Abstract
Invariant NKT (iNKT) cells are an innate type of T cells, which respond rapidly on activation. iNKT cells acquire these innate-like abilities during development; however, the signals driving development and functional maturation remain only partially understood. Because interleukin-15 (IL-15) is crucial for iNKT development and is delivered by transpresentation, we set out to identify the cell types providing IL-15 to developing iNKT cells and determine their role at the various states of development and maturation. We report here that transpresentation of IL-15 by parenchymal cells was crucial for generating normal number of iNKTs in the thymus, whereas both hematopoietic and parenchymal cells regulated iNKT cell numbers in the periphery, particularly in the liver. Specifically, dendritic cells contributed to peripheral iNKT cell numbers by up-regulating Bcl-2 expression and promoting extrathymic iNKT cell ex-pansion and their homeostatic proliferation. Whether IL-15 affects functional maturation of iNKT cells was also examined. In IL-15Rα−/− mice, CD44HighNK1.1+ iNKT cells displayed decreased T-bet expression and in response to α-galactosylceramide, had deficient interferon-γ expression. Such defects could be reversed by exogenous IL-15 signals. Overall, these studies identify stage-specific functions of IL-15, which are determined by the tissue microenvironment and elucidate the importance of IL-15 in functional maturation.
Introduction
CD1-dependent invariant natural killer T (iNKT) cells are a unique population of T cells as these cells are functionally active immediately on stimulation and thus behave in an innate-like fashion.1-3 iNKT cells are defined by the expression of an invariant T-cell receptor-α (TCR-α) chain (Vα14-Jα18 in mice, and Vα24-Jα18 in humans) along with multiple NK cell-surface markers.4-8 Interestingly, iNKT cells display immunoregulatory activity that includes the ability to either enhance or suppress immune responses.9-13 These unique abilities of iNKT cells are acquired during development.
Similar to conventional T cells, iNKT cell development occurs in the thymus; however, the developmental process of iNKT cells does not follow the conventional pathway.14-16 Developing iNKT cells go through multiple transitional stages that can be followed by staining with CD1d tetramers along with other cell-surface markers (eg, CD4, CD44, and NK1.1).17 The earliest detectable stage of iNKT cell development occurs after positive selection by CD1d+ thymocytes and is marked by expression of CD4+CD44LowNK1.1−.18-20 Double-negative (DN, CD4−CD8−) iNKT subsets also exist, but the stage where CD4 is down-regulated giving rise to the DN population is currently an ambiguous developmental event. In addition, the significance of the DN population is unclear. Despite being immature, both DN and CD4+ CD44LowNK1.1− iNKT cells are capable of producing interleukin-4 (IL-4).15,21 These immature iNKT cells expand and differentiate into CD44High cells, which are capable of producing both IL-4 and interferon-γ (IFN-γ) and exiting the thymus to seed the periphery.15,21 The final stage of development or maturation is demarcated by NK1.1 expression, cell expansion, and an enhanced expression of IFN-γ with minimal IL-4 expression.15,21 Again, atypical of normal conventional T-cell development, this final stage of maturation of iNKT cells can occur either in the thymus or the periphery.
Although maturation of iNKT cells is usually defined by the acquisition of NK1.1 expression, functional maturation does not always correlate to NK1.1 expression. Despite the expression of NK1.1, thymic iNKT cells in normal mice do not express IFN-γ in response to α-galactosylceramide (α-GalCer),22 suggesting that these cells are suppressed, inadequately stimulated in the thymus, or perhaps not truly functionally mature. Moreover, it has recently been suggested that the NK1.1− iNKT cells in the periphery may be mature or possibly a subset of iNKT cells that only transiently expresses NK1.1.23 Therefore, functional responsiveness or true maturation of iNKT cells is not inherent to all NK1.1+ iNKT cells and therefore is probably subject to additional regulation. The transcription factor T-bet is one protein known to be important for generating functional iNKT cells.24,25 In iNKT cells, T-bet up-regulates expression of CD122 (ie, IL-2/15Rβ) and allows expression of IFN-γ, granzyme B, and perforin.25 Although it is clear that T-bet is important for iNKT maturation, how T-bet is regulated in developing iNKT cells in vivo is not known.
IL-15, a common γ-chain (γC) cytokine, is a crucial factor for the development of iNKT cells.26,27 Mice deficient for IL-15 lack normal numbers of thymic and peripheral iNKT cells.26,27 In these mice, all differentiation stages are present; however, CD44HighNK1.1+ cells are preferentially lost.27,28 In addition to roles in development, IL-15 is also important for iNKT cell homeostasis.27,28 Although IL-15 is critical for the overall iNKT numbers in the thymus and periphery, the source of IL-15 has not been identified. In addition, whether IL-15 contributes to other aspects of iNKT cell biology, such as functional maturation, has not been investigated.
A distinguishing feature of IL-15 is its unique mode of delivery through a mechanism called transpresentation.29,30 Transpresentation is a process whereby specific cell types expressing surface IL-15, via a high-affinity IL-15 receptor/binding protein (IL-15Rα), present IL-15 to opposing cells. The opposing cells respond through the signaling components of an IL-15 receptor complex (IL-15Rβ/γC).30 Despite studies identifying IL-15 transpresenting cells for NK, memory CD8+ T cells, and intestinal intraepithelial lymphocytes,31-33 the cell type(s) transpresenting IL-15 to iNKT cells has not been described. Prior studies using various IL-15Rα bone marrow (BM) chimeras revealed IL-15Rα expression by either hematopoietic or nonhematopoietic cells partially recovers peripheral NK1.1+ T cell numbers.31 Unfortunately, this study did not specifically investigate effects on iNKT cells as CD1d tetramers were not used. Moreover, neither the thymus nor the functions of iNKT cells were examined.
Here, we show that the IL-15-mediated events in iNKT cell development are mediated by IL-15 transpresented by distinct cell types, depending on the tissue microenvironment. In the thymus, radiation-resistant thymic stroma provided IL-15 for iNKT survival; whereas in the periphery, both radiation-sensitive and -resistant cells provided IL-15 for maturation. Furthermore, IL-15 signaling was shown to impact the functional maturation and activation of iNKT cells. Overall, we define the tissue-specific roles of IL-15 in iNKT cell biology and identify the specific cells transpresenting IL-15, thus regulating the development, homeostasis, and activation of iNKT cells.
Methods
Mice
C57BL/6J (CD45.1 and CD45.2) mice were purchased from The Jackson Laboratory and the National Cancer Institute. IL-15Rα−/− mice34 were generously provided by A. Ma (University of California–San Francisco) and backcrossed to B6 mice 15 generations. CD11c/IL-15Rα transgenic (Tg) mice backcrossed to the IL-15Rα−/− background were generated in our laboratory and described elsewhere.32 Tg founder line 1 was used throughout this study and characterized as having IL-15Rα expression restricted to CD11c+ cells. All mice were maintained under specific pathogen-free conditions at the University of Texas M. D. Anderson Cancer Center in accordance with the Institutional Animal Care and Use Committee guidelines. The described experiments were conducted with M. D. Anderson Cancer Center Institutional Animal Care and Use Committee approval.
Generation of BM chimeras
BM was collected from the tibia and femurs of IL-15Rα−/− (CD45.1 or CD45.1/CD45.2) and WT (CD45.1) mice and processed to deplete T cells as previously described.35 IL-15Rα−/− (CD45.2) and WT (CD45.2) recipients were irradiated with 1000 cGy and injected intravenously with 3 × 106 BM cells. Chimera mice were analyzed for the presence of donor iNKT cells in the thymus, liver, and spleen 8 to 10 weeks later after complete BM reconstitution.
