for general practice and for clinical trials. However, to accelerate progress in research the panel strongly recommends entry of AML patients on clinical trials, and storage of biosamples to enable correlative laboratory studies.
The following statements and recommendations are based on studies that predominantly were performed in the United States and Europe. Specific dosages and interventions may vary among different countries and populations.
2. Methods
2.1 Composition of the panel
The panel included 19 members with recognized clinical and research expertise in AML, of whom 13 came from European Union countries, 5 from the United States, and 1 from Japan. The panel met 4 times.
2.2 Scope of the review
Computerized literature searches of the PubMed, Cochrane, and Medline databases in the English language were conducted using key words relevant to the various sections of this article. Relevant abstracts presented at the 2006, 2007, and 2008 meetings of the American Society of Hematology, the European Hematology Association, and the American Society of Clinical Oncology were also reviewed. The categories of evidence and consensus were those used by the National Comprehensive Cancer Network (NCCN; www.nccn.org/professionals/physician_gls/PDF/aml.pdf). The vast majority of recommendations were category 2A recommendations, that is, they are based on low-level evidence and there is uniform panel consensus. The benefits are closely balanced with the risks and burdens, and the best action may differ depending upon circumstances.
3. WHO classification
The recent WHO classification reflects the fact that an increasing number of acute leukemias can be categorized based upon their underlying cytogenetic or molecular genetic abnormalities, and that these genetic changes form clinico-pathologic-genetic entities (Table 1).3,5 The subgroup “AML with recurrent genetic abnormalities” comprises several primary AML entities. “AML with t(8;21)(q22;q22); RUNX1-RUNX1T1” and “AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11” are considered as AML regardless of bone marrow blast counts. In “APL with t(15;17)(q22;q12); PML-RARA,” RARA translocations with other partner genes are recognized separately. The former category “AML with 11q23 (MLL) abnormalities” was redefined in that “AML with t(9;11)(p22;q23); MLLT3-MLL” is now a unique entity; balanced translocations other than that involving MLLT3 should be specified in the diagnosis. Three new cytogenetically defined entities were incorporated: “AML with t(6;9)(p23;q34); DEK-NUP214”; “AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2); RPN1-EVI1”; and “AML (megakaryoblastic) with t(1;22)(p13;q13); RBM15-MKL1,” a rare leukemia most commonly occurring in infants. Two new provisional entities defined by the presence of gene mutations were added, “AML with mutated NPM1 [nucleophosmin (nucleolar phosphoprotein B23, numatrin)],” and “AML with mutated CEBPA [CCAAT/enhancer binding protein (C/EBP), alpha].” There is growing evidence that these 2 gene mutations represent primary genetic lesions (so-called class II mutations)6 that impair hematopoietic differentiation. Mutations in the fms-related tyrosine kinase 3 (FLT3) gene are found in many AML subtypes and are considered class I mutations conferring a proliferation and/or survival advantage. AML with FLT3 mutations are not considered a distinct entity, although determining the presence of such mutations is recommended by WHO because they have prognostic significance.
The former subgroup termed “AML with multilineage dysplasia” is now designated “AML with myelodysplasia-related changes.” Dysplasia in 50% or more of cells, in 2 or more hematopoietic cell lineages, was the diagnostic criterion for the former subset. However, the clinical significance of this morphologic feature has been questioned.7,8 AMLs are now categorized as “AML with myelodysplasia-related changes” if (1) they have a previous history of myelodysplastic syndrome (MDS) or myelodysplastic/myeloproliferative neoplasm (MDS/MPN) and evolve to AML with a marrow or blood blast count of 20% or more; (2) they have a myelodysplasia-related cytogenetic abnormality (listed in a footnote to Table 1); or (3) if 50% or more of cells in 2 or more myeloid lineages are dysplastic.
“Therapy-related myeloid neoplasms” has remained a distinct entity; however, since most patients have received treatment using both alkylating agents and drugs that target topoisomerase II for prior malignancy, a division according to the type of previous therapy is often not feasible. Therefore, therapy-related myeloid neoplasms are no longer subcategorized. Myeloid proliferations related to Down syndrome are now listed as distinct entities.
4. Diagnostic procedures
4.1 Morphology
A bone marrow aspirate is part of the routine diagnostic work-up of a patient with suspected AML. The panel considers a marrow trephine biopsy optional, but it should be performed in patients with a dry tap (punctio sicca).
Blood and marrow smears are morphologically examined using a May-Grünwald-Giemsa or a Wright-Giemsa stain. It is recommended that at least 200 leukocytes on blood smears and 500 nucleated cells on marrow smears be counted, with the latter containing spicules. For a diagnosis of AML, a marrow or blood blast count of 20% or more is required, except for AML with t(15;17), t(8;21), inv(16) or t(16;16), and some cases of erythroleukemia. Myeloblasts, monoblasts, and megakaryoblasts are included in the blast count. In AML with monocytic or myelomonocytic differentiation, monoblasts and promonocytes, but not abnormal monocytes, are counted as blast equivalents. Erythroblasts are not counted as blasts except in the rare instance of pure erythroid leukemia.
To identify lineage involvement some countries still rely more on cytochemistry, rather than on immunophenotyping (usually by flow cytometry), using myeloperoxidase (MPO) or Sudan black B (SBB) and nonspecific esterase (NSE) stains. Detection of MPO (if present in ≥ 3% of blasts) indicates myeloid differentiation, but its absence does not exclude a myeloid lineage because early myeloblasts and monoblasts may lack MPO. SBB staining parallels MPO but is less specific. NSE stains show diffuse cytoplasmic activity in monoblasts (usually > 80% positive) and monocytes (usually > 20% positive). In acute erythroid leukemia, a periodic acid-Schiff (PAS) stain may show large globules of PAS positivity. Iron stains may allow for the detection of iron stores, normal sideroblasts, and ring sideroblasts.
4.2 Immunophenotyping
Immunophenotyping using multiparameter (commonly at least 3- to 4-color) flow cytometry is used to determine lineage involvement of a newly diagnosed acute leukemia (Table 2).3,9,10 There is no general consensus on the cutoff point for considering an acute leukemia to be positive for a marker. For most markers, a commonly used criterion is 20% or more of leukemic cells expressing the marker,10 whereas for selected markers (eg, cytoplasmic CD3, MPO, TdT, CD34, CD117) a lower cutoff has been applied (10%). Quantification of expression patterns of several surface and cytoplasmic antigens is necessary for lineage assignment, to diagnose mixed phenotype acute leukemia (MPAL), and to detect aberrant immunophenotypes allowing for measurement of minimal residual disease (MRD). Flow cytometry determination of blast count should not be used as a substitute for morphologic evaluation.5
Immunophenotyping is required to establish the diagnosis of AML with minimal differentiation, acute megakaryoblastic leukemia, and acute leukemias of ambiguous lineage.3 AML with minimal differentiation is an AML without morphologic and cytochemical evidence of myeloid differentiation.11 Most cases express early hematopoiesis-associated antigens (eg, CD34, CD38, and HLA-DR) and lack most markers of myeloid and monocytic maturation; while MPO is negative by cytochemistry, detection of intracytoplasmic MPO antigens may be positive by flow cytometry in at least a fraction of blasts. Acute megakaryoblastic leukemia is a leukemia with 20% or more blasts of which 50% or more are of megakaryocytic lineage; megakaryoblasts typically express one or more of the platelet glycoproteins CD41 and/or CD61, and less commonly CD42. Acute leukemias of ambiguous lineage are rare leukemias and comprise those cases that show no evidence of lineage differentiation (ie, acute undifferentiated leukemia [AUL]) or those with blasts that express markers of more than one lineage (ie, MPAL). AULs often express HLA-DR, CD34, and/or CD38, but by definition lack lineage-associated markers. MPAL can either contain distinct blast populations of different lineages, or one blast population with markers of different lineages on the same cell, or a combination of both. MPAL as defined by WHO encompasses several subsets, with or without an underlying genetic abnormality (Table 1).
BCR-ABL1–positive acute leukemia immunophenotypically may present as MPAL (Table 1). Such leukemias should not be treated as AML, but rather as ALL with incorporation of an ABL1 tyrosine kinase inhibitor with chemotherapy. Also, in BCR-ABL1–positive leukemias, the differential diagnosis of chronic myelogenous leukemia (CML) blast crisis should be considered.
