Abstract
The combination of cytotoxic chemotherapy and imatinib has improved the outcome for patients with Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). Dasatinib has significant clinical activity in patients with imatinib resistance. We examined the efficacy and safety of combining chemotherapy with dasatinib for patients with Ph+ ALL. Newly diagnosed patients received dasatinib 50 mg by mouth twice per day (or 100 mg daily) for the first 14 days of each of 8 cycles of alternating hyper-CVAD, and high-dose cytarabine and methotrexate. Patients in complete remission received maintenance daily dasatinib and monthly vincristine and prednisone for 2 years, followed by dasatinib indefinitely. Thirty-five patients with untreated Ph+ ALL with a median age of 53 years (range, 21-79 years) were treated; 33 patients (94%) achieved complete remission. Two patients died of infections before response assessment. Grade 3 and 4 adverse events included hemorrhage and pleural and pericardial effusions. With a median follow-up of 14 months (range, 4-37 months), the median disease-free survival and median overall survival have not been reached, with an estimated 2-year survival of 64%. The combination of chemotherapy with dasatinib is effective in achieving long-term remissions in patients with newly diagnosed Ph+ ALL. This study was registered at www.ClinicalTrials.gov as NCT00390793.
Introduction
Before the introduction of tyrosine kinase inhibitors, the outcome of the majority of patients with Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) was poor. Although complete remission (CR) could be achieved in most patients (60%-90%), CR duration and disease-free survival (DFS) were short, with few longterm survivors. In older patients, particularly those older than 60 years of age, the outcome was particularly dismal, with high treatment-related mortality, low CR rates, and low long-term DFS and survival.1 As a result, allogeneic stem cell transplantation would be offered to all patients in first CR who had a suitable donor. Even with these traditional regimens, the degree of reduction of BCR-ABL transcripts after induction and consolidation was a powerful predictor of disease response and survival.2
However, the success of allogeneic stem cell transplantation has been limited because of its associated toxicity and the limited availability of donors. The introduction of tyrosine kinase inhibitors improved the likelihood of identifying a donor because these agents provide relatively durable responses that allow for the identification of a donor. In vitro studies demonstrated synergistic or additive effects against Ph+ cell lines when imatinib was combined with various cytotoxic agents, which suggests a potential role for these combinations in patients.3-5 Several investigators explored the efficacy of imatinib in combination with chemotherapy for frontline treatment of patients with Ph+ ALL. Initially, the optimal schedule was debated, and both concurrent and sequential schedules were investigated.6-11
In the first clinical trial reporting the combination of imatinib with chemotherapy, a CR rate of 96% with a 2-year DFS rate of 85% was reported, and half of the initial cohort of 20 patients underwent allogeneic stem cell transplantation.6 These results were significantly superior to historical results with the chemotherapy regimen alone. Furthermore, molecular complete responses as analyzed by reverse transcription–polymerase chain reaction were reported in 60% of patients. Importantly, there was no unexpected toxicity related to the addition of imatinib to the regimen.6 Other investigators have also reported the results of studies that incorporated imatinib into chemotherapy regimens designed for ALL, and the initial debates of concurrent versus sequential imatinib have been largely settled by several reports of improved efficacy and low toxicity with the concurrent regimens.9 In the early reports, the outcome of patients treated with such regimens was comparable whether or not the patients underwent allogeneic stem cell transplantation in first CR, which raises the debate of the potential for a “cure” without a transplant.7,11
Both acquired and intrinsic resistance to imatinib have been described in patients with Ph+ ALL. Acquired imatinib resistance may be due to BCR-ABL–dependent mechanisms such as BCR-ABL overexpression or mutations in the kinase domains (KDs).12,13 Other mechanisms of resistance independent of BCR-ABL have also been reported and include pharmacokinetic factors that reduce the availability of imatinib within Ph+ cells and activation of alternative signaling pathways such as the Src-kinase pathways.14-16
Second-generation inhibitors capable of overcoming such resistance are available. Dasatinib is a dual Src and Abl kinase inhibitor that binds both active and inactive moieties of the bcr-abl protein and is approximately 325 times more potent against the kinase in preclinical studies.17 The inhibition of Src may also be important in overcoming imatinib resistance, particularly in lymphoid leukemias, in which Src kinase activity may have pathogenetic importance.18 Dasatinib is active in vitro against all imatinib-resistant BCR-ABL mutants with the exception of T315I.17 Significant activity of dasatinib in patients with Ph+ leukemias who were resistant to or intolerant of imatinib has been reported.19,20 Ottmann et al conducted a phase 2 study of dasatinib in 36 patients with Ph+ ALL after failing imatinib.20 The median age of the patients was 46 years (range, 15-85 years). Major hematologic response was achieved in 15 patients (42%) and cytogenetic CR in 21 (58%). They reported 6 patients with a baseline T315I mutation, none of whom responded to treatment. Response rates were similar in patients with other mutations compared with those with no mutations.20 Recently, administration of dasatinib once daily in patients with Ph+ ALL produced similar responses and was associated with less toxicity, with a lower incidence of myelosuppression or pleural effusions.21
On the basis of the significant activity of dasatinib against BCR-ABL and impressive data in patients with relapsed or imatinib-resistant disease, we hypothesized that the combination of dasatinib and chemotherapy would be effective in treating patients with Ph+ ALL and conducted this phase 2 study to examine the toxicity and efficacy of this regimen.