Lymphocyte isolation and flow cytometry
Lymphocytes were isolated from various tissues as previously described.31 iNKT cells were detected by staining with CD1d-PBS57-tetramer allophycocyanin-conjugated (National Institutes of Health Tetramer Facility) for 30 minutes at 37°C before being stained for other cell-surface markers. The following antibodies were purchased: CD3 (17A2), TCRβ (H57), CD45.2 (104), CD45.1 (A20), CD44 (IM7), CD122 (TM-b1), IFN-γ, NK1.1 (PK136), NK1.1, CD45.2 (104), CD4 (RM4-5) (BD Biosciences); T-bet (eBio4B10) and CD44 (IM7) (eBioscience); and CD45.1 (A20) and TCRβ (BioLegend). Bcl-2 in iNKT cells was detected using the BD Biosciences cytofix/cytoperm kit according to the manufacturer's instructions. T-bet expression was detected using eBioscience Foxp3 staining buffer set protocol. For bromodeoxyuridine (BrdU) labeling, mice were administered water supplemented with BrdU (0.8 mg/mL) that was changed every day for 1 week. BrdU incorporation by iNKT cells was detected using the BrdU Flow kit (BD PharMingen). All samples were collected on a LSR II Flow Cytometry (BD Biosciences), and data were analyzed with FlowJo software Version 8.8.6 (TreeStar).
In vivo α-galactosylceramide stimulation
α-GalCer (2 μg/mouse; Funakoshi) diluted in 1× phosphate-buffered saline was injected intravenously into the various mice. Tissues were collected and processed 2.5 hours after injection. Single-cell suspensions were supplemented with 2 μL of GolgiStop for 1 hour at 37°C, stained for cell-surface markers, and then fixed and permeabilized using BD Cytofix/Cytoperm buffers for staining of intracellular IFN-γ expression. For in vivo IL-15 stimulation, 2.5 μg of recombinant murine IL-15 (PeproTech) was precomplexed to 15 μg of recombinant IL-15Rα/Fc chimera (R&D Systems) as previously described36 and then injected intraperitoneally 1 or 2 days before analysis of T-bet expression or α-GalCer stimulation, respectively.
Adoptive transfer of thymocytes
To enrich for iNKT cells, 10 to 15 thymuses were pooled from WT (CD45.1) mice and incubated with rat anti-CD8 (2.43), anti-CD19 (1D3), anti-MHC II (M5) followed by incubation with goat ant–irat Dynabeads (Dynal) and magnetic separation. For examination of homeostasis, enriched cells were labeled with 2mM carboxyfluorescein succinimidyl ester (CFSE) and injected intravenously into IL-15Rα−/−, Tg, or WT (CD45.2+) mice (∼ 5-8 × 106 cells/mouse). Seven days after transfer, tissues were harvested and analyzed for the presence of donor cells and CFSE dilution by flow cytometry. For α-GalCer stimulation, mice were killed 2 days after transfer of donor cells.
Statistical analysis
Graphs generated display the mean plus or minus SEM and were obtained using GraphPad Prism software Version 5.03. Statistical significance was assessed by performing an unpaired Student t test.
Results
Radiation-resistant cells are crucial in providing IL-15 during intrathymic iNKT cell development
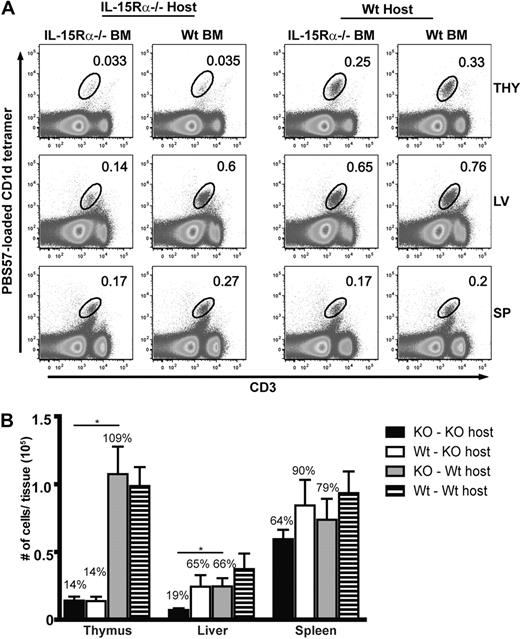
Presently, it is unclear which cells require IL-15Rα expression for iNKT cell development. Therefore, BM chimeras were generated to assess the role of IL-15 transpresentation by hematopoietic or nonhematopoietic cells in restoring thymic and peripheral iNKT cells. Accordingly, iNKT cells were detected in the thymus, liver, and spleen in the 4 groups of BM chimera mice using CD1d tetramers (Figure 1A). IL-15Rα expression by nonhematopoietic cells (IL-15Rα−/−BM → WT host) recovered the frequency and total numbers of iNKT cells in the thymus similar to that found in WT chimeras (WT BM → WT; Figure 1B). Conversely, the frequency and absolute numbers of thymic iNKT cells were still very much deficient when IL-15Rα expression was expressed solely by hematopoietic cells (WT BM → IL-15Rα−/−) to a level that was similar to IL-15Rα−/− chimeras (IL-15Rα−/−BM → IL-15Rα−/−; Figure 1B). Among peripheral iNKT cells in the liver, both IL-15Rα+ hematopoietic or nonhematopoietic cells significantly increased the levels of iNKT cells (∼ 65% of control) compared with that in the complete absence of IL-15Rα (∼ 19% of control; P < .05; Figure 1B). In contrast to hepatic iNKT cells, splenic iNKT cells had minimal requirements for IL-15Rα as the number of iNKT cells in the complete absence of IL-15Rα was only approximately 64% of control levels. Although the numbers of splenic iNKT cells were increased by both IL-15Rα+ hematopoietic or nonhematopoietic cells, these differences were not significant. Surprisingly, IL-15Rα+ hematopoietic cells increased the total number of NKT cells in the liver and spleen, albeit the reduction of iNKT cell numbers in the thymus (Figure 1B). In addition, even though normal numbers of iNKT cells were produced in the thymus by parenchymal cells, expression of IL-15Rα in the periphery by both hematopoietic and parenchymal cells was required to maintain normal levels of iNKT cells (Figure 1B). Regarding CD4+ and DN iNKT subsets, both subsets were equally dependent on IL-15Rα expression despite cell-restricted expression (data not shown). Collectively, these findings suggest that thymic iNKT cell development uses the nonhematopoietic cell compartment for IL-15 transpresentation, whereas peripheral iNKT cells receive IL-15 signals from both nonhematopoietic and hematopoietic cells. Remarkably, peripheral iNKT cell numbers can recover from a defect in thymic iNKT development, indicating the importance of late developmental events occurring postthymically.
Tissue-specific recovery of iNKT cells in IL-15Rα BM chimeras. (A-B) Tissue-resident iNKT cells or iNKT subsets were identified in the thymus, spleen, and liver of IL-15Rα−/− BM chimeras 8 to 10 weeks after irradiation and BM reconstitution by flow cytometric analysis. (A) Representative flow cytometric plots of CD1d tetramer+CD3+ cells from the indicated tissues. (B) Absolute numbers of CD1d tetramer+ cells in the respective tissues from the indicated groups. Error bars represent SEM, and the numbers above bars represent the percentage of WT levels. *P ≤ .05. Data are the average from 3 independent experiments: n = 9 mice per group for the thymus and n = 8 for the liver and spleen. Statistic significance was examined by unpaired Student t test.
