Abstract
Osteolytic bone disease mediated by osteoclasts occurs adjacent to myeloma cells suggesting a tight interrelationship between these cells and their microenvironment. In this issue of Blood, Noonan and colleagues1 challenge this current paradigm by invoking a role for a subpopulation of T cells known as T-helper 17 (Th17) as a key regulator of bone disease in myeloma.
Bone disease represents a major morbidity in multiple myeloma affecting up to 80% of patients during the course of their illness. Myeloma-induced lytic bone disease can manifest as bone pain, pathologic fractures, spinal cord compression, and/or hypercalcemia. Central in the pathogenesis of bone disease is osteoclast (OC) activation which is initiated by myeloma cell–stromal interaction leading to up-regulation of factors such as interleukin-6 (IL-6), interleukin 1α (IL-1α), receptor-activator of nuclear factor–kappa B ligand (RANKL), macrophage inhibitory protein (MIP-1α), and stromal-derived factor-1 (SDF-1) that stimulate OC formation. The additional production of factors that inhibits osteoblast (OB) activity such as Dickkopf-1 (DKK-1), TGF-β, and HGF along with the reduced production of osteoprotegrin (OPG; a soluble decoy receptor secreted by OB and stromal cells that binds RANKL), contributes to bone disease by disrupting the fine balance between OC and OB activity.2
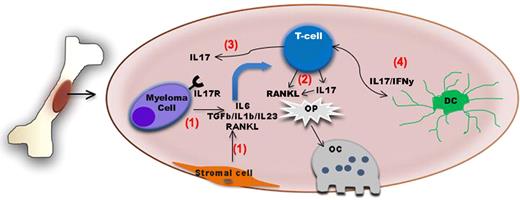
Schematic diagram summarizing the role of IL-17–producing lymphocytes in bone disease in myeloma. The bone and the bone marrow are immunologically active sites. (1) Myeloma cell–stromal interaction leads to up-regulation of factors such as IL-6, TGF-β, IL-1β, IL-23, and RANKL The first 2 factors in particular will induce IL-17–producing lymphocytes which also express RANKL. (2) Both factors in turn will act upon osteoclast precursors (Op) and increase osteoclast generation and/or activity. (3) IL-17 also increases adherence of myeloma cells expressing IL17R to BM stromal elements by functioning as a myeloma growth factor. (4) Dendritic cells might also contribute to the induction of Th17 cells in the BM of myeloma patients.
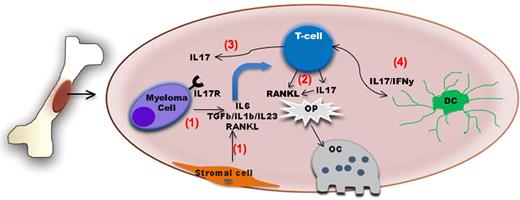
Schematic diagram summarizing the role of IL-17–producing lymphocytes in bone disease in myeloma. The bone and the bone marrow are immunologically active sites. (1) Myeloma cell–stromal interaction leads to up-regulation of factors such as IL-6, TGF-β, IL-1β, IL-23, and RANKL The first 2 factors in particular will induce IL-17–producing lymphocytes which also express RANKL. (2) Both factors in turn will act upon osteoclast precursors (Op) and increase osteoclast generation and/or activity. (3) IL-17 also increases adherence of myeloma cells expressing IL17R to BM stromal elements by functioning as a myeloma growth factor. (4) Dendritic cells might also contribute to the induction of Th17 cells in the BM of myeloma patients.
The bone and the bone marrow (BM) microenvironment are increasingly seen as immunologically active sites. For instance, the BM is a reservoir of memory T cells with heightened antigen specificity and it is a priming site for T-cell responses to blood-borne antigens.3 Not surprisingly, immune cells and/or their products have been shown to also influence bone homeostasis. Indeed, the OC activator, RANKL, has been demonstrated to be expressed on activated T cells. Conversely, IFN-γ secretion can suppress OC formation.4 In this issue, Noonan et al introduce IL-17–producing lymphocytes as critical regulators of the myeloma tumor microenvironment that mediate bone lytic disease.