Methods
Eligibility
Patients with previously untreated Ph+ ALL, determined by the identification of either t(9;22) karyotype or BCR-ABL fusion transcript, were eligible. Furthermore, patients had to be 18 years or older, have an Eastern Cooperative Oncology Group performance status of 2 or less, and have an adequate liver and renal function (with serum bilirubin ≤ 3.0 mg/dL and a serum creatinine ≤ 3.0 mg/dL, unless considered due to tumor). Patients were excluded if they had an active infection not controlled by antibiotics, clinical evidence of grade 3 to 4 heart failure as defined by the New York Heart Association criteria, active second malignancy, or prior history of treatment with dasatinib. Patients were also not eligible to participate if they were pregnant or breastfeeding, had a history of bleeding diathesis, or had a pleural or pericardial effusion thought not to be related to leukemia. All patients signed a consent form in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Texas M. D. Anderson Cancer Center.
Treatment regimen
The details of the hyper-CVAD regimen have been published previously.6,22 Odd courses (1, 3, 5, and 7) of hyperfractionated cyclophosphamide (Cytoxan), doxorubicin (Adriamycin), vincristine (Oncovin), and dexamethasone were given alternately with even courses (2, 4, 6, and 8) of high-dose cytarabine and methotrexate. All even courses were preceded by a chest radiograph to ensure the absence of a significant pleural effusion before the administration of methotrexate. Dasatinib 50 mg orally twice daily (or 100 mg orally daily after an amendment to the study when further data on the best dose and schedule of dasatinib became available21,23 ) was administered in the first 14 days of each of the above 8 courses because of concern about the myelosuppressive property of dasatinib, particularly in combination with intensive chemotherapy. For central nervous system (CNS) prophylaxis, intrathecal therapy with methotrexate and cytarabine was given alternately on days 2 and 7 of each course for a total of 6 or 8 doses, depending on the risk of CNS relapse (based on serum lactate dehydrogenase and the marrow proliferative index). For patients presenting with active CNS disease, confirmed by cytologic examination of the cerebrospinal fluid (CSF), the above regimen was repeated twice weekly until the CSF became clear of leukemic cells and the CSF cell count normalized; then the patients received intrathecal therapy once per week for 4 weeks or until initiation of the next cycle of chemotherapy, when the above-stated prophylactic regimen was resumed. Cranial irradiation was not administered for prophylaxis, but patients presenting with or developing cranial nerve palsies received radiation to the base of the skull in addition to intrathecal therapy.
Maintenance therapy was given for 2 years with monthly courses of intravenous vincristine and 5 days of oral prednisone 200 mg daily; this was initiated after completion of the 8 courses of chemotherapy (or earlier because of poor tolerability and toxicity). Dasatinib, 50 mg orally twice daily or 100 mg orally daily, was administered throughout the planned 2-year maintenance period and was continued indefinitely thereafter. We omitted the antimetabolites 6-mercaptopurine and methotrexate to avoid compromising the dose of dasatinib, which was believed to be the most effective agent to prevent relapse. Maintenance therapy could be interrupted in months 6 and 13 with intensification courses of hyper-CVAD and dasatinib. Patients with no evidence of minimal residual disease (MRD) who were deemed poor candidates for such intensification continued maintenance therapy uninterrupted. Appropriate dose reductions for the cytotoxic agents according to the type and degree of side effects and according to previously published measures were permitted.6,22 For dasatinib, both during initial therapy and during the maintenance period, dose reductions to 70 mg or 50 mg orally daily were allowed for significant drug-related toxicity, and dose escalation to 140 mg daily was permitted for inadequate response. At any time during the intensive or maintenance therapy phases, patients with an available matched donor had the option to proceed to an allogeneic stem cell transplantation procedure.