Tissue-specific recovery of iNKT cells in IL-15Rα BM chimeras. (A-B) Tissue-resident iNKT cells or iNKT subsets were identified in the thymus, spleen, and liver of IL-15Rα−/− BM chimeras 8 to 10 weeks after irradiation and BM reconstitution by flow cytometric analysis. (A) Representative flow cytometric plots of CD1d tetramer+CD3+ cells from the indicated tissues. (B) Absolute numbers of CD1d tetramer+ cells in the respective tissues from the indicated groups. Error bars represent SEM, and the numbers above bars represent the percentage of WT levels. *P ≤ .05. Data are the average from 3 independent experiments: n = 9 mice per group for the thymus and n = 8 for the liver and spleen. Statistic significance was examined by unpaired Student t test.
DCs transpresent IL-15 to peripheral iNKT cells
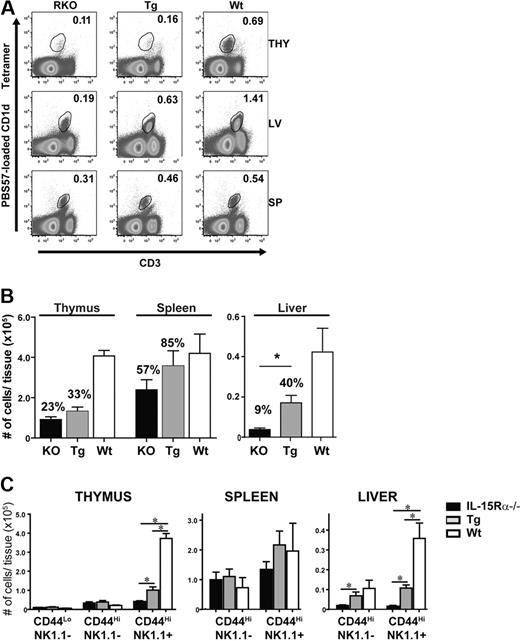
Because IL-15Rα+ hematopoietic cells recover peripheral iNKT cells independent of thymic iNKT cell numbers, the role of dendritic cells (DCs) as a potential IL-15 transpresenting cell was investigated. To examine the role of DCs in iNKT cell development and homeostasis, CD11c/IL-15Rα Tg mice (on an IL-15Rα−/− background) were used as a model where only DCs can transpresent IL-15.32 In these CD11c/IL-15Rα Tg mice, IL-15Rα+ DCs had little effect in recovering thymic NKT cell numbers, leading to only a slight increase over that found in IL-15Rα−/− mice (Figure 2A-B). Interestingly, the loss of CD44highNK1.1+ cells was the major developmental subset affected by the deficiency of IL-15Rα (Figure 2C). In the liver, IL-15Rα expression was most crucial compared with the other tissues as its absence resulted in the most dramatic iNKT cell deficiency (< 90%) affecting both CD44highNK1.1− and NK1.1+ cells (Figure 2B-C). Moreover, IL-15Rα+ DCs significantly increased the frequency and absolute numbers of total CD1d tetramer+, CD44highNK1.1−, and CD44highNK1.1+ cells (P < .05) compared with IL-15Rα−/− mice (Figure 2). The frequency and absolute numbers of splenic iNKT cells in Tg mice were increased compared with IL-15Rα−/− mice, but this increase was not significantly different (Figure 2B). As observed in the chimeras, no preferential effect was observed among either DN or CD4+ iNKT cell subsets (data not shown). These data demonstrate that iNKT cells respond to DCs specifically in the peripheral tissues, indicating that thymic and peripheral iNKT cells respond to distinct IL-15Rα+ cells depending on the tissue microenvironment.
IL-15Rα+ DCs partially recover peripheral iNKT cells. (A) Representative flow cytometric plots of CD1d tetramer+ CD3+ cells in the thymus, liver, and spleen of IL-15Rα−/− (left column), CD11c/IL-15Rα Tg (middle column), and WT (right column) mice. Plots are representative of 3 independent experiments. (B) Absolute numbers of iNKT cells as detected by CD1d tetramer were calculated for each group of mice, IL-15Rα−/− (■), CD11c/IL-15Rα Tg ( ), and WT (□), in the respective tissues. Error bars represent SEM, and the numbers above bars represent the percentage of WT levels. *P ≤ .05. Data are the average of 3 independent experiments; n = 6/group. Tg and WT iNKT cell numbers were statistically compared against total iNKT from IL-15Rα−/− mice. (C) Absolute numbers of iNKT subsets as determined by CD44 and NK1.1 expression in the indicated mice.
), and WT (□), in the respective tissues. Error bars represent SEM, and the numbers above bars represent the percentage of WT levels. *P ≤ .05. Data are the average of 3 independent experiments; n = 6/group. Tg and WT iNKT cell numbers were statistically compared against total iNKT from IL-15Rα−/− mice. (C) Absolute numbers of iNKT subsets as determined by CD44 and NK1.1 expression in the indicated mice.
IL-15Rα+ DCs partially recover peripheral iNKT cells. (A) Representative flow cytometric plots of CD1d tetramer+ CD3+ cells in the thymus, liver, and spleen of IL-15Rα−/− (left column), CD11c/IL-15Rα Tg (middle column), and WT (right column) mice. Plots are representative of 3 independent experiments. (B) Absolute numbers of iNKT cells as detected by CD1d tetramer were calculated for each group of mice, IL-15Rα−/− (■), CD11c/IL-15Rα Tg ( ), and WT (□), in the respective tissues. Error bars represent SEM, and the numbers above bars represent the percentage of WT levels. *P ≤ .05. Data are the average of 3 independent experiments; n = 6/group. Tg and WT iNKT cell numbers were statistically compared against total iNKT from IL-15Rα−/− mice. (C) Absolute numbers of iNKT subsets as determined by CD44 and NK1.1 expression in the indicated mice.
), and WT (□), in the respective tissues. Error bars represent SEM, and the numbers above bars represent the percentage of WT levels. *P ≤ .05. Data are the average of 3 independent experiments; n = 6/group. Tg and WT iNKT cell numbers were statistically compared against total iNKT from IL-15Rα−/− mice. (C) Absolute numbers of iNKT subsets as determined by CD44 and NK1.1 expression in the indicated mice.