Some AMLs with recurrent genetic abnormalities are associated with characteristic immunophenotypic features. For example, AMLs with t(8;21) frequently express the lymphoid markers CD19 or, to a lesser extent, CD7; they may also express CD5612,13 ; AMLs with inv(16) frequently express the T lineage–associated marker CD214 ; and AMLs with NPM1 mutation typically have high CD33 but absent or low CD34 expression.15
4.3 Cytogenetics
Conventional cytogenetics analysis is a mandatory component in the diagnostic evaluation of a patient with suspected acute leukemia. Chromosome abnormalities are detected in approximately 55% of adult AML.16,17 Seven recurrent balanced translocations and inversions, and their variants, are recognized in the WHO category “AML with recurrent genetic abnormalities.” Furthermore, several cytogenetic abnormalities are considered sufficient to establish the WHO diagnosis of “AML with myelodysplasia-related features” when 20% or more blood or marrow blasts are present (Table 1).
A minimum of 20 metaphase cells analyzed from bone marrow is considered mandatory to establish the diagnosis of a normal karyotype, and recommended to define an abnormal karyotype. Abnormal karyotypes may be diagnosed from blood specimens.
4.4 Molecular cytogenetics
Methanol/acetic acid–fixed cell pellets should be stored so if cytogenetic analysis fails, fluorescence in situ hybridization (FISH) is an option to detect gene rearrangements, such as RUNX1-RUNX1T1, CBFB-MYH11, MLL and EVI1 gene fusions, or loss of chromosome 5q and 7q material.18,19 FISH is frequently necessary to identify MLL fusion partners in 11q23 translocations.
4.5 Molecular genetics
A marrow (and blood) specimen should routinely be taken for molecular diagnostics. Ideally, DNA and RNA should be extracted and viable cells stored; if cell numbers are limited, RNA extraction should be a priority, because RNA is suitable for molecular screening for fusion genes and leukemia-associated mutations.
Molecular diagnosis by reverse transcriptase–polymerase chain reaction (RT-PCR) for the recurring gene fusions, such as RUNX1-RUNX1T1, CBFB-MYH11, MLLT3-MLL, DEK-NUP214, can be useful in certain circumstances. RT-PCR, for which standardized protocols were published by the BIOMED-1 group,20 is an option to detect these rearrangements, if chromosome morphology is of poor quality, or if there is typical marrow morphology but the suspected cytogenetic abnormality is not present.21,22
Somatically acquired mutations have been identified in several genes, for example, the NPM1 gene,15 the FLT3 gene,23,24 the CEBPA gene,25 the myeloid/lymphoid or mixed-lineage leukemia (trithorax homolog, Drosophila) (MLL) gene,26 the neuroblastoma RAS viral (v-ras) oncogene homolog (NRAS) gene,27 the Wilms tumor 1 (WT1) gene,28 the v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT) gene,29 the runt-related transcription factor 1 (RUNX1) gene,30 the tet oncogene family member 2 (TET2) gene,31,32 and the isocitrate dehydrogenase 1 (NADP+), soluble (IDH1) gene.33 The frequencies of these gene mutations vary among cytogenetic groups.34
AML with mutations in NPM1 or CEBPA have been incorporated in the WHO classification as provisional entities.3 Screening for these 2 markers as well as for FLT3 mutations should be done in clinical trials. While testing for NPM1, CEBPA, and FLT3 is currently not considered mandatory outside clinical trials, the panel recommends that these 3 mutations be analyzed at least in patients with cytogenetically normal AML (CN-AML) who will receive treatment other than low-dose chemotherapy or best supportive care.
4.6 Genome-wide studies
Recent progress in genomics technology has resulted in the identification of novel genetic abnormalities and holds the promise of making the systematic characterization of cancer genomes feasible.35,36 For example, gene- and microRNA-expression profiling have proven valuable for the discovery of novel leukemia subgroups and of prognostic signatures.37-39 The introduction of genome-wide single nucleotide polymorphism (SNP)–based mapping arrays, providing both copy number and allele-specific information, led to the identification of a novel mechanism involved in the pathogenesis of AML, that is, uniparental disomy (UPD).40 Acquired UPD is due to a mitotic recombination event and may render a cell homozygous for a preexisting mutation located in the affected genomic region. The power of SNP genotyping as a tool for gene discovery is shown by several recent studies.31,32,41,42 While analyses of genomic copy number will continue to be informative with regard to selection of candidate leukemia genes, it is also hoped that high-throughput DNA sequence analysis will become possible at an affordable cost, which may ultimately result in the development of comprehensive, disease- and allele-specific oncogene mutation profiling strategies.33,43 Finally, functional genetic approaches, such as large-scale RNA interference screens, have great potential for the identification of novel cancer genes. An example is a recent study in which graded down-regulation of multiple candidate genes by RNA interference was used to identify RPS14 as a causal gene for the MDS 5q− syndrome.44
4.7 Biobanking
Within clinical trials, we strongly recommend storing patients' pretreatment leukemic marrow and blood within a biobank. A prerequisite for biobanking is the patient's informed consent that ideally should allow a broad spectrum of correlative laboratory studies that also include analysis of germline DNA. Pretreatment samples should include nucleic acid (DNA and RNA, stored at −80°C) and viable cells (stored at −196°C). For further optional storage, we advise saving germline DNA (eg, from a buccal swab, skin biopsy, or sputum), a plasma sample, a methanol/acetic acid–fixed cell pellet (from cytogenetic analysis), and frozen cell pellets from various time points during and after treatment (ie, at the time of complete remission [CR], at relapse; and for MRD monitoring at defined time points during treatment and follow-up), stored under appropriate conditions.
4.8 Other diagnostic tests
Additional diagnostic tests and procedures in the initial work-up of a patient with AML are given in Table 3.
5. Prognostic factors
Prognostic factors may be subdivided into those related to patient characteristics and general health condition and those related to characteristics particular to the AML clone. The former subset usually predicts treatment-related mortality (TRM) and becomes more important as patient age increases while the latter predicts resistance to, at least, conventional therapy.
5.1 Patient-related factors
Increasing age is an adverse prognostic factor.45,46 Even after accounting for risk factors, such as cytogenetics, molecular genetics, type of AML (ie, de novo AML; AML with previous history of MDS or MDS/MPN; therapy-related AML), and performance status, older patients have worse outcomes than younger patients, suggesting the effect of unknown age-related factors. Nonetheless, calendar age alone should not be a reason for not offering potentially curative therapy to an older patient,46 because age is not the most important prognostic factor for either TRM or resistance to therapy. Attention should be given to a careful evaluation and documentation of comorbidities. In a recent study of patients older than 60 years of age receiving induction therapy (idarubicin 12 mg/m2 for 3 days, cytarabine 1.5 g/m2 for 3 days),47 scoring of baseline comorbidities using the hematopoietic cell transplantation comorbidity index (HCTCI)48 was predictive of early death rates and overall survival (OS). Comorbidity scoring is a current field of investigation and should contribute to a better definition of the patient considered “unfit” for intensive chemotherapy.
5.2 AML-related factors
AML-related prognostic factors includes white blood count (WBC), existence of prior MDS, previous cytotoxic therapy for another disorder (see section 9), and cytogenetic and molecular genetic changes in the leukemic cells at diagnosis. Various other factors, such as splenomegaly and elevated serum lactate dehydrogenase (LDH) levels, have been reported to confer some prognostic effect but with variable consistency among studies. The significance of a prognostic factor is always dependent on the therapy given to a patient.
5.2.1 Cytogenetics.
The karyotype of the leukemic cells is the strongest prognostic factor for response to induction therapy and for survival.16,17 Younger adult patients are commonly categorized into 3 risk groups, favorable, intermediate, or adverse.49-52 The most appropriate risk group assignment for a number of the rarer cytogenetic abnormalities, for example, del(7q), isolated trisomy 8, del(9q), t(v;11)(v;q23) other than t(9;11), and del(20q), remains uncertain due to limitations of sample size and differences in treatment schedule among studies. The impact of secondary genetic lesions in cases with balanced translocations or inversions requires further investigation. With the possible exception of trisomy 22 in AML with inv(16) or t(16;16) that has been associated with an improved relapse-free survival (RFS),53,54 no such impact has been shown for other secondary cytogenetic changes.
Complex karyotype, which occurs in 10% to 12% of patients, has consistently been associated with a very poor outcome.55 Complex karyotype has been defined as the presence of 3 or more (in some studies ≥ 5) chromosome abnormalities in the absence of t(8;21), inv(16) or t(16;16), and t(15;17), because in most studies increased karyotype complexity in these subgroups did not adversely affect outcome. As indicated in the new WHO classification, cases with other recurring genetic abnormalities, such as AML with t(9;11) or t(v;11), AML with inv(3) or t(3;3), and AML with t(6;9) should also be excluded,5 because these groups constitute separate entities. The nonrandom pattern of abnormalities within complex karyotypes includes a paucity of balanced rearrangements, and a predominance of chromosomal imbalances; losses most frequently affect 5q, 17p, and 7q, and gains 8q, 11q, and 21q.55-57 Prominent features of complex karyotype cases are the frequent loss of 17p and/or TP53 gene mutation,56,57 occurring in approximately two-thirds of the cases, and a high prevalence of high-level DNA amplifications.57 Recently, a new cytogenetic category has been proposed that distinguishes AML of particularly unfavorable risk, that is, the monosomal karyotype.58 In this study, the monosomal karyotype was defined by the presence of one single monosomy (excluding isolated loss of X or Y) in association with at least one additional monosomy or structural chromosome abnormality (excluding core-binding factor [CBF] AML).
