Abstract
Hematopoietic stem cells (HSCs) are essential for homeostasis and injury-induced regeneration of the vertebrate blood system. Although HSC transplantations constitute the most common type of stem cell therapy applied in the clinic, we know relatively little about the molecular programming of HSCs during vertebrate embryogenesis. In vertebrate embryos, HSCs form in close association with the ventral wall of the dorsal aorta. We have shown previously that in zebrafish, HSC formation depends on the presence of a signaling cascade that involves Hedgehog, vascular endothelial growth factor, and Notch signaling. Here, we reveal that Hey2, a hairy/enhancer-of-split–related basic helix-loop-helix transcription factor often believed to act downstream of Notch, is also required for HSC formation. In dorsal aorta progenitors, Hey2 expression is induced downstream of cloche and the transcription factor Scl/Tal1, and is maintained by Hedgehog and vascular endothelial growth factor signaling. Whereas knockdown of Hey2 expression results in a loss of Notch receptor expression in dorsal aorta angioblasts, activation of Notch signaling in hey2 morphants rescues HSC formation in zebrafish embryos. These results establish an essential role for Hey2 upstream of Notch in HSC formation.
Introduction
During vertebrate embryogenesis, hematopoietic stem cells (HSCs) first form in the definitive wave of hematopoiesis. This wave follows a transient primitive wave that occurs in the mammalian and avian extraembryonic yolk sac and generates embryonic red blood cells (RBCs). HSCs develop inside the embryo, in the aorta-gonads-mesonephros (AGM) region where clusters of hematopoietic cells are found associated with the ventral wall of the dorsal aorta (DA). The clusters, the endothelial lining, and the mesenchyme below express the transcription factor Runx1, which is essential for definitive hematopoiesis. Targeted deletion of runx1 abrogates cluster formation and AGM HSC activity. The HSC activity resides exclusively within the runx1+ fraction of AGM cells.1-3 Specific deletion of runx1 in endothelial cells (ECs) that express VE-cadherin results in loss of HSC formation,4 which demonstrates that runx1 is specifically needed in ECs for cluster formation. This finding supports the idea that HSCs arise from hemogenic endothelium.5,6
In zebrafish embryos, primitive hematopoiesis takes place inside the embryo in 2 locations, the anterior and the posterior lateral mesoderm (ALM and PLM).7 In the ALM, primitive myeloid progenitors form next to ECs and endocardial cells. Cells of the PLM migrate to the midline to form the intermediate cell mass below the notochord. The intermediate cell mass gives rise to angioblasts, endothelial progenitors that form the major axial blood vessels, the DA dorsally and the posterior cardinal vein (PCV) ventrally. It also forms a large number of primitive RBCs in the position of the PCV. Before these RBCs enter the circulation, primary intersomitic vessels (ISVs) sprout from the DA, followed by secondary ISVs that originate from the PCV. The first runx1-expressing HSCs appear just before the onset of blood circulation immediately above the differentiating primitive RBCs in the intermediate cell mass.8 These cells also express other hematopoietic transcription factors such as c-Myb and Ikaros, and like their mammalian counterparts, they are closely associated with the ventral wall to the DA.8-10 The region between the DA and the vein is thus the zebrafish equivalent of the mammalian AGM region. Lineage-tracing experiments have shown that cells of the zebrafish AGM region enter the bloodstream via the vein. They then seed the ventral tail mesenchyme, the caudal hematopoietic tissue (CHT), an intermediate hematopoietic organ, before settling in the adult hematopoietic organ, the kidney, and entering the thymus.11-13 Knockdown of runx1 expression in morpholino (MO)-injected embryos, so-called runx1 morphants, leads to the loss of CHT progenitors and to severely reduced numbers of thymocytes.8,9 All of these findings strongly suggest that the AGM runx1+ cells include HSCs, although formal proof that these cells repopulate over the long term is still needed. We will refer to these runx1+ cells as HSCs.
Zebrafish HSCs share their cellular origin with the ECs of the DA. At 24 hours after fertilization (hpf), runx1 is first expressed in a ventral subpopulation of DA angioblasts that express the vascular endothelial growth factor (Vegf) receptor 2, Kdrl (also known as Flk1).8 Furthermore, DA ECs and HSCs share common signaling requirements. Arterial gene expression and HSC emergence require intact Hedgehog, Vegf, and Notch signaling pathways, whereas venous ECs and primitive RBCs develop normally when these pathways are blocked.8,14,15 Hedgehog that is produced by the notochord and the floor plate induces VegfA secretion by the medioventral somites. VegfA is detected by the Kdrl-expressing DA angioblasts as they reach the midline. This in turn induces Notch signaling within the DA angioblast cord. Activation of Notch receptors by their ligands triggers a cascade of events. First, the ligand undergoes endocytosis in a process that requires ligand ubiquitination, which is mediated by ubiquitin E3 ligases such as Mind bomb (Mib). During endocytosis, the ligand removes the extracellular domain of the Notch receptor. Proteolytic cleavage of the remaining receptor leads to the release of Notch intracellular domain (NICD) and to its transfer to the nucleus, where it forms a complex with Mastermind and Rbpj [also known as CSL or Su(H)]. This complex drives expression of downstream targets by binding to Rbpj binding sites in promoter and enhancer sequences.16 Notch signaling in the DA angioblast cord induces arterial specification and HSC formation, a role that is conserved in all vertebrates.17,18
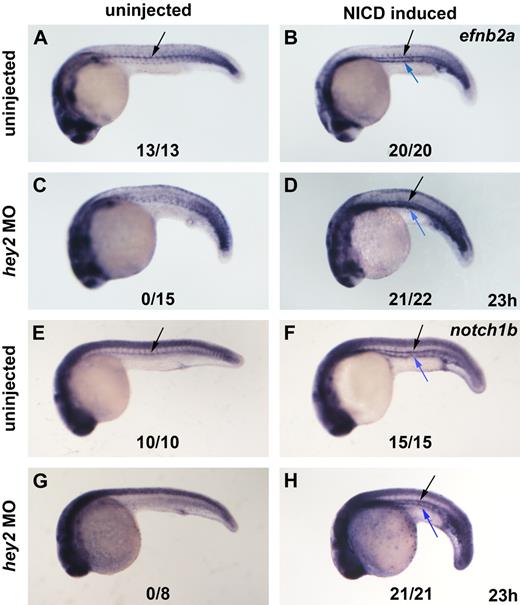
Lineage-labeling experiments suggested that DA and PCV progenitors are already distinct in the PLM.19 A very recent study proposed that the vascular progenitors only segregate into aorta and vein much later, when they are in the midline.20 A transcription factor implicated in the process of committing angioblasts to the DA is Hey2. Hey2 is one of the hairy/enhancer-of-split-related basic helix-loop-helix transcription factors that often act as repressors of gene expression downstream of Notch signaling. Zebrafish hey2 was first identified as the gene mutated in gridlock (grlm145) mutants, which display a specific block in caudal blood circulation. This block is due to the failure to form a proper vessel connection between the anterior bilateral aortae and the single posterior dorsal aorta.21 Formation of collateral vessels eventually reestablishes blood flow to the posterior aorta in the mutants.22 In the mutant allele, the stop codon is replaced with an additional 44 codons, which extends the original protein at the C-terminus. The mutated protein retains some activity in rescue experiments, which suggests that grlm145 is a hypomorph.23 Hey2 morphants were reported to have a more severe vascular defect that is characterized by a complete loss of the DA angioblast cord and an expansion of the PCV, which suggests that the role of Hey2 is to recruit angioblasts in the PLM to the DA.19 The presence of an Rbpj binding site in the hey2 promoter and experiments in which a dominant-negative Rbpj protein was misexpressed in zebrafish embryos suggested that hey2 acts downstream of Notch signaling in this context. However, mib (mibta52b) embryos that are deficient in Notch signaling and are unable to express arterial genes such as efnb2a do retain a DA angioblast cord, initiate blood circulation (only to lose it later), and display hey2 expression in the DA angioblast cord at 24 hpf.24
Here, we show that hey2 not only is needed for arterial gene expression but also is essential for HSC formation in zebrafish embryos. Our expression analyses suggest that hey2 acts upstream rather than downstream of Notch in the gene regulatory network that governs arterial specification and HSC formation. Consistent with this notion, runx1 and efnb2a expression can be rescued in hey2-deficient embryos through activation of the Notch signaling pathway. Our experiments reveal that early endothelial programming is needed for Notch-induced HSC gene expression.