IL-15Rα+ DCs regulate proliferation and Bcl-2 levels in peripheral iNKT cells
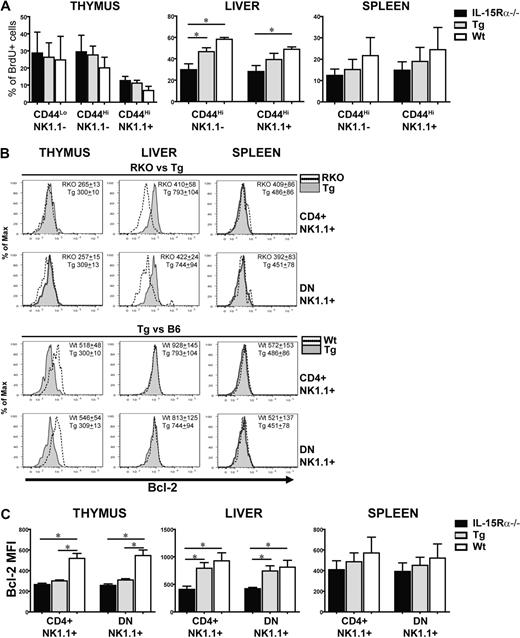
iNKT cell numbers are dictated in part by proliferation and enhanced survival; therefore, these parameters were examined as a means to investigate the mechanism for the increased iNKT cell numbers mediated by IL-15Rα+ DCs. Proliferation is thought to occur simultaneously with differentiation of CD44LowNK1.1− into CD44HighNK1.1− iNKT cells and the maturation into CD44HighNK1.1+ cells.15 To determine whether DCs drive the expansion of iNKT cells via IL-15 transpresentation, all 3 groups of mice were treated with BrdU for 7 days and the BrdU incorporation of iNKT cells at the different developmental stages was analyzed (Figure 3A). Surprisingly, no significant differences in BrdU incorporation by iNKT cells from the 3 groups of mice were observed at any stage of iNKT cells in the thymus (Figure 3A). In contrast, BrdU incorporation by hepatic iNKT cells was congruent with the increase in peripheral iNKT cells observed in Tg and WT mice compared with IL-15Rα−/− mice (Figure 3A). This effect occurred regardless of NK1.1 expression, suggesting that DC-mediated expansion was not restricted to a specific stage of differentiation or maturation (Figure 3A). Overall, peripheral expansion of hepatic and splenic iNKT cells is mediated by DCs transpresenting IL-15.
Proliferation and survival of peripheral iNKT cells are supported by DCs via IL-15 transpresentation. (A) The percentage of BrdU+ iNKT subsets in the thymus, liver, and spleen of IL-15Rα−/−, CD11c/IL-15Rα Tg, and WT mice after given BrdU (0.8 mg/mL) supplemented by drinking water for 7 days. Subsets consisted of the various stages of iNKT cell development as defined by CD44 and NK1.1 expression. Data are mean plus or minus SEM, derived from 2 independent experiments: n = 4 for IL-15Rα−/−, n = 5 for CD11c/IL-15Rα Tg, n = 4 for WT mice. *P ≤ .05. (B) Representative histograms comparing the levels of Bcl-2 expression from NK1.1+ subset-derived IL-15Rα−/− and CD11c/IL-15Rα Tg mice (top 2 panels) or CD11c/IL-15Rα Tg and WT mice (bottom 2 panels) in the various tissues. (C) MFI of Bcl-2 from CD4+ or DN NK1.1+ subsets isolated from the thymus, liver, and spleen of IL-15Rα−/−, CD11c/IL-15Rα Tg, and WT mice. Data are mean ± SEM, derived from 3 independent experiments; n = 6 to 8 mice/group. *P ≤ .05.
Proliferation and survival of peripheral iNKT cells are supported by DCs via IL-15 transpresentation. (A) The percentage of BrdU+ iNKT subsets in the thymus, liver, and spleen of IL-15Rα−/−, CD11c/IL-15Rα Tg, and WT mice after given BrdU (0.8 mg/mL) supplemented by drinking water for 7 days. Subsets consisted of the various stages of iNKT cell development as defined by CD44 and NK1.1 expression. Data are mean plus or minus SEM, derived from 2 independent experiments: n = 4 for IL-15Rα−/−, n = 5 for CD11c/IL-15Rα Tg, n = 4 for WT mice. *P ≤ .05. (B) Representative histograms comparing the levels of Bcl-2 expression from NK1.1+ subset-derived IL-15Rα−/− and CD11c/IL-15Rα Tg mice (top 2 panels) or CD11c/IL-15Rα Tg and WT mice (bottom 2 panels) in the various tissues. (C) MFI of Bcl-2 from CD4+ or DN NK1.1+ subsets isolated from the thymus, liver, and spleen of IL-15Rα−/−, CD11c/IL-15Rα Tg, and WT mice. Data are mean ± SEM, derived from 3 independent experiments; n = 6 to 8 mice/group. *P ≤ .05.
IL-15 up-regulates Bcl-2 expression and is one mechanism by which it increases cell survival.37 In iNKT cells, Bcl-2 expression is also increased on acquisition of NK1.1+ expression.38 To determine whether DC-mediated increases in iNKT cells correlated to Bcl-2 levels, Bcl-2 expression was examined in iNKT cells by quantitating the mean fluorescence intensity (MFI) of Bcl-2 via flow cytometry. In thymic iNKT cells, both CD4+ or DN NK1.1+ cells from WT mice expressed significantly higher levels of Bcl-2 than the analogous subsets from either IL-15Rα−/− and Tg mice (Figure 3B-C). In the liver, Bcl-2 expression in CD4+ and DN iNKT cells from the Tg mice was significantly higher (P < .05) than the same cells from IL-15Rα−/− mice and was quite comparable with that seen in WT mice (Figure 3B-C). In splenic iNKT cells, Bcl-2 had a hierarchal expression among IL-15Rα−/− < Tg < WT mice; however, this trend was not statistically significant (Figure 3B-C). In general, these findings suggest that reduced numbers of iNKT cells observed in the thymus of IL-15Rα−/− and Tg mice are the result of decreased numbers of CD44highNK1.1+ iNKT cells, resulting in part by reduced Bcl-2 but not defective proliferation. In the periphery, particularly in the liver, the partial restoration of iNKT cells in the Tg mice is mediated by DCs transpresenting IL-15, which affect both the proliferation and survival of iNKT cells during late development.
Homeostatic proliferation of iNKT cells is mediated by DCs via IL-15 transpresentation
Similar to memory CD8 T cells, the homeostasis of mature iNKT cells is mediated by basal proliferation and is similarly dependent on IL-1527,28 ; however, the cell type mediating this event for iNKT cells has not been elucidated. To assess the role of IL-15Rα+ DCs in regulating iNKT cell homeostasis, mature iNKT cells (> 94% CD44HighNK1.1+) were enriched from the thymus of WT mice (CD45.1+), CFSE-labeled, and transferred to IL-15Rα−/−, Tg, and WT mice (all CD45.2+). Seven days after transfer, donor-derived CD1d tetramer+ cells were detected in the spleen and liver, but not the thymus of all mice. Among the different tissues, the liver was the major site of iNKT cell homeostatic proliferation. As expected, very little cell division of iNKT cells was observed on transfer into IL-15Rα−/− hosts, whereas a low level of proliferation was observed in WT hosts (Figure 4A). On transfer into Tg mice, iNKT cells underwent a similar level of proliferation as that in WT hosts, indicating that the presence of IL-15Rα+ DCs was sufficient for iNKT cell proliferation (Figure 4A). Furthermore, the number of donor iNKT cells found in the liver was completely restored by the presence of IL-15Rα+ DCs but decreased in IL-15Rα−/− mice (Figure 4B). Interestingly, the maintenance of iNKT cells in the spleen did not depend on IL-15Rα expression (Figure 4B). These findings demonstrate that DCs are a major cell type providing IL-15 in the liver for homeostatic proliferation.
IL-15Rα+ DCs drive the homeostatic proliferation of mature iNKT cells. iNKT cells enriched from the thymus of WT congenic (CD45.1) mice were labeled with CFSE and then transferred to congenic (CD45.2) IL-15Rα−/−, CD11c/IL-15Rα Tg, and WT mice. Seven days after the transfer, mice were killed to assess the recovery and CFSE dilution of donor cells in the various tissues of the 3 groups of mice. (A) Dilution of CFSE by donor iNKT cells (CD45.1+) found in the spleen and liver 7 days after the adoptive transfer into congenic CD45.2+ IL-15Rα−/−, CD11c/IL-15Rα Tg, and WT mice. (B) Absolute number of donor iNKT cells (gated on CD45.1+ CD1d tetramer+ cells) recovered in the liver and spleen 7 days after the adoptive transfer into the 3 groups of mice. Horizontal bar represents the average of 3 independent experiments; n = 5 to 7 mice/group. *P < .001.