One striking observation is the increasing incidence of adverse versus favorable cytogenetic abnormalities with increasing age. This, at least in part, contributes to the poorer outcome of AML in older adults.45,59,60 Several cytogenetic risk classifications have been proposed for elderly patients.61-63
5.2.2 Molecular genetics.
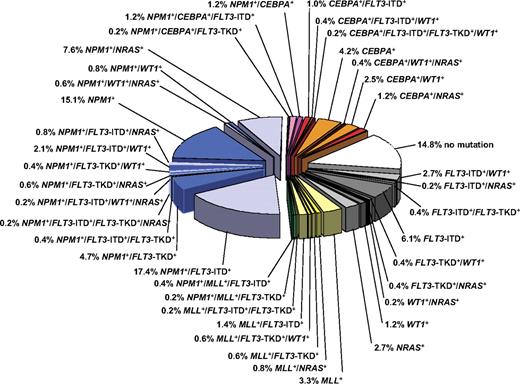
Gene mutations and deregulated gene expression have been identified that allow us to decipher the genetic diversity within defined cytogenetic groups, in particular the large and heterogeneous group of patients with CN-AML (Figure 1).34,64,65 Prognostic significance within CN-AML has consistently been shown for mutations in the NPM1, CEBPA, and FLT3 genes alone or in combination in younger adult patients.
Pie chart illustrating the molecular heterogeneity of cytogenetically normal AML based on mutations in the NPM1, CEBPA, MLL, FLT3 (ITD and TKD mutations at codons D835 and I836), NRAS, and WT1 genes. The bluish colors denote NPM1-mutated subsets, the orange/red colors CEBPA-mutated subsets, and the yellow/green colors MLL-mutated subsets. The gray colors depict subsets without hypothetical class II mutations, and the white sector shows the subset without any mutation in the above-mentioned genes. Data are derived from mutational analysis of 485 younger adult patients with cytogenetically normal AML from AMLSG.
Pie chart illustrating the molecular heterogeneity of cytogenetically normal AML based on mutations in the NPM1, CEBPA, MLL, FLT3 (ITD and TKD mutations at codons D835 and I836), NRAS, and WT1 genes. The bluish colors denote NPM1-mutated subsets, the orange/red colors CEBPA-mutated subsets, and the yellow/green colors MLL-mutated subsets. The gray colors depict subsets without hypothetical class II mutations, and the white sector shows the subset without any mutation in the above-mentioned genes. Data are derived from mutational analysis of 485 younger adult patients with cytogenetically normal AML from AMLSG.
CN-AML patients harboring internal tandem duplication (ITD) of the FLT3 gene have an inferior outcome compared with cases without FLT3-ITD.65-70 There is also evidence that outcome may be more related to the level of the mutated allele,66,67,69,70 rather than its mere presence, or to the insertion site of the ITD.71,72 The prognostic relevance of FLT3 tyrosine kinase domain (TKD) mutations (at codons D835 and I836) remains controversial.65,73-76
In several, but not all studies, the presence of NPM1 mutation in CN-AML has been associated with higher CR rates and better RFS and event-free survival (EFS).77-81 Of note, approximately 40% of patients with NPM1 mutations also carry FLT3-ITD, and multiple studies have shown that the genotype “mutated NPM1 without FLT3-ITD” represents a favorable prognostic marker, with higher CR rates, and better RFS and OS that is reminiscent of that seen in patients with inv(16) or t(8;21).65,77-80 This favorable impact of mutated NPM1 (with or without FLT3-ITD) on survival endpoints also seems to hold up among patients of older age.81-84 CN-AML with mutations in CEBPA is another subset that has been associated with a favorable prognosis.65,85-89 The survival data are very similar to those of AML patients with mutated NPM1 without FLT3-ITD. Two recent studies suggest that there is heterogeneity among mutated CEBPA cases, in that only cases with double mutations, usually biallelic, have a favorable outcome.90,91 It remains an open question whether the presence of a FLT3-ITD impacts on prognosis in patients with mutant CEBPA.65,92 In cytogenetically favorable CBF AML [ie, AML with t(8;21) or inv(16)/t(16;16)], the presence of a KIT mutation has been shown to have an unfavorable influence on outcome in retrospective studies.93-97
There is a growing list of genetic abnormalities that are being investigated (Table 3). These include mutation analyses of the WT1,28,98-101 RUNX1,30,102,103 TET2,31,32 and IDH1 genes,33 and the analyses of gene expression signatures,37,38 or of deregulated expression of single genes, such as EVI1,19 ERG,104 MN1,105,106 and BAALC107 genes.
5.2.3 Monitoring of minimal residual disease.
The monitoring of MRD as determined by RT-PCR detecting leukemia-specific targets (eg, gene fusions, gene mutations, overexpressed genes), or by multiparameter flow cytometry identifying leukemia-associated aberrant phenotypes remains an active field of investigation.108 Despite technical developments, there is still, except for APL,4 a paucity of large prospective trials demonstrating its clinical utility.108 Potentially useful applications of MRD monitoring include early assessment of response to therapy to improve risk stratification and guide postremission therapy; and posttreatment monitoring to detect impending relapse and guide preemptive therapy. The kinetics of RUNX1-RUNX1T1 and CBFB-MYH11 decline has been found to correlate with risk of relapse, or to represent a prognostic factor independent of other pretreatment variables.108-110 After consolidation therapy, low-level PCR-positivity can be detected in patients even in long-term remission of CBF AML. Normalizing to 104 copies of ABL1 in accordance with standardized criteria, transcript levels below 12 to 10 copies appear to predict long-term remission.109,110 Real-time quantitative (RQ)–PCR assays have been developed for other fusion gene targets such as MLLT3-MLL and DEK-NUP214, but data are very scarce due to the low frequencies of these leukemias.108 NPM1 mutations likely provide one of the most promising new targets and studies are ongoing to evaluate the clinical utility of MRD monitoring in AML with NPM1 mutation.111
The major advantage of using flow cytometric detection of MRD lies in its applicability to virtually all patients. Although its sensitivity, as reported in previous studies, is at least 1 log below that of RQ-PCR assays, the sensitivity will likely be improved by the use of 6- to 8-color laser technology. Among 8 studies,108 7 demonstrated that immunophenotypic detection of MRD in AML after induction and consolidation provides independent prognostic information. Validation, using larger patient cohorts and modern technology, is ongoing.
5.3 Standardized reporting system for genetic abnormalities
The panel proposes a standardized reporting system for genetic abnormalities when presenting data correlating genetic findings with clinical outcome allowing for a better comparison of data among studies (Table 4). This standardized report includes data from cytogenetic analysis and from mutation analyses of the NPM1, CEBPA, and FLT3 genes.
6. Response criteria and survival outcomes
Most response criteria and survival measures as described in the previous recommendations have been widely used by clinicians and cooperative groups (for definitions see Tables 5 and 6).2 Response criteria should meet the specific objective of a study, for example, in phase 2 and 3 clinical trials CR or CR with incomplete blood recovery (CRi) should be the appropriate end point, whereas in phase 1 clinical trials, the criteria of partial remission (PR) and morphologic leukemia-free state may also be useful. Other response criteria are cytogenetic CR (CRc)112-115 and molecular CR (CRm; Table 5).
Response assessment.
After conventional induction therapy with 3 days of an anthracycline and 7 days of cytarabine (“3 + 7”) or therapies of comparable intensity, response assessment is commonly performed between day 21 and day 28 after start of therapy. The exact timing may vary among protocols and should meet the specific objectives of the study.
Early response assessment.
Early response assessment may be required in investigational studies to evaluate the antileukemic efficacy of a novel agent, or to guide subsequent treatment, for example, with protocols applying intensified induction regimens. It is made at 7 to 10 days after chemotherapy. Bone marrow at that time is usually hypoplastic or aplastic, documenting the antileukemia effect.
Response assessment during follow-up period.
Within clinical trials, it is usually recommended that repeat marrow aspirates be performed every 3 months for the first 2 years; in some cases, surveillance continues every 6 months for the following 2 to 3 years. Most relapses occur within 1 to 3 years after the end of therapy. Standardized time points are necessary if MRD monitoring is performed. Outside clinical trials, repeat marrow aspirates may not be needed, and should be done only if blood counts become abnormal.116 Blood counts should be done every 1 to 3 months for the first 2 years, then every 3 to 6 months up to 5 years.