Methods
Maintenance of fish stocks
Breeding zebrafish were maintained and embryos were raised and staged according to The Zebrafish Book.25 The mutant alleles used were clos5, mibta52b, and grlm145. The following transgenic lines were used: TG(hsp70:gal4), TG(uas:notch1a-intra), TG(fli1:egfp), and TG(cd41:gfp). The Zebrafish Book, as well as information on all the lines, is available online at zfin.org. All animal work was approved by the ethics review committee of the University of Nottingham and performed under United Kingdom Home Office project license no. 40/2893.
Treatment of embryos with small-molecule inhibitors
Embryos were treated with cyclopamine (Toronto Research Chemicals; stock solution in DMSO [dimethylsulfoxide] 12.5mM) at a concentration of 100μM in fish facility water (60 mg of Instant Ocean salt per liter of H2O) from the 2-8 cell stage or from the 90% epiboly stage (9 hpf). Embryos were treated with the VegfR tyrosine kinase inhibitor 4-[(4′-chloro-2′-fluoro)phenylamino]-6,7-dimethoxyquinazoline (Calbiochem, catalog no. 676475; stock solution in DMSO 12.5mM) at a concentration of 5μM in fish facility water from the 90% epiboly stage (9 hpf). Equivalent amounts of DMSO were added to the media of control embryos. Some of the embryos were grown in the presence of 0.003% phenylthiourea (Sigma) to prevent pigmentation.
MO injections
The following MOs were ordered from Gene-Tools Inc and injected into zebrafish embryos at the 2-8 cell stage to knock down the expression of the following genes: hey2 (5′-CGCGCAGGTACAGACACCAAAAACT-3′),19 scl/tal1 (5′-AATGCTCTTACCATCGTTGATTTCA-3′),26 etsrp (5′-CACTGAGTCCTTATTTCACTATATC-3′),27 and rbpja/b (5′-CAAACTTCCCTGTCACAACAGGCGC-3′).28,29 The following control MO was used: 5′-CCTCTTACCTCAGTTACAATTTATA-3′. Amounts of MO injected are given in the Figure legends.
VegfA21 mRNA injection
Capped zebrafish vegfA mRNA was transcribed in vitro from a NotI-linearized pCS2+vegfA121 template with the SP6 RNA polymerase in the mMessage mMachine kit (Ambion).30
Heat-shock–induced Notch activation
Adult fish that carry the hsp70:gal4 and UAS:notch1a-intra transgenes were identified by polymerase chain reaction amplification of transgene-specific fragments on genomic DNA isolated from their progeny. A UAS:notch1a-intra–specific 450-bp fragment was amplified between the primers 5′-CATCGCGTCTCAGCCTCAC-3′ and 5′-CGGAATCGTTTATTGGTGTCG-3′ as published previously.31 The oligonucleotides 5′-CGATATTTGCCGACTTAAAAAGC-3′ and 5′-TGAAGCCAATCTATCTGTGACG-3′ were newly designed to amplify 307 bp of the hsp70:gal4 transgene. For heat shock–induced Notch activation, embryos from crosses of double-transgenic TG(hsp70:gal4) and TG(UAS:notch1a-intra) adults14 were collected and either injected, treated, or left untreated as indicated. Embryos were then grown overnight at 24°C to the 17-somite (17s) stage, transferred in 5 mL of fish facility water into a 50-mL Falcon tube (Becton Dickinson), and heat shocked at 38°C in a water bath for 20 minutes. Embryos were then transferred back to their original dish and grown to the stage of collection.
Whole-mount in situ hybridization
RNA whole-mount in situ hybridization experiments were performed as described previously.8 Images were acquired on a Nikon SMZ-1500 or a Nikon Eclipse 80i microscope with a Nikon DS-U1 camera and the Nikon Act-2U acquisition software. Figures were collated in Adobe Photoshop CS3.
Results
Hey2 is needed for HSC formation in zebrafish embryos
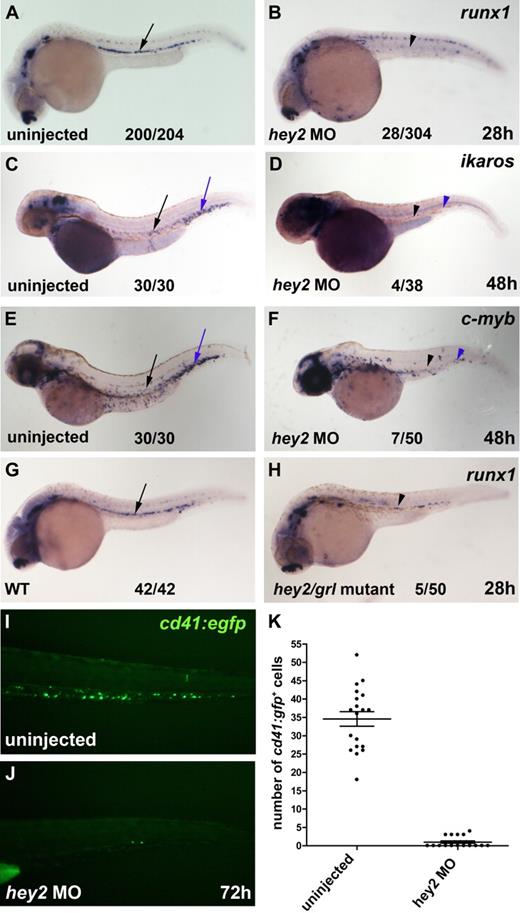
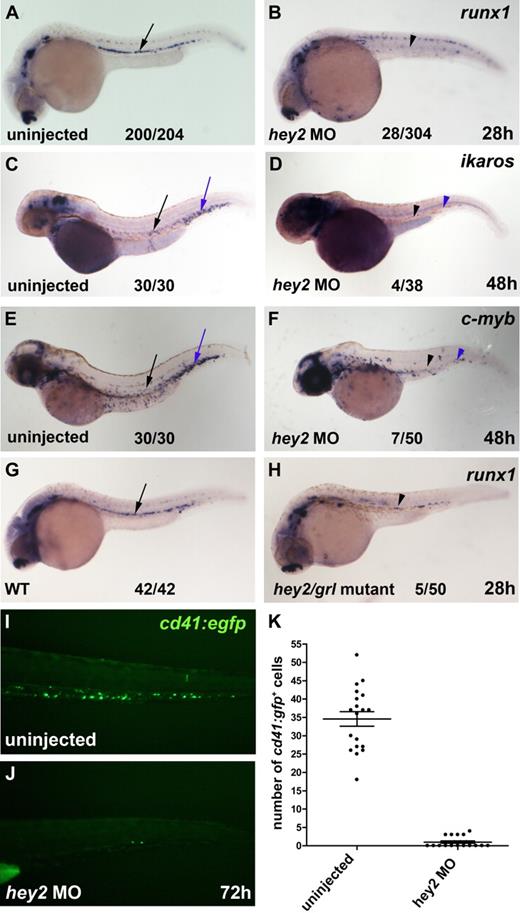
To determine whether Hey2 was required for HSC formation during embryogenesis, we injected zebrafish embryos with a hey2 MO that had been used previously to reveal the role of Hey2 in arterial specification of DA angioblasts.19 We performed in situ hybridization experiments on hey2 morphant embryos to analyze expression of the HSC genes runx1, c-myb, and ikaros in the ventral wall of the DA and in the CHT. These experiments revealed that runx1 expression was severely reduced in the ventral wall of the DA of hey2 morphants at 26 hpf (Figure 1A,B) and that ikaros expression and c-myb expression were strongly diminished in the ventral wall of the DA and in the CHT at 48 hpf (Figure 1C-F). Furthermore, runx1 expression was also reduced in homozygous hey2/grl mutants that carry the hypomorph allele grlm14519 (Figure 1G,H). To quantify the effect of the hey2 MO on definitive hematopoiesis, we injected the hey2 MO into cd41:gfp transgenic embryos that express green fluorescent protein in definitive blood progenitors and thrombocytes in the CHT.11,32 At 72 hpf, the number of cd41:gfp+ cells was significantly lower in hey2 morphants (Figure 1I-K). Altogether, these data suggest that hey2/grl is needed for the emergence of runx1+ HSCs from the ventral wall of the DA.