IL-17, a cytokine induced by TGF-β and IL-6, is mainly produced by a newly defined lineage of T lymphocytes termed Th17. These cells have been implicated as key mediators of pathology in numerous autoimmune conditions. However, the exact role of Th17 cells in tumor immunity remains less well defined. Recently, several groups have begun to unveil the role of Th17 in multiple myeloma. First, myeloma cells have been shown to express the IL17R; in addition, IL-17 stimulation increases myeloma adherence to BM stromal elements by functioning as a myeloma growth factor.5 Second, the induction of Th17 cells in the BM of myeloma patients seems to be mediated by dendritic cells.6 Noonan et al further extended these observations by providing novel insights into the role of IL17 as a key regulator of osteolytic bone disease within the myeloma microenvironment (see figure). First, they found higher levels of cytokines needed for Th17 differentiation in the BM plasma of myeloma patients. These higher cytokines levels were accompanied with an increased percentage of Th17 cells among the CD3+ T cells isolated from the marrow and peripheral blood of myeloma patients compared with cells isolated from normal donors. Given the previously described reciprocal relationship between Th17 and regulatory T cells,7 the IL-6–mediated Th17 phenotype may also explain the paucity of regulatory T cells that Noonan et al observed in the bone marrow of myeloma patients relative to marrow from normal donors. Second, the authors found that treatment of myeloma peripheral blood lymphocytes (PBLs) with the combination of IL-6 and TGF-β induces T cells displaying a Th17 phenotype. Conversely, by adding neutralizing antibodies against IL-6 and TGF-β to myeloma PBLs cultured in the presence of BM plasma from myeloma patients (rich in IL-6 and TGF-β) they showed that such a treatment abrogates the generation of the Th17 phenotype. Third, exposure of bone marrow cells to soluble IL-17 or Th17 cells increased the generation of OC above the levels observed when cells were stimulated with macrophage–colony-stimulating factor (M-CSF) plus RANK-L. Addition of anti–IL-17 neutralizing antibodies to this culture system significantly decreased the generation of OC, supporting a previously unknown role for IL-17 in OC induction. Finally, by analyzing the extent of bone disease in a cohort of 40 myeloma patients and the levels of several cytokines in the BM plasma of these patients, they found a correlation among IL-17 levels and extent of bone disease in this patient population.
In the past several years, we have witnessed significant progress in the treatment of both myeloma and its associated bone disease. Bisphosphonates have dramatically improved the quality of life of many patients through their effective inhibition of osteo-clast activation. Newer antimyeloma agents such as lenalidomide and bortezomib can inhibit osteoclast formation and increase osteoblast numbers, respectively. Most recently, denosumab, a monoclonal antibody targeting RANKL, has shown considerable clinical efficacy in patients with bone metastases in prostate and breast cancer with more effective normalization of markers of bone resorption and fewer skeletal-related events compared with bisphosphonates8 with encouraging results also in myeloma. Another key regulator of bone disease is DKK-1, a protein secreted by myeloma cells capable of inhibiting osteoblast differentiation. Antibodies targeting this protein are currently in early stages of clinical development. It remains to be seen, however, whether the beneficial effects of these novel agents in myeloma-induced lytic bone disease can be explained—at least in part—by a putative influence upon the newly discovered OC-Th17 axis.
The findings by Noonan et al point to manipulation of IL-17–producing cells as an additional therapeutic approach with the potential advantages of not only reducing bone disease but also increasing the endogenous myeloma-specific immune responsiveness and directly inducing anti-myeloma cell killing. However, future translational and clinical studies will ultimately determine whether this new member of the “skull and bones” would receive (or not) full accreditation and be recognized as a key player in this otherwise highly selective “society.”
Conflict-of-interest disclosure: The author declares no competing financial interests. ■