7. Management of younger adults: 18 to 60 years
7.1 Induction therapy
Three days of an anthracycline (eg, daunorubicin, at least 60 mg/m2 [higher doses are being explored], idarubicin, 10-12 mg/m2, or the anthracenedione mitoxantrone, 10-12 mg/m2) and 7 days of cytarabine (100-200 mg/m2 continuous IV) (“3 + 7”) currently remains the standard for induction therapy. With such regimens, CR is achieved in 60% to 80% of younger adults. No other intervention has been convincingly shown to be better.117,118 Induction chemotherapy should be started after the diagnostic work-up has been completed, preferably with minimal delay. Retrospective data suggest that treatment outcome might be adversely impacted when the time from diagnosis to start of treatment increases beyond 5 days.119
Alternative anthracyclines, high-dose cytarabine, additional agents given with conventional induction chemotherapy.
Randomized studies have compared daunorubicin at a dose of 45-60 mg/m2 with other anthracyclines, such as idarubicin120-123 or aclarubicin,124 with amsacrine,125 or with mitoxantrone.126 With respect to OS, it is not clear whether any agent is superior to daunorubicin at equivalent doses. High-dose cytarabine (HiDAC) combined with daunorubicin in induction has been studied by the Southwest Oncology Group (SWOG; 2 g/m2 every 12 hours [q12h] on days 1-6),127 and the Australian Leukemia Study Group (ALSG; 3 g/m2 per q12h on days 1, 3, 5, and 7)128 in prospective randomized trials, and by the Eastern Cooperative Oncology Group (ECOG; 3 g/m2 per q12h on days 1, 3, and 5)129 and SWOG (cytarabine 100 mg/m2 cont. IV on days 1-7, followed by HiDAC 2 g/m2 per q12h on days 8-10; “3 + 7 + 3”)130 in phase 2 trials. Neither randomized trial showed a higher CR rate with HiDAC, and both demonstrated increased toxicity. In a trial by the AML Cooperative Group (AMLCG), 1 versus 2 courses with HiDAC (3 g/m2 per q12h on days 1-3) in induction produced equal CR rates, disease-free survival (DFS) and moderate toxicity.131 Therefore, it is not generally recommended that HiDAC be included in induction regimens outside clinical trials.
Attempts to increase response rates by the use of additional cytotoxic agents (thioguanine, etoposide, fludarabine, topotecan), or modulators of multidrug resistance (MDR) in general have failed.132-137 Sensitization of leukemic cells with hematopoietic growth factors, such as granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage (GM)–CSF, has been studied to increase cytotoxicity of chemotherapy.138 The Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) showed priming with G-CSF resulted in a significantly better DFS, and in the intermediate-risk group also a significantly better OS.139 Similarly, in a study by the Acute Leukemia French Association (ALFA) group, priming with GM-CSF resulted in a higher CR rate and a better EFS, in particular in the intermediate-risk group, albeit without influencing OS.140 In contrast, in a study by the AMLCG, G-CSF priming did not impact OS or RFS.141 Priming with growth factors remains an active field of clinical investigation; it cannot be recommended in routine practice. Another area of induction therapy research is the evaluation of gemtuzumab ozogamicin (GO) administered with conventional chemotherapy (see section 11.1).
7.2 Postremission therapy
7.2.1 Postremission strategies.
High-dose cytarabine.
A landmark study performed by Cancer and Leukemia Group B (CALGB) showed that 4 cycles of HiDAC (3 g/m2 per q12h on days 1, 3, and 5) are superior to 4 courses of intermediate- (400 mg/m2 continuous IV on days 1-5) or standard-dose (100 mg/m2 continuous IV on days 1-5) cytarabine; patients were scheduled to also receive 4 courses of monthly maintenance treatment.142 This beneficial effect of cytarabine dose intensification, however, was restricted to patients with CBF AML and, to a lesser extent, to patients with CN-AML, whereas outcome of patients with other cytogenetic abnormalities was not affected by cytarabine dose.143 There remain open questions regarding the number of cycles, the most appropriate dose and schedule, and the role of combining HiDAC with other agents. Outcome results similar to those after HiDAC consolidation may be obtained using other intense chemotherapy regimens. However, use of prolonged intensive consolidation,144 or of multiagent chemotherapy does not appear to be superior to HiDAC alone.145,146
Maintenance therapy.
In one study there was no benefit in remission duration or OS with 3 years of intensive maintenance compared with autologous HSCT as postremission therapy, whereas maintenance proved superior for DFS to 1 course of consolidation according to the sequential HiDAC and mitoxantrone (S-HAM) protocol (HiDAC 1g/m2 per q12h on days 1, 2, 8, and 9; mitoxantrone 10 mg/m2 on days 3, 4, 10, and 11).147 Maintenance chemotherapy is generally not routinely administered outside of clinical trials for patients with non-APL AML.
Autologous hematopoietic stem cell transplantation.
Autologous HSCT is considered an alternative option for postremission therapy in patients with favorable- and intermediate-risk cytogenetics, whereas it cannot be recommended in patients with high-risk cytogenetics.50,148,149 Outcome after autologous HSCT is at least as good as after the use of postremission chemotherapy; however, there has been no evidence of an improvement in outcome. Autologous HSCT may offer an advantage in specific subsets of AML.150
Allogeneic hematopoietic stem cell transplantation.
Allogeneic HSCT as a postremission strategy is associated with the lowest rates of relapse. This benefit is attributable to both the high-dose therapy of standard conditioning regimens and a potent graft-versus-leukemia (GVL) effect.151 However, benefits of allogeneic HSCT have been limited by the high TRM. Single prospective trials have neither shown a definitive advantage nor disadvantage in OS of allogeneic HSCT for patients with AML in first CR (CR1).152-155 Meta-analyses of clinical trials that prospectively assigned allogeneic HSCT versus alternative consolidation therapies for AML in CR1 on an intent-to-treat donor versus no-donor basis show that allogeneic HSCT offers significant OS benefit for patients with intermediate- and high-risk AML.156-158
The value of allogeneic HSCT needs to be reassessed based on the identification of AML-related genetic changes that profoundly impact on prognosis, on the availability of different transplant sources (bone marrow, blood) and donor types (matched related, unrelated and haploidentical donors, umbilical cord stem cell grafts), and in light of the use of reduced-intensity conditioning (RIC) regimens.65,159 Finally, it is important to consider TRM that may vary between less than 15% and up to 50%. It is essential to assess whether the benefit of the reduced relapse rate outweighs TRM or will be offset by a high TRM. Comorbidity scores, such as the HCTCI,48,160 provide useful guidance in these decisions. Furthermore, a composite risk score, previously established for CML,161 that includes patient age, disease stage, time interval from diagnosis to transplant, donor type, and donor-recipient sex combination, has been shown to be highly predictive of TRM, leukemia-free survival, and OS also in patients with AML.162 Further risk factors include cytomegalovirus (CMV) serum status of recipient and donor,163 and non-HLA genetics, that is, SNPs or microsatellites of cytokines, cytokine receptor genes, or genes associated with innate immunity.164
Thus, for individual clinical decision making, it is recommended to take into account both the disease risk as best assessed by the cytogenetic and molecular genetic profile of the leukemia and the risk associated with the transplant itself as assessed by comorbidity and other transplant-related risk indices.
7.2.2 Postremission therapy according to cytogenetic and molecular genetic risk
Favorable-risk AML.
Postremission therapy with repetitive cycles of HiDAC (3 g/m2 per q12h on days 1, 3, and 5) is considered a reasonable choice for younger adult patients with CBF AML,53,54,165,166 and also for AML with mutated NPM1 without FLT3-ITD and with mutated CEBPA.65
For CBF AML, retrospective studies by CALGB suggest that 3 or more cycles of HiDAC (cumulative dose: 54-72 g/m2) are superior to only one cycle (18 g/m2).165,166 No advantage has been shown for autologous or allogeneic HSCT in frontline treatment.53,156-158,167-169 Nonetheless, there are subsets of CBF AML that do rather poorly (eg, t(8;21) with high WBCs, CBF AML with KIT mutations or molecular disease persistence); allogeneic HSCT may be considered in these patients, especially for those with a low transplant risk (eg, European Bone Marrow Transplant [EBMT] risk 0-1, CMV-negative serostatus, no comorbidity), although such a strategy should be investigated within a clinical trial.
A study by the German-Austrian AML Study Group (AMLSG) provided evidence that those AML patients whose molecular genetic profile predicts a favorable prognosis, such as CN-AML with mutated NPM1 without FLT3-ITD, may also not benefit from allogeneic HSCT.65 Thus, in general, patients with such favorable-risk AML are not considered candidates for allogeneic HSCT, unless they have a very low transplant risk or new transplant strategies (eg, RIC HSCT) are evaluated within a clinical trial.