Hey2 is essential for HSC formation. (A-B) The number of runx1-expressing HSCs (black arrow and arrowhead) was severely reduced in hey2 morphants at 28 hpf. Embryos injected with a control MO had normal runx1 expression at 28 hpf (n = 24/24; data not shown). Ikaros+ (C-D) and c-myb+ (E-F) blood progenitors were missing in the DA (black arrow and arrowhead) and in the CHT (blue arrow and arrowhead) of hey2 morphants at 48 hpf. (G-H) Homozygous hey2/grlm145 mutants possessed fewer runx1+ HSCs (black arrow and arrowhead) than wt control embryos. (I-K) In the CHT, the number of cd41-gfp+ cells was significantly lower in hey2 morphants than in uninjected embryos (Student t test, P < .001). Error bars represent the standard error of the mean for both groups of embryos. In panels A-H, numbers of embryos with normal gene expression are given as a fraction of the number of embryos analyzed. All views of embryos are lateral, with anterior at the left and dorsal at the top. The embryos in panels C-D were treated with phenylthiourea to prevent pigmentation. All morphants were injected with 5 ng of hey2 or control MO.
Hey2 is essential for HSC formation. (A-B) The number of runx1-expressing HSCs (black arrow and arrowhead) was severely reduced in hey2 morphants at 28 hpf. Embryos injected with a control MO had normal runx1 expression at 28 hpf (n = 24/24; data not shown). Ikaros+ (C-D) and c-myb+ (E-F) blood progenitors were missing in the DA (black arrow and arrowhead) and in the CHT (blue arrow and arrowhead) of hey2 morphants at 48 hpf. (G-H) Homozygous hey2/grlm145 mutants possessed fewer runx1+ HSCs (black arrow and arrowhead) than wt control embryos. (I-K) In the CHT, the number of cd41-gfp+ cells was significantly lower in hey2 morphants than in uninjected embryos (Student t test, P < .001). Error bars represent the standard error of the mean for both groups of embryos. In panels A-H, numbers of embryos with normal gene expression are given as a fraction of the number of embryos analyzed. All views of embryos are lateral, with anterior at the left and dorsal at the top. The embryos in panels C-D were treated with phenylthiourea to prevent pigmentation. All morphants were injected with 5 ng of hey2 or control MO.
Hey2 expression is induced downstream of cloche and scl/tal1 in the PLM and is maintained by Hedgehog and Vegf signaling as cells reach the embryonic midline
Next, we wanted to know where Hey2 is placed within the gene regulatory network that controls arterial gene expression and HSC emergence. For this purpose, we sought to determine the genes that (1) induce hey2 expression in the PLM and (2) maintain its expression as the angioblasts migrate to the midline to form the DA angioblast cord. To this end, we analyzed hey2 expression in several morphant and mutant embryos, as well as in inhibitor-treated embryos at the 10-somite stage (10s/14 hpf) and at 24 hpf (Figure 2; Table 1).
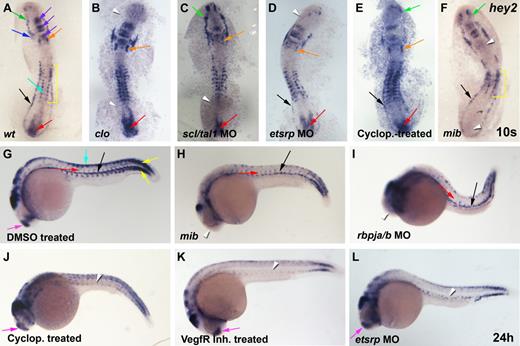
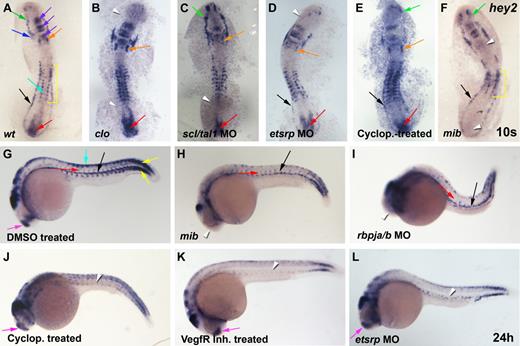
Hey2 expression in the lateral mesoderm is induced by cloche, scl/tal1, and etsrp and is maintained in the DA by Hedgehog and Vegf signaling. (A) At the 10s stage (14 hpf), hey2 was expressed in the ALM (green arrow), the bilateral heart field (blue arrow), the otic vesicle (orange arrow), parts of the midbrain and hindbrain (purple arrow), the neural crest (turquoise arrow), the somites (yellow bracket), the PLM (black arrow), and the tail bud (red arrow). (B) In clo mutants, hey2 expression was lost in the ALM and PLM (white arrowheads). (C) In scl/tal1 morphants (5 ng of MO), PLM expression was lost (white arrowhead). (D) In etsrp morphants (10 ng of MO), hey2 expression in the ALM was lost (white arrowhead). (E) Inhibition of the Hh pathway with cyclopamine (100μM) from the 2-8 cell stage did not eliminate hey2 expression at the 10s stage in any of the tissues analyzed. (F) Hey2 expression was present in the ALM and PLM of mibta52b mutants (green and black arrows) but was absent in the otic vesicle and the tail bud (white arrowheads) and was reduced in the somites (yellow bracket). (G) At 24 hpf, hey2 was expressed in the dorsal aorta (black arrow), the ISVs (red arrow), the somites (yellow arrow), the neural crest (turquoise arrow), and the telencephalon (pink arrow), in addition to parts of the midbrain and hindbrain. In mibta52b mutants (H) and rbpja/b morphants (2 ng of MO; I), hey2 expression in the DA (black arrow) and in ISVs (red arrow) was retained, whereas telencephalon expression was lost (white arrowhead). By contrast, VegfR inhibitor–treated (J; 5μM) and cyclopamine-treated (K; 100μM) and etsrp-morphant (L; 10 ng) embryos displayed normal expression of hey2 in the telencephalon (pink arrow) but failed to express hey2 in the DA and the ISVs (white arrowheads). Embryos in panels J-K were treated from 90% epiboly. All views of embryos are lateral, with anterior at the left and dorsal up.
