Abstract
CD40 is highly expressed on various B-lineage malignancies and represents an attractive immunotherapy target for neoplastic disease. Previous work showed that engineering the Fc domain of an antibody for increased binding to Fcγ receptors (FcγRs) significantly enhanced Fc-mediated immune effector function and antitumor activity in vitro and in vivo. We developed a humanized anti-CD40 antibody similarly Fc-engineered for increased FcγR binding (XmAbCD40) and compared its efficacy with that of an anti-CD40 native IgG1 analog and the anti-CD20 antibody rituximab. XmAbCD40 increased antibody-dependent cell-mediated cytotoxicity (ADCC) up to 150-fold relative to anti-CD40 IgG1 against B-lymphoma, leukemia, and multiple myeloma cell lines, and significantly enhanced ADCC against primary tumors. XmAbCD40 was also superior to rituximab in enhancing ADCC (both in cell lines and primary tumors) and in augmenting antibody-dependent cellular phagocytosis. XmAbCD40 significantly inhibited lymphoma growth in disseminated and established mouse xenografts and was more effective than the IgG1 analog or rituximab. An anti-CD40 antibody constructed to abrogate FcγR binding showed no reduction of tumor growth, indicating that the in vivo antitumor activity of XmAbCD40 is primarily mediated via FcγR-dependent mechanisms. These data demonstrate that XmAbCD40 displays potent antitumor efficacy and merits further evaluation for the treatment of CD40+ malignancies.
Introduction
In the past decade, monoclonal antibodies directed against B-cell antigens have proven beneficial for patients with B-lineage neoplasms. Approved immunotherapeutics include the anti-CD52 antibody alemtuzumab (Campath-1H),1 2 anti-CD20 antibodies, rituximab (Rituxan)2 and most recently ofatumumab (Arzerra),3 and 2 anti-CD20 conjugates carrying radioactive isotopes, 90Y-ibritumomab tiuxetan (Zevalin)4 and 131I-tositumomab (Bexxar).5 In particular, when used as a single agent or in combination with chemotherapy, rituximab has shown efficacy and relative safety in many B-cell non-Hodgkin lymphomas (NHLs) and some B-cell leukemias.2,6 However, some patients do not respond to rituximab, and many of those that do will relapse.2 In addition, some B-cell tumors either lack CD20 expression or lose it during rituximab treatment.7 Thus, antibodies targeting other B-cell antigens are warranted, and several are being explored, including those targeting CD40, a 45- to 50-kDa transmembrane glycoprotein belonging to the tumor necrosis factor receptor family.
CD40 is broadly expressed on hematologic cells, including B cells and on several types of epithelial and endothelial cells.8-10 The ligand of CD40, CD40L (CD154), is preferentially expressed on activated T cells but has also been found on other hematologic cell types. Signaling via the CD40 pathway plays a key role in defining cellular and humoral immune responses.10 CD40 is overexpressed by the malignant counterparts to B-lineage cells and is found on most mature B-cell malignancies, including NHL, Hodgkin disease, and chronic lymphocytic leukemia (CLL), and on some early acute lymphocytic leukemias (ALL)8,11,12 ; it is also expressed on multiple myeloma (MM) cells.13 Many solid tumors express CD40, including melanoma and carcinomas of the kidney, bladder, lung, pancreas, colon, prostate, ovary, breast, head, and neck.14
CD40 agonists have been shown to trigger immune responses against various tumors15,16 and to inhibit the growth of different neoplastic cells, both in vitro and in vivo.17,18 Broad stimulation of the immune system and expression on a wide range of malignancies make CD40 an attractive target for antibody-based immunotherapy. Over the past 2 decades, several CD40-specific antibodies have been tested against B-lineage malignancies in vitro and in animal models, yielding various degrees of success.19-23 Recently, 2 humanized antibodies have emerged for further development: a mild CD40 agonist, SGN-40,20,24,25 and a CD40 antagonist, HCD122 (CHIR-12.12).21,23 These agents were evaluated in phase 1 and 2 trials in patients with relapsed MM, NHL, and CLL. Thus far, they have demonstrated a favorable safety profile and have shown some antitumor activity in NHL and MM.26-28 In addition, a strongly agonistic anti-CD40 antibody, CP-870,893, evaluated in phase 1 trials in patients with melanoma and other solid tumors, was found to be well tolerated and to have antitumor activity.29 Therefore, preclinical and early clinical data with CD40 antibodies have validated CD40 as a target for B-lineage malignancies and solid tumors, and have encouraged continued exploration of CD40-specific immunotherapies for neoplastic disease.
Therapeutic antibodies for oncology treatment elicit their cytotoxic activity via multiple immune effector mechanisms, including antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cell-mediated phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC). Anticancer antibodies may also facilitate apoptosis, resulting in antiproliferative effects that may contribute to therapeutic efficacy. Significant progress has been made in understanding the interactions of antibodies with Fcγ receptors (FcγRs) on immune effector cells, and antibodies have been engineered to enhance engagement with immune effectors, increasing their cytotoxic potential.30 Mutations in the Fc domain have improved binding to FcγRs, and antibodies with dramatically increased affinities for the activating receptors FcγRIIIa and FcγRIIa have been generated, resulting in significant improvements in ADCC and ADCP.31-35
Recently, we showed that an Fc-engineered anti-CD19 antibody markedly enhanced ADCC and ADCP and significantly inhibited tumor growth in human lymphoma xenograft mouse models.36 We concluded that FcγR-dependent mechanisms were primarily responsible for the antitumor activity. These results suggested that engineering an anti-CD40 antibody to increase FcγR binding could also substantially enhance its cytotoxic potential. We were also encouraged by evidence of safety and antitumor efficacy in early clinical trials with the Fc-engineered anti-CD30 antibody XmAb2513.37
Here we describe the characterization of XmAbCD40, a humanized anti-CD40 antibody with increased FcγR binding that facilitates highly enhanced ADCC against B-lymphoma, leukemia, and MM cell lines and against primary tumor cells from patients with CLL and plasma cell leukemia (PCL). XmAbCD40 also increases ADCP, has antiproliferative activity, and inhibits lymphoma growth in Ramos mouse xenograft models. Most importantly, XmAbCD40 exhibits superior antitumor activity relative to both rituximab and an anti-CD40 native IgG1 analog and thus may represent a promising next-generation immunotherapeutic for B-lineage malignancies.
Methods
Antibodies
Mouse anti-CD40 antibody (clone S2C6; Mabtech)38,39 variable region genes were ligated into the vector pTT5 (National Research Council Canada) containing the human light chain κ and heavy chain constant regions to create chimeric antibody constructs. To produce XmAbCD40, the Fv of S2C6 was humanized,40 and standard mutagenesis techniques were used to introduce 2 mutations (S239D/I332E) into the human Fc. An Fv identical to that of XmAbCD40 was used to generate both the XmAbCD40 IgG1 analog (anti-CD40 IgG1) and the anti-CD40 Fc-knockout (anti-CD40 Fc-KO); however, the Fc was wild-type IgG1 for the analog, and for the Fc-KO, 2 Fc substitutions were made (G236R/L328R) to eliminate binding to FcγRs. Construction of the XmAb isotype control, which has the same Fc as XmAbCD40 but an unrelated Fv (from antirespiratory syncytial virus), transfections, and antibody purification were performed as described.36 Rituximab was purchased from RxUSA, and phycoerythrin (PE)–conjugated F(ab′)2 goat secondary antibodies were purchased from Jackson ImmunoResearch Laboratories.