Intermediate-risk AML.
For the remaining patients with CN-AML (intermediate-I) and those with intermediate-II karyotypes (Table 4), repetitive cycles of HiDAC (3-4 cycles; 3 g/m2 per q12h on days 1, 3, and 5) are currently widely used by many cooperative groups; however, outcome for most of the subsets remains unsatisfactory. There is accumulating evidence that allogeneic HSCT is an attractive option for those patients who are at high risk of relapse. The benefit might be highest for patients with a low or intermediate transplant risk. A beneficial effect has been shown for patients with intermediate-risk cytogenetics in general,156-159 and for patients with CN-AML and unfavorable molecular markers, that is, those who lack the favorable genotypes of mutated NPM1 without FLT3-ITD or mutated CEBPA.65 In particular, although evidence from prospective trials is not available, allogeneic HSCT should be considered in patients whose leukemic cells have FLT3-ITD.65,170
Adverse-risk AML.
For most patients with high-risk cytogenetics, outcome remains dismal with conventional consolidation therapy.49-52,143 An allogeneic HSCT from a matched related donor is currently considered the treatment of choice for patients with unfavorable cytogenetics in CR1; this recommendation is based on results from single studies50,155,156,171 as well as from meta-analyses.156-158 The US Intergroup Study demonstrated an advantage for allogeneic HSCT for patients with unfavorable cytogenetics with a survival of 44% versus 15% for patients receiving only a single cycle of HiDAC consolidation chemotherapy, although the number of patients was small and the consolidation limited.50 Data from the European Organization for Research and Treatment of Cancer (EORTC)/Gruppo Italiano Malattie Ematologiche d'Adulto (GIMEMA) AML-10 trial and from 3 consecutive trials of the HOVON-SAKK group with a larger number of patients demonstrate a benefit for allogeneic HSCT, among younger patients with adverse cytogenetics.155,156
The outcome after allogeneic HSCT from fully matched unrelated donors (defined by molecular high-resolution HLA typing) appears to be similar compared with allogeneic HSCT from matched related donors. The Center for International Blood and Marrow Transplant Research (CIBMTR) recently reported a long-term survival probability of 30% for AML patients with adverse cytogenetics transplanted in CR1 from matched unrelated donors.172 Given the dismal results after conventional chemotherapy, allogeneic HSCT from either matched related or unrelated donors in CR1 is therefore considered a reasonable treatment option for patients with unfavorable cytogenetics.
7.3 Primary refractory disease
Several studies have shown that lack of early blast clearance or nonresponse to the first induction cycle are major predictors for poor outcome, and conventional chemotherapy offers almost no chance of cure for these patients.148,173,174 Consequently, allogeneic HSCT has been widely used for these patients. Retrospective studies show outcome is limited by a high relapse rate and a high nonrelapse mortality leading to OS rates of 20% to 30%.175-179 To improve on these results, alternative conditioning regimens are being investigated. One approach evaluated in a multicenter trial is a sequential strategy of intensive chemotherapy followed, after 3 days of rest, by RIC for allogeneic HSCT, and prophylactic administration of donor lymphocyte infusions.180 A prerequisite for success for such a transplant strategy is rapid identification of a suitable matched donor. HiDAC, if not used for first induction, with or without an anthracycline may be considered for salvage therapy before allogeneic HSCT. Patients with induction failure who are not eligible for allogeneic HSCT should be considered for clinical trials evaluating novel agents.
8. Management of older patients: 60 years or older
Although the prognosis of AML probably worsens with each year of increasing age, “older” patients are generally considered those 60 or older. Older patients are more likely to suffer treatment-related early death and to exhibit therapeutic resistance.45,46,181 Increasing age is associated with factors predictive of early death, for example, poor performance status or various comorbidities, and of treatment resistance, for example, adverse cytogenetics, secondary AML, or the MDR phenotype.45,59-63 Even after accounting for these associations, older age remains an important predictor of poor outcome.45,46,83,182 Evidence is accumulating to indicate that in older patients age-dependent leukemia-specific differences also account for reduced treatment response.82 Older age per se, however, should not be a reason to withhold intensive therapy. Studies suggest that remission induction chemotherapy provides better quality of life and longer survival than supportive care only.46,183,184 Thus, these patients often deserve being offered the option of standard chemotherapy. Older patients from a clinical practice perspective may be divided according to whether they are 60 to 74, or 75 years of age or older.
8.1 Patients age 60 to 74
Induction therapy.
For patients with performance status less than 2 and no comorbidities, standard induction therapy is often a plausible option resulting in CR rates averaging 50% and rates of death in aplasia or from indeterminate cause below 15%.45,181 Similar to younger adults, induction therapy generally consists of 3 days of an anthracycline (eg, daunorubicin 45-60 mg/m2 or an alternative anthracycline at equivalent dose), and 7 days of cytarabine (100-200 mg/m2 continuous IV). Dose reduction may be considered for individual patients. Both American185,186 and European187 cooperative group studies have found that the choice of anthracycline (daunorubicin or idarubicin, or the anthracenedione mitoxantrone) is of little consequence, assuming equitoxic doses are administered. The AMLCG over 15 years has used 60 mg/m2 daunorubicin with acceptable toxicity.147 The HOVON/SAKK/AMLSG recently showed that daunorubicin can be dose-intensified to 3 × 90 mg/m2 in older patients up to 65 years with more CRs and better survival, without marked additional toxicity.188
The degree of acceptability of administering standard induction therapy depends greatly on cytogenetics. Adverse cytogenetics is a strong independent prognostic factor for failure to achieve CR and OS.61-63 For this subset of older patients, CR rates are 30% or less, and OS is less than 5%. Thus, although the karyotype may be unknown at diagnosis in most centers, patients known to have adverse cytogenetics, even those with a good performance status and lacking comorbidities, may be considered for investigational therapies, or, if such therapies are unavailable, for mild cytoreductive therapy only. Recent data suggest delays in initiating therapy may not be harmful in older patients, thus allowing individualized approaches.119
Postremission therapy.
Randomized studies in elderly patients achieving CR are biased because only a low proportion of the initial study cohort is randomized and the majority of these patients have intermediate- or favorable-risk cytogenetics and lack significant comorbidities. These studies have generally compared “more” versus “less” postremission therapy. In the British Medical Research Council (MRC) AML11 study, 4 postremission courses of moderate intensity were compared with 1 course, with equivalent survival in the 2 arms.189 The CALGB compared 2 relatively intense cycles (cytarabine 500 mg/m2 per q12h; mitoxantrone 5 mg/m2 per q12h, each for 6 doses) with 4 less intensive cycles (cytarabine 100 mg/m2 continuous IV on days 1-5) and found no differences.190 In the AMLCG92 trial, older patients benefited with longer remission duration from monthly myelosuppressive maintenance (cytarabine 100 mg/m2 per q12h × 10 with an anthracycline or thioguanine) compared with a single course of the S-HAM regimen (cytarabine dosage: 500 mg/m2 per q12h on days 1, 2, 8, and 9).147 The French ALFA 9803 trial found that 6 cycles of outpatient consolidation (daunorubicin 45 mg/m2 or idarubicin 9 mg/m2 on day 1 and cytarabine 60 mg/m2 per q12h subcutaneously [s.c.] on days 1-5) gave superior DFS and OS than 1 cycle of “4 + 7” consolidation.187 Despite its longer duration, the outpatient arm required less time in hospital and fewer red cell and platelet transfusions. In the AMLSG AML HD98B trial, intensive consolidation (idarubicin 12 mg/m2 on days 1 and 3; etoposide 100 mg/m2 on days 1-5) was superior to a mild 1-year oral maintenance therapy (idarubicin 5 mg per os (p.o.) on days 1, 4, 7, 10, and 13; etoposide 100 mg p.o. on days 1 and 13; q4 weeks).191 From these data no clear recommendation can be given. For patients without adverse cytogenetics, good performance status and no significant comorbidity, standard “3 + 7” induction followed by repetitive cycles of modest dose consolidation may be an acceptable norm, with recent results from the Swedish National Registry suggesting that this approach is associated with longer survival than lower doses of similar therapy.46
There is growing evidence that AML with a favorable genetic profile, that is, CBF AML and AML with mutated NPM1 (with or without FLT3-ITD), may benefit from dose escalation during consolidation.61-63,81-84,188 A recent AMLSG study suggests that AML with mutated NPM1 without FLT3-ITD may benefit from the addition of all-trans retinoic acid to intensive induction and consolidation therapy, although this finding awaits confirmation by further studies.81,192
Allogeneic HSCT using reduced-intensity conditioning.
