Abstract
The initiation of immune responses is associated with the maturation of dendritic cells (DCs) and their migration to draining lymph nodes. En route activated DCs encounter cells of the tissue microenvironment, such as fibroblasts. Because we have shown that DCs interact with fibroblasts during immune responses, we studied the impact of skin fibroblasts on human monocyte-derived DC function and subsequent human T-cell (TC) differentiation. We show that fibroblasts support interleukin-23 (IL-23) secretion from DCs preactivated by lipopolysaccharide (DCact) compared with lipopolysaccharide-activated DCs alone. The underlying complex feedback-loop mechanism involves IL-1β/tumor necrosis factor-α (from DCact), which stimulate fibroblasts prostaglandin E2 production. Prostaglandin E2, in turn, acts on DCact and increases their IL-23 release. Furthermore, fibroblast-stimulated DCact are far superior to DCact alone, in promoting the expansion of Th17 cells in a Cox-2-, IL-23-dependent manner. Using CD4+CD45RO+ memory TCs and CD4+CD45RA+ naive TCs, we showed that fibroblasts induce a phenotype of DCact that promotes the expansion of Th17 cells. Moreover, in psoriasis, a prototypic immune response in which the importance of IL-23/Th17 is known, high expression of Cox-2 in fibroblasts was observed. In conclusion, skin fibroblasts are involved in regulation of IL-23 production in DCs and, as a result, of Th17 expansion.
Introduction
Dendritic cells (DCs) are in permanent contact with their tissue microenvironment. For example, niches created by the stromal microenvironment in the bone marrow enable DC precursors to differentiate into DC subpopulations.1,2 In peripheral tissues, DCs interact with a microenvironment composed of the extracellular matrix and stromal cells, such as fibroblasts, macrophages, and endothelial cells. Recently, we demonstrated an interaction of DCs and fibroblasts, resulting in enhanced migration of DCs.3 Several groups reported the effect of stromal cells on the regulation of DC functions under steady-state as well as inflammatory conditions.4,5 Renkl et al6 and Termeer et al7 showed that maturation and differentiation of DCs are regulated by the extracellular matrix components hyaluronic acid and osteopontin. Zhang et al reported splenic stromal cells to drive DCs toward a regulatory phenotype.8 Furthermore, emigration of Langerhans cells from the skin to the lymph node is influenced by a lack of matrix-associated SPARC (secreted protein, acidic and rich in cysteine), resulting in enhanced cutaneous contact hypersensitivity.9
Fibroblasts are thought to be involved in the regulation of DC functions because they synthesize factors known to influence DC function, such as cytokines, chemokines, prostanoids, matrix components, and matrix-degrading enzymes.10-12 Moreover, transcriptome analysis of fibroblasts showed significant differences between fibroblasts from different anatomic regions.12,13 Thereby, fibroblasts could transfer tissue-specific information to DCs.12,13 In skin, fibroblasts regulate the emigration of Langerhans cells from epidermis to dermis via CXCL12 secretion.14 Briard et al showed that fibroblasts can mediate the maturation of DC and natural killer cells in spleen.15
In response to pathogens and under the influence of the microenvironment, DCs secrete specific cytokines, involved in polarization of T-cell (TC) subsets mediating distinct types of inflammation to combat pathogens.16 Interleukin-12 (IL-12) promotes Th1 differentiation, IL-4 is critical for the Th2 lineage.17 IL-23 promotes the development of the novel Th17 population, characterized by the production of IL-17A,17,18 IL-22,18-20 and expression of RORγt.17,21 Th17 cells are important for defense against extracellular pathogens.22,23 On the other hand, the IL-23/IL-17A axis plays a role in different autoimmune diseases, including psoriasis.24-27
Here, we show that skin fibroblasts actively participate in the regulation of an immune response by affecting the IL-23 production of DCs and thus supporting the Th17 cell expansion.
Methods
Antibodies
Antibodies include anti–CD83-fluorescein isothiocyanate (FITC), anti–CD86-FITC, anti–CD80-FITC, anti–CD1a-phycoerythrin (PE), anti–CD3-PE, and anti–Cox-2 (BD Biosciences); anti–HLA-DR-FITC, anti–CD11c-PE, anti–CD3-FITC, anti–CD4-PE, anti–CD45RO-FITC (Beckman Coulter); anti-CD1c, anti–CD45RA-FITC, and anti–IL-17A-PE; anti–INGγ-PE (Miltenyi Biotec); anti–RORγt-PE (eBioscience); and anti-iNOS (Dianova). Secondary goat anti–rabbit FITC was purchased from Santa Cruz Biotechnology, and neutralizing anti–human IL-23p19 antibody, anti–human IL-6, and normal IgG (isotype control) from R&D Systems.
Cell preparation and culture conditions
Human DCs were generated from CD14-positive monocytes and cultured in DC-medium (RPMI 1640, Invitrogen) supplemented with 2% fetal calf serum (Promocell), 1% penicillin/streptomycin (Invitrogen), 1% l-glutamine (Invitrogen), 0.1mM nonessential amino acids (Invitrogen), 10mM HEPES (Invitrogen), 100 U/mL IL-4 (PeproTech), and 1000 U/mL granulocyte-macrophage colony-stimulating factor (Leukine, Berlex).3 Morphologic analysis and high expression of CD1a and CD11c were parameters for quality and purity of DC preparations. An immature phenotype of DCs was verified by low expression of CD80, CD83, CD86, and HLA-DR.
Human Pan TC, CD4+ TCs and memory CD4+ TCs were isolated from PBMC using the Pan T Cell Isolation Kit II, CD4+ T Cell Isolation Kit II and Memory CD4+ T Cell Isolation Kit (Miltenyi Biotec), respectively, according to manufacturer's instructions. Naive CD4+ TCs were gained by 2-step isolation with first using the CD4+ T Cell Isolation Kit II followed by the Naive CD4+ T Cell Isolation Kit (Miltenyi Biotec). Purity of TC populations was verified by flow cytometry with anti-CD3 for Pan TC, anti-CD4 for CD4+ TC as well as anti-CD4, anti-CD45RO, and anti-CD45RA for memory and naive TCs. Cell culture medium was a mixture of DC medium and proliferation medium (DC-RPMI supplemented with 10% fetal calf serum).
Human fibroblasts were isolated from skin biopsies by enzymatic digestion as described and cultured in Dulbecco modified Eagle medium (Biochrom) containing 10% fetal calf serum and 1% penicillin/streptomycin (Biochrom) up to the fourth passage.3 Morphologic analysis and high expression of Thy-128 controlled for purity of fibroblasts. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the University of Leipzig (065-2009).
Coculture experiments and supernatant transfer
DC-fibroblast coculture.
Dermal fibroblasts were cultured in 24-well-plates to confluence. For preactivation, DCs were stimulated with a final concentration of 1 μg/mL lipopolysaccharide (LPS). After 3 hours, DCs were harvested and washed with phosphate-buffered saline (PBS) to remove LPS. For coculture, 4 × 105/mL DCs were added to washed, allogeneic fibroblasts in DC-RPMI and incubated for 18 hours. As control, LPS-stimulated DCs and fibroblasts were cultured alone under identical conditions. Supernatants were collected after 18 hours. For RNA preparation, cells were harvested after 3 hours of coculture. As indicated, 1 μg/mL bioactivity-neutralizing antibodies or 2μM indomethacin (Sigma-Aldrich) were added.
DC-TC coculture.
A total of 3 × 105 TCs and 1.5 × 104 DCs were cocultured in 96-well F-bottom plates in DC-medium containing 10% fetal calf serum. Before DC-TC coculture, fibroblasts were removed from the DC-fibroblast coculture using anti–Thy-1–coupled magnetic beads (Invitrogen).28,29 For control, LPS-stimulated DCs, which had been cultured alone, were used under the same conditions. After 3 days, cells were harvested and used for intracellular flow cytometric analysis. After 6 days, cell culture supernatants were collected. As indicated, 1 μg anti–IL-23p19, anti–IL-6, or isotype control was added to DC-TC coculture.
For experiments with supernatants of DC-fibroblast cocultures, 2 × 105 Pan, CD4+, memory, or naive TCs per well were resuspended in a 1:1 mixture of fresh proliferation medium and coculture-conditioned DC-RPMI in the presence of 1.5 aCD3/CD28 beads (Invitrogen) per TCs. After a proliferation time of 5 days, TC supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA).
Transfection of fibroblasts and DCs with siRNA
Subconfluent fibroblasts were transfected with either 25nM siRNA (primer 1: UCCAGACAAGCAGGCUAAUACUGAU, primer 2: AUCAGUAUUAGCCUGCUUGUCUGGA) specific for human cyclooxygenase (Cox) 2 or 25nM scrambled siRNA (Invitrogen) using Lipofectamine RNAiMax Transfection Reagent (Invitrogen) overnight according to manufacturer's instructions.
DCs were transfected with same siRNA specific for human Cox-2 or scrambled siRNA (Invitrogen) using Amaxa-transfection system (Lonza Germany). A total of 2 × 106 DCs were transfected with 2μM Cox-2 specific siRNA or scrambled siRNA using program X001. Subsequently, 5 × 105 transfected DCs were cultured in DC medium without IL-4 and granulocyte-macrophage colony-stimulating factor overnight. For transfection, control 1 μg/mL LPS was added, prostaglandin E2 (PGE2) secretion was detected after 24 hours by ELISA. For coculture experiments, transfected DCs were stimulated for 3 hours with 1 μg/mL LPS and coculture was performed as described in “Coculture experiments and supernatant transfer.”