FcγRs, cell lines, donors, and patients
Human and mouse Fc receptors for binding studies and FcγRIIIa-158V-glutathione-S-transferase (GST) for antibody cross-linking were obtained or produced as described.36 Raji, RPMI8266, and IM-9 cell lines were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ), and Ramos and MEC-1 cell lines were obtained from ATCC. Peripheral blood samples were obtained from healthy volunteers or from male patients with CLL or PCL. All protocols were performed with written informed consent in accordance with the Declaration of Helsinki and were approved by the Ethics Committee of the Christian-Albrechts-University, Campus Kiel. Percentage of tumor cells and CD40 expression were determined using directly labeled antibodies and analyzed by fluorescence-activated cell sorting on a Cytomics FC500 (Beckman Coulter).
Binding to Fc receptors and CD40 antigen
Binding to human FcγRs was determined using Biacore analysis as described.36 CD40 dissociation constants (Kd) were determined by both Biacore analysis and cell-based binding assays. First, the extracellular domain of CD40 antigen was coupled to a CM5 biosensor chip, and then serially diluted solutions of antibodies were injected into a Biacore 3000 (Biacore) at 30 μL/minute for 2 minutes followed by dissociation for 3 minutes. Data were fit and analyzed to obtain Kd values as described.36
Second, Raji cells were seeded into 96-well plates at 5 × 104 cells/well, antibodies were added in 2-fold serial dilutions starting at 5nM, and the mixture incubated on ice for 30 minutes. After washing with phosphate-buffered saline (PBS), PE-labeled goat secondary antibody was added and the mixture incubated for an additional 20 minutes. Cells were washed twice, fixed with 1% paraformaldehyde, and then analyzed on a FACSCanto II (BD Biosciences). Resulting mean fluorescent intensity (MFI) values were normalized to the maximal observed signal for each antibody and then plotted against the log of the concentrations. Each curve was fitted with a 4-parameter logistic model using Prism (Version 4.03; GraphPad Software), and the relative affinities were determined as EC50 values of each curve.
Cell-surface CD20 and CD40 in tumor cell lines
CD20 and CD40 cell-surface antigen density was determined by quantitative flow cytometry using Dako QIFIKIT (Dako North America). A total of 1 × 106 tumor cells (MEC-1, Ramos, IM-9, or RPMI8226) was incubated with a saturating concentration of unconjugated mouse anti-CD20 antibody (clone Leu-16; BD Biosciences PharMingen) or mouse anti-CD40 antibody (clone S2C6) at 4°C for 1 hour, and the antigen density was determined according to the manufacturer's protocol. The number of binding sites/cell was calculated by comparing the MFI values of stained cells with a standard curve obtained by regression analysis of MFI values of standard beads. Prism (Version 4.03) was used to calculate and plot receptor cell surface density.
ADCC
ADCC for cell lines was measured using a lactate dehydrogenase (LDH) activity assay as described,36 except that natural killer (NK) effector cells were isolated using the EasySep Human NK Cell Enrichment Kit (StemCell Technologies) and tumor target cells were opsonized with antibodies for 20 minutes at 37°C.
Primary tumor cell ADCC was measured with a 51Cr release assay as described,36 except that tumor cells from patients with CLL or PCL were used as targets. Effectors (from healthy donors) were either fresh unstimulated peripheral blood mononuclear cells (PBMCs; CLL assays) or purified unstimulated NK cells (PCL assays). Fresh PBMCs from patients and healthy donors were isolated as described.36 NK cells from donors were isolated using a commercial NK-cell isolation kit (Miltenyi Biotec). NK-cell purity was determined by 2-color flow cytometry using CD16–fluorescein isothiocyanate and CD56-PE antibodies (Beckman Coulter). Routinely, preparations contained 85% to 95% pure NK cells. Donor PBMCs typically contained 60% CD3+ T cells, 10% to 20% CD56+ NK cells, and 10% CD14+ monocytes; PBMC samples from patients with CLL contained more than 90% tumor cells, and those from patients with PCL contained more than 94% tumor cells; average cell viability was more than 95%.
Phagocytosis
Macrophage ADCP was determined by flow cytometry as described,33 except that Ramos or IM-9 cells were used as targets and target cells were labeled with carboxyfluorescein succinimidyl ester (Invitrogen).
CDC
CDC of XmAbCD40, anti-CD40 IgG1, and rituximab was assayed using Ramos as target cells as described.41
Cell viability
Proliferation was assessed with a luminescent cell viability assay in the presence of antibodies cross-linked by FcγRIIIa-GST as described,36 except that cell viability was measured using 5 × 104 Raji or Ramos cells.
In vivo tumor xenograft models
Female C.B-17 severe combined immunodeficient (SCID) mice (Taconic Farms) were maintained in a pathogen-free facility, kept in microisolator cages (Innovive), and used when 6 to 12 weeks old. Xencor's Institutional Animal Care and Use Committee approved all experiments. For the disseminated tumor model, mice were injected intravenously with 5 × 106 Ramos lymphoma cells. Starting 3 days later, mice were injected intraperitoneally with 0.6, 2, or 6 mg/kg antibody or vehicle (PBS) twice per week for a total of 5 injections (n = 10/group).
For the therapeutic tumor model, mice were injected subcutaneously in the right flank with 2.5 × 106 Ramos cells. Seventeen days later, mice bearing tumors between 200 and 350 mm3 were placed into groups such that each group had the same mean tumor volume (± 10%; n = 10-15/group). For the dose-response study, mice were injected intraperitoneally with 0.6, 2, or 6 mg/kg XmAbCD40 or vehicle twice per week for 3 weeks; and in the comparison study, mice were injected intraperitoneally with 6 mg/kg antibody (XmAbCD40, anti-CD40 IgG1, anti-CD40 Fc-KO, or rituximab) or vehicle twice per week for 3 weeks. Tumors were measured twice per week with calipers and volumes calculated as π/6(length × width × height).
Statistical analysis
For in vivo studies, differences between groups were calculated using the nonparametric Mann-Whitney U test. P values less than .05 were considered significant.
Results
Development of XmAbCD40
In earlier studies, we showed that engineering the Fc domain for improved FcγR affinity substantially increased the in vitro ADCC of various antibodies (anti–HER-2, anti-CD20, and anti-CD52).32 We subsequently applied the most effective mutations (S239D/I332E) to the Fc of an anti-CD19 antibody, producing a variant that exhibited markedly enhanced antitumor activity both in vitro and in vivo.36 Here, we characterize an anti-CD40 antibody modified with the same Fc engineering strategy. Initially, a human Fc domain containing the S239D/I332E mutations was combined with the Fv domain from a mouse anti-CD40 antibody (clone S2C638,39 ). The Fv domain was then humanized40 to generate several variants, and the best was selected based on CD40 binding affinity, ADCC potency, thermal and chemical stability, and expression. Here we describe the in vitro and in vivo properties of this humanized Fc-engineered anti-CD40 antibody (termed XmAbCD40).