Supportive care
Supportive care measures were implemented according to standard guidelines. Tumor lysis prophylaxis with allopurinol or alternatives such as rasburicase and appropriate intravenous hydration and alkalinization were administered in the first course to all patients. Prophylactic antibiotic therapy with oral levofloxacin or trimethoprim/sulfamethoxazole, valacyclovir, fluconazole, or equivalent alternatives was provided to all patients during periods of neutropenia. Transfusion of blood, platelets, or other blood products was used according to established guidelines to support periods of cytopenia or coagulopathy.
Follow-up assessments
All patients underwent baseline evaluation, which included history and physical examination, complete blood count with differential, full chemistry panel (including renal and hepatic panel), bone marrow aspirate for histology, flow cytometry, cytogenetics, fluorescent in situ hybridization, and reverse-transcription quantitative polymerase chain reaction (RT-qPCR) for BCR-ABL transcripts, as well as DNA PCR for immunoglobulin heavy-chain (IGH) gene rearrangements.
Bone marrow evaluations were repeated on approximately days 14 and 21 of the first cycle of treatment. Complete blood counts, electrolytes, and renal and hepatic indices were obtained at least weekly during the intensive cycles of chemotherapy. Bone marrow aspiration material was assessed by morphology, cytogenetics, flow cytometry, and BCR-ABL RT-qPCR every 2 to 3 cycles.
CSF assessment was performed at baseline on day 2 of induction chemotherapy at the time of administration of the first intrathecal chemotherapy. Baseline cardiac function was evaluated with a multigated radionuclide ventriculography (MUGA) scan or an echocardiogram, and this assessment was repeated if indicated clinically.
MRD monitoring techniques
Molecular monitoring.
BCR-ABL RT-qPCR was performed on total RNA extracted from leukocytes after red blood cell lysis. Reverse transcription was performed with random hexamers, and PCR was performed with TaqMan primer/probes for the e1a2, e13a2 (b2a2), and e14a2 (b3a2) BCR-ABL transcripts in a single tube with normalization to total ABL transcripts. Post-PCR capillary electrophoresis was used to type splice form, with the method having a sensitivity of approximately 1 in 10 000 BCR-ABL–expressing cells, as established by periodic dilution studies.24 Major molecular response was defined as a BCR-ABL/ABL ratio of less than 0.05%. BCR-ABL KD mutation analysis that covered codons 221 to 500 was performed on cDNA with a nested PCR strategy.25 For case subjects with a T315I mutation, quantitation of mutation levels was performed with a pyrosequencing-based strategy with a sensitivity detection rate of 1%.25
IGH clonality studies were performed on extracted genomic DNA with separate FR1, FR2, and FR3 PCR reactions with a consensus J primer. The sensitivity of detection of this method in a sample with low numbers of polyclonal B cells (such as posttreatment bone marrow and CSF) is approximately 0.2% to 1%.
Multiparameter flow cytometry.
MRD assessment by flow cytometry was performed on whole bone marrow specimens by use of a standard stain-lyse-wash procedure. A total of 1 × 106 cells were stained for each analysis tube, and data were acquired on 2 × 105 cells when specimen quality permitted. In the initial part of the study, data on 4-color staining combinations were acquired on FACSCalibur cytometers with CellQuest software (Version 6.0; BD Biosciences) and analyzed with FlowJo (Version 8.8.6; TreeStar). Beginning in March 2009, data on 6-color stains were acquired on FACSCanto cytometers with FACSDiva software (Version 6.1.2; BD Biosciences) and analyzed with FCS Express (Clinical edition, Version 3; De Novo Software). Four-color combinations contained CD19 conjugated to peridinin chlorophyll protein–cyanine 5.5 (PerCP-Cy5.5) and CD34 conjugated to allophycocyanin (APC) in all tubes, with additional antigens conjugated to fluorescein isothiocyanate (FITC) and phycoerythrin (PE), including CD10, CD13, CD15, CD20, CD22, CD25, CD33, CD38, CD45, CD58, CD66c, and CD81 (all antibodies from BD Biosciences, except CD10 from Beckman Coulter and CD66c from Immunotech). Six-color combinations included CD34-PerCP-Cy5.5, CD10-PE-Cy7, and CD19-APC-H7 in each tube, with the additional antigens listed above conjugated to FITC, PE, and APC. MRD was identified in comparison with the known patterns of antigen expression by normal maturing CD19+ B cells by an approach similar to that described by Weir et al.26 A distinct cluster of at least 20 cells that showed altered antigen expression was regarded as an aberrant population, which yielded a sensitivity for both 4- and 6-color assays of 1 in 10 000 cells (for adequate specimens in which 2 × 105 cells could be collected). We required aberrant expression of at least 2 antigens to make a diagnosis of MRD.