Flow cytometry
For analysis of extracellular epitopes, cells were incubated with indicated labeled antibody for 45 minutes at 4°C. Cells were washed twice with PBS/Gelafusal (Serumwerke Bernburg)/sodium acid. Antibody binding was analyzed by flow cytometry (FC 500, Beckman Coulter). For intracellular flow cytometric staining, TCs were restimulated using 5 ng/mL phorbol myristate acetate (Sigma-Aldrich) and 500 ng/mL ionomycin (Sigma-Aldrich) in the presence of 100 ng/mL brefeldin A (Calbiochem) for 4 hours. Cells were harvested, washed twice with PBS/Gelafusal/sodium acid, and incubated with anti-CD3 for 45 minutes at 4°C for selective recognition of TCs. Afterward, cells were fixed with 4% paraformaldehhyde in PBS and permeabilized with 0.1% saponin. Then, indicated labeled antibody was added for 45 minutes at 4°C, and cells were washed twice with PBS/Gelafusal/sodium acid.
RNA preparation and real-time PCR
Fibroblasts and DCs from coculture experiments were separated using anti–Thy-129 coupled magnetic beads (Invitrogen). Success of the separation was controlled by FACS staining with anti–Thy-1 and anti-CD11c29 and real-time polymerase chain reaction (PCR) analysis of Thy-1 and CD11c. FACS analysis revealed complete depletion of DC from fibroblasts, whereas the more sensitive PCR showed that approximately 1% of DCs could not be removed from the fibroblasts.
After separation of DCs and fibroblasts, total RNA was then isolated with the innuPREP RNA Mini Kit (Analytik Jena), and 0.5 μg of total RNA was used for first-strand cDNA synthesis with M-MLV reverse transcriptase (Promega) according to the manufacturer's protocol. Real-time PCR was performed. The following primers (metabion International) were used: Ribosomal protein S26 (RPS26): forward: 5′-GCAGCAGTCAGGGACATTTCTG-3′, reverse: 5′-TTCACATACAGCTTGGGAAGCA-3′, IL-23p19: forward: 5′-TCCAAGCCTCAGTCCCAG-3′, reverse: 5′-TGGGGTGGTAGATTTATCTTGG-3′, COX2: forward: 5′-TGCTGTGGAGCTGTATCCTG-3′, reverse: 5′-TCATCTAGTCCGGAGCGG-3′, CD11c: forward: 5′-TTCTGACAGCCAATGTGAGC-3′, reverse: 5′-TCCTGGTTCAGCTCCACAG-3′, TNF-α: forward: 5′-GCAATGATCCCAAAGTAGACCTGCCCAGACT-3′, reverse: 5′- GAGTGACAAGCCTGTAGCCCATGTTGTAGCA-3′, IL-1β: forward: 5;-GACACATGGGATAACGAGGC-3′, reverse: 5′-ACGCAGGACAGGTACAGATT-3′. Genes were quantified through a standard curve, normalized to the unregulated housekeeping gene RPS26 and computed as percentage of mRNA expression in DC-LPS. For TNF-α, the relative level of each mRNA was calculated on the basis of ΔCt values. Genes were normalized to the unregulated housekeeping gene RPS26 and computed as percentage of mRNA expression in DC-LPS.
ELISA
IL-23, IL-17, TNF-α, IL-12, IL-6, IL-1β, transforming growth factor-β (TGF-β), interferon-γ (IFN-γ), and PGE2 were detected by ELISA (eBioscience, BD Biosciences, and R&D Systems) according to the manufacturers' instructions.
Immunohistochemistry
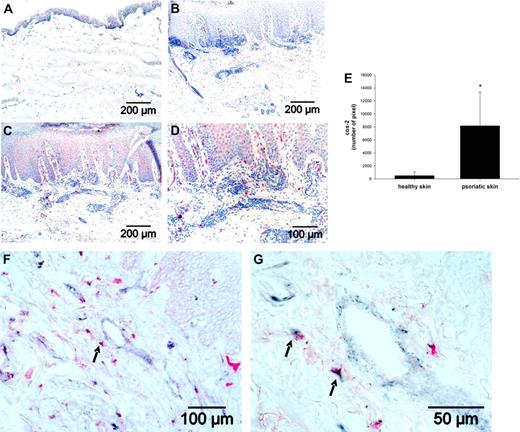
Frozen tissue sections of healthy skin and lesional psoriatic skin (n = 6) were fixed with 4% paraformaldehyde and permeabilized with PBS/0.5% Tween20. Cox-2 expression was detected with an anti–Cox-2 antibody, avidin-biotin-alkaline phosphatase complex, according to the manufacturer's protocol (Biogenix) and visualized colorimetrically using the New Fuchsin substrate system (Dako). For quantification of Cox-2 expression, skin sections were scanned with a digital camera (Carl Zeiss; 5 shots per skin) and analyzed with HistoClick software based on morphometric image analysis developed in our laboratory.30 The degree of Cox-2 expression is expressed by the number of pixels.
For double labeling, Cox-2 expression was detected in the same way. Subsequently, sections were washed and blocked with an avidin/biotin blocking kit (Vector Laboratories). After washing, Thy-1 was detected by a biotinylated anti–Thy-1 antibody and strepavidin-peroxidase (Vector Laboratories). Bound antibodies were detected by ImmuPACT substrate SG resulting in a gray-blue color. Tissue sections were extensively washed with PBS/0.1% Tween20 after every antibody incubation step. In control sections, primary antibodies were replaced by appropriate isotype control antibodies.
Statistical analysis
Distribution of data was assessed by Shapiro-Wilk test; and depending on the normality of the data, analysis was performed using Mann-Whitney rank-sum test or Student t test. Values of P less than .05 were considered to be significant.
Results
Human dermal fibroblasts increase IL-23 production of monocyte-derived DCs
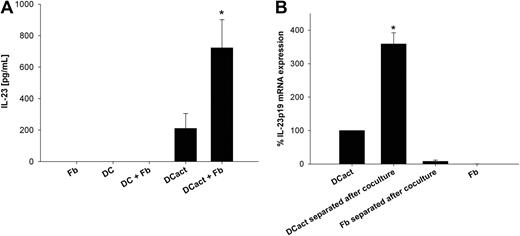
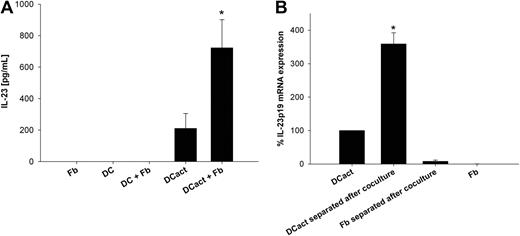
Monocyte-derived DCs were preactivated with LPS (DCact) for 3 hours to imitate DCs within an inflammatory environment. Subsequently, DCact were cocultured with skin-derived fibroblasts, and IL-23 was measured in the coculture supernatants. DCact alone produced intermediate amounts of IL-23, whereas fibroblasts markedly increased IL-23 secretion by DCact (Figure 1A). Because fibroblasts stimulated with LPS did not express or secrete IL-23, we can exclude a direct effect of residual amounts of LPS carried over with DCs into the coculture on IL-23 secretion from fibroblasts (data not shown). In supernatants of unstimulated DCs or fibroblasts alone as well as unstimulated DCs in coculture with fibroblasts, no IL-23 could be detected (Figure 1A). To identify the cellular source of IL-23, we separated DCact and fibroblasts after cocultivation and measured the amount of IL-23p19 transcripts. Figure 1B shows that IL-23p19 mRNA was solely generated by DCact.
Fibroblasts support IL-23 release from DCs. (A) Monocyte-derived DCs were preactivated with LPS for 3 hours. Subsequently, LPS-stimulated DCs (termed DCact in the entire manuscript) were washed extensively and then cocultured with fibroblasts (DCact + Fb) overnight. As control, LPS-stimulated DCs were cultured alone (DCact). Furthermore, we cultured immature DCs without (DC) and with fibroblasts (DC + Fb) or fibroblasts (Fb) alone. IL-23 protein levels were measured by ELISA. *P < .001 compared with DCact (n = 5 independent experiments). (B) DCact and fibroblasts were cocultured for 3 hours; afterward, DCs (DCact separated after coculture) and fibroblasts (Fb separated after coculture) were separated from the coculture by anti–Thy-1–coupled magnetic beads. For comparison, DCact and fibroblasts (Fb) were cultured alone. Subsequently, RNA preparation and real-time PCR of IL-23p19 mRNA were performed. IL-23p19 mRNA expression values were normalized to the unregulated housekeeping gene RPS26 and are given as percentage of IL-23p19 mRNA expression in DCact. *P < .001 compared with DCact (n = 3 independent experiments).