XmAbCD40 has increased binding affinity for human and mouse FcγRs
We first assessed FcγR binding to ensure that the S239D/I332E mutations in XmAbCD40 caused the expected increase in affinity. A Biacore biosensor was used to obtain Kd values, and results were compared with those of a control antibody containing an Fv identical to that of XmAbCD40 but with a native human IgG1 Fc (anti-CD40 IgG1). As shown in Table 1, XmAbCD40 displayed markedly enhanced binding to all human FcγRs, with the greatest increase seen for FcγRIIIa. We also evaluated an anti-CD40 Fc-knockout (anti-CD40 Fc-KO); this variant has the same Fv domain but contains 2 Fc substitutions abrogating Fc interactions (G236R/L328R) and was used as a control devoid of effector function. As expected, the Fc-KO showed no detectable binding to any human FcγRs.
It was critical to determine whether XmAbCD40 also enhanced binding to mouse FcγRs to properly evaluate the antitumor activity of these antibodies in mouse xenografts. As seen with human FcγRs, XmAbCD40 displayed increased affinity for all mouse Fc receptors relative to the IgG1 analog; binding to FcγRI and FcγRIV was enhanced the most (69- and 91-fold, respectively); again, the anti-CD40 Fc-KO showed no detectable binding to any mouse FcγRs. Thus, XmAbCD40 has substantially increased binding affinity for both human and mouse FcγRs.
XmAbCD40 retains high affinity for CD40 antigen
To ensure that humanization of the Fv domain did not compromise XmAbCD40's affinity for the CD40 antigen, we assessed CD40 binding using both cell-free and Raji cell-based binding assays (Table 2). Using Biacore analysis, we demonstrated that all 3 humanized antibodies (XmAbCD40, anti-CD40 IgG1, and the anti-CD40 Fc-KO) bind CD40 antigen with nearly identical Kd values (0.40-0.47nM), similar to the Kd values obtained for the parent mouse S2C6 (0.44nM) and chimeric (0.30nM) antibodies. Similar results were obtained with the cell-based assay. These data are consistent with those for another humanized anti-CD40 antibody, SGN-40,20 and indicate that humanization of the Fv can be achieved without impairing affinity toward CD40.
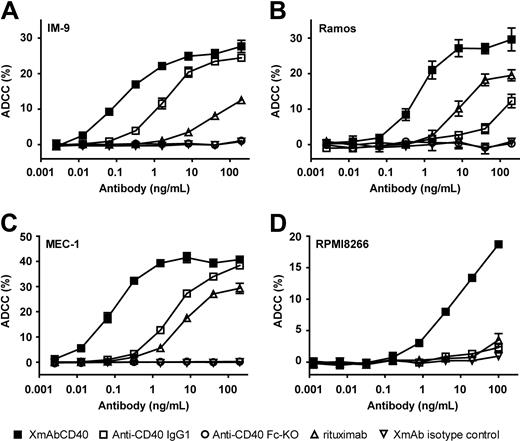
XmAbCD40 augments ADCC in B-lymphoma, leukemia, and MM cell lines
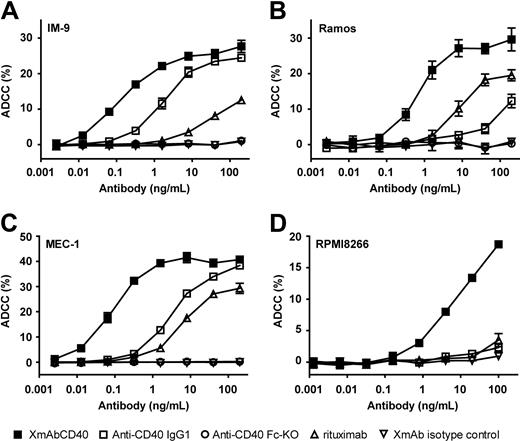
To assess whether XmAbCD40's increased affinity for FcγRs facilitates increased immune effector cell function, we first performed in vitro ADCC assays using purified interleukin-2 (IL-2)–activated NK cells as effectors and cell lines derived from various human B-cell tumors as targets (Burkitt lymphoma, CLL, MM, and a lymphoblastoid tumor). The levels of CD40 antigen expressed by these cell lines varied (3600-92 000 molecules/cell) and were substantially lower than CD20 levels (Table 3). XmAbCD40 markedly increased ADCC relative to the native IgG1 analog in all cell lines tested (Figure 1). EC50 values for XmAbCD40 ranged from 0.08 to 6.0 ng/mL, representing increases in potency up to 150-fold. Efficacy (maximal lysis) for XmAbCD40 ranged from 19% to 41% and was significantly increased for 2 of the 4 cell lines tested (2-fold and 8-fold for Ramos and RPMI8266, respectively). There was no significant correlation between CD40 cell-surface expression and XmAbCD40 ADCC activity. The IgG1 analog was completely inactive in RPMI8266, a cell line with low CD40 expression (∼ 3600 molecules/cell); in contrast, XmAbCD40 induced substantial lysis in this cell line, albeit with lower cytotoxicity than that observed in cell lines with higher CD40 expression. Rituximab exhibited reduced ADCC maximal lysis and 10- to 200-fold lower potency than XmAbCD40. No lysis was seen with the anti-CD40 Fc-KO, confirming that FcγR engagement is critical for antibody effector function. Moreover, no cell lysis was detected with the XmAb isotype control antibody, demonstrating that, in addition to FcγR engagement, specific Fv-antigen interactions are required to elicit ADCC. Thus, XmAbCD40 showed substantially enhanced ADCC relative to the IgG1 analog and rituximab in B-lineage and MM tumor cell lines.
XmAbCD40 enhances ADCC in multiple B-lineage tumor cell lines. ADCC was measured with an LDH release assay using purified IL-2–activated NK cells as effectors and cells from human B-lymphoma, leukemia, or MM cell lines as targets. Target cells were opsonized with antibodies and mixed with NK cells at an effector/target (E/T) ratio of 5:1; LDH release was measured 4 hours later. Percentage ADCC is shown for 4 tumor cell lines: (A) IM-9 (a lymphoblastoid line). (B) Ramos (Burkitt lymphoma), (C) MEC-1 (CLL), and (D) RPMI8266 (MM). Data were obtained in triplicate and represent the mean plus or minus SD. Cell-surface expression of CD40 and CD20 was determined by flow cytometry using QIFIKIT (Dako North America; Table 3). We demonstrate that XmAbCD40 increased ADCC relative to the IgG1 analog in all cell lines tested (potency improved up to 150-fold; maximal lysis increased 2- and 8-fold in Ramos and RPMI266 cell lines, respectively). No correlation is seen between CD40 expression levels and ADCC activity. XmAbCD40 is also superior to rituximab, exhibiting greater maximal lysis and 10- to 200-fold improved potency, although cell-surface CD40 levels are much lower than CD20 levels. No cell lysis is observed with the anti-CD40 Fc-KO or the XmAb isotype control antibodies.