Hey2 expression in the lateral mesoderm is induced by cloche, scl/tal1, and etsrp and is maintained in the DA by Hedgehog and Vegf signaling. (A) At the 10s stage (14 hpf), hey2 was expressed in the ALM (green arrow), the bilateral heart field (blue arrow), the otic vesicle (orange arrow), parts of the midbrain and hindbrain (purple arrow), the neural crest (turquoise arrow), the somites (yellow bracket), the PLM (black arrow), and the tail bud (red arrow). (B) In clo mutants, hey2 expression was lost in the ALM and PLM (white arrowheads). (C) In scl/tal1 morphants (5 ng of MO), PLM expression was lost (white arrowhead). (D) In etsrp morphants (10 ng of MO), hey2 expression in the ALM was lost (white arrowhead). (E) Inhibition of the Hh pathway with cyclopamine (100μM) from the 2-8 cell stage did not eliminate hey2 expression at the 10s stage in any of the tissues analyzed. (F) Hey2 expression was present in the ALM and PLM of mibta52b mutants (green and black arrows) but was absent in the otic vesicle and the tail bud (white arrowheads) and was reduced in the somites (yellow bracket). (G) At 24 hpf, hey2 was expressed in the dorsal aorta (black arrow), the ISVs (red arrow), the somites (yellow arrow), the neural crest (turquoise arrow), and the telencephalon (pink arrow), in addition to parts of the midbrain and hindbrain. In mibta52b mutants (H) and rbpja/b morphants (2 ng of MO; I), hey2 expression in the DA (black arrow) and in ISVs (red arrow) was retained, whereas telencephalon expression was lost (white arrowhead). By contrast, VegfR inhibitor–treated (J; 5μM) and cyclopamine-treated (K; 100μM) and etsrp-morphant (L; 10 ng) embryos displayed normal expression of hey2 in the telencephalon (pink arrow) but failed to express hey2 in the DA and the ISVs (white arrowheads). Embryos in panels J-K were treated from 90% epiboly. All views of embryos are lateral, with anterior at the left and dorsal up.
In 10s/14-hpf wild-type (wt) embryos, hey2 is expressed in the ALM and in the PLM (Figure 2A), the regions that harbor blood and endothelial progenitors. Hey2 expression is also found in the developing somites, the heart field, parts of the midbrain and hindbrain, the otic vesicle, the neural crest, and around the tail bud, as previously reported by others.23,33
Homozygous cloche (clos5) mutant embryos form ALM and PLM, as evidenced by normal expression of the Ets transcription factor gene fli1.34 The cells, however, subsequently fail to develop into blood, endothelial, and endocardial cells.35,36 In clo mutants, hey2 expression was absent in the ALM and in the PLM but was retained in all other tissues, including the bilateral heart fields (Figure 2B). Within the ALM and PLM, clo is required for expression of the transcription factors Scl/Tal1 and Etsrp.37,38 Loss of etsrp causes complete and specific loss of myeloid and endothelial development.26,39-41 In myeloid cells, Scl/Tal1 appears to be an essential mediator of the role of Etsrp.39 Loss of Scl/Tal1 itself abrogates myeloid and erythroid development from the ALM and PLM, as well as HSC formation. It also causes malformation of the endocardium and interferes with normal gene expression in endothelial progenitors, which leads to a loss of arterial gene expression and ISV formation.26,42,43 In clo mutants, forced Scl/Tal1 expression can rescue blood and endothelial gene expression,37 whereas forced expression of Etsrp has been shown to reestablish endothelial gene expression.38 We observed that scl/tal1 and etsrp morphants displayed interesting changes to the wt hey2 expression pattern. Although hey2 expression required scl/tal1 in the PLM (Figure 2C), it needed etsrp in the ALM (Figure 2D). Hey2 expression in the ALM and PLM was dependent on neither Hedgehog nor Notch signaling. Embryos treated with cyclopamine, a specific inhibitor of the Hedgehog signal transducer Smoothened, displayed normal hey2 expression at this stage (Figure 2E). Homozygous mib mutants and rbpja/b morphants possessed normal hey2 expression in the ALM and PLM (Figure 2F and data not shown), which suggests that Notch does not, as previously suggested, induce hey2 expression in the lateral mesoderm. Instead, Notch controls hey2 expression in the otic vesicle, the tail bud, and the somites. In mib mutants and rbpja/b morphants, hey2 expression was lost or reduced in these tissues. These results demonstrate that the involvement of Notch in controlling hey2 expression is context-dependent. In 24-hpf wt embryos, hey2 expression was most prominent in the DA and in the ISVs, in addition to its somitic and neural crest expression (Figure 2G).23,24,33
As in the PLM, hey2 expression is independent of Notch signaling in the DA and in the ISVs. Although hey2 expression was noticeably absent in the telencephalon, its expression in the DA and in the ISVs was completely normal in 24-hpf mib mutants and rbpja/b morphants (Figure 2H,I).24 By contrast, this endothelial hey2 expression was completely lost in embryos treated with cyclopamine or a Vegf receptor tyrosine kinase inhibitor (VegfR inhibitor; Figure 2J,K), which suggests that Hh (hedgehog) and Vegf signaling maintain hey2 expression once the PLM cells reach the midline to form the DA angioblast cord. At that time point, hey2 expression was also found to be dependent on the presence of Etsrp (Figure 2L). Etsrp morphants previously had been shown to lack expression of the VegfR Kdrl.27,40,41 Thus, in the absence of Etsrp, the cells are no longer able to receive a Vegf signal that is required to maintain hey2 expression as the cells reach the embryonic midline. Scl/Tal1 morphants that already lacked hey2 expression at the 10s stage (14 hpf) showed no hey2 expression in the DA angioblasts at 24 hpf either (data not shown; Bussmann et al42 ). Altogether, the present data suggest that (1) Notch signaling is not needed for hey2 expression in the PLM and in DA angioblasts, (2) clo and scl/tal1 are essential for inducing hey2 expression in the PLM, and (3) Hh and Vegf signaling and Etsrp are needed to maintain hey2 expression in DA angioblasts.
Hey2 is needed for Notch receptor expression in the DA angioblast cord
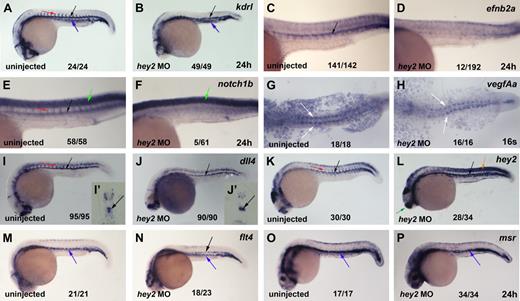
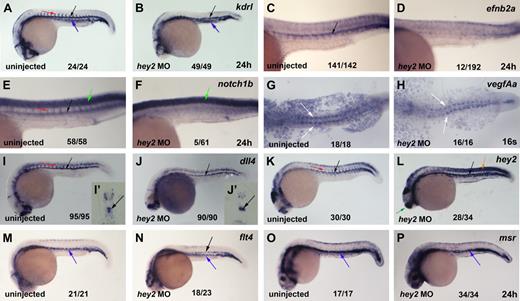
To further examine the role of Hey2 in arterial specification and HSC formation, we analyzed the expression of several arterial-specific, venous-specific, and general endothelial genes in hey2 morphants. The VegfR Kdrl is expressed throughout the endothelium at 24 hpf but displays higher levels of expression in arterial vessels (Figure 3A).35 In the hey2 morphants, kdrl expression in the trunk and tail revealed that the anlagen for both major axial vessels, the DA and the PCV (Figure 3B), were formed in the majority of embryos. Thus, loss of runx1 expression was not due to a complete lack of the DA angioblast cord. The previously described loss of the DA angioblast cord as a consequence of hey2 MO injection19 was observed rarely, required the injection of higher amounts of MO, and was usually associated with drastic overall morphologic abnormalities. Expression of the arterial genes efnb2a, notch1b, and notch5 was eliminated by the hey2 MO (Figure 3C-F; supplemental Figure 1A,B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This loss was probably not due to a defect in the hey2-expressing somites, which continued to express vegfA in the hey2 morphants (Figure 3G,H). Interestingly, DA expression of the genes for the Notch ligands, Dll4 and DeltaC, was not affected in hey2 morphants (Figure 3I,J; supplemental Figure 1C,D). Endogenous hey2 mRNA levels were even found to be drastically elevated in the DA, somites, and telencephalon of the hey2 morphants (Figure 3K,L), which suggests that Hey2 negatively autoregulates its own expression in various tissues. Reduced expression of retained DA markers in the ISVs (Figure 3A,B,I-L; supplemental Figure 1A-D) is due to delayed and irregular development of ISVs in hey2 morphants (supplemental Figure 1E-H), a phenotype that is reminiscent of abnormalities observed in ISV formation in hey1/hey2 double-knockout mice.44 Altogether, these data show that a DA angioblast cord forms under conditions in which neither efnb2a nor runx1 is expressed in hey2 morphants, which suggests a specific role for Hey2 in DA angioblasts. In hey2 morphants, DA angioblasts fail to express Notch receptors but display normal expression of Notch ligands.
