Abstract
Tumor-induced immune defects can weaken host immune response and permit tumor cell growth. In a systemic model of murine acute myeloid leukemia (AML), tumor progression resulted in increased regulatory T cells (Treg) and elevation of program death-1 (PD-1) expression on CD8+ cytotoxic T cells (CTLs) at the tumor site. PD-1 knockout mice were more resistant to AML despite the presence of similar percentage of Tregs compared with wild type. In vitro, intact Treg suppression of CD8+ T-cell responses was dependent on PD-1 expression by T cells and Tregs and PD-L1 expression by antigen-presenting cells. In vivo, the function of adoptively transferred AML-reactive CTLs was reduced by AML-associated Tregs. Anti–PD-L1 monoclonal antibody treatment increased the proliferation and function of CTLs at tumor sites, reduced AML tumor burden, and resulted in long-term survivors. Treg depletion followed by PD-1/PD-L1 blockade showed superior efficacy for eradication of established AML. These data demonstrated that interaction between PD-1 and PD-L1 can facilitate Treg-induced suppression of T-effector cells and dampen the antitumor immune response. PD-1/PD-L1 blockade coupled with Treg depletion represents an important new approach that can be readily translated into the clinic to improve the therapeutic efficacy of adoptive AML-reactive CTLs in advanced AML disease.
Introduction
With standard intensive chemotherapy, the majority of adult acute myeloid leukemia (AML) patients can reach a complete remission, but only 20% to 40% can achieve a disease-free survival of more than 5 years.1,2 Therefore, additional treatment options are needed. The graft-versus-leukemia effect after allogeneic stem cell transplantation, donor lymphocyte infusions, and identification of leukemia-associated antigens has set the foundation of immunotherapy strategies.3-7 Considerable interest and effort have been put in developing alternative strategies, such as the use of adoptive T-cell–based therapy, which can target tumor cells without the toxicity to normal tissues.8-12 However, the outcomes of cytotoxic T lymphocyte (CTL) therapy for hematologic diseases has been disappointing because of poor persistence of T cells in vivo and massive immunosuppression in the tumor microenvironment.11,13-15 We and others have shown that effective CTL therapy can be achieved with reagents targeting the suppressive factors in the tumor microenvironment, such as regulatory T cells (Tregs).12,16 However, the elimination of tumor remained suboptimal, and most mice with advanced AML died of disease despite Treg depletion and CTL infusion.
Programmed death-1 (PD-1) is a recently defined molecule that serves as a negative costimulatory receptor on various cell types, including T and B cells as well as myeloid-derived cells. The PD-1 molecule has been recognized as a hallmark for cell exhaustion, and PD-1 expressing antigen-specific T cells are dysfunctional in cytokine production and proliferation upon antigen restimulation.17-20 There are 2 main ligands for PD-1: PD-L1 (B7-H1) and PD-L2 (B7-DC). Whereas PD-L2 expression is limited to antigen-presenting cells (APCs), PD-L1 is ubiquitously expressed in a large variety of tissues, including liver, lung, spleen, and bone marrow.21-23 Furthermore, PD-L1 can also be found on the surface of multiple tumor cell types,24 including AML, and its expression is elevated after interferon-γ (IFN-γ) exposure.25 Interaction between PD-L1 and PD-1 plays an important role in controlling immune responses and is involved in peripheral tolerance, autoimmunity, allergy, infection, and antitumor immunity.21,26-28 PD-L1 expression is associated with poor prognosis in many cancers, including those of the lung, stomach, colon, breast, cervix, ovary, renal cell, and liver, as well as in adult T-cell leukemia, glioma, and melanoma.21,24,29
In this study, we examined the role of PD-1/PD-L1 interaction in Treg-mediated immune suppression of adoptively transferred CTLs in a murine model of advanced AML, the only setting in which the bona fide role of tumor-induced immunosuppression can be analyzed for its effects on adoptively transferred CTLs and findings best extrapolated to the clinic. Our data demonstrate that interaction between PD-1 and PD-L1 can facilitate Treg-induced suppression of exogenously administered CTLs propagated from non–tumor-bearing mice and dampen the antitumor immune responses in advanced AML disease. Thus, the PD-1 signaling pathway plays an important role in tumor-associated immune dysfunction, and PD-L1 blockade coupled with Treg depletion represents an important new approach that can be translated into the clinic to improve the efficacy of adoptive AML-reactive CTLs under conditions of advanced AML, a setting in which either Treg depletion or PD-1/PD-L1 pathway blockade alone has been unable to rescue mice from tumor lethality.
Methods
Mice
C57BL/6 (B6, H2b, CD45.2) mice and congenic B6-Ly5.2 mice (H2b, CD45.1), 7 to 12 weeks old at study, were obtained from the National Institutes of Health. B6 PD-1 knockout (KO)30 and B6 PD-L1 KO31 mice were maintained in the animal facility at the University of Minnesota. Rag−/− mice were obtained from The Jackson Laboratory. Mice were housed in micro-isolator cages under specific pathogen-free conditions. All experiments were conducted under approved University of Minnesota Masonic Cancer Center Institutional Animal Care and Use Committee protocols.
AML cells
C1498, an AML cell line that developed spontaneously, was obtained from ATCC. C1498FFDsR, stable transfectants of C1498 that express the fluorescent Discoma coral-derived protein DsRed2 and firefly luciferase, were used to assess tumor burden.16
Flow cytometry
Cells were washed and incubated with α-FcR to block nonspecific binding of fluorochromes. The following directly conjugated monoclonal anti-bodies (mAbs; BD Biosciences PharMingen) were incubated at 4°C for 30 minutes: PD-1-fluorescein isothiocyanate (FITC); CD44-peridinin chlorophyll protein (PerCP)-Cy5.5, CD4-PerCP-Cy5.5, CD25-PerCP-Cy5.5, CD62L-allophycocyanin, CD8α-allophycocyanin, Foxp3-allophycocyanin, CD25-allophycocyanin, and Foxp3-phycoerythrin-Cy7, and CD8α-efluro450. For PD-L1 detection, cells were labeled with PD-L1-biotin followed by strepavidin phycoerythrin-Cy7. For detection of intracellular IFN-γ, cells were stimulated in vitro with anti-CD3 (1 μg/mL), anti-CD28 (1 μg/mL), and monensin (eBioscience) for 5 hours. Cells were labeled with CD8α-allophycocyanin and PD-1 FITC mAb, permeabilized, and stained with anti–IFN-γ-PerCP-Cy5.5 (BD Biosciences PharMingen). Cells were washed and analyzed using the FACSCanto. For cell counts, 50 μL of counting beads was added to each tube, ratio of beads to cells was measured, and cell number was calculated.