XmAbCD40 enhances ADCC in multiple B-lineage tumor cell lines. ADCC was measured with an LDH release assay using purified IL-2–activated NK cells as effectors and cells from human B-lymphoma, leukemia, or MM cell lines as targets. Target cells were opsonized with antibodies and mixed with NK cells at an effector/target (E/T) ratio of 5:1; LDH release was measured 4 hours later. Percentage ADCC is shown for 4 tumor cell lines: (A) IM-9 (a lymphoblastoid line). (B) Ramos (Burkitt lymphoma), (C) MEC-1 (CLL), and (D) RPMI8266 (MM). Data were obtained in triplicate and represent the mean plus or minus SD. Cell-surface expression of CD40 and CD20 was determined by flow cytometry using QIFIKIT (Dako North America; Table 3). We demonstrate that XmAbCD40 increased ADCC relative to the IgG1 analog in all cell lines tested (potency improved up to 150-fold; maximal lysis increased 2- and 8-fold in Ramos and RPMI266 cell lines, respectively). No correlation is seen between CD40 expression levels and ADCC activity. XmAbCD40 is also superior to rituximab, exhibiting greater maximal lysis and 10- to 200-fold improved potency, although cell-surface CD40 levels are much lower than CD20 levels. No cell lysis is observed with the anti-CD40 Fc-KO or the XmAb isotype control antibodies.
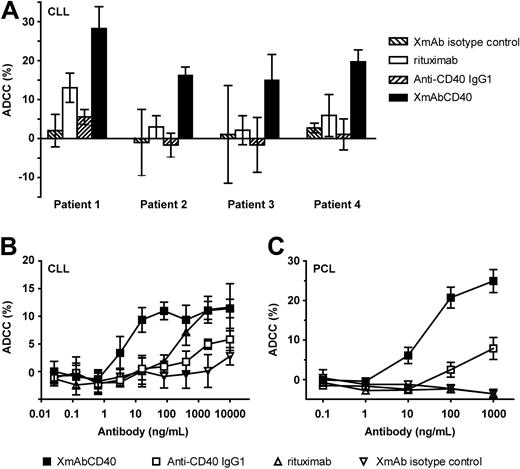
XmAbCD40 enhances ADCC against primary tumors
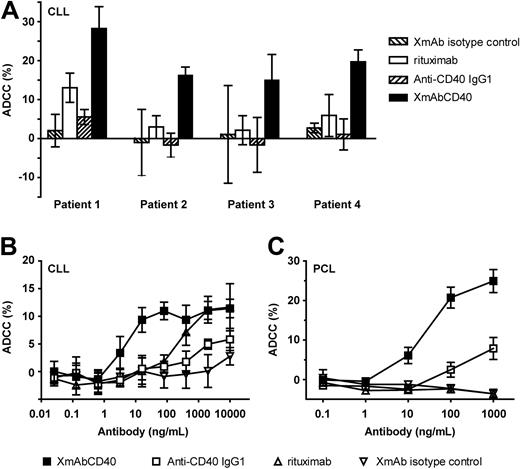
We further determined the ADCC of XmAbCD40 in patient-derived primary tumors. Assays were performed using tumor cells from patients with CLL or PCL (an aggressive form of MM) as targets. Effector cells were either fresh unstimulated PBMCs (CLL assays) or purified unstimulated NK cells (PCL assays). Figure 2A shows responses for 4 individual CLL samples; assays were performed using antibodies at 10 μg/mL. In each case, XmAbCD40 increased maximal lysis relative to both the IgG1 analog and to rituximab. Dose-response experiments with samples from CLL or PCL patients confirmed these results, with XmAbCD40 producing marked enhancements in both potency and efficacy (Figure 2B-C). The EC50 values for XmAbCD40 were improved 160-fold and 4-fold relative to the IgG1 analog for CLL and PCL samples, respectively, and maximal lysis was enhanced 2- to 3-fold. XmAbCD40 was also more potent than rituximab in CLL (by ∼ 50-fold). Differences were even more pronounced in PCL, where rituximab showed no detectable ADCC, whereas both anti-CD40 antibodies were efficacious, reflecting the fact that the PCL samples expressed CD40 but not CD20.
XmAbCD40 enhances ADCC against primary tumor cells. ADCC was determined with a 51Cr release assay using primary tumor cells from patients with CLL or PCL as targets. Effector cells were fresh unstimulated PBMCs (CLL assays) or purified unstimulated NK cells (PCL assays). Tumor cells were labeled with 51Cr (100 μCi/106 cells), opsonized with antibodies, and mixed with PBMCs or NK cells at an E/T ratio of 80:1 and 10:1, respectively; 51Cr release was measured 4 hours later. (A) Maximal lysis against samples from 4 individual CLL patients (assays used antibodies at 10 μg/mL). In each case, XmAbCD40 markedly increases maximal lysis relative to both the IgG1 analog and to rituximab. Data were obtained in triplicate and represent the mean ± SD. (B) Percentage ADCC against primary CLL. XmAbCD40 markedly improves potency and efficacy relative to the IgG1 analog (160- and 2-fold, respectively) and is approximately 50-fold more potent than rituximab. (C) Percentage ADCC against primary PCL. XmAbCD40 exhibits enhanced potency and efficacy relative to the IgG1 analog (4- and 3-fold, respectively), whereas rituximab has no effect; note that these target cells did not express CD20 as evidenced by flow cytometry (data not shown). (B-C) Data represent the mean ± SD of triplicate experiments for each of 2 (B) or 3 (C) PBMC donors.
XmAbCD40 enhances ADCC against primary tumor cells. ADCC was determined with a 51Cr release assay using primary tumor cells from patients with CLL or PCL as targets. Effector cells were fresh unstimulated PBMCs (CLL assays) or purified unstimulated NK cells (PCL assays). Tumor cells were labeled with 51Cr (100 μCi/106 cells), opsonized with antibodies, and mixed with PBMCs or NK cells at an E/T ratio of 80:1 and 10:1, respectively; 51Cr release was measured 4 hours later. (A) Maximal lysis against samples from 4 individual CLL patients (assays used antibodies at 10 μg/mL). In each case, XmAbCD40 markedly increases maximal lysis relative to both the IgG1 analog and to rituximab. Data were obtained in triplicate and represent the mean ± SD. (B) Percentage ADCC against primary CLL. XmAbCD40 markedly improves potency and efficacy relative to the IgG1 analog (160- and 2-fold, respectively) and is approximately 50-fold more potent than rituximab. (C) Percentage ADCC against primary PCL. XmAbCD40 exhibits enhanced potency and efficacy relative to the IgG1 analog (4- and 3-fold, respectively), whereas rituximab has no effect; note that these target cells did not express CD20 as evidenced by flow cytometry (data not shown). (B-C) Data represent the mean ± SD of triplicate experiments for each of 2 (B) or 3 (C) PBMC donors.