Response and outcome definitions
CR was defined as the presence of fewer than 5% blasts in the bone marrow, with more than 1 × 109/L neutrophils and more than 100 × 109/L platelets in the peripheral blood and no extramedullary disease. Relapse was defined by recurrence of more than 5% lymphoblasts in a bone marrow aspirate unrelated to recovery or by the presence of extramedullary disease. CR duration was calculated from the time of CR until relapse. DFS was calculated from the time of CR until relapse or death due to any cause. Event-free survival was calculated from the beginning of treatment until an event occurred, including relapse, death during induction, or death during CR. Overall survival was calculated from the time of diagnosis until death.
Statistical methods
Survival curves were plotted by the Kaplan-Meier method and compared with the log-rank test. Differences in subgroups by different covariates were evaluated with the χ2 test for nominal values and the Mann-Whitney U and Fisher exact tests for continuous variables.
Results
Patients and treatment
From September 28, 2006, to July 15, 2009, 35 patients with untreated Ph+ ALL were enrolled in the study and treated. The study has completed accrual, and this is the first report of the patients' outcome. The pretreatment characteristics of the patients are given in Table 1. Their median age at presentation was 53 years (range, 21-79 years); 20 patients (57%) were older than 50 years, and 11 (31%) were older than 60 years. Their median white blood cell count at diagnosis was 17.4 × 109/L (range, 1.7-284.3 × 109/L). Four patients (11%) had CNS involvement at presentation. Twenty-four patients (69%) had the e1a2 transcript, 5 (14%) had the e13a2 transcript, 3 (9%) had both the e13a2 and e14a2 transcripts, 1 (3%) had the e14a2 transcript, and 1 (3%) had the e1a3 transcript; 1 patient (3%) in whom the breakpoint was not detected by the RT-qPCR assay had a BCR-ABL fusion by fluorescent in situ hybridization.
Pretreatment patient characteristics
| Characteristic . | Value . |
|---|---|
| Patients, N | 35 |
| Median age, y (range) | 53 (21-79) |
| Median WBC, ×109/L (range) | 17.4 (1.7-284.3) |
| Performance status | |
| 0-1 | 33 (94) |
| 2 | 2 (6) |
| CNS disease at start | 4 (11) |
| Cytogenetics | |
| Ph+ | 9 (25) |
| Ph+ plus other | 24 (69) |
| IM/ND (BCR-ABL+) | 2 (6) |
| Molecular | |
| e1a2 or e1a3 | 25 (71) |
| e13a2/e14a2 | 9 (26) |
| Unknown | 1 (3) |
| CD20 expression ≥20% | 17 (49) |
| Characteristic . | Value . |
|---|---|
| Patients, N | 35 |
| Median age, y (range) | 53 (21-79) |
| Median WBC, ×109/L (range) | 17.4 (1.7-284.3) |
| Performance status | |
| 0-1 | 33 (94) |
| 2 | 2 (6) |
| CNS disease at start | 4 (11) |
| Cytogenetics | |
| Ph+ | 9 (25) |
| Ph+ plus other | 24 (69) |
| IM/ND (BCR-ABL+) | 2 (6) |
| Molecular | |
| e1a2 or e1a3 | 25 (71) |
| e13a2/e14a2 | 9 (26) |
| Unknown | 1 (3) |
| CD20 expression ≥20% | 17 (49) |
Values are n (%) unless otherwise indicated.
WBC indicates white blood cell count; IM, insufficient metaphases; and ND, not done.
Overall, these 35 patients received a total of 187 courses of therapy, and the median number of courses per patient was 6 (range, 1-8 courses). To date, 23 patients have received maintenance therapy, and 4 have completed all 24 courses of maintenance.
Response to induction
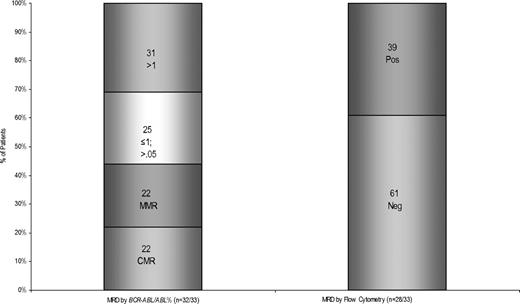
All but 2 patients were evaluable for assessment of response to induction (Table 2). Thirty-three patients (94%) achieved CR after the first course of therapy. Two patients (6%) died before response assessment due to infections; in both patients, bone marrow examination on day 14 showed no detectable disease. The median time to achievement of CR was 23 days (range, 16-43 days). Among the patients who achieved CR, 27 (82%) achieved cytogenetic CR after 1 course of therapy; 4 (12%) had persistent Ph+ metaphases (3 had 5%, and 1 had 15%), and 2 (6%) had insufficient metaphases. Overall, 31 patients (94%) eventually achieved cytogenetic CR. One patient remained persistently Ph+, and a second patient's last bone marrow examination before death had insufficient metaphases. Of the 33 patients who achieved CR, 20 (61%) achieved complete molecular remission at a median of 14 weeks (range, 2-59 weeks), and 7 additional patients (21%) achieved major molecular response at a median of 11 weeks (range, 2-51 weeks). MRD assessment by flow cytometry was negative in 29 (88%) of 33 patients at a median of 3 weeks (range, 2-18 weeks) Figure 1 shows the levels of residual disease after 1 cycle of protocol therapy in CR for the entire cohort as measured by BCR-ABL/ABL RT-qPCR or by flow cytometry.