Bone marrow–derived DC isolation and AML lysate pulsing
Bone marrow was harvested as described.16 A single-cell suspension was incubated in Dulbecco modified Eagle medium complete media with murine granulocyte-macrophage colony-stimulating factor for 10 or 7 days. Dendritic cells (DCs) were cultured for 2 additional days with murine interleukin-4 (IL-4) and CpG for activation. Day 12 and day 9 DCs were incubated with freeze-thawed tumor lysate as described.11 After 4 hours, DCs were harvested and used for T-cell priming.
In vitro generation, activation, and expansion of AML-reactive CTLs
CTLs were generated as described.16 Briefly, naive splenocytes were cocultured with DCs pulsed with C1498 AML lysate in 24-well tissue culture plates with murine IL-15 (Amgen) and refreshed after 7 days. After 12 days, cells were activated with anti-CD3ϵ/anti-CD28 mAb-coated beads at a 1:1 bead-to-cell ratio. IL-2 and IL-7 were added. Two days later, beads were removed, and cells were further expanded with IL-15, IL-2, and IL-7 for an additional 2 days and harvested 16 days after culture initiation.
In vivo Treg depletion and mAb treatment
Anti–PD-L1 (clone MIH732 ) or control rat IgG at 200 μg/dose were given to mice by intraperitoneal injections every other day from day 10 to day 20. Tregs were depleted with IL-2 diphtheria toxin (IL-2DT, 1 μg/dose) intraperitoneally on days 4 and 13 after AML injection.16 For some experiments, AML-reactive CTLs (30 × 106 cells/dose) were infused 14 days after AML challenge.
Treg suppression assay
Tregs were isolated from spleens of naive wild-type (WT) or PD-1 KO mice by MACs column selection to more than 90% purity. CD8+ T cells from WT or PD-1 KO mice were isolated by MACs column selection and labeled with 1μM carboxyfluorescein succinimidyl ester (CFSE). T and NK cell-depleted splenocytes from WT or PD-L1 KO mice were used as APCs. A total of 5 × 104 CFSE-labeled CD8+ T responder cells were stimulated with 1.5 × 105 APCs in RPMI-c and 1 μg/mL purified anti-CD3. Where indicated, 1 × 105 Tregs from WT or PD-1 KO mice and/or anti–PD-L1 or control rat IgG at 0.5 μg/mL was added to the culture. Four days later, cells were harvested and proliferation was determined by CFSE dilution. Cell supernatant was harvested for IFN-γ levels as determined by IFN-γ enzyme-linked immunosorbent assay.
To assess in vivo suppression, 106 CTLs ± 106 Tregs isolated from AML-bearing mice (25 days after C1498FFDsR injection) were transferred into Rag−/− mice that had been injected with 1 × 104 C1498 cells 7 days before the adoptive transfer. Anti–PD-L1 or control rat IgG (200μg/dose) was given every other day from day 3 to day 14 after C1498 injection. Thirteen days after the CTL injection (20 days after C1498 injection), spleens and livers were harvested. Leukocytes from livers were isolated with discontinuous Percoll gradient centrifugation. Cells were stimulated for 5 hours in vitro with α = CD3 (1 μg/ml) and α = CD28 (1 μg/ml), and labeled with CD8α-FITC, IFN-γ–PerCP, and CD45.1-allophycocyanin, and 100 000 live events were gated to determine the percentage of IFN-γ–producing CD8+ CTLs.
In vivo CTL adoptive transfer and assessment of proliferation
C1498FFDsR cells were given intravenously at a lethal dose (1 × 106/mouse) on day 0. Anti–PD-L1 or control IgG was given at 200 μg/dose intraperitoneally every other day starting from day 10 to day 20 after AML injection. CTLs were infused intravenously into the leukemia-bearing animals on day 14 at a dose of 30 × 106/mouse. Mice were monitored for survival or killed on day 20 or day 25 for immune parameter analysis.
In vivo CTL proliferation of liver leukocytes was measured by 5-bromo-2-deoxyuridine (BrdU) incorporation according to the manufacturer's specifications. BrdU was added to the drinking water and provided throughout the duration of the study. Four mice per group were killed, and spleens and livers were harvested 20 days after C1498FFDsR injection.
Immunofluorescence staining
Livers from naive and AML-bearing mice (25 days after AML injection) were cryopreserved at −80°C; 6-μm frozen sections were mounted and fixed in acetone. Slides were blocked with normal donkey serum and then incubated with an AB blocking kit (Vector Laboratories). The livers were labeled with primary mAbs biotin rat anti–mouse PD-1 or rabbit anti–mouse DsRed, CD8 FITC. Appropriate isotype controls were used. Secondary antibody streptavadin Cy5 and donkey anti–rabbit Cy3 were used. For FoxP3 staining, slides were blocked with undiluted normal horse serum and incubated with an AB blocking kit. Primary antibodies biotin rat anti–mouse FoxP3 (eBioscience) and rabbit anti–mouse DsRed (Clontech) were used. CD8 FITC and secondary antibodies, including streptavidin Cy5 and donkey anti–rabbit Cy3, were applied. Slides were examined using an Olympus BX51 FluoView 500 confocal microscope and FluoView software (Version 4.3). All images were taken at 40×/1.45 oil objective.
BLI studies
A Xenogen IVIS imaging system was used for live animal imaging. Firefly luciferin substrate (0.1 mL; 30 mg/mL) was injected intraperitoneally, and the IVIS imaging was performed immediately after substrate injection. Data were analyzed and presented as photon counts per area.
Statistics
The Kaplan-Meier product-limit method was used to calculate survival. Differences between groups were determined using log-rank statistics. One-way analysis of variance with post hoc Tukey test and Student t test unpaired comparison were used to determine significant differences between each group in bar graph. P values less than .05 were considered to be significant.