We also measured ADCC against primary CLL using purified IL-2–stimulated NK cells as effectors. Results were similar, except that efficacy was much higher, with maximal lysis for XmAbCD40 increasing from 12% to 60% in nonstimulated versus stimulated NK cells, respectively (data not shown). Thus, XmAbCD40 displayed substantially superior cytotoxic activity against primary tumor cells relative to the IgG1 analog and rituximab.
XmAbCD40 enhances ADCP
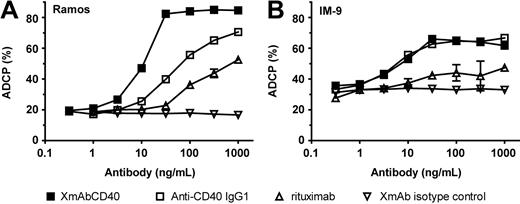
Macrophage phagocytosis has been implicated as an important effector function in mediating the cytotoxic activity of therapeutic antibodies, including those targeting CD40.42 We therefore assessed the impact of XmAbCD40 on ADCP. Assays were performed with monocyte-derived macrophages using a Burkitt lymphoma cell line (Ramos) and a lymphoblastoid cell line (IM-9) as targets. With Ramos cells, XmAbCD40 displayed approximately 5-fold greater potency relative to the IgG1 analog; maximal phagocytosis was also enhanced (from 73% to 87%; Figure 3A). Relative to rituximab, XmAbCD40 exhibited a 15-fold greater potency and almost 2-fold improved efficacy. No differences were observed between XmAbCD40 and the IgG1 analog with IM-9 cells, but both anti-CD40 antibodies exhibited enhanced ADCP efficacy and potency relative to rituximab (Figure 3B).
XmAbCD40 enhances macrophage phagocytosis. ADCP was determined by flow cytometry. Purified CD14+ monocytes were cultured with macrophage colony-stimulating factor for 5 days, and differentiated macrophages were identified by a combination of anti-CD11b–allophycocyanin (APC) and anti-CD14–APC. Target Ramos or IM-9 cells were fluorescently labeled with carboxyfluorescein succinimidyl ester, opsonized with antibodies, and incubated with the monocyte-derived macrophages for 4 hours at an E/T ratio of 4:1. Cells were double-stained with anti-CD11b–APC and anti-CD14–APC and evaluated by fluorescence-activated cell sorting; percentage ADCP was calculated as the number of double-positive cells divided by the total number of tumor cells. With Ramos cells (A), XmAbCD40 markedly enhances ADCP potency and efficacy relative to both the IgG1 analog and rituximab. With IM-9 cells (B), XmAbCD40 and the IgG1 analog are equally effective, and both demonstrate greater ADCP than rituximab. Data were obtained in triplicate and represent the mean ± SD.
XmAbCD40 enhances macrophage phagocytosis. ADCP was determined by flow cytometry. Purified CD14+ monocytes were cultured with macrophage colony-stimulating factor for 5 days, and differentiated macrophages were identified by a combination of anti-CD11b–allophycocyanin (APC) and anti-CD14–APC. Target Ramos or IM-9 cells were fluorescently labeled with carboxyfluorescein succinimidyl ester, opsonized with antibodies, and incubated with the monocyte-derived macrophages for 4 hours at an E/T ratio of 4:1. Cells were double-stained with anti-CD11b–APC and anti-CD14–APC and evaluated by fluorescence-activated cell sorting; percentage ADCP was calculated as the number of double-positive cells divided by the total number of tumor cells. With Ramos cells (A), XmAbCD40 markedly enhances ADCP potency and efficacy relative to both the IgG1 analog and rituximab. With IM-9 cells (B), XmAbCD40 and the IgG1 analog are equally effective, and both demonstrate greater ADCP than rituximab. Data were obtained in triplicate and represent the mean ± SD.
XmAbCD40 inhibits proliferation and facilitates CDC of B-lymphoma cells
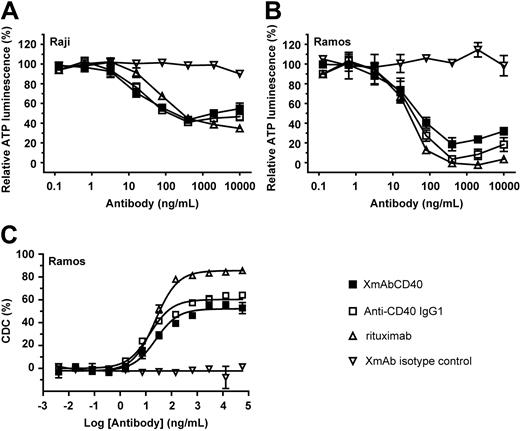
CD40 ligation by anti-CD40 antibodies or by recombinant soluble CD40 ligand has been shown to significantly inhibit the growth of neoplastic B cells in vitro, and cross-linking the CD40 antibodies with a secondary antibody can enhance this effect.19,20,22 We therefore explored the effect of XmAbCD40 on cell viability using 2 Burkitt lymphoma cell lines (Ramos and Raji). To enable antibody cross-linking and better simulate in vivo effects, we used a human FcγRIIIa-GST fusion receptor in which the GST domain facilitates dimerization of the FcγRIIIa.36 Tumor cells were incubated with cross-linked antibodies for 72 hours, and cell titers were analyzed using a cell viability assay. Responses to XmAbCD40 and the anti-CD40 IgG1 analog were similar, with both antibodies producing a dose-dependent inhibition of cell growth in both cell lines (Figure 4A-B). EC50 values ranged from approximately 15 to 30 ng/mL, and proliferation was inhibited 60% to 95%, with the Ramos cell line inhibited the most. Rituximab exhibited about the same level of inhibition as the 2 anti-CD40 antibodies. To determine CDC facilitated by anti-CD40 antibodies and rituximab, both were incubated with human serum complement for 2 hours, Alamar Blue was added, and cell lysis was quantitated after overnight culturing. CDC activity by XmAbCD40 and native anti-CD40 IgG1 was very similar and approximately 30% less than that facilitated by rituximab (Figure 4C). The lack of distinction between XmAbCD40 and its IgG1 analog suggests that the antiproliferative activity and CDC of these antibodies are unaffected by increased FcγR binding.
XmAbCD40 exhibits antiproliferative and CDC activity. Proliferation assays were performed on Raji (A) and Ramos (B) cells using a human FcγRIIIa-GST fusion receptor to provide antibody cross-linking. Cross-linked antibodies were incubated with 6 × 103 cells for 72 hours, and cell titers were analyzed using a luminescent cell viability assay. XmAbCD40, the IgG1 analog, and rituximab all show similar antiproliferative activity; cell growth is inhibited 60% to 95% relative to vehicle controls, and the inhibition is more pronounced in Ramos cells. (C) CDC assay was performed on Ramos cells using human serum complement. Antibodies were incubated with cells and complement for 2 hours at 37°C, Alamar Blue was added, cells were cultured overnight, and fluorescence was measured. XmAbCD40 and the IgG1 analog showed similar CDC that was reduced relative to rituximab by 30%. Data for all experiments were obtained in triplicate and represent the mean ± SD.