Fibroblasts support IL-23 release from DCs. (A) Monocyte-derived DCs were preactivated with LPS for 3 hours. Subsequently, LPS-stimulated DCs (termed DCact in the entire manuscript) were washed extensively and then cocultured with fibroblasts (DCact + Fb) overnight. As control, LPS-stimulated DCs were cultured alone (DCact). Furthermore, we cultured immature DCs without (DC) and with fibroblasts (DC + Fb) or fibroblasts (Fb) alone. IL-23 protein levels were measured by ELISA. *P < .001 compared with DCact (n = 5 independent experiments). (B) DCact and fibroblasts were cocultured for 3 hours; afterward, DCs (DCact separated after coculture) and fibroblasts (Fb separated after coculture) were separated from the coculture by anti–Thy-1–coupled magnetic beads. For comparison, DCact and fibroblasts (Fb) were cultured alone. Subsequently, RNA preparation and real-time PCR of IL-23p19 mRNA were performed. IL-23p19 mRNA expression values were normalized to the unregulated housekeeping gene RPS26 and are given as percentage of IL-23p19 mRNA expression in DCact. *P < .001 compared with DCact (n = 3 independent experiments).
Characterization of DCs cocultured with fibroblasts
Next, we investigated the attributes of DCact that were cocultured with fibroblasts compared with DCs preactivated by LPS alone (Table 1). Flow cytometry revealed both DCact and DCact in coculture with fibroblasts expressed similar amounts of HLA-DR, CD1c, and inducible nitric oxide synthase (iNOS) (Table 1). To further characterize fibroblast-stimulated DCact, cytokine secretion was analyzed by ELISA. As summarized in Table 1, supernatants of fibroblast-stimulated DCact contained higher amounts of IL-23 and IL-6. IL-12 was expressed at low levels, but fibroblasts stimulated IL-12 production 2-fold in DCact compared with DCact alone. TGF-β and IL-1β amounts were similar in DCact and in fibroblast-DCact cocultures. Level of TNF-α was less in fibroblast-DCact coculture than in supernatants of DCact.
Blocking of TNF-α and IL-1β prevents fibroblast-stimulated DCs from increased IL-23 secretion
We next investigated the mechanisms of the fibroblast-stimulated IL-23 secretion in DCact. Supernatants of fibroblasts did not stimulate IL-23 release from DCact (Figure 2A). Moreover, fixed fibroblasts allowing exclusively cell-cell interactions did not increase IL-23 secretion in DCact (Figure 2A). These results suggested a more complex feedback loop mechanism between DCact and fibroblasts. Thus, we first investigated potential mediators produced by DCs in coculture. The proinflammatory cytokines TNF-α and IL-1β are known to be present abundantly in inflamed skin31,32 and to be produced by activated DCs,16 including fibroblast-stimulated DCact (Table 1; Figure 2C-D). In addition, supernatants of fibroblasts activated with TNF-α and IL-1β also increased IL-23 release of DCact compared with DCact (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
DC-derived TNF-α and IL-1β are involved in a feedback loop mechanism that causes increased IL-23 production in cocultures of DCs and fibroblasts. (A) DCact were cultured in the presence of fibroblast supernatants (DCact + [Fb-sn]) or PFA-fixated fibroblasts (DCact + Fb-fix). As controls, DCact either cultured alone (DCact) or cocultured with fibroblasts (DCact + Fb) were used. IL-23 was detected by ELISA. *P < .001 compared with DCact (n = 3 independent experiments). (B) Neutralizing anti–TNF-α or anti–IL-1β antibodies were added to coculture of DCact and fibroblasts separately (DCact + Fb + aTNF-α and DCact + Fb +aIL-1beta) or in combination (DCact + Fb + aTNFalpha/aIL-1beta). IL-23 levels were compared with coculture with isotype control (DCact + Fb + iso) and preactivated DCs cultured alone (DCact). #,*P < .05 (n = 4 independent experiments). (C-D) DCact and fibroblasts were cocultured for 3 hours and then separated (DCact separated after coculture and Fb separated after coculture). For comparison, preactivated DCs (DCact) and fibroblasts (Fb) were cultured alone. Subsequently, RNA preparations and real-time PCR were performed. (C) Relative level of TNF-α mRNA was calculated on the basis of ΔCt values. mRNA expression was normalized to the unregulated housekeeping gene RPS26 and computed as percentage of TNF-α mRNA expression in DCact. *P < .01 compared with DCact (n = 3 independent experiments). (D) IL-1β mRNA was quantified through a standard curve, normalized to the unregulated housekeeping gene RPS26, and computed as percentage of mRNA expression in DCact (n = 3 independent experiments).
DC-derived TNF-α and IL-1β are involved in a feedback loop mechanism that causes increased IL-23 production in cocultures of DCs and fibroblasts. (A) DCact were cultured in the presence of fibroblast supernatants (DCact + [Fb-sn]) or PFA-fixated fibroblasts (DCact + Fb-fix). As controls, DCact either cultured alone (DCact) or cocultured with fibroblasts (DCact + Fb) were used. IL-23 was detected by ELISA. *P < .001 compared with DCact (n = 3 independent experiments). (B) Neutralizing anti–TNF-α or anti–IL-1β antibodies were added to coculture of DCact and fibroblasts separately (DCact + Fb + aTNF-α and DCact + Fb +aIL-1beta) or in combination (DCact + Fb + aTNFalpha/aIL-1beta). IL-23 levels were compared with coculture with isotype control (DCact + Fb + iso) and preactivated DCs cultured alone (DCact). #,*P < .05 (n = 4 independent experiments). (C-D) DCact and fibroblasts were cocultured for 3 hours and then separated (DCact separated after coculture and Fb separated after coculture). For comparison, preactivated DCs (DCact) and fibroblasts (Fb) were cultured alone. Subsequently, RNA preparations and real-time PCR were performed. (C) Relative level of TNF-α mRNA was calculated on the basis of ΔCt values. mRNA expression was normalized to the unregulated housekeeping gene RPS26 and computed as percentage of TNF-α mRNA expression in DCact. *P < .01 compared with DCact (n = 3 independent experiments). (D) IL-1β mRNA was quantified through a standard curve, normalized to the unregulated housekeeping gene RPS26, and computed as percentage of mRNA expression in DCact (n = 3 independent experiments).
To study the role of TNF-α and IL-1β in the up-regulation of IL-23 secretion in DCact on coculture with fibroblasts, neutralizing antibodies were used. Anti–TNF-α reduced IL-23 production of fibroblast-stimulated DCact by approximately 30% compared with isotype control, whereas anti–IL-1β alone led to a significant decrease (P < .05) of IL-23 at least to the level of IL-23 in DCact. The combination of anti–TNF-α and anti–IL-1β caused no further reduction (Figure 2B). To identify the cellular source of TNF-α and IL-1β, DCact and fibroblasts were separated from the coculture and quantitative reverse-transcribed (RT) PCR was performed. Neither in fibroblasts alone nor in fibroblasts cocultured with DCact, TNF-α– and IL-1β–mRNA could be detected; by contrast, DCact expressed high levels of TNF-α– and IL-1β–mRNA (Figure 2C-D).
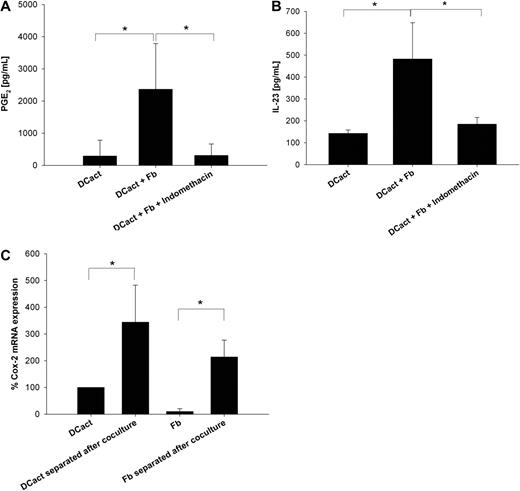
PGE2 derived from fibroblasts supports IL-23 production of activated DCs
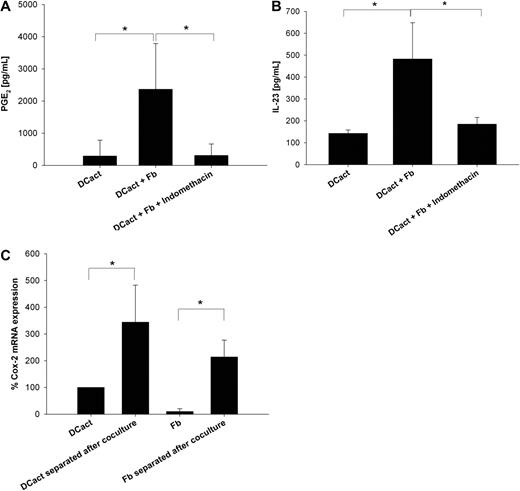
Activation of fibroblasts with TNF-α and IL-1β results in PGE2 secretion.3 In addition, we showed that PGE2 in concentrations measured in DCact-fibroblast cocultures drives DCact to secrete increased amounts of IL-23 compared with DCact alone (supplemental Figure 2). Therefore, we added indomethacin to DCact-fibroblast cocultures to inhibit Cox-1 and Cox-2. Coculture of DCact and fibroblasts led to an increase in PGE2 compared with DCact alone, which was completely prevented by indomethacin (Figure 3A). Importantly, indomethacin reduced IL-23 secretion in DCact-fibroblast cocultures to the level of DCact alone (Figure 3B), indicating that PGE2 is an important mediator in the feedback loop mechanism that drives the fibroblast-induced IL-23 secretion in DCact.