Allogeneic HSCT in older patients has become an active promising field of investigation.193-201 Nonmyeloablative or RIC regimens have been developed to reduce TRM in older or medically less fit patients. A retrospective study from the Cooperative German Transplant Study Group of 368 patients (median age, 57 years; range, 50-73) suggests that matched unrelated and matched sibling donor allogeneic HSCT (72% had received RIC regimens) result in comparable survival in older AML patients.202
Nevertheless, current data are difficult to interpret due to small patient cohorts, heterogeneity of conditioning regimens applied, and, most importantly, the considerable inherent patient selection bias in the higher age segment.200 Therefore, allogeneic HSCT should be performed within clinical trials. A prospective comparison of allogeneic HSCT from matched related and unrelated donors using RIC with conventional consolidation therapy has been launched by the EBMT group together with several cooperative groups (ClinicalTrials.gov Identifier: NCT00766779).
8.2 Patients age 75 or older
An alternative to standard-dose induction should be sought for patients 75 or older (and probably ≥ 65) with a performance status of 2 or 3, comorbidities, or organ dysfunction. In a randomized trial,203 low-dose cytarabine (LDAC; 20 mg twice daily s.c. for 10 days) was associated with longer survival than hydroxyurea (sufficient dose to keep WBC < 10 × 109/L) and thus might be considered for such patients, but the magnitude of benefit is not so great as to make hydroxyurea or supportive care an unreasonable option. Even with this low-intensity approach, there was a 30-day mortality of 26%. Furthermore, there is no benefit of LDAC in patients with adverse cytogenetics.203,204 The choice of therapy very much depends on a patient's wishes. Any discussion of choice of therapy must refer to observations that 74% of older patients estimated that their chances of cure with “3 + 7” were 50% or more; in contrast, 85% of their physicians estimated this chance to be less than 10%.205
For patients age 75 or older but with a good performance status and no comorbidities, selection of treatment may again be contingent on cytogenetics and to a lesser extent on type of AML (de novo vs secondary after MDS or MDS/MPN). Patients age 66 or older with CBF AML have a 75% CR rate and only a 16% death rate with cytarabine-containing therapy, making standard therapy a very plausible option.206 Furthermore, some such patients if particularly healthy might be candidates for more aggressive consolidation including moderate-dose cytarabine.61-63,83 Recent data suggest that patients age 60 or older with CN-AML and mutated NPM1 (with or without FLT3-ITD) benefit from standard “3 + 7” regimens.81-84
8.3 Cautions and future directions
Patients entered onto clinical trials of either standard or investigational therapy represent a very small, and likely biased, subset,207 and reported results overestimate the effectiveness of therapy in the general population of older AML.46,208 Physicians decide to give treatment to some patients but not others based on generally accurate perceptions of how patients will fare after such treatment,203 or on strict protocol exclusion criteria.207 Examining 2657 Medicare beneficiaries older than 65 in American Surveillance, Epidemiology, and End Results (SEER) registries, Menzin et al208 found that 70% did not receive “chemotherapy,” ranging from 56% of patients age 65 to 74, to 94% of patients age 85 or older. In the Swedish Adult Acute Leukemia Registry, a true population-based registry, 198 of 727 (27%) patients age 65 to 74 and 800 of 1 115 (72%) patients age older than 74 did not receive intensive chemotherapy but palliation only.46
A second problem is the heterogeneity present in any sizable defined group, but hidden by the terms “older patient,” “medically unfit patient,” or “patient ineligible for standard intensive chemotherapy.” Thus, there is a need to consider multiple variables. Besides age, the most important covariates are cytogenetics and secondary AML (following MDS or MDS/MPN), WBC, performance status, and comorbidities.209 No specific comorbidity index has yet been developed for older patients with AML; thus, only organ dysfunctions are currently taken into account.
As is apparent from the many new induction therapies being studied in older patients, it is easier to recognize that a patient needs investigational therapy than it is to specify what that therapy should be. Part of this difficulty reflects the tendency to report results of single-arm trials without reference to even a historical control group.210 Current examples of investigational therapies include clofarabine, cloretazine, azacitidine (or decitabine) ± histone deacetylase inhibitors or GO, “3 + 7” or LDAC combined with GO, and FLT3 inhibitors in patients with activating FLT3 mutations (see section 11). Tipifarnib has been compared with best supportive care in older patients and was found not beneficial.211
9. Therapy-related AML
Therapy-related AML (t-AML) is a recognized clinical syndrome occurring as a complication after cytotoxic and/or radiation therapy. With emerging new therapies, for example, treatment with monoclonal antibodies, small molecules, antihormone agents and growth factors, the term “t-AML” has blurred and its definition needs to be revisited. Therefore, it is important that previous therapies be meticulously documented and reported.
The etiology and specific factors that predispose to therapy-related myeloid neoplasms largely remain elusive.212,213 Various genetic pathways and cooperating mutations are involved in its pathogenesis.214 Two main groups have been described, one comprising leukemias arising 5 to 7 years after therapy with alkylating agents or irradiation and associated with abnormalities of chromosome arms 5q and/or 7q, and a second occurring with a shorter latency, within 2 to 3 years, after therapy with agents targeting topoisomerase II that are often associated with a translocation involving bands 11q23 (MLL) or 21q22 (RUNX1). However, there appear to be more pathways comprising chromosomal rearrangements and mutations in multiple genes (eg, TP53, RUNX1, RAS).214
Survival of t-AML patients has been poor compared with that of patients with de novo AML.212,215-221 Several factors may explain the poor outcome, such as persistence of the primary malignant disease, injury to organs from prior therapy, depletion of normal hematopoietic stem cells, damage to marrow stroma (in particular by radiation therapy), chronic immunosuppression or dysfunctional phagocytes leading to colonization with pathogenic or antibiotic-resistant bacteria and fungi, and refractoriness to transfusion support. Finally, t-AML is associated with a higher frequency of unfavorable cytogenetics.216,217,220-223 In multivariable analyses, however, t-AML appears to remain an independent adverse prognostic factor.221,222 Scarce data are available regarding whether t-AML changes outcome in general, or only in specific subsets.220,221,224-227 A recent study suggests that survival is also poorer in patients with therapy-related CBF AML compared with de novo CBF AML.227
There is a paucity of prospective treatment data because patients with t-AML have often been excluded from frontline clinical trials. There are no randomized studies comparing standard AML therapy to other forms of treatment. In a review of 644 t-AML patients treated with various standard AML chemotherapy regimens, only 182 (28%) achieved a CR.218 Individual small series report CR rates of 40% to 50%. The treatment most likely to cure t-AML is allogeneic HSCT. In case series, OS appears to be approximately 20% to 30%.228-230 Nonmyeloablative, RIC allogeneic HSCT is under investigation for those who are not eligible for standard myeloablative HSCT. The EBMT Registry reported on 65 t-AML patients who underwent autologous HSCT; the 3-year OS was 35%.231
Thus, primary considerations for treatment of t-AML patients should include the status of the primary cancer, the patient's performance status, the presence of complications from primary therapy, and the leukemic karyotype. Patients with t-AML should be encouraged to participate in prospective trials that are designed for other AML patients with similar genetic changes. Patients who have an HLA-matched donor should be considered for allogeneic HSCT.
10. Relapsed AML
In the majority of patients with AML who achieve a CR, the leukemia will recur within 3 years after diagnosis. In general, the prognosis of patients after relapse is poor and treatment options unsatisfactory.232-235
10.1 Prognostic factors in relapsed AML
Because of the poor prognosis of patients in relapse, it would be useful to be able to assess whether treatment with curative intent is a realistic possibility for a particular patient. Long-term survival will depend on the ability to successfully induce a remission and the probability to consolidate with HSCT. Patients with an early relapse (ie, duration of CR1 < 6 months), adverse cytogenetics, or older age have a poor outcome. An estimate of long-term survival can be obtained by assessing a defined set of prognostic factors as shown in Table 7.234 The prognostic score furnishes a basis for recommendations regarding treatment with curative objectives, or palliative therapy, or therapy in the context of a phase 1/2 study.
10.2 Reinduction of remission
There is a lack of prospective controlled studies evaluating different treatments in relapsed AML, and therefore no generally established standard. A commonly accepted approach is to define a treatment that is directed at achieving a new remission and that leads to HSCT. One mainstay of second-line treatment is cytarabine which can be used at intermediate (1 g/m2) and high-dose (2-3 g/m2) levels. An example of a salvage regimen is cytarabine (3 g/m2 per q12h on days 1, 3, 5, and 7) combined with daunorubicin (50 mg/m2) or idarubicin (10 mg/m2) on days 2, 4, and 6.236 Another schedule is single agent cytarabine for 6 days (2-3 g/m2 per q12h).237 Regimens that include cytarabine at high doses cannot be safely applied in patients aged 60 years or older because of an unacceptably high risk of toxicity. Another schedule is mitoxantrone (10 mg/m2 per day) and etoposide (100 mg/m2 per day) given for 5 days.238,239 GO (see section 11.1) can produce remissions in older patients, especially those relapsing after a prolonged (> 6 months) CR1. Patients with short initial first remissions may be candidates for an investigational drug.