XmAbCD40 exhibits antiproliferative and CDC activity. Proliferation assays were performed on Raji (A) and Ramos (B) cells using a human FcγRIIIa-GST fusion receptor to provide antibody cross-linking. Cross-linked antibodies were incubated with 6 × 103 cells for 72 hours, and cell titers were analyzed using a luminescent cell viability assay. XmAbCD40, the IgG1 analog, and rituximab all show similar antiproliferative activity; cell growth is inhibited 60% to 95% relative to vehicle controls, and the inhibition is more pronounced in Ramos cells. (C) CDC assay was performed on Ramos cells using human serum complement. Antibodies were incubated with cells and complement for 2 hours at 37°C, Alamar Blue was added, cells were cultured overnight, and fluorescence was measured. XmAbCD40 and the IgG1 analog showed similar CDC that was reduced relative to rituximab by 30%. Data for all experiments were obtained in triplicate and represent the mean ± SD.
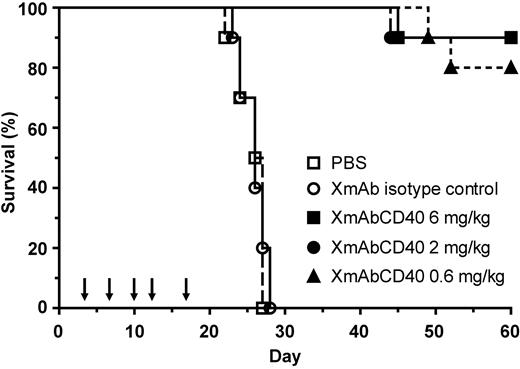
XmAbCD40 improves survival of mice bearing disseminated Ramos lymphoma
Mice with disseminated Ramos tumors were treated twice per week for a total of 5 injections with 0.6, 2, or 6 mg/kg XmAbCD40, XmAb isotype control antibody, or vehicle (PBS). XmAbCD40 significantly enhanced survival at all doses compared with either isotype control or vehicle (Figure 5). Whereas all the mice treated with vehicle or isotype control died by day 28, 80% to 90% of the XmAbCD40-treated mice were alive at study end on day 60 (80% for the 0.6 mg/kg group and 90% for 2 mg/kg and 6 mg/kg groups). These results suggest that XmAbCD40 may be effective for the treatment of disseminated human lymphoma.
XmAbCD40 improves survival of mice bearing disseminated Ramos lymphoma. Ramos cells (5 × 106) were injected intravenously into SCID mice. Starting 3 days later, 0.6, 2, or 6 mg/kg XmAbCD40, XmAb isotype control, or vehicle (PBS) was administered intraperitoneally twice per week for a total of 5 injections, as indicated by arrows (n = 10/group). XmAbCD40 significantly enhances survival at all doses relative to both vehicle and isotype controls (P < .0001); 80% of the 0.6-mg/kg group and 90% of the 2-mg/kg and 6-mg/kg groups continued to survive at study end (day 60), whereas all the control mice had died by day 28.
XmAbCD40 improves survival of mice bearing disseminated Ramos lymphoma. Ramos cells (5 × 106) were injected intravenously into SCID mice. Starting 3 days later, 0.6, 2, or 6 mg/kg XmAbCD40, XmAb isotype control, or vehicle (PBS) was administered intraperitoneally twice per week for a total of 5 injections, as indicated by arrows (n = 10/group). XmAbCD40 significantly enhances survival at all doses relative to both vehicle and isotype controls (P < .0001); 80% of the 0.6-mg/kg group and 90% of the 2-mg/kg and 6-mg/kg groups continued to survive at study end (day 60), whereas all the control mice had died by day 28.
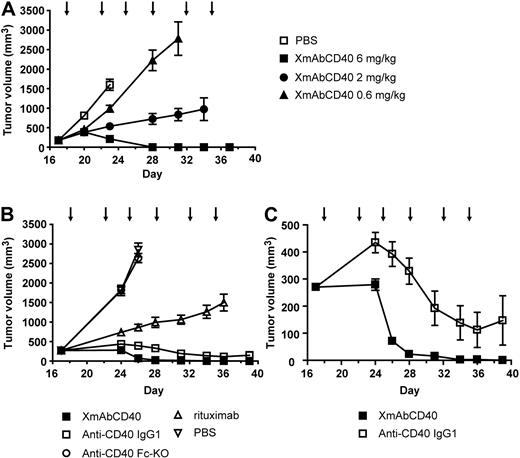
XmAbCD40 inhibits growth of established subcutaneous Ramos tumors
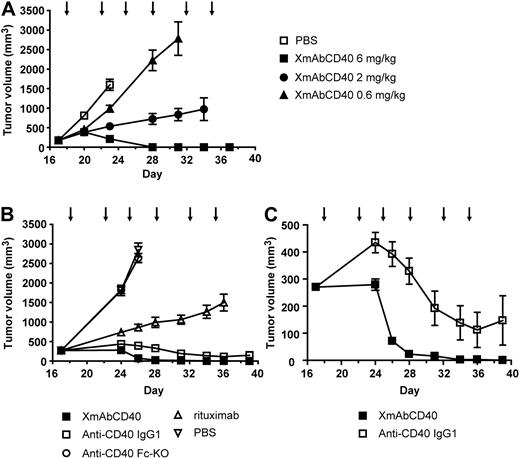
To identify the optimal treatment dose of XmAbCD40, mice bearing established human Ramos tumors were treated twice per week for 3 weeks with vehicle or the antibody at 0.6, 2, or 6 mg/kg. A clear dose-response is evident (Figure 6A). On day 28, mice treated with 0.6, 2, or 6 mg/kg of XmAbCD40 had mean tumor volumes (MTVs) of 2225, 723, and 0 mm3, respectively. Notably, 80% of the mice treated with the highest dose (6 mg/kg) showed complete tumor regression, whereas no regression was observed in the mice treated with the lower doses. These results demonstrated a dose-response for therapeutic treatment with XmAbCD40 and established 6 mg/kg as the optimal dose for further studies.