Results
PD-1 is up-regulated on endogenous CD8+ T cells in the liver of AML-bearing mice
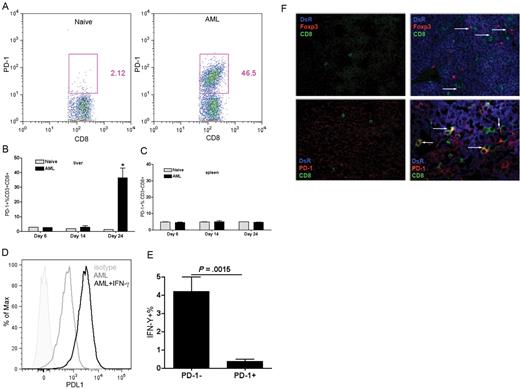
PD-1 overexpression on immune effector cells has shown a strong correlation with a suppressive microenvironment that favors tumor growth, including melanoma, chronic myeloid leukemia, and prostate cancer.33-35 Previous studies by our laboratory and others have demonstrated liver to be one of the major sites for C1498FFDsR dissemination.16,25 As AML burden progressed in the liver, the percentage of CD8+ T cells that expressed PD-1 was significantly elevated 24 days after AML injection (Figure 1A-B). PD-1 expression was not detected on the CD8+ T cells in the spleen (Figure 1C), a site with much less AML invasion.16
PD-1 expressing CD8+ T cells in the liver of AML-bearing mice displayed impaired function. B6 mice were injected intravenously with 106 C1498FFDsR cells and killed 14, 20, or 25 days after tumor injection. Flow cytometric analysis was performed on liver leukocytes and splenocytes (A-C). (A) Flow dot plot of PD-1 expression on liver CD8+ T cells 25 days after AML injection. (B) PD-1 was up-regulated on liver CD8+ T cells at late phase of AML progression. (C) PD-1 expression was not found on the spleen CD8+ T cells of AML-bearing mice. (D) PD-L1 expression on C1498FFDsR left untreated or treated with IFN-γ for 48 hours was assessed by flow cytometric analysis. PD-L1 expression was found on C1498FFDsR, and mean fluorescent intensity was increased by IFN-γ treatment. (E) Percentage of IFN-γ–producing PD-1+/PD-1− CD8+ T cells was determined by flow cytometry analysis. Percentage of IFN-γ–secreting cells. PD-1+ fraction was much lower than the PD-1− fraction. (F) Liver samples from naive mice (left side) or AML-bearing mice 25 days after C1498FFDsR injection (right side) were evaluated by immunofluorescence staining. Slides were mounted with VECTASHIELD (Vector Laboratories) and images were taken at 40×/1.30 oil objective through Olympus UPlanApo oil lens and an Olympus FV500 camera, compiled with Fluoview software (Version 4.3), then cropped in Adobe Photoshop CS3. (Top panels) Colocalization of tumor cells (DsR+, blue), CD8+ T cells (CD8+, green), and Tregs (Foxp3+, red) is designated by white arrows. (Bottom panels) Colocalization of tumor cells, CD8+ T cells, and PD-1 (red) is designated by white arrows. Results from one of 5 representative experiments are shown. Bar graphs represent mean ± SD.
PD-1 expressing CD8+ T cells in the liver of AML-bearing mice displayed impaired function. B6 mice were injected intravenously with 106 C1498FFDsR cells and killed 14, 20, or 25 days after tumor injection. Flow cytometric analysis was performed on liver leukocytes and splenocytes (A-C). (A) Flow dot plot of PD-1 expression on liver CD8+ T cells 25 days after AML injection. (B) PD-1 was up-regulated on liver CD8+ T cells at late phase of AML progression. (C) PD-1 expression was not found on the spleen CD8+ T cells of AML-bearing mice. (D) PD-L1 expression on C1498FFDsR left untreated or treated with IFN-γ for 48 hours was assessed by flow cytometric analysis. PD-L1 expression was found on C1498FFDsR, and mean fluorescent intensity was increased by IFN-γ treatment. (E) Percentage of IFN-γ–producing PD-1+/PD-1− CD8+ T cells was determined by flow cytometry analysis. Percentage of IFN-γ–secreting cells. PD-1+ fraction was much lower than the PD-1− fraction. (F) Liver samples from naive mice (left side) or AML-bearing mice 25 days after C1498FFDsR injection (right side) were evaluated by immunofluorescence staining. Slides were mounted with VECTASHIELD (Vector Laboratories) and images were taken at 40×/1.30 oil objective through Olympus UPlanApo oil lens and an Olympus FV500 camera, compiled with Fluoview software (Version 4.3), then cropped in Adobe Photoshop CS3. (Top panels) Colocalization of tumor cells (DsR+, blue), CD8+ T cells (CD8+, green), and Tregs (Foxp3+, red) is designated by white arrows. (Bottom panels) Colocalization of tumor cells, CD8+ T cells, and PD-1 (red) is designated by white arrows. Results from one of 5 representative experiments are shown. Bar graphs represent mean ± SD.
PD-L1 is ubiquitously expressed on various types of cells, including B cells, APCs, and most T cells36 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Several tumor cell lines, including C1498, can express PD-L1.25,29 To determine whether C1498FFDsR maintains PD-L1 expression, cells were left untreated or treated with recombinant mouse IFN-γ for 48 hours, and PD-L1 expression was measured by flow cytometry. Consistent with the findings of others,25 PD-L1 was expressed by the leukemia cell line, and the level was elevated by IFN-γ treatment (Figure 1D). Ligation of PD-L1 to PD-1 can program T cells for exhaustion and predispose T cells to apoptosis.20,33 Up-regulation of PD-1 is correlated with the suppression of IFN-γ production by antigen-specific CD8+ T cells in the liver microenvironment.37 We next measured the percentage of liver CD8+ T cells that were able to produce IFN-γ as a readout for their function. Leukocytes were isolated from livers of mice 25 days after AML injection and stimulated in vitro with anti-CD3 and IL-2. The percentage of IFN-γ–producing cells was significantly decreased in the PD-1+– expressing CD8+ T-cell fraction compared with PD-1− fraction (Figure 1E).
Multiple immunosuppressive mechanisms are often assembled and used by tumor to counteract host immune clearance. Coinfiltration of Tregs and PD-1+ T cells was found in high-risk breast cancer patients.38 Previous studies by us have demonstrated increased percentage and number of Tregs found in the liver of AML-bearing mice 25 days after tumor injection.16 We next determined whether there is a connection between the induction/infiltration of AML-associated Tregs and PD-1 expression. Liver samples from AML-bearing mice were sectioned and labeled for tumor cells (DsR+), Tregs (Foxp3+), and PD-1-expressing CD8+ T cells. Confocal microscopy showed that a significant increase in Treg numbers in tumor-bearing mice compared with naive mice (Figure 1F). Tregs were found to be in association with CD8+ T cells, suggesting they were eliciting their suppressive effects directly on the CD8+ T cells (Figure 1F).
PD-1 KO mice were more resistant to AML
We next investigated whether interference with PD-1 signaling could promote host antitumor immune response by comparing AML progression in PD-1 KO mice to WT mice. Whole body imaging was used to monitor tumor burdens at different time points (days 14 and 20) after AML injection. PD-1 KO mice had significantly less AML burden at 14 and 20 days after AML injection compared with WT mice (Figure 2A). Consequently and confirming a recently published report,25 PD-1 KO mice exhibited prolonged survival compared with WT mice (mean survival time [MST] 70 days vs 20 days) resulting in 20% survival (Figure 2B).
PD-1 KO mice were more resistant to AML. B6 PD-1 KO and B6 WT mice were injected intravenously with 106 C1498FFDsR cells. Mice (8-10 mice/group) were monitored using BLI, analyzed for survival, or killed (3 or 4 mice/group) at 25 days after AML injection for immune parameter determination. (A) B6 PD-1 KO versus WT mice had much lower tumor burdens (A) and significantly prolonged survival time (B) compared with WT mice (C). The number of Foxp3+ Tregs per liver in WT naive mice was significantly lower than in mice with AML. The total number of Tregs in naive PD-1 KO mice was significantly higher than WT mice and did not further increase by AML challenge. (D) The ratio of CD8+ T cells to Tregs was significantly reduced in AML-bearing WT versus naive WT mice, whereas the CD8+ T cell-to-Treg ratio was significantly higher in naive PD-1 KO versus WT mice because of a proportionately higher number of CD8+ T cells in PD-1 KO versus WT mice (data not shown). Challenging PD-1 KO with AML did not significantly alter this ratio. Results from one of 3 representative experiments are shown. Bar graphs represent mean ± SD.