Hey2 is essential for expression of Notch receptors in the DA angioblast cord. (A) Kdrl was expressed in the DA (black arrow), PCV (blue arrow), and ISVs (red arrow) of the trunk and tail of 24-hpf control embryos. (B) Separate cords of DA and PCV kdrl-expressing angioblasts were present in hey2 morphant embryos. Expression of the arterial genes efnb2a (C-D) and notch1b (E-F) was lost in hey2 morphants. Note that spinal cord expression of notch1b was unaffected (E-F green arrow). VegfAa expression in somites was normal in hey2 morphant embryos (G-H white arrows). The Notch ligand gene dll4 was expressed in the DA of hey2 morphants (Ii-Jii black arrows). Loss of Hey2 protein caused increased endogenous hey2 mRNA expression in the DA and in somites (K-L orange arrow) of hey2 morphants, which suggests the presence of a negative autoregulatory loop. In kdrl- and dll4-stained hey2 morphant embryos, ISV staining was reduced (B,J; n(kdlr) = 41/49; n(dll4) = 51/90). (M) At 24 hpf, flt4 expression was restricted to the PCV in control embryos. (N) In the DA of hey2 morphants, it was retained in addition to normal venous expression. (O-P) Another venous marker, msr, was expressed in the vein of control and hey2 morphant embryos. All views of embryos in panels A-F and I-P are lateral, with anterior left and dorsal up. Dorsal views of embryos with anterior left are provided in panels G-H. Close-ups of the trunk of embryos are shown in C-F. The inserts (Ii and Ji) show transverse sections through the posterior trunk. Numbers of embryos with normal gene expression in the DA (A-F,I-P) or in the somites (G-H) are given as a fraction of the number of embryos analyzed. All morphants were injected with 5 ng of hey2 MO.
Hey2 is essential for expression of Notch receptors in the DA angioblast cord. (A) Kdrl was expressed in the DA (black arrow), PCV (blue arrow), and ISVs (red arrow) of the trunk and tail of 24-hpf control embryos. (B) Separate cords of DA and PCV kdrl-expressing angioblasts were present in hey2 morphant embryos. Expression of the arterial genes efnb2a (C-D) and notch1b (E-F) was lost in hey2 morphants. Note that spinal cord expression of notch1b was unaffected (E-F green arrow). VegfAa expression in somites was normal in hey2 morphant embryos (G-H white arrows). The Notch ligand gene dll4 was expressed in the DA of hey2 morphants (Ii-Jii black arrows). Loss of Hey2 protein caused increased endogenous hey2 mRNA expression in the DA and in somites (K-L orange arrow) of hey2 morphants, which suggests the presence of a negative autoregulatory loop. In kdrl- and dll4-stained hey2 morphant embryos, ISV staining was reduced (B,J; n(kdlr) = 41/49; n(dll4) = 51/90). (M) At 24 hpf, flt4 expression was restricted to the PCV in control embryos. (N) In the DA of hey2 morphants, it was retained in addition to normal venous expression. (O-P) Another venous marker, msr, was expressed in the vein of control and hey2 morphant embryos. All views of embryos in panels A-F and I-P are lateral, with anterior left and dorsal up. Dorsal views of embryos with anterior left are provided in panels G-H. Close-ups of the trunk of embryos are shown in C-F. The inserts (Ii and Ji) show transverse sections through the posterior trunk. Numbers of embryos with normal gene expression in the DA (A-F,I-P) or in the somites (G-H) are given as a fraction of the number of embryos analyzed. All morphants were injected with 5 ng of hey2 MO.
Expression of venous genes like flt424 and msr26 was normal in the vein of hey2 morphants (Figure 3M-P). In wt embryos, flt4 is initially expressed in all ECs and by 24 hpf becomes restricted to the vein. Notch is known to repress flt4 expression in the DA.24 In hey2 morphants, flt4 expression was not down-regulated in the DA, possibly because of a lack of Notch signaling in the hey2 morphants, which fail to express Notch receptors. Ectopic expression of the venous marker msr was not observed in the hey2 morphants. Together with the finding that hey2, deltaC, and dll4 expression was retained in the DA, this suggests that the DA angioblast cord had not been reprogrammed to express venous genes in the hey2 morphant.
Rescue experiments confirm that hey2 is expressed downstream of Vegf in the DA angioblast cord
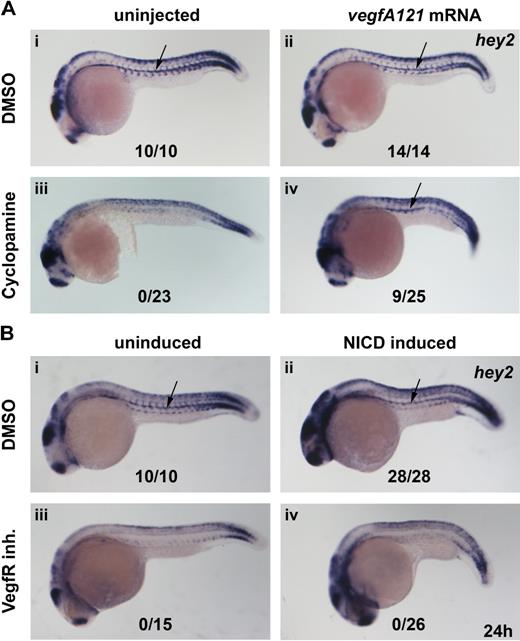
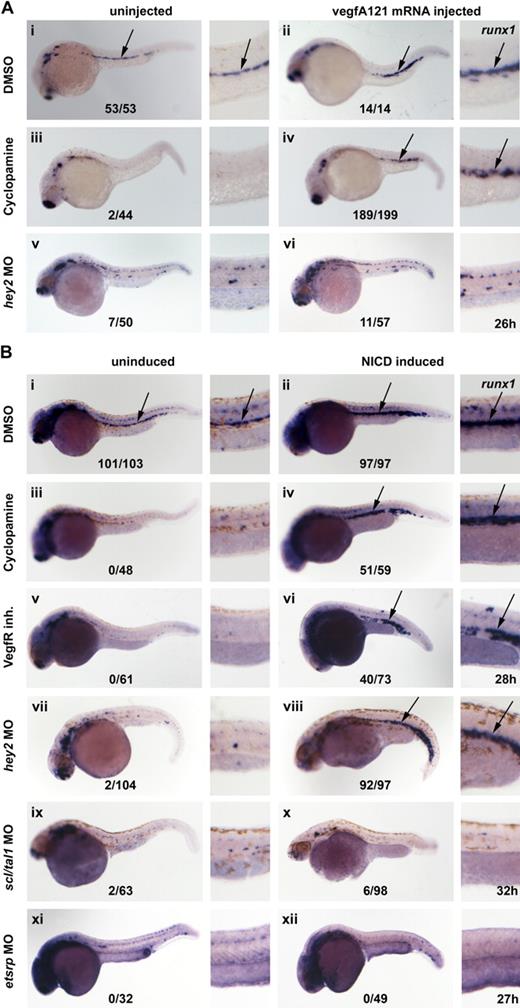
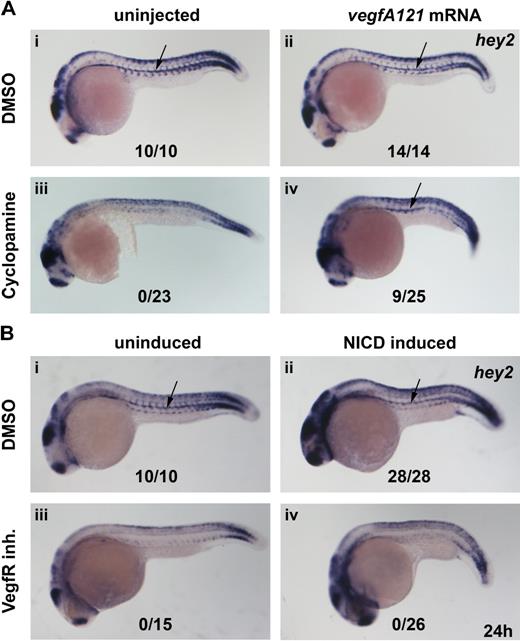