XmAbCD40 inhibits growth of established large Ramos tumors. Ramos cells (2.5 × 106) were injected subcutaneously into SCID mice. Starting on day 17 after tumor cell injection, mice bearing large tumors (200-350 mm3) were injected intraperitoneally with vehicle (PBS) or antibodies twice per week for 3 weeks, as indicated by arrows. (A) Dose-response study showing effects of XmAbCD40 at 0.6, 2, or 6 mg/kg (n = 10/group). A dose-dependent antitumor effect is evident (day 28 MTV = 2225, 723, and 0 mm3 for 0.6, 2, and 6 mg/kg, respectively; P = .0007 for 0.6 vs 2 mg/kg and P = .0004 for 2 vs 6 mg/kg), with complete tumor regression occurring in 80% of mice treated with the highest dose. (B) Study comparing antitumor activity of XmAbCD40 with anti-CD40 IgG1, rituximab, and the anti-CD40 Fc-KO, all at 6 mg/kg (n = 15/group). XmAbCD40 dramatically inhibits tumor growth compared with vehicle (day 26 MTV = 70 mm3 vs 2849 mm3, P = .000076) and is significantly more effective than rituximab (day 36 MTV = 3 mm3 vs 1500 mm3, P = .000076). By day 36, 67% of the XmAbCD40-treated mice became completely tumor-free (vs none of the rituximab-treated mice); and by day 39, 100% were tumor-free. The IgG1 analog also shows potent antitumor activity but is significantly less effective than XmAbCD40 at all but the last 2 time points (P ≤ .007 for days 24-34). The anti-CD40 Fc-KO shows no antitumor activity, indicating the importance of FcγR engagement. (C) The y-axis magnification of panel B illustrates the difference between the anti-CD40 IgG1 and XmAbCD40 responses and demonstrates the improved efficacy of XmAbCD40 mediated by increased affinity to FcγRs. Data are mean ± SEM.
XmAbCD40 inhibits growth of established large Ramos tumors. Ramos cells (2.5 × 106) were injected subcutaneously into SCID mice. Starting on day 17 after tumor cell injection, mice bearing large tumors (200-350 mm3) were injected intraperitoneally with vehicle (PBS) or antibodies twice per week for 3 weeks, as indicated by arrows. (A) Dose-response study showing effects of XmAbCD40 at 0.6, 2, or 6 mg/kg (n = 10/group). A dose-dependent antitumor effect is evident (day 28 MTV = 2225, 723, and 0 mm3 for 0.6, 2, and 6 mg/kg, respectively; P = .0007 for 0.6 vs 2 mg/kg and P = .0004 for 2 vs 6 mg/kg), with complete tumor regression occurring in 80% of mice treated with the highest dose. (B) Study comparing antitumor activity of XmAbCD40 with anti-CD40 IgG1, rituximab, and the anti-CD40 Fc-KO, all at 6 mg/kg (n = 15/group). XmAbCD40 dramatically inhibits tumor growth compared with vehicle (day 26 MTV = 70 mm3 vs 2849 mm3, P = .000076) and is significantly more effective than rituximab (day 36 MTV = 3 mm3 vs 1500 mm3, P = .000076). By day 36, 67% of the XmAbCD40-treated mice became completely tumor-free (vs none of the rituximab-treated mice); and by day 39, 100% were tumor-free. The IgG1 analog also shows potent antitumor activity but is significantly less effective than XmAbCD40 at all but the last 2 time points (P ≤ .007 for days 24-34). The anti-CD40 Fc-KO shows no antitumor activity, indicating the importance of FcγR engagement. (C) The y-axis magnification of panel B illustrates the difference between the anti-CD40 IgG1 and XmAbCD40 responses and demonstrates the improved efficacy of XmAbCD40 mediated by increased affinity to FcγRs. Data are mean ± SEM.
In a subsequent study on the relative effectiveness of XmAbCD40, mice bearing large established subcutaneous Ramos tumors of 200 to 350 mm3 were treated twice per week for 3 weeks with 6 mg/kg XmAbCD40, anti-CD40 IgG1, the anti-CD40 Fc-KO, rituximab, or vehicle. Vehicle-treated mice had an MTV of 2849 mm3 on day 26 and were euthanized because of large tumor burden (Figure 6B). In mice treated with rituximab, tumors also continued to grow; on day 26, MTV was 859 mm3, but by day 36, MTV increased to 1500 mm3 and the mice were euthanized (none became tumor-free). In contrast, mice treated with XmAbCD40 had a significantly lower MTV of 70 mm3 on day 26; by day 36, tumors had shrunk to 3 mm3; by day 39, 100% of mice were tumor-free (3 of 15 mice were euthanized on day 24 because their tumors, although in the range of 300-400 mm3, had become ulcerated). The anti-CD40 IgG1 analog also exhibited strong antitumor activity; however, MTV values were substantially larger than for the XmAbCD40-treated group (Figure 6C; 392 vs 70 mm3, P < .0001; and 147 vs 0 mm3, P = .08 on days 26 and 39, respectively); in addition, fewer mice were tumor-free at study end (60% vs 100%). Mice treated with the anti-CD40 Fc-KO showed no reduction of tumor growth, with an MTV virtually identical to vehicle-treated controls.
Thus, in a xenograft tumor model, therapy with Fc-engineered anti-CD40 antibody in either disseminated or established human lymphoma resulted in a dramatic inhibition of tumor growth. XmAbCD40 exhibited dose dependence and was significantly better at reducing tumor growth than rituximab. The ineffectiveness of the anti-CD40 Fc-KO indicates that the in vivo antitumor activity of XmAbCD40 is primarily mediated via FcγR-dependent mechanisms.
Discussion
NHL is one of the most common hematologic malignancies, and the majority of NHLs (85%) are B-cell disorders, with T-cell neoplasms accounting for the rest. In 1997, the anti-CD20 monoclonal antibody rituximab was approved in combination with chemotherapeutic agents for the treatment of B-cell NHL. Its remarkable success initiated a dramatic change in hematologic cancer treatment, with the biologics, especially monoclonal antibodies, emerging as key therapeutic agents. However, the extended use of rituximab also pointed to its limitations: some tumors were either not susceptible or developed resistance to it. Consequently, several other cell-surface antigens have been explored as alternatives to CD20, including CD19, CD22, CD23, CD70, CD80, HLA-DR, and CD40.2,36,43 Although CD40 was identified 25 years ago and antibodies to this cell-surface marker were developed shortly thereafter, only recently have anti-CD40 antibodies been shown to be potent modulators of neoplastic and in particular lymphoproliferative disease.20,21,23-25,29 Although B cells express less CD40 than CD20, CD40 expression begins earlier and is maintained longer through B-cell maturation, from pro-B cells to plasma cells. Accordingly, the spectrum of lymphoid malignancies expressing CD40 is broader.8,11-13 Therefore, in addition to mature B-cell tumors, anti-CD40 antibodies may be efficacious against MM, PCL, ALL, and other CD20− tumors that are not amenable to treatment with rituximab. Thus far, 2 anti-CD40 antibodies have been evaluated in clinical trials.26-28
Here, we present the in vitro and in vivo characterization of an Fc-engineered anti-CD40 antibody (XmAbCD40). We demonstrate that XmAbCD40 shows nearly 2 orders of magnitude increased binding to FcγRIIIa and 1 order of magnitude increased binding to FcγRIIa. The increased affinity for FcγRIIIa translated into dramatically increased NK cell–mediated ADCC. Results were consistent in several cell lines expressing different levels of CD40 antigen as well as in patient-derived primary tumors. XmAbCD40 also produced a smaller and less consistent increase in ADCP by monocyte-derived macrophages. Given the prominent role FcγRIIa plays in macrophage-mediated cellular immunity,33,44 this lesser effect on ADCP is consistent with XmAbCD40's relatively modest enhancement of FcγRIIa binding (∼ 10-fold less than to FcγRIIIa).