PD-1 KO mice were more resistant to AML. B6 PD-1 KO and B6 WT mice were injected intravenously with 106 C1498FFDsR cells. Mice (8-10 mice/group) were monitored using BLI, analyzed for survival, or killed (3 or 4 mice/group) at 25 days after AML injection for immune parameter determination. (A) B6 PD-1 KO versus WT mice had much lower tumor burdens (A) and significantly prolonged survival time (B) compared with WT mice (C). The number of Foxp3+ Tregs per liver in WT naive mice was significantly lower than in mice with AML. The total number of Tregs in naive PD-1 KO mice was significantly higher than WT mice and did not further increase by AML challenge. (D) The ratio of CD8+ T cells to Tregs was significantly reduced in AML-bearing WT versus naive WT mice, whereas the CD8+ T cell-to-Treg ratio was significantly higher in naive PD-1 KO versus WT mice because of a proportionately higher number of CD8+ T cells in PD-1 KO versus WT mice (data not shown). Challenging PD-1 KO with AML did not significantly alter this ratio. Results from one of 3 representative experiments are shown. Bar graphs represent mean ± SD.
Responsible mechanisms for PD-1 resistance to AML to date have focused on the expression of IFN-γ by ELISPOT in PD-1 KO versus WT mice.25 To explore in greater depth the mechanisms responsible for tumor resistance in PD-1 KO mice, flow cytometric analysis for activation determinants was performed on liver CD8+ T cells and Tregs from naive or AML-bearing PD-1 KO and WT mice. The majority of CD8+ T cells from WT mice without tumor showed a phenotype of CD44−CD62L+ naive T cells (50%), whereas most of the CD8+ T cells from PD-1 KO mice have a phenotype of CD44+CD62L− effector memory T cells (60%, supplemental Figure 2A). This alteration in phenotype may result in an enhanced and accelerated T-cell response to leukemia cells. AML progression caused significant accumulation of CD4+25+FoxP3+ Tregs in the liver of WT mice (P < .05, Figure 2C; supplemental Figure 2B). Intriguingly, an increased percentage and number of Tregs were also found in the liver of non–tumor-bearing, naive PD-1 KO mice to a similar level of AML-bearing WT mice (P < .05, Figure 2C). However, PD-1-KO mice had a constitutively elevated level of Tregs, and this was not altered by the presence of tumor (Figure 2C, supplemental Figure 2B). This raised an interesting question because the PD-1 KO mice were more resistant to tumor, yet they appeared to have higher levels of immunosuppressive Tregs, although the ratio of CD8+ T cells to Tregs in the liver of AML-bearing WT mice was still lower than in PD-1 KO mice (Figure 2D). To determine whether diminished Treg function in PD-1 KO mice also may have contributed to the increased resistance to AML, subsequent studies were performed.
Interaction of PD-1 and PD-L1 contributes to immunosuppressive function of Tregs
The evidence of substantial Treg infiltration and PD-1+ CD8+ T cells at sites of PD-L1+ AML (Figure 1E) indicates that PD-1 signaling might be one of the suppressive mechanisms exploited by Tregs in the tumor environment. To further investigate whether ligation of PD-1 is important for the suppressive function of Tregs, in vitro suppression assays were performed. CFSE dilution was used to measure CD8+ T-cell proliferation and IFN-γ level in the supernatant was used to measure their function. We first evaluated the suppressive ability of WT Tregs to PD-1 KO and WT CD8+ T cells. Proliferation of responder T cells and IFN-γ secretion were notably reduced (P < .001, Figure 3A-B). Although blocking PD-1/PD-L1 ligand signaling has been shown to increase Treg expansion,39 blockade also decreases Treg suppression of alloreactive CD4+CD25− T cells in a graft-versus-host disease model.40 To further examine the suppressive function of Tregs in PD-1 KO mice, the suppressive function of Tregs from PD-1 KO mice was compared with WT mice. Similar to WT mice, more than 90% of the CD4+CD25+ population in the PD-1 KO splenocytes expresses Foxp3 (supplemental Figure 3), indicating that Treg isolation can be achieved by CD4+CD25+ selection from PD-1 KO splenocytes. Tregs from PD-1 KO mice were unable to suppress CD8+ T-cell proliferation or IFN-γ secretion (Figure 3C-D).
Blocking PD-1/PD-L1 interaction reduced Treg-mediated suppression of CD8+ T cells in vitro. Treg suppression assay was performed as described. Proliferating CD8+ T cells were quantified by the dilution of CFSE resulting in a CFSElow population and IFN-γ secretion was measured by enzyme-linked immunosorbent assay. CFSE-labeled CD8+ T cells were cocultured with syngeneic, anti-CD3ϵ mAb-loaded APCs with or without Tregs and/or anti–PDL1 mAb as indicated. CD8+ T cells, Tregs, and APCs were obtained from either WT or B6 PD-1 KO mice, as indicated. PD-1 KO CD8+ T cells are more resistant to WT Treg-mediated suppression of proliferation (A) and IFN-γ secretion (B) than WT CD8+ T cells. Compared with WT Tregs, PD-1 KO Tregs have a defective capacity to suppress the proliferation (C) and IFN-γ secretion (D) of CD8+ T cells. Anti–PD-L1 mAb prevents WT Treg suppression of proliferation (E) and IFN-γ secretion (F) of WT CD8+ T cells. Neither PD-1 KO nor WT Tregs are able to suppress proliferation of CD8+ T cells in the presence of PD-L1 KO APCs (G). Results from one of 3 representative experiments are shown. Bar graphs represent mean ± SD.
Blocking PD-1/PD-L1 interaction reduced Treg-mediated suppression of CD8+ T cells in vitro. Treg suppression assay was performed as described. Proliferating CD8+ T cells were quantified by the dilution of CFSE resulting in a CFSElow population and IFN-γ secretion was measured by enzyme-linked immunosorbent assay. CFSE-labeled CD8+ T cells were cocultured with syngeneic, anti-CD3ϵ mAb-loaded APCs with or without Tregs and/or anti–PDL1 mAb as indicated. CD8+ T cells, Tregs, and APCs were obtained from either WT or B6 PD-1 KO mice, as indicated. PD-1 KO CD8+ T cells are more resistant to WT Treg-mediated suppression of proliferation (A) and IFN-γ secretion (B) than WT CD8+ T cells. Compared with WT Tregs, PD-1 KO Tregs have a defective capacity to suppress the proliferation (C) and IFN-γ secretion (D) of CD8+ T cells. Anti–PD-L1 mAb prevents WT Treg suppression of proliferation (E) and IFN-γ secretion (F) of WT CD8+ T cells. Neither PD-1 KO nor WT Tregs are able to suppress proliferation of CD8+ T cells in the presence of PD-L1 KO APCs (G). Results from one of 3 representative experiments are shown. Bar graphs represent mean ± SD.