PGE2, which is produced because of coculturing of activated DCs and fibroblasts, is also involved in IL-23 up-regulation. (A) Indomethacin's PGE2 inhibiting efficiency was verified by detection of PGE2 levels in DCact cultured alone (DCact) and in coculture of DCact and fibroblasts with and without 2μM indomethacin (DCact + Fb and DCact + Fb + indomethacin, respectively). *P < .01 (n = 7 independent experiments). (B) DCs were preactivated with LPS and subsequently cocultured with fibroblasts (DCact + Fb) or without fibroblasts (DCact). Indomethacin (2μM) was added to the cocultures (DCact + Fb + indomethacin) and IL-23 was measured by ELISA. *P < .001 (n = 7 independent experiments). (C) DCs and fibroblasts were separated using anti–Thy-1 coated microbeads after a coculture time of 3 hours (DCact separated after coculture and Fb separated after coculture). Controls were again LPS-stimulated DC (DCact) and fibroblasts (Fb) cultured alone. Then, RNA was prepared and quantitative RT-PCR was performed. Cox-2 mRNA expression values were normalized to the unregulated housekeeping gene RPS26 and are given as percentage of Cox-2 mRNA expression in DCact. *P < .01 (n = 3 independent experiments).
PGE2, which is produced because of coculturing of activated DCs and fibroblasts, is also involved in IL-23 up-regulation. (A) Indomethacin's PGE2 inhibiting efficiency was verified by detection of PGE2 levels in DCact cultured alone (DCact) and in coculture of DCact and fibroblasts with and without 2μM indomethacin (DCact + Fb and DCact + Fb + indomethacin, respectively). *P < .01 (n = 7 independent experiments). (B) DCs were preactivated with LPS and subsequently cocultured with fibroblasts (DCact + Fb) or without fibroblasts (DCact). Indomethacin (2μM) was added to the cocultures (DCact + Fb + indomethacin) and IL-23 was measured by ELISA. *P < .001 (n = 7 independent experiments). (C) DCs and fibroblasts were separated using anti–Thy-1 coated microbeads after a coculture time of 3 hours (DCact separated after coculture and Fb separated after coculture). Controls were again LPS-stimulated DC (DCact) and fibroblasts (Fb) cultured alone. Then, RNA was prepared and quantitative RT-PCR was performed. Cox-2 mRNA expression values were normalized to the unregulated housekeeping gene RPS26 and are given as percentage of Cox-2 mRNA expression in DCact. *P < .01 (n = 3 independent experiments).
To differentiate between DCs and fibroblasts as PGE2 producers, DCact and fibroblasts were separated after coculture to perform quantitative RT-PCR. Both DCact and fibroblasts showed increases in Cox-2 mRNA levels compared with their counterparts cultured alone (Figure 3C). Specifically, Cox-2 mRNA increased 3-fold in DCact, whereas it increased more than 10-fold in fibroblasts on coculture with DCact.
To discriminate whether DCact- or fibroblast-derived PGE2 promotes IL-23 secretion in fibroblast-stimulated DCact, Cox-2 expression in fibroblasts or DCs was inhibited by siRNA silencing before setting up DCact-fibroblast coculture. To evaluate efficiency of Cox-2 silencing, transfected fibroblasts were incubated with TNF-α/IL-1β and transfected DCs were activated by LPS overnight, both known stimulators of PGE2 production.3 Cox-2 siRNA transfection dramatically reduced PGE2 secretion of stimulated fibroblasts as well as stimulated DCs (P < .05; Figure 4A) compared with the scrambled siRNA control. However, PGE2 decline was not complete because approximately 10% to 20% of PGE2 could still be detected in Cox-2–silenced cells (Figure 4A-B). Interestingly, IL-23 secretion was significantly decreased (P < .001) in DCact cocultured with Cox-2–silenced fibroblasts compared with cocultures with scrambled-transfected fibroblasts (Figure 4B). In contrast, silencing of Cox-2 in DCs did not affect their IL-23 secretion on coculture with fibroblasts. Thus, fibroblast-derived PGE2 is essential to stimulate IL-23 production of DCact.
Fibroblast-derived PGE2 is the critical PGE2 for stimulation of IL-23 secretion from DCact. (A) Fibroblasts or DCs were transfected with Cox-2 siRNA or scrambled siRNA. To induce PGE2 expression, Cox-2– and scrambled-transfected fibroblasts were stimulated with TNF-α/IL-1β (Fb[Cox-2si]+TNFalpha/IL-1beta; Fb[scr] + TNFalpha/IL-1beta). Cox-2– and scrambled-transfected DCs (DC[Cox-2si] + LPS; DC[scr] + LPS) were activated with LPS. PGE2 release was measured by ELISA. *P < .05 compared with scrambled-transfected cells (n = 3 independent experiments). (B) LPS-preactivated DCs (DCact) were cocultured with scrambled-siRNA-transfected fibroblasts (DCact + Fb[scr]) or with Cox-2-siRNA transfected fibroblasts (DCact + Fb[Cox-2si]). As control, DCact were cultured alone. IL-23 production was measured by ELISA. *P < .001 (n = 5 independent experiments). (C) Cox-2– or scrambled siRNA–transfected DCs were preactivated with LPS and subsequently cocultured with fibroblasts (DCact[scr] + Fb; DCact[Cox-2si] + Fb). As control, transfected DCact were cultured alone (DCact[scr]; DCact[Cox-2si]). *P < .001.
Fibroblast-derived PGE2 is the critical PGE2 for stimulation of IL-23 secretion from DCact. (A) Fibroblasts or DCs were transfected with Cox-2 siRNA or scrambled siRNA. To induce PGE2 expression, Cox-2– and scrambled-transfected fibroblasts were stimulated with TNF-α/IL-1β (Fb[Cox-2si]+TNFalpha/IL-1beta; Fb[scr] + TNFalpha/IL-1beta). Cox-2– and scrambled-transfected DCs (DC[Cox-2si] + LPS; DC[scr] + LPS) were activated with LPS. PGE2 release was measured by ELISA. *P < .05 compared with scrambled-transfected cells (n = 3 independent experiments). (B) LPS-preactivated DCs (DCact) were cocultured with scrambled-siRNA-transfected fibroblasts (DCact + Fb[scr]) or with Cox-2-siRNA transfected fibroblasts (DCact + Fb[Cox-2si]). As control, DCact were cultured alone. IL-23 production was measured by ELISA. *P < .001 (n = 5 independent experiments). (C) Cox-2– or scrambled siRNA–transfected DCs were preactivated with LPS and subsequently cocultured with fibroblasts (DCact[scr] + Fb; DCact[Cox-2si] + Fb). As control, transfected DCact were cultured alone (DCact[scr]; DCact[Cox-2si]). *P < .001.
However, knockdown of Cox-2 in fibroblasts did not completely inhibit the stimulation of IL-23 secretion in DCact observed after coculture with fibroblasts, supposedly because of the incomplete Cox-2 knockdown in fibroblasts. Indeed, stimulation of DCact with PGE2 concentrations present in cocultures on transfection of fibroblasts with Cox-2 siRNA still caused a duplication of IL-23 compared with DCact alone, which was also seen in coculture of DCact with Cox-2–silenced fibroblasts (data not shown).
These results support the notion that mainly fibroblast-derived PGE2 is responsible for enhanced IL-23 secretion in DCact on interaction with fibroblasts.
Fibroblasts support Th17 development via stimulation of IL-23 secretion from DCs
To analyze the consequences of the up-regulated IL-23 secretion of fibroblast-stimulated DCact for TC function, DCact and fibroblast-stimulated DCact were harvested after 18 hours of coculture. Fibroblasts were removed from the coculture using anti–Thy-1–coupled magnetic beads as described.29 Thereafter, CD3+ (Pan) or CD4+ TCs were cocultured with the harvested DCact or DCact purified from DCact-fibroblast coculture in the presence of respective supernatants of the DCact culture or DCact-fibroblast coculture. As control, TCs were cultured alone. CD3+ as well as CD4+ TCs cocultured with fibroblast-stimulated DCact produced significantly (P < .01) higher amounts of IL-17A compared with TCs that were stimulated with DCact (Figure 5A). Intracellular flow cytometric staining of CD3+ TCs revealed that augmented IL-17 secretion was associated with an increased number of IL-17A–producing TCs (Figure 5B). RORγt-positive TCs were also elevated through fibroblast-stimulated DCact compared with DCact alone (Figure 5C). In addition, we showed that fibroblast-conditioned DCact also enhanced IL-22 secretion of CD3+ TCs compared with TCs cultured with DCact (P < .05; Figure 5D). Interaction of CD3+ TCs with fibroblast-stimulated DCact also enhanced IFN-γ release (Figure 5E).