The in vivo evaluation in mouse xenograft models demonstrated that XmAbCD40 was more effective at inhibiting tumor growth than its native IgG1 analog. However, the extent of this increased inhibition, although statistically significant, was less pronounced than might have been expected from the in vitro results, particularly the significant increase in ADCC. A possible explanation is the remarkable potency of the IgG1 analog. In vitro both XmAbCD40 and the native IgG1 display similar antiproliferative and CDC activities. In vivo, anti-CD40 IgG1 showed strong antitumor activity and was substantially more robust than the IgG1 analog of another antibody (anti-CD19) in a similar model,36 causing not only slowed growth, but tumor shrinkage. Thus, the inherently high antitumor activity of the IgG1 analog reduced the dynamic range in the xenograft model; and although XmAbCD40 treatment resulted in almost complete tumor regression, the extent of increased antitumor activity relative to the IgG1 analog was limited. This antitumor activity by the native antibody is consistent with reports for other agonistic anti-CD40 antibodies in mouse models and clinical trials (anti-CD40 IgG1 and XmAbCD40 are both mildly agonistic; J.O.R., unpublished data, March 2008). In mice, CD40 ligation by agonistic antibodies eradicated CD40− tumors by inducing strong systemic immunity mediated by CD8+ cytotoxic T cells.16 Agonistic anti-CD40 antibodies were also reported to activate NK cells by inducing IL-12 and interferon-γ production, and the antitumor response elicited by these antibodies was reduced by NK-cell depletion.45 In a phase 1 melanoma trial, a strongly agonistic anti-CD40 antibody, CP-870,893, exhibited antitumor activity that was partly attributed to systemic immune stimulation.29 Thus, systemic activation of the immune system and non–cell-mediated cytotoxicity appear to be important for the antitumor activity of anti-CD40 agonistic antibodies.
Because the immune system of cancer patients may be impaired by the disease or by cancer treatments, it is difficult to predict which aspect of immune stimulation will be most responsible for clinical improvements. Although earlier trials in NHL and MM were promising, a phase 2 NHL trial comparing therapy with the anti-CD40 antibody SGN-40 with existing treatments alone was stopped because of lack of efficacy. However, an antibody capable of both systemic immune stimulation and enhanced effector function, resulting from Fc optimization, could be more efficacious in the clinic than a more limited SGN-40 with a native Fc. The data presented here support this hypothesis, as evidenced by the substantially improved cytotoxicity displayed in vitro and in vivo by the Fc-engineered XmAbCD40 relative to the native IgG1 analog.
Although we observed enhanced antitumor activity in the mouse, the impact of Fc optimization may prove to be more pronounced in humans. Significant differences between human and mouse immune systems complicate comparisons of human in vitro and mouse in vivo results. Extrapolating from mouse models to the clinic is equally fraught with caveats. XmAbCD40 enhances binding to the mouse-activating receptors FcγRI and FcγRIV by nearly 2 orders of magnitude. Both of these receptors are expressed on mouse macrophages and dendritic cells, and FcγRIV is also expressed on neutrophils; however, neither are expressed on NK cells.30,46 As in humans, mouse NK cells exclusively express FcγRIII. XmAbCD40 binding to mouse FcγRIII is enhanced approximately 3-fold, which is more than 10-fold less than the enhancement seen for human FcγRIIIa. Therefore, NK cells may be less important in mediating the cytotoxic effects of XmAbCD40 in mice than in humans. In mice, macrophages could be major effectors responsible for the enhanced activity of our Fc-engineered antibody. This hypothesis is supported by 2 reports: a recent xenograft study demonstrating that the antitumor activity of the anti-CD40 antibody SGN-40 occurs via Fc-FcγR interactions and is primarily mediated by macrophages,42 and by studies implicating macrophages in antibody-mediated B-cell depletion.44
However, the role of NK cells in mediating antibody-induced antitumor responses in humans should not be underestimated. FcγRIII polymorphism studies show that some cancer patients expressing the allele with higher affinity to IgG (V158) respond better to rituximab therapy,47,48 and NK cells from patients with this allele exhibit enhanced antibody-induced NK-cell activation.49 Previously, we demonstrated a transient reduction in peripheral NK cells associated with the B cell–depleting activity of an anti-CD19 antibody in cynomolgus monkeys, a species exhibiting FcγR binding affinities and immune system biology very similar to that of humans.50 Similarly, treatment with IL-2 elicits a transient reduction of NK cells in the blood, and it was proposed that NK-cell activation can lead to NK-cell margination and trafficking from the circulation.51
Our results demonstrate that Fc engineering to increase binding for FcγRIIIa and FcγRIIa substantially increased the cell-mediated antitumor activity of XmAbCD40, making it more potent and efficacious than rituximab. Both in vitro and in vivo studies illustrated the superiority of XmAbCD40 over rituximab in a broad range of tumor cells, independent of the relative expression of CD20 and CD40. The optimized interactions of XmAbCD40 with FcγR may activate immune cells so that differences in cell surface expression of the 2 antigens become secondary to the effects of increased FcγR affinity. The most dramatic difference between XmAbCD40 and rituximab was observed in the mouse xenograft study: at study end, XmAbCD40 cured 100% of mice of tumors, whereas rituximab cured none. The robust antitumor profile demonstrated by XmAbCD40 warrants its further evaluation for the treatment of CD40+ B-lineage malignancies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Araz Eivazi, Bally Randhawa, and Chi-Ming Yu for technical contributions, Marie Ary for her contribution to writing the manuscript, and David Szymkowski for helpful discussions.
Authorship
Contribution: E.A.Z. designed the research; H.M.H., M.J.B., M.P., E.P., S.K., S.Y.C., J.O.R., and H.C. performed the research and collected data; E.A.Z., H.M.H., R.R., and J.R.D. analyzed and interpreted the data; and E.A.Z. drafted the manuscript.
Conflict-of-interest disclosure: The authors are current or former employees of Xencor Inc, with the exceptions of M.P. and R.R. This research was completed by E.A.Z. and J.O.R. while they were employed at Xencor Inc, and no confidential information belonging to Boehringer Ingelheim Pharmaceuticals Inc or Aurora HealthCare is disclosed in the publication. The remaining authors declare no competing financial interests.
The current affiliation for J.O.R. is Immunotherapy Program, Aurora HealthCare, Milwaukee, WI. The current affiliation for E.A.Z. is Biotherapeutics and Integrative Biology, Boehringer Ingelheim Pharmaceuticals Inc, Ridgefield, CT.
Correspondence: John R. Desjarlais, Xencor Inc, 111 W Lemon Ave, Monrovia, CA 91016; e-mail: jrd@xencor.com; and Eugene A. Zhukovsky, Boehringer Ingelheim Pharmaceuticals Inc, 175 Briar Ridge Rd, Ridgefield, CT 06877; e-mail: eugene.zhukovsky@boehringer-ingelheim.com.