The present in situ data suggested that Hey2 acts downstream of Hedgehog and Vegf and upstream of Notch in arterial specification and HSC formation in the zebrafish embryo. We assumed that Hhg signaling was relayed by VegfA. To confirm this, we performed rescue experiments in which cyclopamine-treated embryos were injected with vegfA121 mRNA to determine whether it could rescue hey2 expression. Although misexpression of VegfA121 did not change the wt hey2 expression pattern in control embryos (Figure 4Ai,ii), it rescued hey2 expression in the DA of cyclopamine-treated embryos (Figure 4Aiii,iv). Next, we tried to rescue hey2 expression in embryos that had been treated with the VegfR inhibitor by activating the Notch pathway. We used a Gal4-UAS system to misexpress constitutively active zebrafish NICD in zebrafish embryos.24 Heat-shock induction of NICD expression at the 17s (17.5 hpf) stage caused a mild shortening of the yolk cell extension and gave the embryo a slightly stocky appearance. It did not alter hey2 expression in DMSO-treated embryos (Figure 4Bi,ii), but it caused ectopic runx1 expression in the dorsolateral aspect of the DA and in the vein (data not shown), as reported previously.15 In the same batch of transgenic embryos, NICD induction caused the typical phenotypic alterations in overall morphology but failed to rescue hey2 expression after VegfR inhibitor treatment (Figure 4Biii,iv). Altogether, these results are consistent with the idea that Hey2 is maintained in the DA angioblast cord by Vegf signaling downstream of Hh signaling and that it acts upstream rather than downstream of Notch.
Hey2 is expressed downstream of Vegf signaling in the DA. Inhibition of the Hh signaling pathway with the Smoothened inhibitor cyclopamine (100μM) or of the Vegf signaling pathway with a VegfR inhibitor (5μM) from 90% epiboly (9 hpf) caused a loss of hey2 expression in the DA (Ai,iii,Bi,iii arrow). Although injection of 100 pg of vegfA mRNA did not change hey2 expression in control embryos, it rescued hey2 expression in cyclopamine-treated embryos (Aii,iv). In a similar rescue experiment, heat-shock–induced ubiquitous expression of NICD at the 17s (17.5-hpf) stage failed to rescue hey2 expression. All views of embryos are lateral, with anterior left and dorsal up. Numbers of embryos with hey2 gene expression in the DA are given as a fraction of the number of embryos analyzed. inh. indicates inhibitor.
Hey2 is expressed downstream of Vegf signaling in the DA. Inhibition of the Hh signaling pathway with the Smoothened inhibitor cyclopamine (100μM) or of the Vegf signaling pathway with a VegfR inhibitor (5μM) from 90% epiboly (9 hpf) caused a loss of hey2 expression in the DA (Ai,iii,Bi,iii arrow). Although injection of 100 pg of vegfA mRNA did not change hey2 expression in control embryos, it rescued hey2 expression in cyclopamine-treated embryos (Aii,iv). In a similar rescue experiment, heat-shock–induced ubiquitous expression of NICD at the 17s (17.5-hpf) stage failed to rescue hey2 expression. All views of embryos are lateral, with anterior left and dorsal up. Numbers of embryos with hey2 gene expression in the DA are given as a fraction of the number of embryos analyzed. inh. indicates inhibitor.
Notch signaling rescues arterial gene expression in hey2 morphants
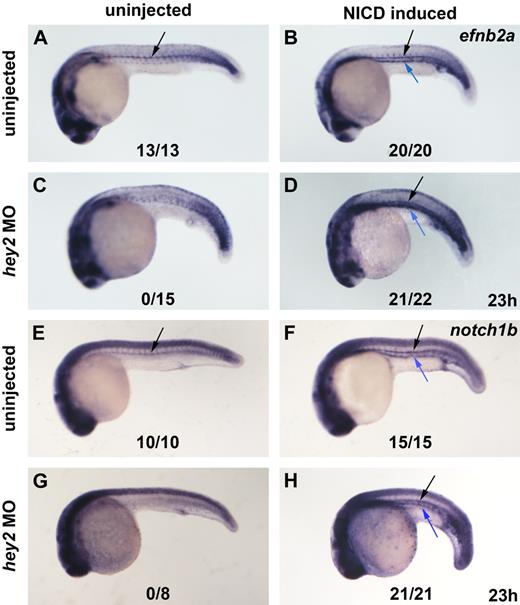
Next, we wanted to see whether Notch is able to rescue arterial gene expression in hey2 morphants. For this purpose, NICD expression was induced at the 17s stage in hey2 morphant embryos that were subsequently analyzed for efnb2a and notch1b expression by in situ hybridization. In control embryos, we observed that Notch induction caused ectopic expression of both arterial genes in the vein in addition to their normal expression in the DA (Figure 5A,B,E,F). In hey2 morphants that usually fail to express efnb2a and notch1b in the DA, expression of efnb2a and notch1b was rescued by forced expression of NICD (Figure 5C,D,G,H). This confirms that Notch acts downstream of Hey2 in the induction of arterial gene expression.
Notch signaling acts downstream of Hey2 in arterial gene expression. Hey2 morphants failed to express the arterial genes efnb2a and notch1b in the DA angioblast cord (A,C,E,G black arrow). Heat-shock–induced ubiquitous activation of the Notch pathway rescued efnb2a (D) and notch1b (H) expression in the DA of hey2 morphants and caused ectopic induction of arterial gene expression in all embryos analyzed (B,D,F,H blue arrow). All views of embryos are lateral, with anterior left and dorsal up. Numbers of embryos with arterial gene expression in the DA are given as a fraction of the number of embryos analyzed. All morphants were injected with 4 ng of hey2 MO.
Notch signaling acts downstream of Hey2 in arterial gene expression. Hey2 morphants failed to express the arterial genes efnb2a and notch1b in the DA angioblast cord (A,C,E,G black arrow). Heat-shock–induced ubiquitous activation of the Notch pathway rescued efnb2a (D) and notch1b (H) expression in the DA of hey2 morphants and caused ectopic induction of arterial gene expression in all embryos analyzed (B,D,F,H blue arrow). All views of embryos are lateral, with anterior left and dorsal up. Numbers of embryos with arterial gene expression in the DA are given as a fraction of the number of embryos analyzed. All morphants were injected with 4 ng of hey2 MO.