IL-15Rα+ DCs drive the homeostatic proliferation of mature iNKT cells. iNKT cells enriched from the thymus of WT congenic (CD45.1) mice were labeled with CFSE and then transferred to congenic (CD45.2) IL-15Rα−/−, CD11c/IL-15Rα Tg, and WT mice. Seven days after the transfer, mice were killed to assess the recovery and CFSE dilution of donor cells in the various tissues of the 3 groups of mice. (A) Dilution of CFSE by donor iNKT cells (CD45.1+) found in the spleen and liver 7 days after the adoptive transfer into congenic CD45.2+ IL-15Rα−/−, CD11c/IL-15Rα Tg, and WT mice. (B) Absolute number of donor iNKT cells (gated on CD45.1+ CD1d tetramer+ cells) recovered in the liver and spleen 7 days after the adoptive transfer into the 3 groups of mice. Horizontal bar represents the average of 3 independent experiments; n = 5 to 7 mice/group. *P < .001.
IL-15 is important for functional maturation of IFN-γ–producing hepatic iNKT cells
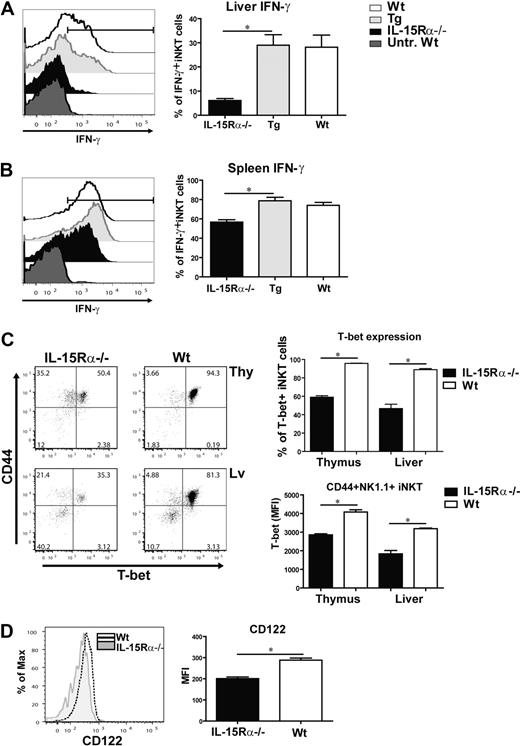
A distinguishing feature of iNKT cells is their ability to rapidly produce IFN-γ on stimulation with α-GalCer.22,39 Therefore, to assess the functional responsiveness of iNKT cells in vivo, IFN-γ expression by iNKT cells was analyzed 2.5 hours after intravenous injection of α-GalCer in the various mice (IL-15Rα−/−, Tg, and WT). Previous studies have reported that thymic iNKT cells in normal mice do not express IFN-γ in response to α-GalCer.22 This finding was also found to be true in our hands (data not shown). In the liver, few iNKT cells produced IFN-γ in IL-15Rα−/− mice, whereas a significant proportion of iNKT cells expressed IFN-γ in response to α-GalCer in Tg and WT mice (Figure 5A-B). Interestingly, iNKT cells in the spleen of all 3 groups of mice produced IFN-γ after α-GalCer stimulation, although the percentage of iNKT cells producing IFN-γ in IL-15Rα−/− mice was significantly reduced compared with WT and Tg mice (Figure 5B). This finding demonstrates that functional maturation of splenic iNKT cells is not heavily dependent on IL-15Rα and therefore may be mediated by other factors. IL-4 was also expressed by iNKT cells at low levels after α-GalCer stimulation but was not affected by IL-15Rα expression (data not shown). Nevertheless, functional maturation of hepatic iNKT cells is critically dependent on IL-15 signaling, which can be provided by DCs.
IL-15 signaling optimizes T-bet–induced gene products in iNKT cells. Peripheral iNKT cells were activated by administering α-GalCer to each group of mice. Two and half hours later, CD1d tetramer+ cells from the liver and spleen were analyzed for IFN-γ production via intracellular cytokine staining. iNKT cells from untreated WT mice were used as a negative control. Representative histograms showing the percentage of hepatic (A) and splenic (B) iNKT cells producing IFN-γ after α-GalCer administration from treated mice (IL-15Rα−/−, CD11c/IL-15Rα Tg, and WT mice) and untreated WT mice. The graph shows the average percentage of IFN-γ+ iNKT cells from the liver (A) and spleen (B) from all treated groups. Error bars represent SEM, derived from combined data from 2 independent experiments; n = 4 or 5 mice/group. *P ≤ .005. (C) Flow cytometric plots displaying CD44 and T-bet expression in CD1d tetramer+ cells from the thymus and liver of IL-15Rα−/− and WT mice. (Upper right) Percentage of T-bet+ iNKT cells from the thymus and liver of the 2 groups. *P < .001. (Bottom right) Average MFI of T-bet expression in CD44+NK1.1+ iNKT cells from IL-15Rα−/− and WT mice isolated from the thymus and liver. *P < .001. (D) Representative histogram of CD122 expression in thymic CD44+NK1.1+ iNKT cells from IL-15Rα−/− and WT mice; the average MFI of CD122 in thymic CD44+NK1.1+ iNKT cells from both groups. *P < .001. (C-D) Combined data from 2 independent experiments; n = 6 for both IL-15Rα−/− and WT mice. Error bars represent SEM.
IL-15 signaling optimizes T-bet–induced gene products in iNKT cells. Peripheral iNKT cells were activated by administering α-GalCer to each group of mice. Two and half hours later, CD1d tetramer+ cells from the liver and spleen were analyzed for IFN-γ production via intracellular cytokine staining. iNKT cells from untreated WT mice were used as a negative control. Representative histograms showing the percentage of hepatic (A) and splenic (B) iNKT cells producing IFN-γ after α-GalCer administration from treated mice (IL-15Rα−/−, CD11c/IL-15Rα Tg, and WT mice) and untreated WT mice. The graph shows the average percentage of IFN-γ+ iNKT cells from the liver (A) and spleen (B) from all treated groups. Error bars represent SEM, derived from combined data from 2 independent experiments; n = 4 or 5 mice/group. *P ≤ .005. (C) Flow cytometric plots displaying CD44 and T-bet expression in CD1d tetramer+ cells from the thymus and liver of IL-15Rα−/− and WT mice. (Upper right) Percentage of T-bet+ iNKT cells from the thymus and liver of the 2 groups. *P < .001. (Bottom right) Average MFI of T-bet expression in CD44+NK1.1+ iNKT cells from IL-15Rα−/− and WT mice isolated from the thymus and liver. *P < .001. (D) Representative histogram of CD122 expression in thymic CD44+NK1.1+ iNKT cells from IL-15Rα−/− and WT mice; the average MFI of CD122 in thymic CD44+NK1.1+ iNKT cells from both groups. *P < .001. (C-D) Combined data from 2 independent experiments; n = 6 for both IL-15Rα−/− and WT mice. Error bars represent SEM.