To determine whether specifically blocking the relevant PD-1/PD-L1 pathway would affect Treg-mediated suppression, studies were performed using anti–PD-L1 mAb. Selective PD-1/PD-L1 blockade completely abrogated the ability of WT Tregs to suppress CD8+ T-cell proliferation IFN-γ production (Figure 3E-F). In addition, APCs from PD-L1 KO mice were completely unable to support Treg-mediated suppression by WT Tregs, similar to using anti–PD-L1 mAb with WT Tregs and WT APCs or PD-1 KO Tregs with WT APCs (Figure 3G). Moreover, the proliferation of CD8+ T cells remained the same when adding anti–PD-L1 mAb to block Treg/CD8+ T-cell interaction under conditions in which PD-L1 KO APCs were used. Collectively, these data support the notion that both enhanced CD8+ T-cell response as a result of diminished suppressive function of PD-1 KO Tregs coupled with augmented PD-1 KO CD8+ T cells contribute to the resistance of tumor growth in PD-1 KO mice. Because anti–PD-L1 mAb added to WT Tregs, WT CD8+ T cells, and WT APCs simulate findings with PD-1 KO Tregs or PD-1 KO CD8+ T cells, blockade of the PD-1/PD-L1 pathway with anti–PD-L1 mAb in vivo may be beneficial in augmenting the immune response to AML cells in WT mice.
Anti–PD-L1 mAb treatment promotes an endogenous T-cell mediated antitumor response
We next used an anti–PD-L1 mAb to block the interaction between PD-1 and PD-L1 in an aggressive, established AML model, beginning 10 days after intravenous challenge with AML. Compared with controls, mice treated with anti–PD-L1 mAb exhibited significantly prolonged survival from AML (MST, 35 vs 26 days, Figure 4A). To study the mechanism of anti–PD-L1 mAb treatment, BrdU was given in the drinking water and the proliferation of CD8+ T cells was measured by BrdU incorporation. Anti–PD-L1 mAb significantly increased the proliferation of CD8+ T cells in the liver of treated mice with AML (30% vs 20%, Figure 4B). Apoptosis of CD8+ T cells in the liver was unaffected by PD-1/PD-L1 blockade (data not shown).
Anti–PD-L1-blocking mAb restored endogenous T-cell function and prolonged surviving time of AML-bearing mice. B6 mice (10 mice/group) were injected with 106 C1498FFDsR cells followed by anti–PD-L1 mAb (□) or rat IgG (■) treatment as described. In some studies, mice (3 or 4 mice /group) were killed 20 days after AML injection for the determination of proliferation by BrdU incorporation or 25 days after AML injection for determination of function by flow cytometric analysis. (A) Anti–PD-L1 mAb-treated mice had significantly prolonged survival compared with rat IgG control (□ vs ■, P = .029). (B) Anti–PD-L1 mAb treatment significantly increased the proliferation of CD8+ T cells in the liver of AML-bearing mice. (C) Anti–PD-L1 mAb treatment significantly increased the percentage of IFN-γ–secreting cells in the liver. (D) Anti–PD-L1 mAb treatment enhanced IFN-γ production by PD-1+ (left graph) but not PD-1− (right graph) cells in the liver. Results from one of 3 representative experiments are shown. Bar graphs represent mean ± SD.
Anti–PD-L1-blocking mAb restored endogenous T-cell function and prolonged surviving time of AML-bearing mice. B6 mice (10 mice/group) were injected with 106 C1498FFDsR cells followed by anti–PD-L1 mAb (□) or rat IgG (■) treatment as described. In some studies, mice (3 or 4 mice /group) were killed 20 days after AML injection for the determination of proliferation by BrdU incorporation or 25 days after AML injection for determination of function by flow cytometric analysis. (A) Anti–PD-L1 mAb-treated mice had significantly prolonged survival compared with rat IgG control (□ vs ■, P = .029). (B) Anti–PD-L1 mAb treatment significantly increased the proliferation of CD8+ T cells in the liver of AML-bearing mice. (C) Anti–PD-L1 mAb treatment significantly increased the percentage of IFN-γ–secreting cells in the liver. (D) Anti–PD-L1 mAb treatment enhanced IFN-γ production by PD-1+ (left graph) but not PD-1− (right graph) cells in the liver. Results from one of 3 representative experiments are shown. Bar graphs represent mean ± SD.
Exhausted T cells are known to have impaired production of IFN-γ, and this can be rescued by PD-1 blockade.18,41,42 To determine whether anti–PD-L1 mAb treatment can rescue the exhaustion phenotype of the CD8+ T cells in AML-bearing mice, the percentage of IFN-γ–producing CD8+ T cells was measured 25 days after AML injection. Anti–PD-L1 mAb treatment significantly increased IFN-γ production by CD8+ T cells in the liver (Figure 4C); however, this effect was not observed in the spleen (not shown). To examine the specificity of anti–PD-L1 mAb treatment on T-cell function, liver CD8+ T cells were gated on their PD-1 expression and the percentage of IFN-γ–secreting cells in PD-1+ and PD-1− fraction was measured. PD-1 expression on CD8+ T cells was unaffected by anti–PD-L1 mAb treatment (not shown). The data illustrated that the anti–PD-L1 mAb treatment selectively enhanced the percentage of IFN-γ–producing cells in the PD-1+ but not the PD-1− fraction (Figure 4D).
Anti–PD-L1 mAb administration enhances the efficacy of adoptive CTL therapy
Previous studies by us and others have shown that in vitro generated tumor-reactive CTLs have poor efficiency in vivo, especially in preexisting tumor conditions.16,43 In an attempt to enhance the efficacy of adoptive CTL therapy in a murine-advanced AML model using an approach that can be readily translated into the clinic, C1498FFDsR cells were injected into mice 14 days before infusion of AML-specific polyclonal CTLs. Anti–PD-L1 mAb was administered to mice every other day from days 10 to 20. Figure 5A shows that CTL therapy alone was ineffective, and anti–PD-L1 mAb treatment alone modestly prolonged the survival of but did not rescue AML-bearing mice (MST, 31 vs 21 days, Figure 5A). However, combined CTL and anti–PD-L1 mAb therapy in mice with advanced AML had an additive effect over either therapy alone and importantly resulted in 20% long-term survival (MST, 57 vs 31 and 20 days, Figure 5A).
