To the editor:
In AL amyloidosis, amyloid fibril deposits, derived from immunoglobulin light chains produced by a clonal plasma cell dyscrasia, accumulate in extracellular tissues and damage vital organs.1 Novel therapies used in multiple myeloma2 can be effective in AL amyloidosis. Separately in the same issue of Blood, our group and the Mayo Clinic described similar results of phase 2 trials of the use of lenalidomide for the treatment of AL amyloidosis.3,4 Here we provide updated information on the durability of complete hematologic response (CR) after treatment with lenalidomide and dexamethasone in AL amyloidosis.
Between 2004 and 2009, 69 patients with AL amyloidosis were treated with lenalidomide and dexamethasone (ClinicalTrials.gov: NCT00091260) at Boston Medical Center. Approval was obtained from the institutional review board of the Boston Medical Center for this study. Informed consent was provided according to the Declaration of Helsinki. The median age was 62 years (range, 42-84 years), and 70% were male. Fifty patients had lambda clonal plasma cell dyscrasia, 36 (52%) had multiorgan involvement, and 31 (45%) had cardiac involvement. Sixty-five patients (94%) had received previous therapy (94% previous melphalan-based therapy, 71% high-dose melphalan/stem cell transplantation, 10% thalidomide, and 7% bortezomib). All patients received lenalidomide and dexamethasone as described in our previous report.3 CR was defined as absence of monoclonal gammopathy in serum and urine by immunofixation electrophoresis, less than 5% clonal plasma cells in the bone marrow biopsy, and normalization of serum-free light chain concentration and ratio.5 Eleven of the 69 patients (16% by intention-to-treat analysis) enrolled on the trial achieved a CR. Fifty-three patients were evaluable after completion of 3 cycles of treatment on the protocol and CR for evaluable patients was 21%. The median dose of lenalidomide at the time of achievement of a CR was 10 mg/d (range, 5-15 mg/d). The median time to achievement of a CR was at 6 cycles. CR occurred by 6 months in 92% of the patients. However, delayed CR occurred in 2 patients at 18 months after initiation of therapy. CR occurred in 2 patients with lenalidomide alone and in 9 patients with lenalidomide plus dexamethasone.
Of the 11 patients with CR, 1 patient died from rejection after orthotopic heart transplantation while on cycle 3 of lenalidomide. Of the 10 surviving patients with CR, 6 (60%) have maintained a CR. Of these 6 patients with continued CR, 2 remain on treatment at 6 and 9 months since initiation and 4 remain off treatment after receiving 9 to 19 cycles. The median duration of CR for patients off treatment is 24 months (range, 9-36 months).
Four of the 10 surviving patients with CR (40%) have relapsed off treatment. All four of these patients are alive and have been treated with combinations of other agents.
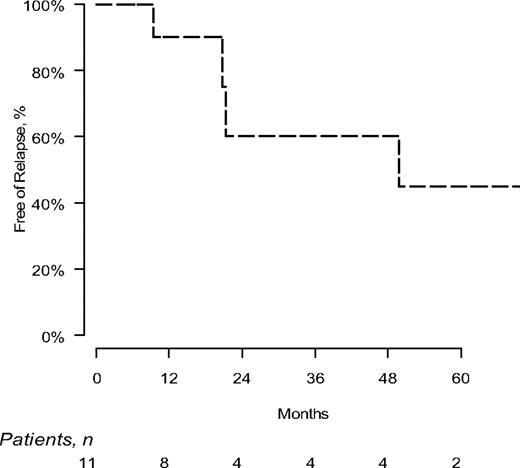
Progression on this clinical trial was defined as hematologic relapse after achievement of a CR and/or time to next treatment. The median progression free interval is 49.8 months (Figure 1).
In summary, lenalidomide, alone and in combination with dexamethasone, can induce hematologic complete responses in 16% of previously treated patients with AL amyloidosis. Most CRs occur by 6 months of treatment and 60% of CRs are durable, even off treatment.
Authorship
Acknowledgment: This work was supported by Celgene Corporation, Summit, NJ.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vaishali Sanchorawala, MD, Section of Hematology/Oncology, FGH 1007, 820 Harrison Ave, Boston, MA 02118; e-mail: Vaishali.Sanchorawala@bmc.org.