10.3 Salvage consolidation treatment including stem cell transplantation
Allogeneic HSCT is the preferred consolidation therapy once a new remission has been attained.234 This can be a transplant from an HLA identical sibling donor, or a matched unrelated donor. If such a donor is not available, an alternative donor can be considered, that is, an umbilical cord blood unit, or a haploidentical donor. In this situation, the risk of the disease has to be balanced with the risk of the transplant.234 The long-term value of allogeneic HSCT after RIC regimens remains to be evaluated but current experience suggests a greater relapse incidence compared with transplants after high-dose preparative regimens. If an allogeneic HSCT is not possible (eg, due to lack of a suitable donor), an autologous HSCT is often regarded as the second best option.149,240 However, often it is not possible to collect an adequate leukemia-free autograft at this phase of the disease. Retrospective studies show that patients who achieve second CR and proceed to autologous HSCT have a probability of long-term survival in the range of 20% to 50%.149 The outcome obviously applies to a highly selected minority of patients. Patients who have already undergone an allogeneic HSCT before relapse generally have immunoprophylaxis discontinued, and they may receive donor lymphocyte infusions.241-243 These interventions are not an option in patients who already have active graft-versus-host disease (GVHD). Finally, transplant recipients with a late relapse (> 1 year after allogeneic HSCT) may be offered a second transplant.244
11. Molecularly targeted therapy
Progress has been made in deciphering the molecular pathogenesis of AML, and in a few instances this has led to the development of molecularly targeted approaches. Genetic alterations comprise mutations in genes that activate signal transduction cascades (eg, FLT3, KIT, RAS), gene fusions or mutations resulting in enhanced or repressed transcriptional activity (eg, PML-RARA, RUNX1-RUNX1T1, CBFB-MYH11, CEBPA), or altered function of genes involved in nuclear cytoplasmic shuttling (eg, NPM1, NUP98, NUP214).245,246 Inhibition of deregulated transcriptional activity has, with the exception of the use of all-trans retinoic acid and arsenic trioxide in APL, proved to be a less tractable pharmacologic goal compared with inhibition of constitutive tyrosine kinase activity. Finally, targeting the myeloid-associated antigen CD33 by the use of anti-CD33 monoclonal antibody has become an interesting new approach.
It is commonly accepted that the AML phenotype results from multiple genetic/epigenetic lesions affecting differentiation, proliferation, and apoptosis. Consequently, targeting of a single aberrant protein is unlikely to eradicate the leukemic clone. Furthermore, although several molecularly targeted therapies have been shown to be active in AML, it is clear from early clinical studies that most of these novel agents will need to be used in combination with conventional cytotoxic therapy. In the following sections, only 3 modalities are considered that have reached phase 3 clinical trial development for frontline therapy of AML.
11.1 Gemtuzumab ozogamicin
GO is a humanized anti-CD33 antibody chemically linked to the cytotoxic agent calicheamicin that inhibits DNA synthesis and induces apoptosis.247-249 GO is approved for relapsed AML (currently in the United States and Japan, but not in Europe) in older patients who are not considered candidates for other cytotoxic therapies.249 GO can produce remissions in 15% to 35% of older patients in first relapse. In a feasibility study in younger adults, the addition of GO to standard induction therapy led to a promising 91% CR rate.250 Randomized trials evaluating the addition of GO to conventional chemotherapy have been completed (eg, MRC AML 15 trial; final results are pending) or are ongoing (eg, SWOG Protocol S0106; ClinicalTrials.gov Identifier: NCT00085709).
11.2 FLT3 inhibitors
Several FLT3-selective tyrosine kinase inhibitors (eg, midostaurin [PKC412],251 lestaurtinib [CEP-701],252 sunitinib [SU11248]253 ) have in vitro cytotoxicity to leukemia cells. These inhibitors have activity as a single agent in relapsed AML with FLT3 mutations, although usually only a transient reduction of blasts in blood, and, to a lesser extent, in marrow, has been observed.254-256 Pilot studies combining intensive induction and consolidation therapy with FLT3 inhibitors have shown promising response rates in patients with FLT3 mutations. A randomized phase 3 trial has been initiated evaluating midostaurin as frontline therapy of younger adult AML patients with activating FLT3 mutations (ClinicalTrials.gov Identifier: NCT00651261).
11.3 Demethylating agents
Two demethylating agents, the cytosine analogs azacitidine and decitabine,257-259 have been approved for the treatment of MDS, azacitidine by the Food and Drug Administration (FDA) and the European Medicine Agency (EMEA) and decitabine by the FDA. In a phase 3 randomized trial, azacitidine prolonged OS compared with conventional care regimens in patients with intermediate-2 or high-risk MDS.260 Approximately one-third of these patients (n = 113) were classified as having AML under current WHO criteria (blast counts 20%-30%). Of note, in these patients, the 2-year OS was 50% with azacitidine compared with 16% with conventional treatment regimens.261 Based on these results, azacitidine has been approved for older AML with 20% to 30% blasts. While these results are promising, data for more proliferative AML are currently not available. Randomized trials comparing demethylating agents with conventional cytotoxic agents in older AML patients with blast percentages more than 30% are underway.
12. Management of special situations
12.1 Hyperleukocytosis
Hyperleukocytosis, generally defined as a WBC more than 100 × 109/L, is associated with increased induction mortality mainly due to hemorrhagic events, tumor lysis syndrome, and infections.262 Hyperleukocytosis with leukostasis and, for example, pulmonary infiltrates or retinal and cerebral hemorrhages requires immediate medical treatment. Leukapheresis is an option for the initial management of hyperleukocytosis; however, no impact on long-term outcome has been shown.263,264 In general, the recommended therapy to lower WBC is hydroxyurea, given at dosages up to 50 to 60 mg/kg per day, until WBCs are less than 10-20 × 109/L. Until the WBC has been reduced, excessive red blood cell transfusions can lead to increased blood viscosity. Special attention should be given to the prevention of tumor lysis syndrome (eg, hydration, control of uric acid production using allopurinol or rasburicase, control of urine pH).
12.2 Central nervous system involvement
Initial involvement of the central nervous system (CNS) in AML occurs in less than 5% of patients.265 There is no indication for intrathecal prophylaxis in patients without CNS symptoms,265,266 although it may be considered in special situations (eg, hyperleukocytosis).
In patients with CNS involvement, 40 to 50 mg of cytarabine should be administered intrathecally, 2 to 3 times per week until clearance of blasts, followed by 3 further injections with the same dosage. Alternatively, liposomal cytarabine (50 mg every other week) may be given for approximately 6 cycles. For prevention of arachnoiditis, dexamethasone (4 mg three times a day [tid] p.o.) may be considered on the days of intrathecal application. Prolonged application of intrathecal therapy does not appear to be justified, given that such therapy carries the risk of complications (eg, leukencephalopathy). In patients with a CNS recurrence, craniospinal irradiation with or without intrathecal chemotherapy has also been shown to be effective; however, its impact on long-term outcome is unknown.267
12.3 Myeloid sarcoma
Myeloid sarcoma (synonyms: extramedullary myeloid tumor, granulocytic sarcoma, chloroma) is a tumor mass consisting of myeloid blasts in which the tissue architecture is effaced, occurring at an anatomical site other than the bone marrow,3 most commonly in skin, lymph nodes, gastrointestinal tract, bone, soft tissue, and testis. Myeloid sarcoma may present de novo preceding AML, concurrently with AML, or as blastic transformation of MDS, MPN, or MDS/MPN. Diagnosis is ascertained by cytochemical and/or immunohistochemical analyses. Often myeloid sarcomas have myelomonocytic or monoblastic morphology. Myeloid sarcomas should be evaluated for genetic and phenotypic features that allow for their classification in the appropriate WHO entity. Myeloid sarcomas have been associated with hyperleukocytosis, t(8;21), and CD56 positivity.268,269
Myeloid sarcoma occurring de novo should be considered as AML and treated as such. Data on the prognostic impact of myeloid sarcoma are limited. Whereas some studies reported a negative impact in selected subgroups,269,270 others suggest that outcome of patients with myeloid sarcoma after conventional chemotherapy or allogeneic HSCT may not be inferior.271,272 Involved field radiation therapy may be considered to enhance local tumor control.
12.4 Pregnancy
Acute leukemia during pregnancy should be managed jointly by the hematologist, obstetrician, and neonatologist. Consideration must be given to both the health of the mother and to the immediate as well as long-term consequences for the fetus exposed to cytotoxic agents. Pregnancy does not appear to alter the course of AML, with more than 75% of patients achieving CR after standard chemotherapy.273-275 Treatment should be started immediately because delays may compromise maternal outcome.274
Leukemia in a pregnant woman bears an increased risk for abortion, intrauterine growth restriction, and perinatal mortality.276-278 The earlier in gestation the diagnosis of leukemia, the higher the incidence of spontaneous abortion, prematurity, and low birthweight. The risk of teratogenicity is highest during weeks 2 to 8 after conception, the period of organogenesis.278 Malformations after exposure to anthracyclines and cytarabine in the first trimester have been reported.278 Idarubicin differs from the other anthracyclines because it is more lipophilic favoring an increased placental transfer and has a higher DNA affinity; thus, daunorubicin should be given rather than idarubicin.279 Given the risk of teratogenicity, the option of therapeutic termination during the first trimester of pregnancy should be submitted for consideration by the mother. Chemotherapy delivered during the second and third trimester of pregnancy has been reported as safe, although stillbirths and low birthweight have also been observed.273,275-277 Delivery while patient and fetus may be cytopenic should be avoided.
13. Supportive care
13.1 Prophylactic anti-infectious treatment
For prophylaxis and treatment of infectious diseases, prevailing institutional infectious organisms and their drug-resistance pattern should primarily be considered. Personal hygiene, dental care, and vigorous hand washing (the latter also for family and caregivers) are very important for prevention of infections. Reasonable precautions should be undertaken to protect patients from bacteria or fungi in their environment. Although eating fresh fruits and/or vegetables is often discouraged, there is little evidence that adherence to such a “neutropenic diet” prevents infections.280
Fungal prophylaxis.
Invasive fungal infections are a major cause of morbidity and mortality in patients with prolonged neutropenia.281 A review of randomized trials in AML found a significant reduction in fungal infection–related mortality and invasive fungal infection in patients given antifungal prophylaxis rather than placebo while the difference in all-cause mortality was borderline significant.282 Prophylaxis with itraconazole, posaconazole, or amphotericin, that is, drugs with antimold activity, reduced the risk of documented aspergillus infection and likely had some effect on mortality. A recent trial found that patients randomized to posaconazole had fewer invasive fungal infections than patients randomized to either fluconazole or itraconazole according to institutional practice.283 Survival was longer in the posaconazole group, although it is unclear how much of the difference was due to the superiority of posaconazole to itraconazole rather than the superiority of posaconazole to fluconazole. In addition, prophylaxis may not be as efficacious if other modes of prophylaxis are used, for example, high-efficiency particulate (HEPAR) air filtration. Nonetheless, the limited data suggest that patients should receive an antimold agent, rather than fluconazole, during remission induction therapy.
Antibiotic prophylaxis.
Bacterial infections are an important cause of morbidity and mortality in neutropenic patients after chemotherapy for AML.284 A Cochrane review compared antibiotic prophylaxis with placebo or no intervention in afebrile neutropenic patients.285 Antibiotic prophylaxis significantly decreased the risk of death and the risk of infection-related death. The most significant reduction in risk for all-cause mortality was observed in trials testing prophylaxis with quinolones, despite the occurrence of adverse effects and development of resistance.286 Thus, antibiotic prophylaxis should be given after chemotherapy for AML with a preference for a quinolone.
13.2 Growth factors
Numerous studies138,185,287-303 have shown that myeloid growth factors, either GM-CSF or G-CSF, accelerate neutrophil recovery by 2 to 5 days, can reduce antibiotic use, duration of fever, and number of days spent in hospital, and do not retard platelet recovery, or have a detrimental effect by stimulation of leukemic cell growth. However, the use of growth factors does not translate into a survival benefit. The cost effectiveness of growth factors is difficult to assess and has been inconsistently reported, but there does not seem to be significant cost savings. Thus, the general use of growth factors in AML cannot be recommended. However, in individual cases (eg, severe infection before expected neutrophil recovery), growth factor use can be considered.
13.3 Transfusion support
Platelet transfusion.
The introduction of platelet transfusions has dramatically reduced mortality from hemorrhage in AML.304 For many years, platelet transfusions were given to keep platelet counts above 20 × 109/L. However, in 3 randomized studies, no significant differences in severe bleeding were shown if a threshold of less than 10 × 109/L rather than less than 20 × 109/L for prophylactic platelet transfusion was used.305-307 American Society of Clinical Oncology guidelines recommend a threshold of 10 × 109/L for prophylactic platelet transfusions.308 Besides the platelet count, mucosal bleeding, infection, severe mucositis, and fever should be considered in the assessment of bleeding risk and should increase the transfusion threshold.
To prevent alloimmunization, removal of contaminating leukocytes is advised. Nevertheless, alloimmunization remains a major obstacle to effective transfusion due to antibodies to HLA class I antigens in many women with prior pregnancies or patients with prior transfusions.309 Such alloimmunized patients are transfused with either HLA-matched platelets or crossmatch-compatible platelets. Both are equally effective.310 Although single-donor platelets are often thought preferable to platelets obtained from multiple donors, a randomized trial showed that they were no more effective in reducing alloimmunization or refractoriness to transfusions.311 The antifibrinolytic agent tranexamic acid can be useful in reducing bleeding and platelet transfusion.312 Anecdotal reports suggest a role for the use of recombinant human activated factor VII in the treatment of bleeding in AML patients refractory to platelet transfusions; however, well-designed clinical trials are lacking.313
Red blood cell transfusion.
Although evidence is lacking, it is generally accepted to keep the hemoglobin level above 8 g/dL, especially in thrombocytopenic patients.
Granulocyte transfusion.
No good evidence exists to recommend granulocyte transfusions in the treatment of AML. A multicenter randomized trial to address the utility of such transfusions in the setting of infections is being conducted in the United States (ClinicalTrials.gov Identifier: NCT00627393).
Transfusion practice before, during and after HSCT.
Only leukocyte-depleted erythrocyte and platelet components should be given to decrease the risk of HLA-associated alloimmunization and reduce the risk of CMV transmission.314 Both autologous and allogeneic stem cell recipients are at risk for transfusion-associated GVHD. Gamma irradiation (at least 25 Gy) is the only reliable method to prevent transfusion-associated GVHD. There is no general consensus when to begin with the transfusion of irradiated blood products. Commonly used practice is to transfuse irradiated blood products from start of conditioning until 6 months after transplant (in the absence of chronic GVHD; allogeneic HSCT), and from 7 days before stem cell harvest until 3 months post transplant for autologous HSCT. Some centers start as soon as a patient is identified to be a potential HSCT candidate. All CMV-seronegative HSCT recipients with seronegative donors should receive CMV-seronegative blood products.
Acknowledgments
We gratefully acknowledge Rüdiger Hehlmann for his generous support of the initiative on behalf of the European LeukemiaNet, Michael Borowitz for reviewing the section on immunophenotyping, and Krzysztof Mrózek and Alois Gratwohl for critically reviewing the manuscript.
This work was supported by the European Union, Sixth Framework Programme, contract no. LSHC-CT-2004-503216 (European LeukemiaNet).
Authorship
Contribution: All authors reviewed the literature and wrote first drafts of specific sections; H. Döhner and C.D.B. assembled the sections and wrote the final version of the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: H. Döhner serves as a consultant to Celgene. A.K.B. receives research funding from Cephalon, Genzyme, Novartis, and Cyclacel. H. Dombret serves as a consultant to Genzyme, Celgene, Sanofi-Aventis, Chugai, Vion, and Bristol-Myers Squibb, and receives lecture fees from speaking at the invitation of sponsors Bristol-Myers Squibb, Celgene, Genzyme, Novartis, and GlaxoSmithKline. P.F. receives research funding from Amgen, Celgene, and Roche, and serves as a consultant to Celgene, Novartis, Merck, Ortho Biotech, Cephalon, and Amgen. R.A.L. receives research funding from Amgen, Bristol-Myers Squibb, Celgene, Novartis, Gemin X, Sanofi-Aventis, Chroma Therapeutics, and Ambit Biosciences, and serves as a consultant to Novartis, Hana Biosciences, Antisoma, Caremark, Trubion, and Celgene. J.S. receives research funding from Amgen, Celgene, and Novartis, and serves as a consultant to Wyeth and Genzyme. M.S.T. serves as a consultant to Genzyme. The remaining authors declare no competing financial interests.
Correspondence: Dr. Hartmut Döhner, Department of Internal Medicine III, University of Ulm, Albert-Einstein-Allee 23, 89081, Ulm, Germany; e-mail: hartmut.doehner@uniklinik-ulm.de.