Anti–PD-L1 mAb treatment enhanced the resistance of adoptively transferred anti-AML CTLs to Treg-induced suppression, resulting in increased long-term survival. B6 mice (10 mice/group) were injected with 106 C1498FFDsR cells followed by anti–PD-L1 mAb (every other day from days 10-20) and CTL treatment (day 14) as described. (A) Anti–PD-L1 mAb treatment alone significantly prolonged the survival of mice compared with either control mice or mice treated with CTLs alone, although all mice died of AML (▵ vs ■ or ▿, P < .01). Combined anti–PD-L1 mAb and anti-AML CTLs had a significantly greater survival benefit (P < .005) compared with either control mice (■) or mice receiving anti–PD-L1 mAb (▵) or CTL alone (▿). Combined therapy permitted 30% of mice with advanced AML to survive long-term. (B-C) B6 mice were injected with 106 C1498FFDsR cells. A total of 30 × 106 congenic B6-ly5.2 (CD45.1+) CTLs and anti–PD-L1 mAb treatment were given as described. BrdU was added to the drinking water to track proliferation. On days 20 or 25 after tumor injection, 4 mice per group were killed. Flow cytometry was done with liver leukocytes (B-C). (B) Anti–PD-L1 mAb treatment significantly augmented the percentage of BrdU+ adoptively transferred CTLs in the liver of mice compared with control mice. (C) Intracellular IFN-γ was determined for adoptively transferred CTLs in the liver of mice with advanced AML. Anti–PD-L1 mAb treatment significantly increased the percentage of IFN-γ–secreting CD8+ T cells. (D) Anti–PD-L1 mAb treatment did not alter the percentage of Foxp3+ Tregs found in the liver of AML-bearing mice. (E) A total of 106 CTLs and 106 Tregs isolated from AML-bearing mice were adoptively transferred to AML-bearing Rag−/− mice. Anti–PD-L1 mAb was given as described. Thirteen days after CTL transfer, flow cytometric analysis was performed on adoptively transferred CTLs in the liver. Intracellular IFN-γ expression was measured on gated, transferred CTLs. Tregs obtained from AML-bearing primary recipients significantly reduced the percentage of IFN-γ, producing adoptively transferred CTLs in AML-bearing secondary recipients. Anti–PD-L1 mAb treatment significantly increased the percentage of IFN-γ–secreting transferred CTLs, similar to a level of transferred CTLs without Treg cotransfer. Results from one of 3 representative experiments are shown. Bar graphs represent mean ± SD.
Anti–PD-L1 mAb treatment enhanced the resistance of adoptively transferred anti-AML CTLs to Treg-induced suppression, resulting in increased long-term survival. B6 mice (10 mice/group) were injected with 106 C1498FFDsR cells followed by anti–PD-L1 mAb (every other day from days 10-20) and CTL treatment (day 14) as described. (A) Anti–PD-L1 mAb treatment alone significantly prolonged the survival of mice compared with either control mice or mice treated with CTLs alone, although all mice died of AML (▵ vs ■ or ▿, P < .01). Combined anti–PD-L1 mAb and anti-AML CTLs had a significantly greater survival benefit (P < .005) compared with either control mice (■) or mice receiving anti–PD-L1 mAb (▵) or CTL alone (▿). Combined therapy permitted 30% of mice with advanced AML to survive long-term. (B-C) B6 mice were injected with 106 C1498FFDsR cells. A total of 30 × 106 congenic B6-ly5.2 (CD45.1+) CTLs and anti–PD-L1 mAb treatment were given as described. BrdU was added to the drinking water to track proliferation. On days 20 or 25 after tumor injection, 4 mice per group were killed. Flow cytometry was done with liver leukocytes (B-C). (B) Anti–PD-L1 mAb treatment significantly augmented the percentage of BrdU+ adoptively transferred CTLs in the liver of mice compared with control mice. (C) Intracellular IFN-γ was determined for adoptively transferred CTLs in the liver of mice with advanced AML. Anti–PD-L1 mAb treatment significantly increased the percentage of IFN-γ–secreting CD8+ T cells. (D) Anti–PD-L1 mAb treatment did not alter the percentage of Foxp3+ Tregs found in the liver of AML-bearing mice. (E) A total of 106 CTLs and 106 Tregs isolated from AML-bearing mice were adoptively transferred to AML-bearing Rag−/− mice. Anti–PD-L1 mAb was given as described. Thirteen days after CTL transfer, flow cytometric analysis was performed on adoptively transferred CTLs in the liver. Intracellular IFN-γ expression was measured on gated, transferred CTLs. Tregs obtained from AML-bearing primary recipients significantly reduced the percentage of IFN-γ, producing adoptively transferred CTLs in AML-bearing secondary recipients. Anti–PD-L1 mAb treatment significantly increased the percentage of IFN-γ–secreting transferred CTLs, similar to a level of transferred CTLs without Treg cotransfer. Results from one of 3 representative experiments are shown. Bar graphs represent mean ± SD.
We next measured the proliferation of CTLs after anti–PD-L1 treatment (day 20 after AML) by the assessment of BrdU incorporation. Adoptively transferred congenic CD45.1+ CTLs had a significant increase in proliferation compared with rat IgG-treated controls (45% vs 20%, Figure 5B). The percentage of IFN-γ–secreting CTLs was also measured 25 days after AML injection. In addition to the augmented proliferation, anti–PD-L1 mAb treatment significantly increased IFN-γ secretion by infused CTLs in the livers (35% vs 15%, Figure 5C). The percentage of Tregs in the AML-bearing mice remained unaffected by anti–PD-L1 mAb treatment (Figure 5D).
To determine whether the effect of PD-1/PD-L1 blockade on adoptively transferred CTLs might be mediated in part through the inhibition of Treg-induced suppression, an in vivo suppression assay with Tregs isolated from AML-bearing mice (25 days after AML injection) and AML-reactive CTLs was performed. Anti–PD-L1 or control rat IgG was given 4 days before the CTL transfer. AML-associated Tregs significantly inhibit the function of anti-AML reactive CTLs as measured by IFN-γ secretion (15% vs 45%, Figure 5E). Anti–PD-L1 mAb increased the percentage of IFN-γ–secreting CTLs in the presence of AML-associated Tregs (37% vs 15%, Figure 5E).
Combined Treg depletion and PD-1 blockade resulted in a robust T cell–mediated antitumor response and elimination of AML
It is worth noting that Treg induction/recruitment was a fairly early event as tumor progressed,16 whereas PD-1 up-regulation on liver CD8+ T cells was a seemingly late event (Figure 1B). We therefore asked whether active Treg depletion with IL-2DT immunotoxin given beginning 4 days after AML was established, followed by sustained PD-1 blockade from days 10 to 20, would have mutually enhancing effects. IL-2DT was administered on days 4 and 13. Tumor burdens were recorded by bioluminescent imaging (BLI) at different time points (days 19, 26, and 33) after C1498FFDsR injection.