The low level of IFN-γ expression in IL-15Rα−/− iNKT cells after α-GalCer stimulation suggests these cells are functionally immature or unprimed. The transcription factor, T-bet, drives the functional maturation of iNKT cells by regulating the expression of several genes, including CD122 and IFN-γ.24,25 Therefore, T-bet expression was analyzed in iNKT cells from both IL-15Rα−/− and WT mice via flow cytometry. Because iNKT cells in thymus and liver are heavily dependent on IL-15Rα, we specifically analyzed iNKT cells from these 2 tissues (Figure 5C). Although T-bet was expressed by IL-15Rα−/− iNKT cells, only 59% and 47% of the population expressed high levels of T-bet in the thymus and liver, respectively, which was significantly less than the observed among iNKT cells in the thymus (96%) and liver (89%) of WT mice (Figure 5C). High T-bet expression was relevant as IFN-γ expression was restricted to this population (data not shown). Among CD44HighNK1.1+ iNKT cells, the level of T-bet expression was significantly reduced in both the thymus and liver, but not spleen, of IL-15Rα−/− mice indicating the overall, reduced T-bet was not just the result of disproportional subsets of developing iNKT cells (Figure 5C; data not shown). In addition to T-bet, CD122 expression by thymic CD44HighNK1.1+ iNKT cells was notably reduced in IL-15Rα−/− mice (Figure 5D). Although T-bet and CD122 are both reduced in the absence of IL-15Rα, double staining for CD122 and T-bet was unable to directly demonstrate a correlation in expression, which may be an indication that T-bet is not the only regulator of CD122. Thus, the reduced number of IFN-γ+ iNKT cells responding to α-GalCer stimulation in IL-15Rα−/− mice suggests that these iNKT cells are not fully functional, which could be in part the result of the lack of IL-15-mediated expression of T-bet and/or CD122.
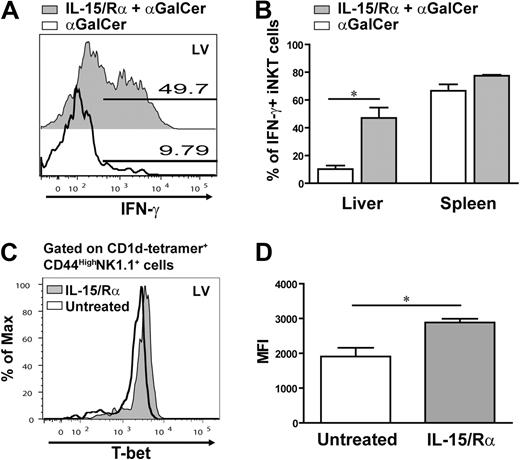
IL-15/IL-15Rα complex enhances IFN-γ and T-bet expression in IL-15Rα−/− iNKT cells
These observations suggest that IL-15 contributes to the functional maturation of iNKT cells. To determine whether IL-15 signaling could recover the defect observed in the absence of IL-15Rα, IL-15Rα−/− mice were treated with the IL-15/IL-15Rα complex. Past studies have shown that the IL-15/IL-15Rα complex provides a potent IL-15 signal independent of transpresentation36 and does not influence the production of IFN-γ by iNKT cells.40 Because the activity of the complex declines considerably within 24 hours,36 possible effects of IL-15 stimulation on maturation can be separated from those during activation by stimulating mice with α-GalCer 2 days after the initial IL-15 treatment. Accordingly, IL-15Rα−/− mice were pretreated with the IL-15/IL-15Rα complex and then injected intravenously with α-GalCer followed by analysis of IFN-γ expression 2.5 hours later. Interestingly, pretreatment with IL-15/IL-15Rα significantly amplified the percentage of hepatic iNKT cells expressing IFN-γ in response to α-GalCer compared with those iNKT cells in IL-15Rα−/− mice not receiving a prior IL-15 signal (Figure 6A). Pretreatment with IL-15/IL-15Rα also increased the expression of IFN-γ in splenic iNKT cells; however, this effect was not statistically significant (Figure 6B). This recovery in IFN-γ expression by IL-15/IL-15Rα pretreatment supports the notion that IL-15 primes hepatic iNKT cells for IFN-γ production.
Pretreatment with the IL-15/IL-15Rα complex restores iNKT cell responses in IL-15Rα−/− mice. Analysis of iNKT cells producing IFN-γ after α-GalCer administration in IL-15Rα−/− mice pretreated with or without the IL-15/IL-15Rα complex. (A) Representative histogram overlaying the percentage of hepatic iNKT cells producing IFN-γ from treated and untreated IL-15Rα−/− mice. (B) Average percentage of iNKT cells in the liver and spleen expressing IFN-γ in response to α-GalCer in mice either pretreated or untreated with the IL-15/IL-15Rα complex. (C-D) Effect of IL-15 signaling on T-bet expression in IL-15Rα-deficient mice. The IL-15/IL-15Rα complex was injected into IL-15Rα−/− mice; and one day later, T-bet expression in iNKT cells was analyzed by flow cytometry. (C) Representative histogram showing an overlay of the level of T-bet expressed in CD1d tetramer+ CD44HighNK1.1+ cells isolated from liver of treated and untreated IL-15Rα−/− mice. (D) Combined data are the average MFI of T-bet in CD44HighNK1.1+ iNKT cells from the liver of IL-15/IL-15Rα complex-treated or untreated mice. (B,D) Data are generated from 2 independent experiments; n = 4 mice. Error bars represent SEM. *P < .05.
Pretreatment with the IL-15/IL-15Rα complex restores iNKT cell responses in IL-15Rα−/− mice. Analysis of iNKT cells producing IFN-γ after α-GalCer administration in IL-15Rα−/− mice pretreated with or without the IL-15/IL-15Rα complex. (A) Representative histogram overlaying the percentage of hepatic iNKT cells producing IFN-γ from treated and untreated IL-15Rα−/− mice. (B) Average percentage of iNKT cells in the liver and spleen expressing IFN-γ in response to α-GalCer in mice either pretreated or untreated with the IL-15/IL-15Rα complex. (C-D) Effect of IL-15 signaling on T-bet expression in IL-15Rα-deficient mice. The IL-15/IL-15Rα complex was injected into IL-15Rα−/− mice; and one day later, T-bet expression in iNKT cells was analyzed by flow cytometry. (C) Representative histogram showing an overlay of the level of T-bet expressed in CD1d tetramer+ CD44HighNK1.1+ cells isolated from liver of treated and untreated IL-15Rα−/− mice. (D) Combined data are the average MFI of T-bet in CD44HighNK1.1+ iNKT cells from the liver of IL-15/IL-15Rα complex-treated or untreated mice. (B,D) Data are generated from 2 independent experiments; n = 4 mice. Error bars represent SEM. *P < .05.
To determine whether the IL-15 signals affect the key regulator in iNKT functional maturation, T-bet was analyzed in IL-15Rα−/− mice treated with just the IL-15/IL-15Rα complex. A day after injection of the IL-15/IL-15Rα complex, hepatic CD44HighNK1.1+ iNKT cells had a significant increase in T-bet expression compared with the corresponding population in untreated IL-15Rα−/− mice (Figure 6C-D). So, although T-bet expression is low in iNKT cells in IL-15Rα−/− mice, encountering IL-15 in vivo increases the level of T-bet expression in mature iNKT cells. Collectively, these data demonstrate that IL-15 signaling not only boosts the iNKT cells' ability to produce IFN-γ in response to α-GalCer but also augments T-bet expression.
IL-15 transpresentation in periphery promotes functional responsiveness in vivo
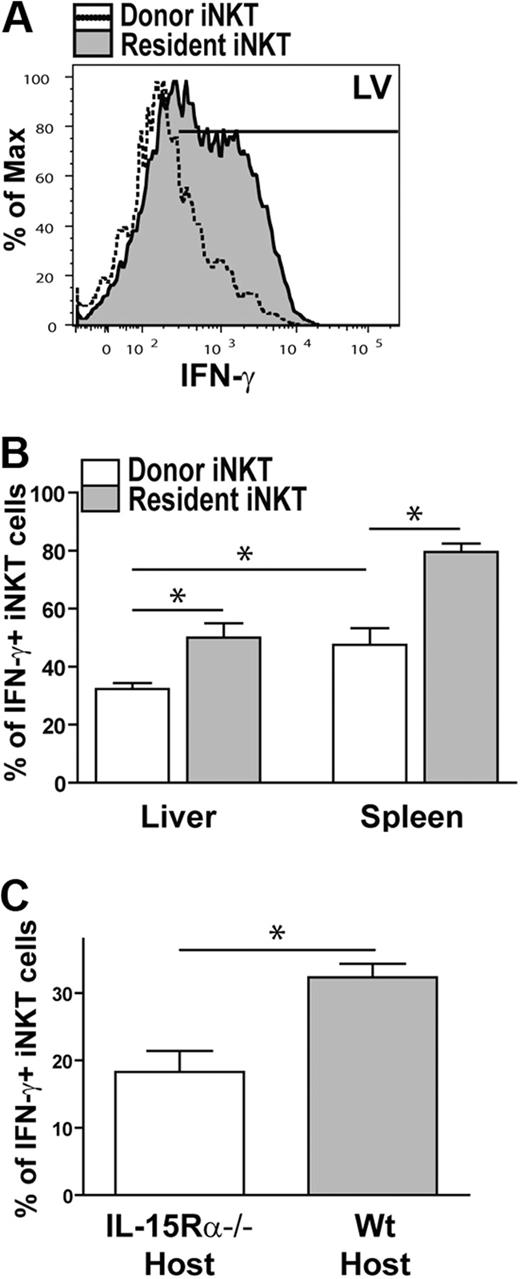
As previously mentioned, thymic iNKT do not respond to α-GalCer in vivo22 ; however, thymic and hepatic iNKT cells express IFN-γ to a similar degree when stimulated with α-GalCer in the presence of DCs.11 Therefore, we asked whether thymic iNKT cells become functionally responsive in a different in vivo tissue microenvironment and whether this is dependent on IL-15Rα expression. Therefore, iNKT cells were enriched from the thymus of WT (CD45.1) mice, adoptively transferred into WT or IL-15Rα−/− (CD45.2) mice, and then analyzed for IFN-γ expression in response to α-GalCer. In WT mice, donor thymic iNKT cells became responsive to α-GalCer in the liver and spleen; however, the donor iNKT cells were not as responsive as the resident iNKT cells (Figure 7A-B). In addition, IFN-γ expression by donor iNKT cells was increased in the spleen compared with the liver (Figure 7A-B). In the absence of host IL-15Rα, the percentage of iNKT cells expressing IFN-γ in response to α-GalCer was further decreased to a significant degree (Figure 7C), suggesting that IFN-γ responses in the liver were dependent on IL-15Rα. Collectively, these results suggest that encountering IL-15 in the periphery promotes and enhances the ability of iNKT cells to express IFN-γ in response to stimulation, thus highlighting an important role for IL-15 in iNKT cell maturation and activation.
Thymic iNKT cells respond to α-GalCer in the periphery in an IL-15Rα-dependent manner. iNKT cells were enriched from the thymus of congenic (CD45.1) WT mice and transferred into a congenic (CD45.2) WT host to seed the periphery. Two days after the cell transfer, mice were injected with α-GalCer to activate iNKT cells in vivo. (A) Representative histogram showing the level of IFN-γ being produced by donor (open histogram) and resident iNKT cells (shaded histogram) in liver of mice that received α-GalCer 2 days after the adoptive transfer. (B) Bar graph of the combined data of IFN-γ+ donor (□) and resident ( ) iNKT cells isolated from the liver or spleen of mice stimulated with α-GalCer in vivo. Data are generated from 2 independent experiments; n = 4. (C) Bar graph of the combined data of donor IFN-γ+ iNKT cells isolated from the liver of IL-15Rα−/− (□) and WT (
) iNKT cells isolated from the liver or spleen of mice stimulated with α-GalCer in vivo. Data are generated from 2 independent experiments; n = 4. (C) Bar graph of the combined data of donor IFN-γ+ iNKT cells isolated from the liver of IL-15Rα−/− (□) and WT ( ) mice. Data are generated from 3 independent experiments; n = 6 for IL-15Rα−/− host and n = 4 for WT host. Error bars represent SEM. *P < .05.
) mice. Data are generated from 3 independent experiments; n = 6 for IL-15Rα−/− host and n = 4 for WT host. Error bars represent SEM. *P < .05.
Thymic iNKT cells respond to α-GalCer in the periphery in an IL-15Rα-dependent manner. iNKT cells were enriched from the thymus of congenic (CD45.1) WT mice and transferred into a congenic (CD45.2) WT host to seed the periphery. Two days after the cell transfer, mice were injected with α-GalCer to activate iNKT cells in vivo. (A) Representative histogram showing the level of IFN-γ being produced by donor (open histogram) and resident iNKT cells (shaded histogram) in liver of mice that received α-GalCer 2 days after the adoptive transfer. (B) Bar graph of the combined data of IFN-γ+ donor (□) and resident ( ) iNKT cells isolated from the liver or spleen of mice stimulated with α-GalCer in vivo. Data are generated from 2 independent experiments; n = 4. (C) Bar graph of the combined data of donor IFN-γ+ iNKT cells isolated from the liver of IL-15Rα−/− (□) and WT (
) iNKT cells isolated from the liver or spleen of mice stimulated with α-GalCer in vivo. Data are generated from 2 independent experiments; n = 4. (C) Bar graph of the combined data of donor IFN-γ+ iNKT cells isolated from the liver of IL-15Rα−/− (□) and WT ( ) mice. Data are generated from 3 independent experiments; n = 6 for IL-15Rα−/− host and n = 4 for WT host. Error bars represent SEM. *P < .05.
) mice. Data are generated from 3 independent experiments; n = 6 for IL-15Rα−/− host and n = 4 for WT host. Error bars represent SEM. *P < .05.
Discussion
Preferences in cell-specific IL-15 transpresentation by hematopoietic or nonhematopoietic cells have been shown for memory CD8+ T cells, NK cells, and intraepithelial lymphocytes, but not for iNKT cells.31,41 Moreover, although IL-15 has a clear role in iNKT cell development and homeostasis,26-28 the role of IL-15 transpresentation in these processes had not been previously investigated. Herein, we show that the cell type transpresenting IL-15 to iNKT cells as well as the function of IL-15 were dependent on the tissue site. Furthermore, we reveal a new role for IL-15 in the functional maturation and activation of iNKT cells. Taken together, our work highlights how IL-15 regulates the development, functional maturation, and homeostasis of iNKT cells through multiple mechanisms, which are mediated by distinct cell types in a tissue-specific fashion.