Hey2 acts upstream of Notch in HSC formation
We previously proposed that as in arterial specification of the DA, Hh, VegfA, and Notch acted in a cascade during HSC formation.8 To provide formal proof of this, embryos were treated with cyclopamine and injected with zebrafish vegfAa121 mRNA30 in an attempt to rescue runx1 expression. Embryos were collected at 26 hpf, and runx1 expression was examined. Although vegfAa121 mRNA–injected embryos displayed the normal runx1 expression pattern, embryos treated with cyclopamine had no runx1 expression (Figure 6Ai-iii). By contrast, a significant number of embryos treated with cyclopamine and injected with vegfA121 mRNA regained runx1 expression in the midline (Figure 6Aiv), which demonstrates that VegfA can induce runx1 expression in the absence of Hh signaling. By contrast, forced expression of VegfA in hey2 morphants failed to rescue runx1 expression (Figure 6Av-vi). From these experiments, we conclude that VegfA acts downstream of Hh but not downstream of Hey2 to specify HSC formation.
Hey2 acts upstream of Notch in HSC specification. (A) Expression of runx1 in the DA was lost in cyclopamine (100μM)-treated and hey2 (4 ng of MO)–morphant embryos (i,iii,v). Although 80 ng of vegfA mRNA expression convincingly rescued runx1 expression in cyclopamine-treated embryos (iv), it failed to do so in hey2 morphants (vi). Ectopic expression of runx1 was observed in 4/14 wt (ii) and in 75 of 199 cyclopamine-treated (iv) VegfAa121 mRNA-injected embryos. (B) Expression of runx1 in the DA was lost in cyclopamine-treated (iii; 100μM, treated from 9 hpf), VegfR inhibitor–treated (v; 5μM, treated from 9 hpf), hey2-morphant (vii; 4 ng), scl/tal1-morphant (ix; 5 ng), and etsrp-morphant (xi; 10 ng) embryos. Heat-shock–induced NICD expression at the 17s (17.5 hpf) stage caused ectopic expression of runx1 in control embryos (ii). Furthermore, it rescued and caused ectopic expression of runx1 in cyclopamine-treated (iv), VegfR inhibitor–treated (vi), and hey2-morphant (viii) embryos. By contrast, it failed to induce runx1 expression in tal1/scl-morphant (x) and etsrp-morphant (xii) embryos. All views of embryos are lateral, with anterior left and dorsal up. High-magnification images of the posterior trunk region of all embryos are provided. Numbers of runx1-expressing embryos are given as a fraction of the number of embryos analyzed. inh indicates inhibitor.
Hey2 acts upstream of Notch in HSC specification. (A) Expression of runx1 in the DA was lost in cyclopamine (100μM)-treated and hey2 (4 ng of MO)–morphant embryos (i,iii,v). Although 80 ng of vegfA mRNA expression convincingly rescued runx1 expression in cyclopamine-treated embryos (iv), it failed to do so in hey2 morphants (vi). Ectopic expression of runx1 was observed in 4/14 wt (ii) and in 75 of 199 cyclopamine-treated (iv) VegfAa121 mRNA-injected embryos. (B) Expression of runx1 in the DA was lost in cyclopamine-treated (iii; 100μM, treated from 9 hpf), VegfR inhibitor–treated (v; 5μM, treated from 9 hpf), hey2-morphant (vii; 4 ng), scl/tal1-morphant (ix; 5 ng), and etsrp-morphant (xi; 10 ng) embryos. Heat-shock–induced NICD expression at the 17s (17.5 hpf) stage caused ectopic expression of runx1 in control embryos (ii). Furthermore, it rescued and caused ectopic expression of runx1 in cyclopamine-treated (iv), VegfR inhibitor–treated (vi), and hey2-morphant (viii) embryos. By contrast, it failed to induce runx1 expression in tal1/scl-morphant (x) and etsrp-morphant (xii) embryos. All views of embryos are lateral, with anterior left and dorsal up. High-magnification images of the posterior trunk region of all embryos are provided. Numbers of runx1-expressing embryos are given as a fraction of the number of embryos analyzed. inh indicates inhibitor.
Next, we tested whether activation of Notch could rescue runx1 expression in Hh-depleted, Vegf-depleted, or hey2 morphant embryos. Embryos that carried the hsp-gal4 and uas-nicd transgenes were first treated with cyclopamine or the VegfR inhibitor or were injected with the hey2 MO. At the 17s (17.5 hpf) stage, the embryos were subjected to heat shock to induce NICD expression. Our experiments revealed that Notch reliably rescued runx1 expression in all of these manipulated embryos (Figure 6Biii-viii). This confirms that Notch acts downstream of Hh, Vegf, and Hey2 to specify HSC formation.
Endothelial programming is an obligate prerequisite for Notch-induced HSC formation
We also tested whether Notch activation could rescue runx1 expression in etsrp or scl/tal1 morphants that have an earlier block in endothelial development than Hh-, Vegf-, or Hey2-depleted embryos. Our experiments showed that although the typical morphologic changes accompanied heat-shock induction of NICD expression at the 17s (17.5 hpf) stage in etsrp and scl/tal1 morphants, Notch was not able to induce runx1 expression in these morphant embryos (Figure 6Bix-xii). These experiments showed that proper early endothelial programming is a prerequisite to enable cells to respond to forced Notch activation by inducing runx1 expression.
Discussion
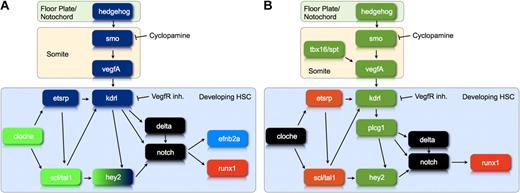
We previously proposed that the Hh-Vegf-Notch signaling cascade, which had been shown to control arterial gene expression in zebrafish embryos,14 was also involved in the emergence of HSCs.8 This was based on the shared need for Hh, Vegf, and Notch signaling in arterial specification and HSC emergence, as well as the identical timing of the Hh requirement for the 2 processes. Here, we provide formal proof by demonstrating both that runx1 expression can be rescued in cyclopamine-treated embryos through the injection of zebrafish vegfA121 mRNA or the induction of NICD expression and that Notch can rescue runx1 expression in embryos treated with a VegfR inhibitor. The latter finding is consistent with a recent publication in which Notch was shown to rescue runx1 expression in plcg1 and spt/tbx16 morphants, both of which have a defect in vegfA signaling.45 These data show that the Hh-Vegf-Notch signaling cascade controls the onset of definitive hematopoiesis (Figure 7A).
The gene regulatory network controlling the differentiation of developing HSC. (A) This diagram illustrates the position of hey2 within the gene regulatory network that controls arterial gene expression and HSC development during zebrafish embryogenesis. Genes shown in green are required for hey2 expression in the PLM at the 10s stage. Genes shown in blue are needed for maintenance of hey2 expression in the DA angioblast cord. Please note that our data neither clarify the position of individual Scl/Tal1 isoforms in the network relative to Hey2 nor provide insights into a specific late role of Scl/Tal1 in HSC formation. (B) This diagram illustrates whether or not runx1 expression can be rescued by activation of the Notch pathway in embryos that lack particular gene products. Loss of genes/gene products in green can be compensated by activation of the Notch pathway, whereas loss of genes/gene products in orange cannot. The diagram summarizes data from the present report and the literature.45 These data define a regulatory state, best described as early endothelial, that a cell needs to reach if it is to respond to a Notch signal by activating runx1 expression.
The gene regulatory network controlling the differentiation of developing HSC. (A) This diagram illustrates the position of hey2 within the gene regulatory network that controls arterial gene expression and HSC development during zebrafish embryogenesis. Genes shown in green are required for hey2 expression in the PLM at the 10s stage. Genes shown in blue are needed for maintenance of hey2 expression in the DA angioblast cord. Please note that our data neither clarify the position of individual Scl/Tal1 isoforms in the network relative to Hey2 nor provide insights into a specific late role of Scl/Tal1 in HSC formation. (B) This diagram illustrates whether or not runx1 expression can be rescued by activation of the Notch pathway in embryos that lack particular gene products. Loss of genes/gene products in green can be compensated by activation of the Notch pathway, whereas loss of genes/gene products in orange cannot. The diagram summarizes data from the present report and the literature.45 These data define a regulatory state, best described as early endothelial, that a cell needs to reach if it is to respond to a Notch signal by activating runx1 expression.