On day 19, mice treated with IL-2DT had decreased tumor burdens compared with nontreated controls (Figure 6A; P < .05), although this benefit was only transient. Neither anti–PD-L1 mAb nor CTLs alone had a significant effect on tumor burden. However, combined IL-2DT and anti–PD-L1 mAb treatment induced a greater reduction compared with either treatment alone (Figure 6A; P < .05). Adding CTLs to IL-2DT or anti–PD-L1 mAb did not reduce AML tumor burden more than IL-2DT alone (Figure 6A). Strikingly, combined IL-2DT and anti–PD-L1 with CTL infusion had markedly less tumor burden compared with nontreated controls (P < .01). None of the mice receiving IL-2DT, anti–PD-L1 mAb, and CTLs that were sequentially imaged developed detectable tumor at any of the 3 time points for BLI in contrast to 2 of 5 mice receiving the next best therapy of IL-2DT and anti–PD-L1 mAb without CTLs that had tumor burden evident on day 19 (Figure 6A-B). Thus, each of 3 components (IL-2DT, anti–PD-L1 mAb, and CTLs) was necessary for rapid and optimal AML elimination.
Combined IL-2DT and anti–PD-L1 mAb had superior antitumor effect to either treatment alone and, when coupled with anti-AML CTLs, more rapidly and uniformly reduced AML tumor burden. B6 mice (10 mice/group) were injected with 106 C1498FFDsR cells followed by IL-2DT, anti–PD-L1 mAb, and/or anti-AML CTL treatment as described. Whole body imaging was performed to determine tumor burden 19, 26, and 33 days after AML injection. (A) Tumor burden was decreased by combination therapy. IL-2DT, but not anti–PD-L1 mAb alone, significantly reduced tumor burden at an early time point (day 19). Combined IL-2DT and anti–PD-L1 had superior effect compared with either treatment alone. Although CTL therapy alone did not significantly decrease AML tumor burden at any time point, adding CTL therapy to IL-2DT and anti–PD-L1 mAb treatment resulted in the greatest and most consistent decrease in AML tumor burden throughout the 33-day post-AML challenge observation period. *P < .05 compared with nontreated AML controls. Error bars represent SEM. (B) BLI studies of 5 mice per group receiving AML with or without IL-2DT, anti–PD-L1 mAb, and CTL therapy are shown. On day 19, 2 of 5 mice receiving IL-2DT and anti–PD-L1 mAb had detectable tumor versus 0 of 5 mice also receiving CTLs. (C) IL-2DT or anti–PD-L1 mAb treatment alone significantly prolonged the survival mice compared with either control mice or mice treated with CTLs alone (♦ and ▴ vs ■ or ▾, P < .05). Combined IL-2DT with CTLs or anti–PD-L1 mAb with CTLs had superior effect compared with either control mice (■, P < .05) or mice receiving IL-2DT (♦, P < .05), anti–PD-L1 mAb (▴, P < .05), or CTL single treatment (▾, P < .05). Combined IL-2DT and anti–PD-L1 mAb with (●) or without CTLs (▿) had the best survival outcome compared with single treatment or in combination with CTLs.
Combined IL-2DT and anti–PD-L1 mAb had superior antitumor effect to either treatment alone and, when coupled with anti-AML CTLs, more rapidly and uniformly reduced AML tumor burden. B6 mice (10 mice/group) were injected with 106 C1498FFDsR cells followed by IL-2DT, anti–PD-L1 mAb, and/or anti-AML CTL treatment as described. Whole body imaging was performed to determine tumor burden 19, 26, and 33 days after AML injection. (A) Tumor burden was decreased by combination therapy. IL-2DT, but not anti–PD-L1 mAb alone, significantly reduced tumor burden at an early time point (day 19). Combined IL-2DT and anti–PD-L1 had superior effect compared with either treatment alone. Although CTL therapy alone did not significantly decrease AML tumor burden at any time point, adding CTL therapy to IL-2DT and anti–PD-L1 mAb treatment resulted in the greatest and most consistent decrease in AML tumor burden throughout the 33-day post-AML challenge observation period. *P < .05 compared with nontreated AML controls. Error bars represent SEM. (B) BLI studies of 5 mice per group receiving AML with or without IL-2DT, anti–PD-L1 mAb, and CTL therapy are shown. On day 19, 2 of 5 mice receiving IL-2DT and anti–PD-L1 mAb had detectable tumor versus 0 of 5 mice also receiving CTLs. (C) IL-2DT or anti–PD-L1 mAb treatment alone significantly prolonged the survival mice compared with either control mice or mice treated with CTLs alone (♦ and ▴ vs ■ or ▾, P < .05). Combined IL-2DT with CTLs or anti–PD-L1 mAb with CTLs had superior effect compared with either control mice (■, P < .05) or mice receiving IL-2DT (♦, P < .05), anti–PD-L1 mAb (▴, P < .05), or CTL single treatment (▾, P < .05). Combined IL-2DT and anti–PD-L1 mAb with (●) or without CTLs (▿) had the best survival outcome compared with single treatment or in combination with CTLs.
Although anti–PD-L1 mAb or IL-2DT as individual therapies prolonged survival (P < .05 vs controls), all mice died of AML, whereas CTL alone did not confer a significant survival advantage to recipients (Figure 6C). Anti-AML CTLs did have a biologic effect because combining CTLs with either IL-2DT or anti–PD-L1 mAb led to a significant increase in survival compared with either therapy alone. The most striking survival advantage was seen when IL-2DT and anti–PD-L1 mAb formed part of the treatment regimen, with 70% of mice surviving long-term. Although CTLs did not further increase this high survival rate, the addition of CTLs provides a more rapid and uniform control of tumor burden (Figure 6B-C).
Discussion
The obstacles of successful antitumor immune responses include suppressive factors in tumor environment that inhibit the function of a sufficient immune response, as well as defeated immune effector components that are unable to control tumor growth. Efforts have been made to eliminate mechanisms that down-regulate the immune system and promote antitumor activity of effectors, respectively. In this study, we focused on the link between an extrinsic component, Tregs in the tumor microenvironment, and an intrinsic factor, PD-1 on the CTLs in regulating antitumor immune response. We show that PD-1–expressing CD8+ T cells found in the liver of AML-bearing mice at a late stage of tumor progression are functionally impaired in both proliferation and IFN-γ production. Disruption of the interaction between PD-1 and PD-L1 reduced Treg-mediated suppression and restored the function of both endogenous CD8+ T-cell and exogenous adoptively transferred CTLs for tumor abolition. In a well-established, aggressive AML model, long-term survival in the majority of mice was only possible with combined Treg depletion and PD-1/PD-L1 blockade with an additional benefit derived from the infusion of anti–AML-reactive CTLs in rapidly and more uniformly controlling AML tumor burden. This clinically translatable regimen suggests a novel therapeutic approach to treating advanced AML in patients.