Fibroblasts promote Th17 development from TCs via the stimulation of IL-23 from activated DCs. LPS-preactivated DCs were cocultured with fibroblasts. After 18 hours, fibroblasts were removed from the coculture using anti–Thy-1–coupled magnetic beads. As control, LPS-preactivated DCs (DCact) were cultured alone. Subsequently, CD3+ TCs or CD4+ were cultured alone (TC) or with either LPS-preactivated DCs (TC + DCact) or LPS-preactivated DCs isolated from the coculture with fibroblasts (TC + [DCact + Fb]) in the presence of respective coculture supernatants. (A,D-E) After 6 days of coculture, IL-17A (A), IL-22 (D), and IFN-γ (E) production was measured by ELISA. *P < .01, compared with TC + DCact (n = 5 independent experiments). (B-C) After 3 days of coculture, TCs were restimulated with phorbol myristate acetate/ionomycin in the presence of brefeldin A and IL-17A expression (B) and transcription factor RORγt expression (C) were measured by intracellular flow cytometric staining. One representative experiment of 3 is shown. (F) Coculture of CD3+ TCs and DCact (TC + DCact) or with fibroblast-stimulated DCact (TC + [DCact + Fb]) were performed without antibody (TC + [DCact + Fb]) in the presence of a control antibody (TC + [DCact + Fb + ctr ab]), a neutralizing anti–IL-6 (TC + [DCact + Fb + aIL-6]), or anti–IL-23 antibody (TC + [DCact + Fb + aIL-23]). After 5 days, IL-17A was quantified by ELISA. *#P < .05 (n = 7 independent experiments). (G) IL-17A secretion of CD3+ Pan TCs, CD4+ TCs, CD4+CD45RO+ memory TCs, and CD4+CD45RA+ naive TCs was compared by culturing the different TC types with medium (aTC), supernatants of LPS-preactivated DC (aTC + [DCact]sn), or supernatants of DCact-fibroblast coculture (aTC + [DCact + Fb]sn) in the presence of CD3/CD28 beads for 5 days. *P < .05, compared with aTC. #P < .01, compared with aTC[DCact]sn (n = 4 independent experiments).
Fibroblasts promote Th17 development from TCs via the stimulation of IL-23 from activated DCs. LPS-preactivated DCs were cocultured with fibroblasts. After 18 hours, fibroblasts were removed from the coculture using anti–Thy-1–coupled magnetic beads. As control, LPS-preactivated DCs (DCact) were cultured alone. Subsequently, CD3+ TCs or CD4+ were cultured alone (TC) or with either LPS-preactivated DCs (TC + DCact) or LPS-preactivated DCs isolated from the coculture with fibroblasts (TC + [DCact + Fb]) in the presence of respective coculture supernatants. (A,D-E) After 6 days of coculture, IL-17A (A), IL-22 (D), and IFN-γ (E) production was measured by ELISA. *P < .01, compared with TC + DCact (n = 5 independent experiments). (B-C) After 3 days of coculture, TCs were restimulated with phorbol myristate acetate/ionomycin in the presence of brefeldin A and IL-17A expression (B) and transcription factor RORγt expression (C) were measured by intracellular flow cytometric staining. One representative experiment of 3 is shown. (F) Coculture of CD3+ TCs and DCact (TC + DCact) or with fibroblast-stimulated DCact (TC + [DCact + Fb]) were performed without antibody (TC + [DCact + Fb]) in the presence of a control antibody (TC + [DCact + Fb + ctr ab]), a neutralizing anti–IL-6 (TC + [DCact + Fb + aIL-6]), or anti–IL-23 antibody (TC + [DCact + Fb + aIL-23]). After 5 days, IL-17A was quantified by ELISA. *#P < .05 (n = 7 independent experiments). (G) IL-17A secretion of CD3+ Pan TCs, CD4+ TCs, CD4+CD45RO+ memory TCs, and CD4+CD45RA+ naive TCs was compared by culturing the different TC types with medium (aTC), supernatants of LPS-preactivated DC (aTC + [DCact]sn), or supernatants of DCact-fibroblast coculture (aTC + [DCact + Fb]sn) in the presence of CD3/CD28 beads for 5 days. *P < .05, compared with aTC. #P < .01, compared with aTC[DCact]sn (n = 4 independent experiments).
Next, we were interested in the mechanism of the IL-17A boost in TCs mediated through the action of fibroblasts on DCs. Analysis of the cytokine secretion in DCact and fibroblast-stimulated DCact revealed an enhanced IL-6 and IL-23 release in the DCact-fibroblast coculture, whereas IL-1β and TGF-β were not differentially secreted (Table 1). Therefore, we studied whether IL-6 or IL-23 are the responsible factors mediating the enhanced IL-17 secretion of TCs on interaction with fibroblast-stimulated DCact. Coculture of CD3+ TCs and fibroblast-stimulated DCact was performed in the presence of neutralizing antibodies. Neutralizing of IL-23 by an anti–IL-23p19 antibody almost completely abrogated, whereas an anti–IL-6 antibody only slightly reduced, the boost of IL-17A achieved by fibroblast-primed DCact (P < .05; Figure 5F). Thus, IL-23 seems to be the most important factor responsible for the increase of IL-17A in TCs cultured with fibroblast-stimulated DCact.
Next, we were interested whether fibroblast-stimulated DCact support the differentiation or expansion of Th17 cells. Therefore, CD4+ TC, CD4+CD45RO+ memory, or CD4+CD45RA+ naive TCs were cultured with aCD3/CD28 beads in the presence of supernatants of either DCact or fibroblast-stimulated DCact. Supernatants of fibroblast-stimulated DCact induced significantly higher IL-17A production in CD4+ and memory TCs than DCact supernatants did (Figure 5G). In contrast, naive TCs released only minimal amounts of IL-17A, and no significant difference between DCact- or DCact-fibroblast supernatants could be observed (Figure 5G). Thus, under our experimental conditions, differentiation of Th17 cells from naive TCs did not take place, whereas expansion of Th17 cells from memory TCs could be achieved.
Cox-2 expression in fibroblasts is enhanced in psoriatic skin lesions.
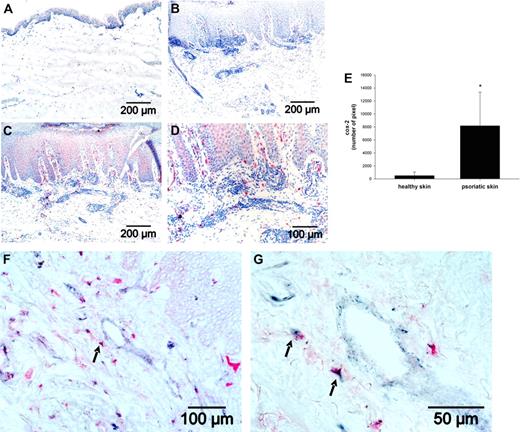
To demonstrate whether the fibroblast-mediated stimulation of IL-23 in DC via PGE2 may play a role during inflammation, we analyzed Cox-2 expression by immunohistochemistry in healthy skin and in psoriatic skin, a chronic inflammatory skin disease where the importance of the IL-23/Th17 axis was already shown.18 In healthy skin samples, no or only a very rare Cox-2 expression was detectable in the epidermis (Figure 6A). In contrast, in lesional psoriatic skin, considerable Cox-2 expression was seen in the epidermis as well as in the dermal compartment (Figure 6C-D). Quantitative analysis revealed a significantly higher (P < .001) Cox-2 expression in psoriatic skin (Figure 6E). To explore whether fibroblasts are involved in PGE2 secretion in psoriatic skin, tissue sections were stained with an anti–Thy-1 antibody to identify fibroblasts (blue-gray) and an anti–Cox-2 antibody (red; Figure 6F-G). We observed single Cox-2- and Thy-1-positive cells underlining the specificity of the double-labeling procedure. In addition, we observed double-positive cells (arrows) indicating that fibroblasts are one source of PGE2 in psoriatic skin.
Cox-2 expression is enhanced in lesional psoriatic skin. Cox-2 expression (red) was detected by immunohistochemistry in (A) healthy skin and (C-D) in psoriatic skin. Nuclei were stained by hematoxylin (blue). (B) An isotype control antibody in lesional psoriatic skin served as negative control. (E) For quantification of Cox-2 expression, morphometric image analysis was performed by scanning 5 images per sections. The sum of pixels was evaluated, and the mean plus or minus SD of the number of pixels from 6 different psoriatic skin samples and 6 healthy skin samples is shown. (F-G) In lesional psoriatic skin, fibroblasts were detected by an anti–Thy-1 antibody (blue-gray) and Cox-2 expression by the anti–Cox-2 antibody (red). Arrows indicate Thy-1/Cox-2 double-positive cells.
Cox-2 expression is enhanced in lesional psoriatic skin. Cox-2 expression (red) was detected by immunohistochemistry in (A) healthy skin and (C-D) in psoriatic skin. Nuclei were stained by hematoxylin (blue). (B) An isotype control antibody in lesional psoriatic skin served as negative control. (E) For quantification of Cox-2 expression, morphometric image analysis was performed by scanning 5 images per sections. The sum of pixels was evaluated, and the mean plus or minus SD of the number of pixels from 6 different psoriatic skin samples and 6 healthy skin samples is shown. (F-G) In lesional psoriatic skin, fibroblasts were detected by an anti–Thy-1 antibody (blue-gray) and Cox-2 expression by the anti–Cox-2 antibody (red). Arrows indicate Thy-1/Cox-2 double-positive cells.
Discussion
In recent years, the influence of the stromal microenvironment on function and cytokine production of DCs was documented in several systems.6-9 Regulation of these processes is carried out through tissue stromal cells, such as fibroblasts, macrophages, and endothelial cells.4 Recently, we demonstrated a close colocalization of fibroblasts and DCs during cutaneous immune responses in situ,33 and several effects of fibroblasts on the function of DCs were reported.3
The influence of the stromal microenvironment on DC function is exerted under both steady-state and inflammatory conditions.4 To imitate inflammatory conditions, DCs were preactivated with LPS for 3 hours (DCact) in our study and subsequently cocultured with skin-derived fibroblasts. Interestingly, fibroblasts augmented IL-23 release of preactivated DCs. In contrast, immature DCs alone or in coculture with fibroblasts did not secrete IL-23, suggesting that under steady-state conditions IL-23 production of DCs may not be affected by fibroblasts. Although knowing that fibroblasts per se do not secrete any IL-23,26 we confirmed that also in our cocultures IL-23 expression was restricted to DCact and was not found in fibroblasts. Fibroblasts also stimulated IL-23 mRNA expression in DCact, indicating that the boost of IL-23 secretion in DCact on fibroblast-stimulation is transcriptionally regulated.
Fixation of fibroblasts revealed that cell-cell contacts were not responsible for fibroblast-stimulated IL-23 release. However, we cannot completely exclude an involvement of cellular interactions because fixation could destroy functional epitopes on fibroblast surface. In addition, supernatants from fibroblasts were not able to substitute for the effect of fibroblasts in the coculture with DCact. Thus, a more complex network seems to be responsible for fibroblast-stimulated IL-23 release from DCact, including the secretion of factors from DCs resulting in a subsequent stimulation of fibroblasts, which then in turn boost IL-23-release in DCs.
Because the proinflammatory cytokines TNF-α and IL-1β are known to be present in inflamed skin31,32 and are produced by activated DCs,16 we studied the role of these cytokines in the stimulation of IL-23 secretion from DCact on coculture with fibroblasts. PCR analyses of coculture DCact and coculture fibroblasts demonstrated that both cytokines are exclusively produced by DCact. We could show that IL-1β and, to a lesser extent, TNF-α secreted by DCact account for the first step of the IL-23-promoting mechanism because anti–IL-1β led to a significant decrease of IL-23 secretion of fibroblast-stimulated DCact. Notably, blocking of TNF-α alone led to a small reduction of IL-23 production, whereas neutralization of IL-1β alone was sufficient to inhibit IL-23 secretion completely. Because the combination of both blocking antibodies had no significant additive effect, we assume that IL-1β is the major mediator to trigger IL-23 release in DCact. It is however possible that TNF-α stimulates IL-1β production of DCact in an autocrine fashion.16,34
Next, we explored which mediator released by fibroblasts on stimulation by DCact in turn up-regulates IL-23 secretion in DCact. Previously, we had shown that stimulation of fibroblasts with TNF-α and IL-1β results in PGE2 secretion.3 Furthermore, stimulation of DCact with PGE2 resulted in increased IL-23 secretion. This is in accordance with Sheibanie et al who suggested that PGE2 induces IL-23 production in DCs.35 Indeed, blocking of PGE2 secretion by indomethacin completely blocked IL-23 release in the coculture of DCact and fibroblasts to IL-23 levels produced by DCact alone. Moreover, Cox-2 mRNA was increased 10-fold in fibroblasts cocultured with DCact.
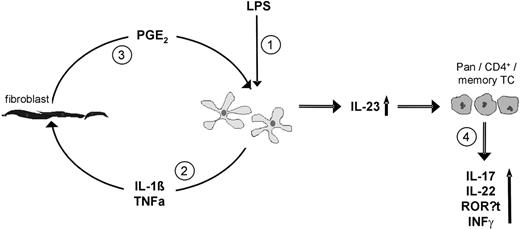
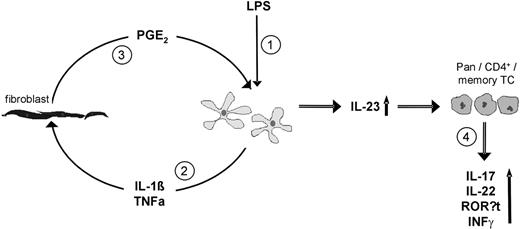
Although both DCact as well as fibroblasts in our cocultures expressed Cox-2, we identified fibroblast-derived PGE2 as the responsible PGE2 for stimulation of IL-23 from DCact. Specifically, a marked suppression of IL-23 secretion in DCact cocultured with Cox-2–silenced fibroblasts was observed. In contrast, inhibition of PGE2 secretion from DCact by siRNA silencing did not affect IL-23 secretion from DCact on interaction with fibroblasts. The control of transfection efficiency confirmed a similar effectiveness of inhibition of PGE2 secretion in DCact and fibroblasts. We realize that IL-23 release in DCact cocultured with Cox-2 siRNA transfected fibroblasts could not be blocked completely to levels observed in DCact. We suppose that this is because of the incomplete Cox-2 mRNA knockdown in fibroblasts. This notion is supported by the finding that addition of indomethacin to DCact-fibroblast coculture was sufficient to completely block PGE2 and decreasing IL-23 levels to that of DCact cultured alone. In summary, our data indicate that preactivation of DC by a 3-hour pulse with LPS induces IL-1β in DCs, which (possibly in combination with TNF-α) promotes PGE2 release in fibroblasts resulting in an up-regulation of IL-23 in DCact (Figure 7).
Model how fibroblasts could stimulate IL-23 secretion from preactivated DCs resulting in expansion of Th17 cells. (1) Preactivation of DC (LPS). (2) Then, DCs secrete IL-1β and TNF-α, which stimulate fibroblasts. (3) Stimulated fibroblasts secrete PGE2, which acts on DCs and therewith increases their IL-23 production. (4) Through the action of elevated IL-23, CD3+ (Pan), CD4+, and CD4+CD45RO+ (memory) TCs are supported to produce IL-17A, IL-22, and to express RORγt as well as to secrete IFN-γ.
Model how fibroblasts could stimulate IL-23 secretion from preactivated DCs resulting in expansion of Th17 cells. (1) Preactivation of DC (LPS). (2) Then, DCs secrete IL-1β and TNF-α, which stimulate fibroblasts. (3) Stimulated fibroblasts secrete PGE2, which acts on DCs and therewith increases their IL-23 production. (4) Through the action of elevated IL-23, CD3+ (Pan), CD4+, and CD4+CD45RO+ (memory) TCs are supported to produce IL-17A, IL-22, and to express RORγt as well as to secrete IFN-γ.
Finally, we looked at the consequences of the enhanced IL-23 secretion by fibroblast-stimulated DCact on the differentiation/polarization of TCs. In accordance with Acosta-Rodriguez et al36 and Zenaro et al,37 DCact alone induced a remarkable Th1 response characterized by IFN-γ secretion but a weak Th17 response as shown by IL-17 and IL-22 secretion. In sharp contrast, fibroblast-stimulated DCact supported the emergence of IL-17A–producing TCs as shown by an increase in IL-17A secretion, an expansion of IL-17A–secreting cells, as well as an enhanced secretion of IL-22 and up-regulated expression of RORγt. Because TGF-β and IL-1β were not differentially expressed in fibroblast-stimulated DCact versus DCact cultured alone, we excluded these mediators as the responsible factors for the fibroblast-driven enhancement of the Th17 response. Romagnani et al21 described that IL-23 and IL-1β seem to drive development of human naive TCs from cord blood or memory TC into Th17 cells, but the function of TGF-β and IL-6 in this process is discussed controversially.21,27,36 The central role of fibroblast-stimulated IL-23 secretion of DCact in the support of Th17 expansion was confirmed by specific antibody blocking of IL-23 in the coculture of fibroblasts and DCact, which almost completely prevented the boost of IL-17A release in TCs. In addition, blocking IL-6 only slightly reduced IL-17A secretion from TCs cocultured with fibroblast-stimulated DCact. We conclude that, in our system, IL-23 secreted by fibroblast-stimulated DCact is the major cytokine-promoting elevated IL-17A production in TCs. However, fibroblast-stimulated DCact also promoted an increased IFN-γ secretion in TCs cocultured with fibroblast-stimulated DCact. IL-12 is the most important factor known to stimulate IFN-γ expression in TCs. Indeed, in our experiments, IL-12 release was also up-regulated in fibroblast-stimulated DCact. The simultaneous stimulation of a Th1- and Th17-type response was also described by other investigators.37
In accordance with our data, Sheibanie et al showed that PGE2 induced IL-23 up-regulation in DCs, resulting in increased production of IL-17 from activated CD4+ T cells.35 Khayrullina et al reported that bone marrow-derived DCs generated in the presence of PGE2 promote Th17 differentiation in vitro and in vivo via IL-23.38 On the other hand, several reports show that PGE2 directly increases IL-17A production in activated TCs.39-41 It should be noted, however, that the direct effect of PGE2 on Th17 differentiation was only achieved by concentrations of PGE2 10- to 1000-fold higher than those detected in our cocultures of fibroblasts and DCact. Another piece of evidence also indicates that, in our system, IL-17A is not the result of a direct effect of PGE2 on TCs. Notably, anti–IL-23p19 blocked almost completely IL-17A release of TCs cocultured with fibroblast-stimulated DCact, although not affecting PGE2 levels.
Whether IL-23 is able to induce IL-17A secretion in both CD4+CD45RO+ (memory) and CD4+CD45RA+ (naive) TCs or only in memory TCs is still debated.27,36 We could show that our cell culture supernatants of fibroblast-stimulated DCact or DCact alone containing IL-23, IL-1β, IL-6, and TGF-β support the emergence of Th17 cells from Pan TC, CD4+ TCs as well as memory TCs. In naive TCs, only minute amounts of IL-17A could be detected. Evidently, under our experimental conditions, DCs activated by LPS with and without fibroblasts do not drive Th17 polarization from naive TCs. This is in accordance with Acosta-Rodriguez et al36 who failed to induce Th17 differentiation from naive TCs with LPS-stimulated monocyte-derived DCs.
Finally, we could demonstrate that this mechanism may be involved in psoriasis, a prototypic immune response for which the importance of IL-23/Th17 immune axis has been shown.18 Specifically, in psoriasis, overexpression of IL-23p19 mainly by monocytes and DCs and expansion of Th17 cells have been reported.42,43 The IL-23/Th17 axis is involved in the development of the psoriatic phenotype, including infiltration of leukocytes, epidermal hyperplasia, acanthosis, and hyperkerathosis.18,44 We observed a significantly higher Cox-2 expression in psoriatic skin. By double-labeling, fibroblasts were identified as one source of PGE2 in psoriasis. Taken together with our previous finding of close apposition of fibroblasts and DCs in lesional psoriatic skin,33 we speculate that fibroblasts, via the secretion of PGE2, are involved in the perpetuation of the immune response in psoriasis by promoting IL-23 secretion of DCs and a subsequent expansion of Th17 cells. In addition, keratinocytes and other yet unidentified dermal cells also express Cox-2 and may act via similar mechanisms in stimulation of IL-23 secretion from inflammatory DCs. As of yet, little information about the effectiveness of Cox inhibition as an antipsoriatic treatment exist (ie, one study reported no improvement of skin lesions); on the other hand, Cox inhibitors are used widely and successfully to treat psoriatic arthritis.45-47 Bearing in mind that Cox-blocking agents drive arachidonate metabolism into the lipoxygenase pathway resulting in enhanced production of leukotrienes, including potent chemoattractants for inflammatory cells in psoriasis,48 it is possible that blocking of just the Cox pathway is not sufficient for successful treatment.
In conclusion, our results support a model (Figure 7) in which under inflammatory conditions DCs produce TNF-α and Il-1β, which in turn activate resident fibroblasts. Via the secretion of different factors, these fibroblasts modulate DC functions and finally the polarization of the TC response. In particular, we demonstrated that PGE2 released by activated fibroblasts stimulates IL-23 secretion from activated DCs, which results ultimately in a prominent expansion of Th17 cells from the memory pool of TCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Heidi Gedicke for technical assistance.
This work was kindly supported by Helmholtz Impulse and Networking Fund through the Helmholtz Interdisciplinary Graduate School for Environmental Research (HIGRADE) and in part by the German Research Council (TRR 67 Si 397/15-1).
Authorship
Contribution: C.S., C.K., and A.S designed and performed experiments and analyzed data; C.S., A.S., and J.C.S. wrote the paper; and C.S., C.K., A.S., J.C.S, and M.V.B. discussed the data and read and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anja Saalbach, Department of Dermatology, Venerology and Allergology, Medical Faculty of the University Leipzig, Johannisallee 30, 04103 Leipzig, Germany; e-mail: anja.saalbach@medizin.uni-leipzig.de.
References
Author notes
J.C.S. and A.S. contributed equally to this study and share the senior authorship.

![Figure 2. DC-derived TNF-α and IL-1β are involved in a feedback loop mechanism that causes increased IL-23 production in cocultures of DCs and fibroblasts. (A) DCact were cultured in the presence of fibroblast supernatants (DCact + [Fb-sn]) or PFA-fixated fibroblasts (DCact + Fb-fix). As controls, DCact either cultured alone (DCact) or cocultured with fibroblasts (DCact + Fb) were used. IL-23 was detected by ELISA. *P < .001 compared with DCact (n = 3 independent experiments). (B) Neutralizing anti–TNF-α or anti–IL-1β antibodies were added to coculture of DCact and fibroblasts separately (DCact + Fb + aTNF-α and DCact + Fb +aIL-1beta) or in combination (DCact + Fb + aTNFalpha/aIL-1beta). IL-23 levels were compared with coculture with isotype control (DCact + Fb + iso) and preactivated DCs cultured alone (DCact). #,*P < .05 (n = 4 independent experiments). (C-D) DCact and fibroblasts were cocultured for 3 hours and then separated (DCact separated after coculture and Fb separated after coculture). For comparison, preactivated DCs (DCact) and fibroblasts (Fb) were cultured alone. Subsequently, RNA preparations and real-time PCR were performed. (C) Relative level of TNF-α mRNA was calculated on the basis of ΔCt values. mRNA expression was normalized to the unregulated housekeeping gene RPS26 and computed as percentage of TNF-α mRNA expression in DCact. *P < .01 compared with DCact (n = 3 independent experiments). (D) IL-1β mRNA was quantified through a standard curve, normalized to the unregulated housekeeping gene RPS26, and computed as percentage of mRNA expression in DCact (n = 3 independent experiments).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/10/10.1182_blood-2010-01-263509/4/m_zh89991057080002.jpeg?Expires=1766534965&Signature=SZF4nuBdC8PwicX2u73cn3Bs46QuIdLyjQo8gBGXYpE7ZqIFAmhJc~JaSTr3A-bciBNoGDyt6vwjbrVhZnwAkwMyy9S76C0Dax~3O7dPEKUpXdWXBizszPBsiMqf6crMOWfzh8l9q0zHufEr2vvJATrTIojlpfka4VVPsVnO8349rNv84Z3cQ5jsorXtBl1oBniJad-A1DtWnxOiv6fBijWHN5ErSW01nGZZwfaViDEYPynIC39mqfSV2TRmx8ZpBmWtl86kLDrGbNssfRRMwdn4zh7xFpPWA8gWV~fAOPYqizm69CVtoTSAI7wzfXynRrSNTYmM6Bt7P4B3rQljhg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Fibroblast-derived PGE2 is the critical PGE2 for stimulation of IL-23 secretion from DCact. (A) Fibroblasts or DCs were transfected with Cox-2 siRNA or scrambled siRNA. To induce PGE2 expression, Cox-2– and scrambled-transfected fibroblasts were stimulated with TNF-α/IL-1β (Fb[Cox-2si]+TNFalpha/IL-1beta; Fb[scr] + TNFalpha/IL-1beta). Cox-2– and scrambled-transfected DCs (DC[Cox-2si] + LPS; DC[scr] + LPS) were activated with LPS. PGE2 release was measured by ELISA. *P < .05 compared with scrambled-transfected cells (n = 3 independent experiments). (B) LPS-preactivated DCs (DCact) were cocultured with scrambled-siRNA-transfected fibroblasts (DCact + Fb[scr]) or with Cox-2-siRNA transfected fibroblasts (DCact + Fb[Cox-2si]). As control, DCact were cultured alone. IL-23 production was measured by ELISA. *P < .001 (n = 5 independent experiments). (C) Cox-2– or scrambled siRNA–transfected DCs were preactivated with LPS and subsequently cocultured with fibroblasts (DCact[scr] + Fb; DCact[Cox-2si] + Fb). As control, transfected DCact were cultured alone (DCact[scr]; DCact[Cox-2si]). *P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/10/10.1182_blood-2010-01-263509/4/m_zh89991057080004.jpeg?Expires=1766534965&Signature=WOsm7aweC0VPZSNTqG9DsrnCBNd6lMh~B9vIaM5y9jcAeUkC~G6VLydfH5b5Pwz-YxcKuUT7LoGTb97f-hZNQY78qXyzOcpXurLRg03C3v~mwp-HDLI6zaO4cIIwrfTfiKZ1-62SrivVtVWJj9-zR52xYT5aqgG1APUBvYc~oGQ7njLKcF6v1WQcq2vOvGNd6zhSlBHTZmdhxsfF~Cjj75UqjBabXbGAS7bvhrJAIVB~VnwBYiLJwtiJzVHO78aLvknqmL1Gw48oIIm3W6XpfWj895Tv4jgK-YdcNsKHq4OfNkkQ8ZztUY3aFqYcVZ1NVycFij~0Yp2G5GACagICsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Fibroblasts promote Th17 development from TCs via the stimulation of IL-23 from activated DCs. LPS-preactivated DCs were cocultured with fibroblasts. After 18 hours, fibroblasts were removed from the coculture using anti–Thy-1–coupled magnetic beads. As control, LPS-preactivated DCs (DCact) were cultured alone. Subsequently, CD3+ TCs or CD4+ were cultured alone (TC) or with either LPS-preactivated DCs (TC + DCact) or LPS-preactivated DCs isolated from the coculture with fibroblasts (TC + [DCact + Fb]) in the presence of respective coculture supernatants. (A,D-E) After 6 days of coculture, IL-17A (A), IL-22 (D), and IFN-γ (E) production was measured by ELISA. *P < .01, compared with TC + DCact (n = 5 independent experiments). (B-C) After 3 days of coculture, TCs were restimulated with phorbol myristate acetate/ionomycin in the presence of brefeldin A and IL-17A expression (B) and transcription factor RORγt expression (C) were measured by intracellular flow cytometric staining. One representative experiment of 3 is shown. (F) Coculture of CD3+ TCs and DCact (TC + DCact) or with fibroblast-stimulated DCact (TC + [DCact + Fb]) were performed without antibody (TC + [DCact + Fb]) in the presence of a control antibody (TC + [DCact + Fb + ctr ab]), a neutralizing anti–IL-6 (TC + [DCact + Fb + aIL-6]), or anti–IL-23 antibody (TC + [DCact + Fb + aIL-23]). After 5 days, IL-17A was quantified by ELISA. *#P < .05 (n = 7 independent experiments). (G) IL-17A secretion of CD3+ Pan TCs, CD4+ TCs, CD4+CD45RO+ memory TCs, and CD4+CD45RA+ naive TCs was compared by culturing the different TC types with medium (aTC), supernatants of LPS-preactivated DC (aTC + [DCact]sn), or supernatants of DCact-fibroblast coculture (aTC + [DCact + Fb]sn) in the presence of CD3/CD28 beads for 5 days. *P < .05, compared with aTC. #P < .01, compared with aTC[DCact]sn (n = 4 independent experiments).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/10/10.1182_blood-2010-01-263509/4/m_zh89991057080005.jpeg?Expires=1766534965&Signature=NB4keRWKh3LDqyrpv~VAE2698O-NYsKvmc5xyumUs6mKzSMq51QVLiR3DGwduRZ6xmmwLVT9djItk1fedt19~E09JWFer-yboam89R0t2NENeRkrYh-HljQWdeYNufArpZZjS5MkqXos5ERUjE9Q~s6RSLmW~3boL1lPigRbdi3bhMflZo-7qpddLliAi5mlEJk69wlq0--9E9SoYi2e6X2HHMwclt6IuyNkp3yo2W4AV1ilVx9VhpAy52R4hxSWXuJWIZyusa3JgdXMXMFCtXhivZXgErGRhcIHl~Q8U6XTITAUKr6Oqljd2pfCjtYq6lsxRnJoUK9C1rr7r17t4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. DC-derived TNF-α and IL-1β are involved in a feedback loop mechanism that causes increased IL-23 production in cocultures of DCs and fibroblasts. (A) DCact were cultured in the presence of fibroblast supernatants (DCact + [Fb-sn]) or PFA-fixated fibroblasts (DCact + Fb-fix). As controls, DCact either cultured alone (DCact) or cocultured with fibroblasts (DCact + Fb) were used. IL-23 was detected by ELISA. *P < .001 compared with DCact (n = 3 independent experiments). (B) Neutralizing anti–TNF-α or anti–IL-1β antibodies were added to coculture of DCact and fibroblasts separately (DCact + Fb + aTNF-α and DCact + Fb +aIL-1beta) or in combination (DCact + Fb + aTNFalpha/aIL-1beta). IL-23 levels were compared with coculture with isotype control (DCact + Fb + iso) and preactivated DCs cultured alone (DCact). #,*P < .05 (n = 4 independent experiments). (C-D) DCact and fibroblasts were cocultured for 3 hours and then separated (DCact separated after coculture and Fb separated after coculture). For comparison, preactivated DCs (DCact) and fibroblasts (Fb) were cultured alone. Subsequently, RNA preparations and real-time PCR were performed. (C) Relative level of TNF-α mRNA was calculated on the basis of ΔCt values. mRNA expression was normalized to the unregulated housekeeping gene RPS26 and computed as percentage of TNF-α mRNA expression in DCact. *P < .01 compared with DCact (n = 3 independent experiments). (D) IL-1β mRNA was quantified through a standard curve, normalized to the unregulated housekeeping gene RPS26, and computed as percentage of mRNA expression in DCact (n = 3 independent experiments).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/10/10.1182_blood-2010-01-263509/4/m_zh89991057080002.jpeg?Expires=1766567019&Signature=2~R0y4SxDjXGMF3bQdFaCs6StLl6RCBaofqozH~TF1JLHKqJWX5wbO4q7qu5hmL4ucyMwCbyRO3p3SS~SGts7NnDPlY9dYvjUO9~BSeqhR0caZOfL8LUfm~zIck98Mo8d0W7K6jvDF4VW73EyFtygQ4LLjRzAVp0tI0FhOZWdUg-DNaC6i4M3IdMHmjBRmPYTpv1Yc-hJbURiwUGKolQnMF2rQ-p7Yszx9ABR~jp31GGIVVNN0lBMZmINCLIuJc8wg576YBOmDGtsmDk1GDvXH7qY95OVwUiR7ZdoxX6tLO97O078~EAlCDVJBIDyO3r4TgITE9Gze5~Ral8sDgCag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Fibroblast-derived PGE2 is the critical PGE2 for stimulation of IL-23 secretion from DCact. (A) Fibroblasts or DCs were transfected with Cox-2 siRNA or scrambled siRNA. To induce PGE2 expression, Cox-2– and scrambled-transfected fibroblasts were stimulated with TNF-α/IL-1β (Fb[Cox-2si]+TNFalpha/IL-1beta; Fb[scr] + TNFalpha/IL-1beta). Cox-2– and scrambled-transfected DCs (DC[Cox-2si] + LPS; DC[scr] + LPS) were activated with LPS. PGE2 release was measured by ELISA. *P < .05 compared with scrambled-transfected cells (n = 3 independent experiments). (B) LPS-preactivated DCs (DCact) were cocultured with scrambled-siRNA-transfected fibroblasts (DCact + Fb[scr]) or with Cox-2-siRNA transfected fibroblasts (DCact + Fb[Cox-2si]). As control, DCact were cultured alone. IL-23 production was measured by ELISA. *P < .001 (n = 5 independent experiments). (C) Cox-2– or scrambled siRNA–transfected DCs were preactivated with LPS and subsequently cocultured with fibroblasts (DCact[scr] + Fb; DCact[Cox-2si] + Fb). As control, transfected DCact were cultured alone (DCact[scr]; DCact[Cox-2si]). *P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/10/10.1182_blood-2010-01-263509/4/m_zh89991057080004.jpeg?Expires=1766567019&Signature=kEIT3EAJbGX~M07mbBap-9we5nq9itdAQhzjd5DmePIENdTATBJHRYRYYX3fdhCkK3ssuJJ0Xu0TcMv8YbETiapjxlau~qwKO7vJQpT6oUYtrIoI~o2J4dMtOmawhaSrEojIhyO4aWJy~a6DIvCM1AYPWXCytho3wGqqoajPJjPYIQ2JFt6poSz0TZA9okMgR9RaNpH4rGw5FVm-06ZOzcdYEvW4ECYIjoNADRtWOQVFf3wOG4vICAcy5ti-4CC1dheDfeNpR7m7DiVUItIY11gkazyl2dTAV2OS3kNJXkpvYFgTJChBijjv~6JZdlf6rapAyffg5kqvo9rdudZBaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Fibroblasts promote Th17 development from TCs via the stimulation of IL-23 from activated DCs. LPS-preactivated DCs were cocultured with fibroblasts. After 18 hours, fibroblasts were removed from the coculture using anti–Thy-1–coupled magnetic beads. As control, LPS-preactivated DCs (DCact) were cultured alone. Subsequently, CD3+ TCs or CD4+ were cultured alone (TC) or with either LPS-preactivated DCs (TC + DCact) or LPS-preactivated DCs isolated from the coculture with fibroblasts (TC + [DCact + Fb]) in the presence of respective coculture supernatants. (A,D-E) After 6 days of coculture, IL-17A (A), IL-22 (D), and IFN-γ (E) production was measured by ELISA. *P < .01, compared with TC + DCact (n = 5 independent experiments). (B-C) After 3 days of coculture, TCs were restimulated with phorbol myristate acetate/ionomycin in the presence of brefeldin A and IL-17A expression (B) and transcription factor RORγt expression (C) were measured by intracellular flow cytometric staining. One representative experiment of 3 is shown. (F) Coculture of CD3+ TCs and DCact (TC + DCact) or with fibroblast-stimulated DCact (TC + [DCact + Fb]) were performed without antibody (TC + [DCact + Fb]) in the presence of a control antibody (TC + [DCact + Fb + ctr ab]), a neutralizing anti–IL-6 (TC + [DCact + Fb + aIL-6]), or anti–IL-23 antibody (TC + [DCact + Fb + aIL-23]). After 5 days, IL-17A was quantified by ELISA. *#P < .05 (n = 7 independent experiments). (G) IL-17A secretion of CD3+ Pan TCs, CD4+ TCs, CD4+CD45RO+ memory TCs, and CD4+CD45RA+ naive TCs was compared by culturing the different TC types with medium (aTC), supernatants of LPS-preactivated DC (aTC + [DCact]sn), or supernatants of DCact-fibroblast coculture (aTC + [DCact + Fb]sn) in the presence of CD3/CD28 beads for 5 days. *P < .05, compared with aTC. #P < .01, compared with aTC[DCact]sn (n = 4 independent experiments).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/10/10.1182_blood-2010-01-263509/4/m_zh89991057080005.jpeg?Expires=1766567019&Signature=r6X-x6E8caEBtTX7Ql6Yq3QD37-308Kv8mfr2pGTmj1lIY74rbHT22xXM4rMomNUjeqOcr2hWJsVFu33--MPlhQXBPVc8nL4JjNNKE57mIyUBdqyuvdL9bow0gxmXAubzVrItBSsGbk9WeeUks3oPT5k01N~C5nzvdhZS~rSC0iUyE9J90e9frZyb9MFSqsXG~5NLDoj91GhSFmLAfoU53ugNXOMZ5n8fbrRXhYSEmMnBHizhvAY1IAVMYMF4r2QeMzvKfJ3H19e08utD4KFk3uzu~pQaY9VusvFpR0h~rHA2LsJRhJNxj4Bkgit3xX0~6YaipEivJKu-NXIDNe5CQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)