Responses assessed after induction cycle
| Response . | n (%) . |
|---|---|
| Complete hematological response | 33 (94) |
| Early death | 2 (6) |
| Complete cytogenetic response | 27 (77) |
| Undetectable by flow cytometry | 17 (49) |
| Complete molecular response | 7 (20) |
| Major molecular response (other than complete) | 6 (17) |
| Response . | n (%) . |
|---|---|
| Complete hematological response | 33 (94) |
| Early death | 2 (6) |
| Complete cytogenetic response | 27 (77) |
| Undetectable by flow cytometry | 17 (49) |
| Complete molecular response | 7 (20) |
| Major molecular response (other than complete) | 6 (17) |
N = 35 patients.
Levels of residual disease after 1 cycle of protocol therapy in CR. MRD after 1 cycle at CR by (A) BCR-ABL/ABL percentage and (B) flow cytometry.
Levels of residual disease after 1 cycle of protocol therapy in CR. MRD after 1 cycle at CR by (A) BCR-ABL/ABL percentage and (B) flow cytometry.
Follow-up and outcome
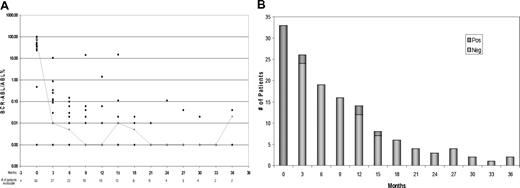
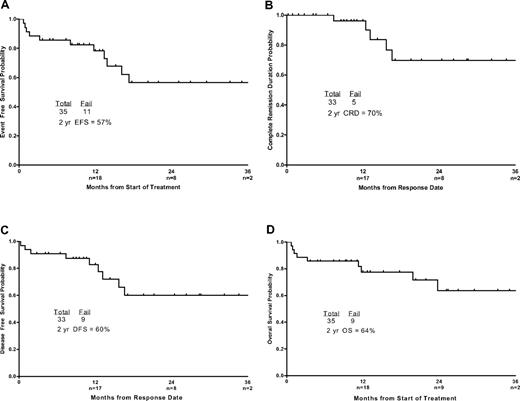
Figure 2A and B demonstrates MRD status by PCR and by flow cytometry with follow-up. All patients who remained in CR became negative for minimal residual leukemia by flow cytometry, but some had persistent low-level BCR-ABL transcripts. With a median follow-up of 14 months (range, 4-37 months), 26 patients were alive and 24 were in CR, for an estimated 2-year survival rate of 64% (95% confidence interval 38%-81%) and an event-free survival rate of 57% (95% confidence interval 34%-74%). Figure 3A and D demonstrates the event-free survival, CR duration, DFS, and overall survival of the patients. Four patients died in CR, 1 of an unrelated cardiac event and 3 of infections. Five patients relapsed, with a median response duration of 57 weeks (range, 32-72 weeks), and 3 of those patients died. Two of the relapsed patients were noncompliant with dasatinib during maintenance therapy. Two patients who relapsed were still alive after receiving further chemotherapy in the form of hyper-CVAD with nilotinib followed by nilotinib or imatinib maintenance. Neither underwent an allogeneic stem cell transplantation, and both remain in complete morphologic and cytogenetic remission with a major molecular response. Three of 4 patients with detectable minimal residual leukemia by flow cytometry at 3 months or beyond eventually relapsed. Furthermore, recurrence of a positive flow cytometry value after a previous negative value was a predictor of relapse. In 4 patients, morphologic relapse was preceded by flow cytometry and molecular relapse (Figure 2). Three of 5 patients with relapse had ABL mutations (1 T315I, 1 F359V, and 1 V299L); no mutations were detected in 1 other patient, and 1 patient was not tested. Overall, only 4 patients underwent allogeneic stem cell transplantation while in first CR (3 with major molecular response and 1 with no molecular response before transplantation), and all are alive and disease-free after transplantation. At any time during the intensive and maintenance phases, patients with an available donor had the option to proceed to allogeneic stem cell transplantation. Many patients, however, chose to continue on study despite the assertion that transplantation in first CR continues to be the standard of care.
MRD status by PCR and by flow cytometry with follow-up. (A) MRD by time from therapy according to BCR-ABL/ABL percentage. The line connects the median values of the patients at the stated time points. Several patients at different time intervals had overlapping values. In 1 patient, BCR-ABL was undetectable at presentation by RT-qPCR and was detected by fluorescence in situ hybridization. (B) MRD by time from therapy according to multiparameter flow cytometry.
MRD status by PCR and by flow cytometry with follow-up. (A) MRD by time from therapy according to BCR-ABL/ABL percentage. The line connects the median values of the patients at the stated time points. Several patients at different time intervals had overlapping values. In 1 patient, BCR-ABL was undetectable at presentation by RT-qPCR and was detected by fluorescence in situ hybridization. (B) MRD by time from therapy according to multiparameter flow cytometry.
Event-free survival, CR duration, DFS, and overall survival of the patients. (A) Event-free survival, (B) CR duration, (C) DFS, and (D) overall survival. Numbers of patients at risk are indicated on the horizontal axis.
Event-free survival, CR duration, DFS, and overall survival of the patients. (A) Event-free survival, (B) CR duration, (C) DFS, and (D) overall survival. Numbers of patients at risk are indicated on the horizontal axis.
Three patients were removed from the study for reasons other than death, transplantation, or relapse. Two were removed from the study because of toxicity, and they were given imatinib. In 1 patient, the leukemic blasts were CD20+, and rituximab was added to his therapy, hence removal from the study.
Toxicity
The median time to neutrophil and platelet recovery for cycle 1 was 18 and 23 days, respectively. The median time to platelet and neutrophil recovery for subsequent courses was 20 and 15 days, respectively (ranges, 0-37 and 0-35 days, respectively). Toxicity included 16 episodes of bleeding (11 gastrointestinal, 2 genitourinary, 1 soft tissue hematoma, and 2 subdural hematomas) and 8 episodes of pleural effusions. These included 2 episodes in the induction cycle and 6 in the subsequent cycles in 8 different patients; 6 episodes were of grade 1/2 severity, and 2 were of grade 3/4 severity (Table 3). Other adverse events included infections; deep vein thromboses and pulmonary emboli; diarrhea; and metabolic abnormalities, including hypophosphatemia, hypokalemia, hypocalcemia, hyperglycemia, elevated transaminases, and a reversible rise in creatinine unrelated to treatment. Adverse events during the study are summarized in Table 3.
Treatment-related toxicities encountered during induction and consolidation intensive chemotherapy cycles
| . | Induction number (%), n = 35 . | Subsequent cycles number (%), n = 31 . | ||
|---|---|---|---|---|
| Grade 1/2 . | Grade 3/4 . | Grade 1/2 . | Grade 3/4 . | |
| Infections | 1 (3) | 24 (69) | 1 (3) | 26 (84) |
| Pleural effusions | 1 (3) | 1 (3) | 5 (16) | 1 (3) |
| Hemorrhage | NA | 4 (11) | 1 (3) | 11 (35) |
| Gastrointestinal | 14 (40) | 1 (3) | 3 (10) | NA |
| Cardiac | 3 (9) | 1 (3) | 2 (6) | 1 (3) |
| Renal failure | NA | 6 (17) | NA | 4 (13) |
| Metabolic | 22 (63) | 21 (60) | 9 (29) | 11 (35) |
| Increased AST/ALT | 4 (11) | 3 (9) | 2 (6) | 1 (3) |
| Neurological | 2 (6) | NA | 2 (6) | 1 (3) |
| DVT/PE | 1 (3) | NA | 3 (10) | 4 (13) |
| . | Induction number (%), n = 35 . | Subsequent cycles number (%), n = 31 . | ||
|---|---|---|---|---|
| Grade 1/2 . | Grade 3/4 . | Grade 1/2 . | Grade 3/4 . | |
| Infections | 1 (3) | 24 (69) | 1 (3) | 26 (84) |
| Pleural effusions | 1 (3) | 1 (3) | 5 (16) | 1 (3) |
| Hemorrhage | NA | 4 (11) | 1 (3) | 11 (35) |
| Gastrointestinal | 14 (40) | 1 (3) | 3 (10) | NA |
| Cardiac | 3 (9) | 1 (3) | 2 (6) | 1 (3) |
| Renal failure | NA | 6 (17) | NA | 4 (13) |
| Metabolic | 22 (63) | 21 (60) | 9 (29) | 11 (35) |
| Increased AST/ALT | 4 (11) | 3 (9) | 2 (6) | 1 (3) |
| Neurological | 2 (6) | NA | 2 (6) | 1 (3) |
| DVT/PE | 1 (3) | NA | 3 (10) | 4 (13) |
AST/ALT indicates aspartate aminotransferase/alanine aminotransferase; DVT/PE, deep vein thrombosis/pulmonary embolism; and NA, not applicable.
During induction and consolidation courses, all patients received dasatinib at the prescribed 100 mg daily; 2 patients received a reduced dose of dasatinib (70 mg daily) for 3 days while they had an infection. During maintenance therapy, 22 patients began taking dasatinib at 100 mg daily, and 1 at 50 mg daily. Of these 22 patients, 14 continued taking 100 mg daily, and 7 had the dose reduced (4 to 70 mg daily, 2 to 50 mg daily, and 1 to 50 mg every other day), whereas 1 patient's dose was increased to 140 mg daily owing to an increasing PCR value for BCR-ABL. The reasons for dose reduction were cytopenia, pleural effusion, diarrhea, elevation of alanine aminotransferase, pericardial effusion, worsening renal function, and infections.
Discussion
Several recent reports have better defined the outcome of patients with Ph+ ALL who underwent an allogeneic stem cell transplantation procedure in the pre-imatinib era.27,28 Fielding et al reported that only 28% of patients in their study actually underwent allogeneic stem cell transplantation as designed by the protocol.27 Therefore, although durable remissions are possible with allogeneic stem cell transplantation performed in first CR, alternative strategies are needed for the majority of patients with this disease who are not candidates for or who are unable to undergo transplantation. Initial studies combining imatinib and chemotherapy have clearly established the feasibility and efficacy of this strategy, with some of the early reports suggesting a possible advantage for patients who did not undergo transplantation in first CR6,8 ; however, late relapses and death due to toxicity may be noted with further follow-up, and the initial advantage may not be maintained.7,29
Mutations in the KD of BCR-ABL have been reported to be an important mechanism of resistance to imatinib, and their role in primary resistance and relapse in patients with ALL who receive imatinib-containing therapy regimens is becoming clearer.25,30-32 Whether these mutations exist before the initiation of therapy or develop as a result of treatment-related clonal selection remains unclear.25,31 Early studies of patients with advanced Ph+ lymphoid leukemias identified KD mutations in most patients with acquired imatinib resistance; however, KD mutations that occurred before initiation of therapy with imatinib and that accounted for primary resistance were not identified.33 More recently, with the use of more sensitive techniques, the presence of low-level KD mutations in imatinib-naive patients has been reported.31 The frequency of the mutant allele at the time of diagnosis was always below the level of detection by direct cDNA sequencing, and adenosine triphosphate–binding P-loop mutations were the dominant type, accounting for 83% of the mutations, with the other 17% being T315I.31 Remarkably, pre-existence of mutations, including T315I, did not adversely affect the CR rate or the achievement of molecular CR compared with patients who only had unmutated BCR-ABL at diagnosis.31 However, other investigators were unable to detect such pre-existing mutations in their cohort.25 It is clear, however, that such mutations occur commonly in relapse. This was further confirmed in the present study, in which the majority of patients in relapse had mutations, including T315I.
Dasatinib is a more potent inhibitor of the tyrosine kinase activity of BCR-ABL in vitro and overcomes the majority of BCR-ABL resistance mutations.17 Furthermore, phase 2 trials have demonstrated significant activity of dasatinib in patients with Ph+ ALL for whom prior imatinib therapy has failed.20 We therefore hypothesized that the addition of dasatinib to chemotherapy would be more effective in maintaining responses than imatinib. Because of the concern about myelosuppression, we opted to give dasatinib 50 mg twice daily and only for 14 days each cycle to allow for the recovery of blood counts. With the release of data on the enhanced safety and equivalent efficacy of once-daily dosing, the protocol was amended, and all subsequent patients received a once-daily dose of 100 mg with chemotherapy and during maintenance.21,34
Overall, the regimen was well tolerated, although grade 3 and 4 side effects did occur, which included several bleeding episodes and pleural effusions that required treatment with steroids or discontinuation of therapy. Dasatinib has a reported deleterious effect on platelet function that is likely to be exacerbated by chemotherapy-induced thrombocytopenia.35,36 Furthermore, we avoided the use of proton pump inhibitors during the administration of dasatinib because of concern about drug interactions. The incidence of other adverse events, such as pleural effusions, was not significantly higher than that reported in single-agent studies of dasatinib in Ph+ ALL and advanced-phase chronic myeloid leukemia.37 Furthermore, the combination of chemotherapy and dasatinib did not result in unacceptable myelosuppression, with the median time to platelet and neutrophil recovery for the induction course being 23 and 18 days, respectively (ranges, 18-44 and 14-22 days, respectively). The regimen was better tolerated after achievement of CR, with rapid recovery of blood counts and fewer grade 3 and 4 adverse events during the subsequent courses of therapy. The length of follow-up for the majority of patients on the study was limited; therefore, the extent of toxicities, and particularly any toxicity related to prolonged therapy with dasatinib, is not yet clear.