Mechanisms exploited by tumor cells to inhibit CD8+ T cell–mediated killing include disruption of antigen presentation of tumor-associated antigens, down-regulation of human leukocyte antigen molecules, and induction/recruitment of immune suppressors, such as Tregs. Several studies have found PD-1 expression on Tregs and that blockade of PD-1/PD-L1 signaling could increase Treg proliferation.39 Our in vitro findings support the idea that PD-1 signaling controls both Treg- and CTL-mediated response. First, we have shown that PD-1 KO Tregs have an impaired suppressive capacity for WT CD8+ T cells. Second, anti–PD-L1 mAb blocks WT Treg-mediated suppression. Both PD-1 KO mice and anti–PD-L1 mAb-treated mice are able to resist but not uniformly eliminate AML. Third, WT Treg suppression of CD8+ T-cell responses in the presence of PD-L1 KO APCs is defective. Fourth, anti–PD-L1 mAb abrogated Treg suppression of CD8+ T-cell responses. These data provide new insights as to the resistance mechanisms seen in PD-1 KO mice and provide a direct link between the PD-1/PD-L1 pathway and Tregs in AML-mediated suppression. In the liver, the colocalization of PD-1+ Tregs,44,45 with PD-L1+ AML cells and CD8+ T cells, provides an ideal microenvironment for Treg-mediated suppression of CD8+ T-cell function. However, it remains unclear and for future studies to determine whether PD-1 signaling of Tregs directly influences Treg suppression and/or indirectly affects Treg suppression via engagement of PD-1 on Tregs with PD-L1 expressed on CD8+ T cells or APCs that then increase Treg suppression46 or decrease CD8+ T-cell function.
In vivo, the increased percentage and number of Tregs at sites of AML may be the consequence of reduced apoptosis and/or increased proliferation in the presence of excessive cytokines, such as IL-2, when PD-1/PD-L1 signaling is blocked. Recently, several antibodies designed to target PD-1/PD-L1 interaction, including human anti–PD-L1 mAb MDX-1105, have been evaluated in clinical trials for selected tumor treatments. A recent study reported that enhanced tumor rejection by anti–PD-L1 mAb treatment was correlated with an increased CD8+ T cell-to-Treg ratio in a murine B16 melanoma model.47 Consistent with these studies, our data extend these findings to an AML model and suggest that the reduction in Treg-mediated suppression by PD-1/PD-L1 blockade also contributes to the enhanced T cell-mediated antitumor immune response.
Compromised tumor-specific killing by CD8+ T cells can result from 2 main mechanisms: defective cytolytic machinery and an increase in inhibitory molecules, such as PD-1. Interestingly, a high frequency of PD-1-expressing CD8+ T cells seems restricted to the tumor-infiltrating CD8+ T-cell population in sites of AML, including the liver and the less infiltrated bone marrow site (range, 2%-51%; data not shown).25 Blockade of the PD-1/PD-L1 pathway prolonged survival of mice with established AML, although as a single agent was not curative. Despite the potent in vitro inhibition of Treg-mediated suppression of CD8+ T-cell responses by anti–PD-L1 mAb, as a single agent in vivo, survival was prolonged but mice were not cured, consistent with a recent report.25 Although an incremental survival benefit was seen by adding anti-AML CTLs, only Treg depletion using IL-2DT permitted long-term survival in the majority of recipients. Studies have shown that Treg depletion must be done prophylactically or very early in the course of disease.8,12,48 Here, we confirm that later Treg depletion alone was ineffective at providing long-term survival. However, an important extrapolation from this study would be consideration for combining anti–PD-L1 mAb with a Treg depletionary approach that can be given at the time of well-established disease as neither approach alone may be sufficient to reverse tumor-mediated immune suppressive mechanisms. We conclude that a reduction in Treg number is essential and speculate that anti–PD-L1 mAb may inhibit the suppressive cell capacity of the remaining Tregs as well as preventing the exhaustion of endogenous and adoptively transferred anti–AML-reactive CD8+ T cells in mice with advanced AML.
PD-L1 signaling is also important for peripheral tolerance.49 Fatal autoimmune pathology was observed in PD-L1 KO mice infected with LCMV clone 13, which causes a chronic infection in WT mice.41 This was not observed in our studies. The cohort of mice given anti–PD-L1 therapy with or without IL-2DT was tolerated in mice. PD-1 KO mice were more resistant to AML than WT mice treated with anti–PD-L1 mAb. The difference in survival may be partially explained by the ligation of PD-1 with other ligands, such as PD-L2, and duration and persistence of anti–PD-L1 mAb treatment.
The process of generating tumor-reactive CTL ex vivo is often time-consuming, leading to a major concern that CTLs may have intrinsic defect after long exposure to excessive cytokine and activation reagents. Multiple studies have focused on elucidating the extrinsic mechanism that limits CTL function in vivo to improve the efficacy of adoptive transferred CTL therapy. In the current study, CTL therapy alone was ineffective in curing mice. However, when combined with the short-lived IL-2DT given in 2 doses before CTL transfer, survival was improved. When combining IL-2DT and anti–PD-L1 mAb with CTLs, tumor burden was more rapidly and uniformly eliminated over the first 33 days of imaging than therapy without CTLs. Compared with the high survival rate of IL-2DT and anti–PD-L1 mAb, CTLs did not further improve outcome perhaps because of the already vigorous endogenous T cell-mediated antitumor response after IL-2DT and anti–PD-L1 treatment. In clinical settings where intensive chemotherapy is given to AML patients and the endogenous T-cell responses are extremely limited because of poor recovery after bone marrow transplantation, infusion of AML-reactive CTLs would be an exceptionally beneficial adjuvant therapy in eliminating the residual disease.
In conclusion, we have developed a novel approach to treat mice with established AML disease using IL-2DT and anti–PD-L1 mAb along with the adoptive transfer of CTLs that can be extrapolated into the clinic in disease settings where Treg suppression and endogenous antitumor CD8+ T-cell responses are insufficient to control tumor progression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Tasuku Honjo and Hiroyuki Nishimura for PD-1 KO mice and Dr Patricia A. Taylor for technical assistance.
This work was supported in part by the National Institutes of Health (grants R01 CA72669, R01AI34495, and P01056299) and the Children's Cancer Research Fund.
National Institutes of Health
Authorship
Contribution: Q.Z. designed, organized, and supervised research, performed experiments, analyzed data, designed the figures, and wrote the paper; M.E.M. performed experiments; S.L.H. performed experiments and edited the paper; J.T. designed and supervised immunofluorescent imaging; B.J.W., D.H.M., and W.J.M. designed research and edited the manuscript; M.R. performed immunofluorescent staining and analyzed data; D.A.V. made the IL-2DT reagent; A.H.S. provided the PD-L1 KO mice; M.A. made the PD-L1 mAb; B.L.L. and C.H.J. made the anti-CD3/28 beads, designed research, and edited the manuscript; and B.R.B. designed, organized, and supervised research and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, Department of Pediatrics, MMC 109, University of Minnesota, Twin Cities, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal