Abstract
Cells from the mononuclear phagocyte system (MPS) act as systemic and local amplifiers that contribute to the progression of chronic inflammatory disorders. Transforming growth factor-β–activated kinase 1 (TAK1) is a pivotal upstream mitogen-activated protein kinase-kinase-kinase acting as a mediator of cytokine expression. It remains critical to determine in vivo the implication of TAK1 in controlling the innate immune system. Here, we describe a vehicle tailored to selectively deliver siRNAs into MPS cells after intravenous administration, and validate in vivo the potential of the RNAi-mediated TAK1 knock down for immunomodulation. In a mouse model of immune-mediated inflammatory disorder, we show that anti-TAK1 siRNA lipoplexes efficiently alleviate inflammation, severely impair the downstream c-Jun N-terminal kinase and nuclear factor-κB signaling pathways, and decrease the expression of proinflammatory mediators. Importantly, the systemic TAK1 gene silencing decreases the frequency of Th1 and Th17 cells, both mediating autoimmunity in experimental arthritis, demonstrating the immunomodulatory potential of TAK1. Finally, in vitro inhibition of TAK1 in myeloid cells decreases interferon-γ–producing T cells, suggesting that a delivery sys-tem able to target MPS cells and to silence TAK1 impacts on pathogenic T effector cells in autoimmunity.
Introduction
Cells from the mononuclear phagocyte system (MPS) play a key role in innate immunity for effective defense against infections. This family of phagocytic cells, derived from a common precursor in the bone marrow, comprises primarily monocytes and macrophages that circulate in the bloodstream and accumulate in organs, such as lymph nodes and the spleen, but also dendritic cells, as well as the Kupffer cells of the liver.1 Cells from the MPS are critical effectors and regulators of inflammation and the innate immune response, but also contribute to shaping T-cell responses. In the context of immune-mediated inflammatory disorders, myeloid cells act as systemic and local amplifiers that contribute to disease progression. Indeed, such cells are abundant in the inflamed tissues; they have an activated phenotype, produce a broad range of proinflammatory and catabolic mediators, and clearly play a central role in acute and chronic inflammation that results in the destruction of tissues injured in various autoimmune and chronic inflammatory disorders. All these observations provide the rationale for optimizing anti-inflammatory therapeutic strategies tailored to selectively target both tissue resident and circulating cells of the MPS, using agents able to specifically interfere with their activated status.
In mononuclear phagocytes, several signals trigger the production of proinflammatory mediators through serine or threonine kinases. Accumulating evidence shows that transforming growth factor-β–activated kinase 1 (TAK1) represents a key mediator of proinflammatory signals. Indeed, many of the signaling pathways triggered by multiple extracellular stimuli converge at the level of TAK1, and its subsequent activation leads to the expression of genes encoding proinflammatory molecules. Therefore, TAK1 appears essential in regulating the balance between inflammatory and anti-inflammatory responses of MPS cells. However, no in vivo functional data exist to support this hypothesis. TAK1 is a mitogen-activated protein-kinase-kinase-kinase (MAP3K) and has initially been identified as a cytosolic component of MAPK pathways activated by ligands of the transforming growth factor (TGF)–β/bone morphogenic protein (BMP) superfamily of secreted factors.2 As a key regulator of the TGF/BMP downstream signaling pathways, TAK1 acts in a number of physiologic processes where it is involved in the control of morphogenesis and tissue homeostasis.3-8 In addition, TAK1 plays a critical role in innate and adaptive immune responses and in inflammation by integrating signaling pathways downstream of interleukin-1 (IL-1), IL-17, IL-18, tumor necrosis factor (TNF)–α, Toll-like receptor (TLR), and NOD-like receptor, as well as the receptor activator of nuclear factor (NF)–κB ligand (RANKL).2,6,9-15
TAK1 deficiency in mice results in embryonic lethality, and therefore, conditional knockout systems have been used to reveal additional in vivo roles of TAK1 in T- and B-cell differentiation and activation.13,16-20 TAK1 interacts with other cytosolic factors, in particular, it forms a complex with TAK1-binding protein 1 and 2 (TAB1/2) to activate MAPK kinase 3/6 (MKK3/6) and p38, MKK4 and c-Jun N-terminal kinase (JNK), as well as the NF-κB pathways.15,21 Because the molecular basis of TAK1 involvement in controlling the innate immune system in vivo is not yet fully understood, RNAi-based approaches might represent powerful, suitable tools to investigate its functions. In vivo application, however, requires the development of efficient vehicles tailored to efficiently target the cellular component of interest. In the present study, using a delivery system able to inhibit TAK1 gene expression within mononuclear phagocytes, we investigated the role of TAK1-dependent cascades in a preclinical mouse model of autoimmune and inflammatory disease.
Methods
Animals
DBA/1 and BALB/c mice (Harlan) were bred in our facility under specific pathogen-free conditions and used at the age of 8-9 weeks. All animal experiments were conducted in accordance with the National Guidelines for Animal Care and approved by the Ethic Committee on Animal Research of the Languedoc-Roussillon (CE-LR-0505).
CIA model and clinical evaluation
Collagen-induced arthritis (CIA) was induced in DBA/1 mice at 9 week of age, as described previously,22 using bovine type II collagen from MD Biosciences, acetic acid from Sigma-Aldrich, and Freund adjuvant from Pierce. Starting at day 21, paw thickness was measured 3 times a week with a Mitutoyo micrometer (Sigma-Aldrich), and the severity of arthritis was graded by macroscopic examination as previously described.23 After euthanasia, the knee joints were collected and incubated for 24 hours in RPMI 1640 (300 μL). Supernatants were stored until quantification of cytokines by enzyme-linked immunosorbent assay (ELISA). The hind paws were collected, fixed in 4% paraformaldehyde, EDTA (ethylenediaminetetraacetic acid) decalcified and embedded in paraffin. Ankle-joint sections (5 μm) were stained with hematoxylin/eosin (HE) or safranin O/Fast green, scanned using the NDP Nanozoomer HT (Hamamatsu Photonics), and scored blindly by 2 independent observers for histopathologic examination of synovial inflammation, cartilage erosion, and proteoglycan loss as follows: For inflammation, score 0 = no inflammation; score 1 = slight thickening of lining layer or some infiltrating cells in sublining layer; score 2 = slight thickening and some infiltrating cells; score 3 = thickening of lining layer, influx of cells in sublining layer, and cells in the synovial space; and score 4 = highly infiltrated synovium and many inflammatory cells. For cartilage erosion, score 0 = no destruction; score 1 = slight erosion limited to single spots; score 2 = moderate erosion in a limited area; score 3 = extended erosions; and score 4 = general destruction. Sections were also stained with Safranin O/Fast green to determine the loss of proteoglycans. Safranin O staining was scored using a semiquantitative scoring system (0-4), where 0 represents no loss of proteoglycans, while a score of 4 indicates complete loss of staining for proteoglycans.
siRNA lipoplexes treatment
From arthritis onset, CIA mice were randomized and injected intravenously through the tail vein with 10 μg of siRNAs formulated as lipoplexes with the cationic liposome RPR209120/DOPE and a carrier DNA, as previously described.24 In vivo delivery experiment using fluorescently labeled Cy3 siRNA (Dharmacon) was carried out by administering formulated siRNA intravenously (0.5 mg/kg). Mice were killed at defined time points, and fluorescence uptake was examined by flow cytometry for indicated phenotypic markers.
Cell preparation and culture
The mouse monocytic cell line, J774A.1, was maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2mM glutamine, and 50 μg/mL penicillin/streptomycin. Mouse bone marrow–derived macrophages (BMMs) were generated by flushing BM cells from femurs and tibiae of Balb/c mice. Briefly, cells grown in mouse granulocyte macrophage colony-stimulating factor (GM-CSF; R&D Systems; 10 ng/mL) were generated as previously described.25 On day 7, adherents GM-BMM (3 × 105 cells/mL) were used for RNAi experiments.
siRNA transfections
Nontargeting control siRNAs (siCT) and siRNAs against TAK1 (siTAK1) were chemically synthesized by Dharmacon and received as desalted, preannealed duplexes in either standard or polyacrylamide gel electrophoresis–purified formats. Cells were seeded in 24-well format plates (3 × 105 cells/well) and transfected with siRNAs using Lipofectamine 2000 according to the manufacturer's conditions (Invitrogen). Twenty-four hours after transfection, cells were stimulated with fresh medium containing TNF-α (R&D Systems; 20 ng/mL) or lipopolysaccharide (LPS; Sigma-Aldrich; 1 μg/mL) for indicated time points before subsequent experiments.
Coculture of CD4+ T cells with GMBMMs
CD4+ T cells were enriched from spleen mononuclear cells of naïve DBA/1 mice by magnetic cell sorting using a mouse CD4+ T-cell isolation kit II (Miltenyi Biotec). The purity of CD4+ T cells was > 92%. T cells were cultured with GM-BMMs at a ratio of 5:1, and cells were stimulated with 1 μg/mL of anti-CD3 in complete medium. Supernatants or cells were analyzed on day 5 of culture for IFN-γ and IL-17A production by CD4+ T cells.
Quantitative reverse transcription polymerase chain reaction
Total RNA was isolated using the RNeasy mini kit (QIAGEN) according to the manufacturer's protocol. Total RNA (1 μg) was reverse transcribed using MultiScribe reverse transcriptase (Applied Biosystems). TaqMan primers specific for TNF-α, IFN-γ, TAK1 and GAPDH, and TaqMan Universal PCR Master Mix (Applied Biosystems) were used. Analysis was performed using ABI Prism 7000 Sequence Detection System software (Applied Biosystems). The relative content of cDNA samples was normalized by subtracting the threshold cycle (Ct) of the endogenous GAPDH reference gene to the target gene (ΔCt = Ct of target gene – Ct of GAPDH). Values were expressed as the relative mRNA level of specific gene expression as obtained using the following formula: 2−(ΔCt).
Western blotting
Immunoblotting analyses were performed following standard procedures using antibodies against TAK1 (Santa Cruz Biotechnology), p-TAK1, JNK, p-JNK, P38, p-P38, ERK, p-ERK, IκBα (Cell Signaling), and peroxidase-coupled antibodies as the secondary antibody (eBioscience). Bands were visualized with enhanced chemiluminescence substrate (Amersham Life Science), and densitometric analysis was performed with ImageJ 1.40g (National Institutes of Health).
Antibodies and FACS analyses
The following antibodies (Abs) were used: fluorescein isothiocyanate-labeled anti-CD4, anti-CD146; PerCP-Cy5.5-labeled anti-CD11c; allophycocyanin (APC)–Cy7-labeled anti-CD11b; V450-labeled anti-B220; and phycoerythrin-labeled anti-IL17, anti-Foxp3, and APC-conjugated anti-interferon (IFN)–γ, anti-CD25 antibodies. All Abs were purchased from BD Pharmingen, except CD146 and FoxP3 (Miltenyi Biotec). Mononuclear cells from blood and specimens from spleen and draining lymph nodes were collected and prepared according to standard procedures. Lymphocytes from liver were obtained as previously reported.26 For cell isolation from arthritic joints, knees, the hind and front paws were cut open and digested with 1 mg/mL of collagenase D (Roche) and 0.2 mg/mL DNase I (Roche) for 60 minutes at 37°C. Cells were then filtered and used for fluorescence-activated cell sorting (FACS) analyses. For IFN-γ and IL17A intracellular cytokine staining, cells (1 × 106 cells/well in a 96-well plate) were activated for 24 hours with plate-bound anti-CD3 monoclonal antibody (mAb; 2 μg/mL; BD Pharmingen) and anti-CD28 mAb (4 μg/mL; BD Pharmingen). Brefeldin A (5 μg/mL; Sigma-Aldrich) was added for the last 4 hours. The Cytofix/Cytoperm kit (BD Pharmingen) was used according to the protocols provided by the manufacturer. Intracellular detection of TAK1 and p-TAK1 expression was performed using rabbit anti-TAK1 and -pTAK1 Abs (Cell Signaling), followed by Alexa Fluor 647–-conjugated anti–rabbit secondary Ab staining (Invitrogen). The levels of intracellular proteins were detected by flow cytometry comparing the APC intensity in the different cell populations investigated, as described elsewhere.19 Data acquisition and analyses were performed on a FACSCalibur or a FACSCanto II flow cytometer (BD Biosciences).