After a median follow-up of 14 months (range, 4-37 months), 26 patients (74%) were alive and 24 (69%) were leukemia free. This compares favorably with historical data concerning use of the hyper-CVAD regimen without a tyrosine kinase inhibitor and is similar to our previous regimen of hyper-CVAD plus imatinib (p = NS).29 In the present study, only 4 patients (10%) underwent allogeneic stem cell transplantation in first CR compared with 16 (20%) of 53 of patients in the imatinib study. Despite this, the outcomes appear comparable in the 2 trials. Furthermore, survival and event-free survival at 2 to 3 years were of similar magnitude as reported for the Medical Research Council (MRC)/Eastern Cooperative Oncology Group (ECOG) trial and the City of Hope for patients undergoing transplantation during the first CR (Figure 4A-B).
CR duration and overall survival by age and transplant status. (A) CR duration, excluding patients who underwent allogeneic stem cell transplantation in first CR. (B) Overall survival, excluding patients who received transplants in first CR. (C) CR duration by age. (D) Overall survival by age. Numbers of patients at risk are indicated on the horizontal axis.
CR duration and overall survival by age and transplant status. (A) CR duration, excluding patients who underwent allogeneic stem cell transplantation in first CR. (B) Overall survival, excluding patients who received transplants in first CR. (C) CR duration by age. (D) Overall survival by age. Numbers of patients at risk are indicated on the horizontal axis.
The degree of reduction of disease burden at CR and with follow-up as measured by RT-qPCR was superior for the dasatinib-containing regimen compared with our historical cohorts (data not shown). Previous reports have suggested that achievement of molecular responses in patients with Ph+ ALL is associated with a superior outcome.2 Therefore, the use of a regimen that is consistently associated with a reduction of disease burden at remission and at follow-up may be associated with an improved outcome. It has been shown that a greater total number of days of tyrosine kinase inhibitor administration in induction and consolidation may be associated with an improved outcome. We administered dasatinib for only 14 days during each cycle of chemotherapy, and because of infections and toxicity, significant delays between periods of dasatinib treatment were possible. Future studies in which dasatinib is administered continuously throughout consolidation will demonstrate whether this strategy can improve the outcome further. Similarly, other strategies, such as the administration of monoclonal antibodies like rituximab or risk-adapted therapy based on the level of MRD, are being pursued in an attempt to reduce relapse and toxicity and improve survival.
Recently, the Gruppo Italiano Malattie Ematologiche dell' Adulto (GIMEMA) reported the results of the LAL1205 study using dasatinib with steroids and intrathecal chemotherapy for the frontline treatment of adult patients (median age 54 years; range, 24-76 years) with Ph+ ALL.38 A CR rate of 100% after 57 days of therapy with an overall survival rate of 81% at 10 months was reported. There were no induction deaths, and only 2 patients discontinued therapy because of toxicity. At the time of the report, 9 of 35 patients enrolled had relapsed after a median of 72 days after the end of induction. No data were provided on the postremission therapy received. The authors concluded that the use of chemotherapy for induction of older patients with Ph+ ALL is questionable. We evaluated the outcome of patients enrolled in the present study by age, and as expected, the overall survival was superior for younger patients (Figure 4C-D). It is arguable that older patients may benefit from a less intensive regimen, but the ideal strategy remains to be defined.
In conclusion, we have demonstrated the feasibility of combining chemotherapy with dasatinib in patients with Ph+ ALL. The regimen is effective in achieving long-term leukemia-free survival even without an allogeneic stem cell transplant in first CR. Other strategies, including continuous dosing of dasatinib or the addition of monoclonal antibodies, may help to further improve the outcome.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by a research grant from Bristol-Myers Squibb.
Authorship
Contribution: F.R. designed and conducted the trial, provided patients, analyzed data, and wrote the manuscript; S.O., D.T., S.F., D.J., J.J., P.K., R.C., G.B., J.B., A.F., G.G.-M., W.W., and J.C. provided patients and material and reviewed the manuscript; R.G. analyzed the data and reviewed the manuscript; S.D. collected the data; and H.K. designed the trial, provided patients, and reviewed the manuscript.
Conflict-of-interest disclosure: F.R. received research support and honoraria from and has been a member of advisory boards of Bristol-Myers Squibb; and J.C. and H.K. received research funding from Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Farhad Ravandi, MD, Department of Leukemia, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: fravandi@mdanderson.org.