IL-15 is capable of mediating differentiation, proliferation, and survival; however, the role of IL-15 on iNKT cells was different between tissues. In the thymus, IL-15 transpresentation was essential for survival but not so much for the expansion of developing iNKT cells. Other studies have found that CD28/B7 and ICOS/B7h costimulation drives intrathymic iNKT cell expansion.42 Thus, the proliferation observed in thymus of IL-15Rα-deficient mice could be the result of costimulation and other γC cytokines. In contrast to the thymus, IL-15 influenced both the proliferation and survival of iNKT cells in the periphery. Our finding of IL-15-mediated proliferation is in contrast to the lack of proliferation of iNKT cells observed in IL-15 Tg mice.43 In this IL-15 Tg model, the excess IL-15 produced probably binds to the many cell types expressing IL-15Rα, which do not normally transpresent IL-15. Therefore, situations of excess IL-15 may yield different outcomes because IL-15 is not provided by specific cell types. We provide evidence that IL-15 regulates cell survival as peripheral iNKT cells displayed reduced levels of Bcl-2 and were not sustained in IL-15Rα−/− mice. Although IL-15 notoriously drives proliferation of mature CD44HighNK1.1+ cells,27 we found that the presence of IL-15Rα also increased the proliferation of immature hepatic CD44HighNK1.1− cells. Because proliferation of immature CD44HighNK1.1− cells was modulated by IL-15 in the liver but not in the thymus, it suggests that the type of IL-15 response is better dictated by the microenvironment than the stage of differentiation.
In contrast to the thymus and liver, splenic iNKT cells in IL-15Rα−/− mice were only minimally deficient in numbers and responded well to α-GalCer. Altogether, these findings could suggest that another factor is substituting for IL-15 in the spleen. Because IL-7 shares many of the same signals as IL-15 and has been shown to be important for splenic iNKT cells,27 we think that IL-7 is a good candidate. Interestingly, as IL-15 is provided to other cell types in the spleen, it is unclear why splenic iNKT cells do not access these IL-15-producing cells. We speculate that other mechanisms determine the niche where specific cells reside or mediate specific cell-cell interactions. Alternatively, iNKT cells in the spleen and liver may be distinct populations that have obtained differential requirements for the respective cytokines.
It is often assumed that the function of IL-15 in lymphocyte development and homeostasis is to induce survival and proliferation; however, our analyses of iNKT cell responses provide evidence that the functions of IL-15 go beyond regulating cell numbers. Somewhere along the developmental pathway, iNKT cells acquire the ability to rapidly respond to TCR stimulation by producing both IL-4 and IFN-γ. As IL-4 and IFN-γ expression is regulated by GATA3 and T-bet, respectively, the expression of these transcription factors is important for functional maturation of iNKT cells.25 Therefore, it was interesting to find that T-bet expression was decreased in iNKT cells in IL-15Rα−/− mice. Although this could be the result of a preferential loss of T-bet+ cells, T-bet was up-regulated in a short-term response to IL-15.
More importantly, the ability of iNKT cells to express IFN-γ in response to α-GalCer was virtually lost in IL-15Rα−/− mice but restored by prior exposure to IL-15. In Th1 cells, signaling through the γC subunit via IL-2 revealed that the JAK3/STAT5 pathway is required for IFN-γ production.44 Essentially, STAT5 grants T-bet access to the IFN-γ promoter region by promoting chromatin accessibility. As IL-15 and IL-2 both use the same receptor subunits and signaling (JAK3/STAT5) pathway, IL-15 could be the physiologic factor activating STAT5 in iNKT cells rather than IL-2. In addition, because repetitive administration with α-GalCer impairs IFN-γ production in iNKT cells,45,46 it would be interesting to investigate whether IL-15/IL-15Rα treatment can reverse this defect. Overall, our findings suggest that IL-15 is important for promoting IFN-γ production in iNKT cells; therefore, treatment of IL-15/IL-15Rα before α-GalCer might generate a more potent IFN-γ response.
We noted that the function of IL-15 during iNKT cell development varied in different tissue microenvironment, which may be credited to the cell type transpresenting IL-15. DCs, macrophages, and thymic stromal cells are all present and capable of transpresenting IL-15 in the thymus; however, despite this, thymic iNKT cells respond only to IL-15 in the nonhematopoietic compartment, again supporting the idea that the niches or cell-cell interactions are tightly regulated. Medullary thymic epithelial cells are probably the nonhematopoietic cell type transpresenting IL-15 to iNKT cells as prior studies found that RelB-deficient mice lack normal medullary thymic epithelial cells and have defective iNKT cell development and reduced levels of IL-15 transcript.47,48 Surprisingly, despite our finding that parenchymal expression of IL-15Rα was completely sufficient to restore iNKT cell numbers in the thymus, a deficiency in iNKT cells in the liver was still apparent. Moreover, a complete deficiency of iNKT cells in the thymus did not translate to a major deficiency in either the liver or the spleen. This demonstrates the unappreciated importance of postthymic development and maturation and illustrates the dependence on the peripheral micro-environment for completing development and maintaining mature iNKT cells.
Unlike thymic iNKT cells, peripheral iNKT cells receive IL-15 transpresented by either hematopoietic or nonhematopoietic cells. Here, we identify DCs as a major cell type regulating Bcl-2 and mediating homeostatic proliferation while having less of an impact on total cell numbers; this could be indicative that DCs have a more minor role in late postthymic expansion and more important role in homeostasis. More surprisingly, DCs were able to restore functional maturation of iNKT cells in the liver, thus identifying a new role for DCs in iNKT cell biology. This ability to induce IFN-γ expression in iNKT cells may provide another mechanism by which DCs dictate the fate of an immune response. IL-15Rα BM chimeras also revealed that hepatic iNKT cell numbers were controlled by IL-15Rα+ nonhematopoietic cells, which are probably hepatic stellate cells (Ito cells). It would be interesting to determine whether Ito cells are also capable of promoting functional maturation.
In conclusion, the tissue microenvironment influences iNKT cells at different stages of development as well as regulates their function. By restricting IL-15Rα expression to certain cellular compartments, we were able to better define the cell types transpresenting IL-15 to iNKT cells in the various tissues. Depending on the tissue, IL-15 regulates the survival and/or proliferation of iNKT cells as well as impacts the functional status of iNKT cells. Overall, identification of the various cell types providing IL-15 to iNKT cells could prove invaluable for improving iNKT cell adoptive immunotherapy and manipulating the immune response in specific tissue microenvironments.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kimberlyn Acklin for technical assistance, the Genetically Engineered Mouse Facility at the University of Texas M. D. Anderson Cancer Center, and the National Institutes of Health tetramer facility for providing CD1d tetramer.
This work was supported by the National Institutes of Health (grant AI070910) and the M. D. Anderson Trust Fellowship (K.S.S). E.F.C. is a PhD candidate at the University of Texas Graduate School of Biomedical Sciences at Houston and the University of Texas M. D. Anderson Cancer Center, and this work was performed in partial fulfillment of the requirements for the PhD degree.
National Institutes of Health
Authorship
Contribution: E.F.C. designed and performed all experiments, analyzed and interpreted data, and cowrote the manuscript; L.F.A. and S.W.S. assisted with some experiments; D.Z. contributed critical reagents and assisted in editing the manuscript; and K.S.S. directed the study, interpreted data, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kimberly S. Schluns, Department of Immunology, M/C 901, University of Texas M. D. Anderson Cancer Center, PO Box 301429, Houston, TX 77030; e-mail: kschluns@mdanderson.org.