We show for the first time in a vertebrate embryo that Hey2 plays an essential role in HSC formation. Such a role is consistent with its expression in the DA angioblast cord before HSC emergence19,24,33 and with the published hypothesis that Hey2 acts downstream of Notch during arterial specification.19 Although we could confirm that Hey2 is required for arterial specification of the DA, we found no evidence for Hey2 activation downstream of Notch signaling in the process. In mibta52b mutants, Hey2 expression was lost neither in the PLM nor in the DA angioblast cord (present data; Lawson et al24 ). Hey2 expression was furthermore unaffected in rbpja/b morphants, and attempts to rescue hey2 expression in embryos treated with a VegfR inhibitor by activating the Notch pathway failed. Interestingly, the mib mutants and rbpja/b morphants in the present study did lose hey2 expression in several other tissues, which demonstrates that whether hey2 expression is activated downstream of Notch signaling is context-dependent.
Analysis of hey2 expression in several mutant, morphant, and inhibitor-treated embryos has helped us to establish a better understanding of hey2 regulation during embryogenesis. The present data suggest that hey2 expression in the ALM and PLM requires cloche, a gene needed for all blood, endothelial, and endocardial development in zebrafish.35,36 Downstream of cloche, different transcription factors are needed for hey2 expression in the ALM and the PLM (Figure 7A). Although the etsrp morphants lacked hey2 expression in the ALM, scl/tal1 morphants had no expression of hey2 in the PLM. This reveals that the wiring of the gene regulatory network that controls hey2 expression is slightly different in the ALM and the PLM. The loss of PLM expression in the scl/tal1 morphants underlines the important role that Scl/Tal1 plays in early vasculogenesis.26,42
After the PLM cells have reached the midline to form the DA angioblast cord, hey2 expression is maintained by Vegf signaling (Figure 7A). Embryos treated with a VegfR inhibitor failed to express hey2 in the DA. Likewise, embryos treated with the Smoothened inhibitor cyclopamine expressed hey2 in the PLM at the 10s stage but failed to maintain it in the DA. Because Hh secreted by the notochord and the floor plate is known to drive VegfAa secretion from the medioventral somites,14 it appears likely that Hh induces hey2 expression indirectly via VegfA signaling. Consistent with this idea, we found that VegfA mRNA injected into cyclopamine-treated embryos rescued hey2 expression in a subset of embryos. The lack of complete rescue may reveal imperfect distribution of the injected mRNA. However, the finding that the same mRNA convincingly rescued runx1 expression in cyclopamine-treated embryos suggests that Hh could play a direct role in inducing hey2 expression in the DA angioblast cord. A direct role for Hh in DA gene expression has been suggested recently by Wilkinson et al.46 Like the cyclopamine-treated embryos, etsrp morphants first expressed hey2 in the PLM and then were unable to maintain its expression. This is likely to be a result of the loss of kdrl expression and consequent lack of Vegf signaling in the etsrp-depleted embryos.27,39-41
Hey2 acts upstream of Notch signaling in arterial specification of DA angioblasts and in HSC formation (Figure 7A). Expression of the Notch receptors Notch1b and Notch5 was lost in the hey2 morphants, which abrogates Notch pathway activation in the DA. Consequently, efnb2a is not expressed, flt4 is not down-regulated, and runx1+ cells do not emerge from the DA angioblast cord. Forced activation of the Notch pathway in hey2 morphants rescued arterial and HSC gene expression, which suggests that the gene regulatory network is a linear cascade that involves Vegf, Hey2, and Notch upstream of arterial and HSC gene expression. However, 2 findings show that Hey2 is not the only transcription factor activated downstream of Vegf and that the gene regulatory network is not a simple linear cascade. First, Delta expression is retained in hey2 morphants but lost in Vegf signaling-depleted embryos; thus, Hey2 expression is likely to be insufficient to induce Notch pathway activation downstream of Vegf. Consistent with this idea, attempts to rescue runx1 expression in Hh- or Vegf-depleted embryos by injection of hey2 mRNA were unsuccessful (J.M.R. and M.G., unpublished data, February 2009), although defects in early embryonic development caused by injection of even relatively moderate amounts of Hey2 mRNA complicate the interpretation of the data. Second, although Notch activation could rescue runx1 expression in VegfR inhibitor–treated embryos, it failed to rescue expression of notch1b and efnb2a in our experiments (supplemental Table 1). We assume that previous reports of successful rescues of arterial gene expression, for example, in VegfA morphants,14 relied on some residual Vegf signaling. The successful Notch-mediated rescue of arterial gene expression in hey2 morphants was probably due to retention of expression of some VegfA-dependent genes in the hey2 morphants.
Embryonic development in general and differentiation of individual cells into mature cell types in particular can be described as a progression of cells through consecutive regulatory states. Each state is defined by the presence and activity of particular sets of transcription factors in the cell nucleus.47 This in turn is a direct consequence of the molecular programming that the cell has undergone. The ability of a cell to activate runx1 expression in response to Notch pathway activation depends on its regulatory state. Although Hh-, Vegf-, and Hey2-depleted embryos expressed runx1 in response to NICD induction, scl/tal1 and etsrp morphants failed to do so (Figure 7B). In scl/tal1 and etsrp morphants, endothelial development is arrested at stages earlier than in Hh-, Vegf-, or Hey2-depleted embryos. Thus, the present findings strongly suggest that normal early endothelial programming is needed to allow cells to reach the regulatory state required for runx1 expression in response to activation of the Notch signaling pathway.
Although there is only 1 arterially expressed hey gene in zebrafish,33 2 hey genes are expressed in overlapping patterns in early arterial vessels of the mouse embryo. Unlike their zebrafish counterpart, their expression is induced by Notch signaling. Mouse hey1 and hey2 expression was lost in the yolk sac vasculature of notch1b- or mib1-deficient embryos.44,48 Mouse hey1 expression was also lost in the DA of mib1 knockout embryos.48 Although the timing of their expression and the details of regulation may differ from that of zebrafish hey2, the role of Hey proteins in the specification of arterial vessels is conserved from fish to mouse. Like hey2-depleted zebrafish embryos, hey1/hey2 double-knockout mice failed to express arterial genes and displayed vascular remodeling defects and vascular degeneration.44,49 It will be interesting to see whether Hey1 and Hey2 are also needed in the mouse for HSC formation. The finding that zebrafish Hey2 acts upstream rather than downstream of Notch signaling raises the question of what acts downstream of Notch signaling in arterial gene expression and HSC formation. In the human microvasculature EC line HMEC1, efnb2a was shown to be a direct Notch target gene.50 In HSC formation, there is no evidence for direct regulation of runx1 by Notch. Instead, gata2 appears to be a direct target of Notch51 and a potential mediator for runx1 regulation.52 Further work is required to elucidate the steps downstream of Notch.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Kathryn McMahon for critical reading of the manuscript. We are grateful to Rouwen Ge for the zebrafish Vegf121 expression plasmid, Julian Lewis for the Gal4-UAS line, Roger Patient for the fli1-egfp line, Philippe Herbomel for the cd41:gfp line, Didier Stainier for the cloche mutant line, and Tim Chico for fixed grl mutant embryos.
The work was supported by a Biotechnology and Biological Sciences Research Council studentship to J.M.R. and a Medical Research Council research grant (no. G0601134) to M.G.
Authorship
Contribution: J.M.R designed, performed, and analyzed the experiments; and M.G. conceived the project, supervised the experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin Gering, Institute of Genetics, School of Biology, University of Nottingham, Queen's Medical Centre, Nottingham, NG7 2UH, United Kingdom; e-mail: martin.gering@nottingham.ac.uk.