CII-specific T-cell response
Immune cells from either draining lymph node or spleen T cells collected from injected mice were seeded into a 96-well plate in triplicate at 8 × 105 cells per well and mixed with serial dilutions of bCII ranging from 0 to 20 μg/well. After 72 hours of incubation, T-cell proliferative responses to bCII were measured by quantitative analysis of cell viability/proliferation using the CellTiter-Glo luminescence assay (Promega).
Anti–type II collagen assays
Serum samples were obtained from retro-orbital puncture of anesthetized animals at indicated times. Serum levels of anti–type II collagen antibodies were measured by ELISA during disease course. Ab units for bCII were determined using a standard curve reference generated from pooled sera of arthritic mice and assigned an arbitrary level of antigen-specific Abs as previously described.27
Cytokine quantification
Supernatants were stored until quantification of mouse IFN-γ, TNF-α, IL-1β, IL-12p70, IL-23, IL-17A (R&D Systems), and IL-6 (eBioscience) using specific ELISA kits.
TUNEL apoptosis
Cell death was determined by TdT-mediated dUTP nick-end labeling (TUNEL) assay using an In Situ Cell Death Detection Kit (Roche).
Statistical analysis
Results were presented as means (± SD) for each sample. Statistical analyses were performed using the Mann-Whitney-Wilcoxon test, as appropriate, according to the distribution of the data. An F test was used to examine the significance of differences in the expression of the signaling mediators in Western blots. Differences were considered significant when the P value was less than .05.
Results
TAK1 gene silencing interferes with LPS- and TNF-induced MAPK signaling in monocytes
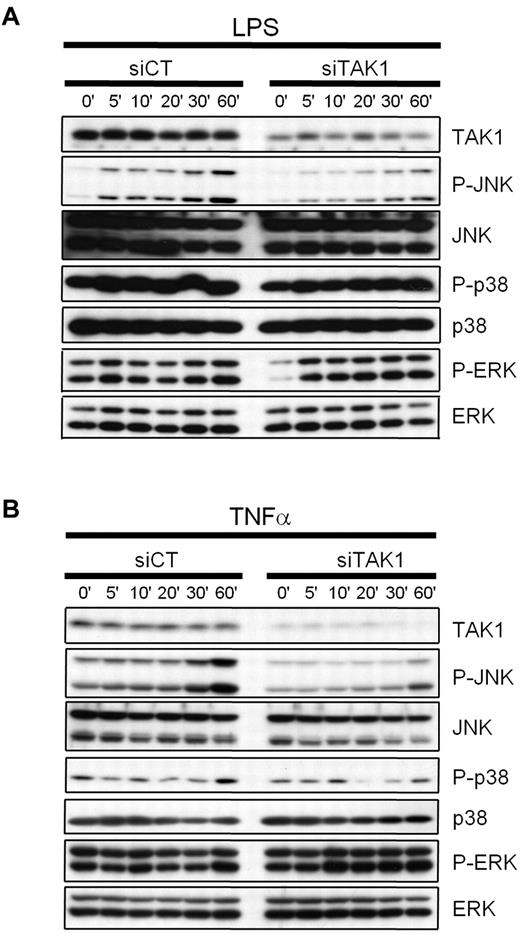
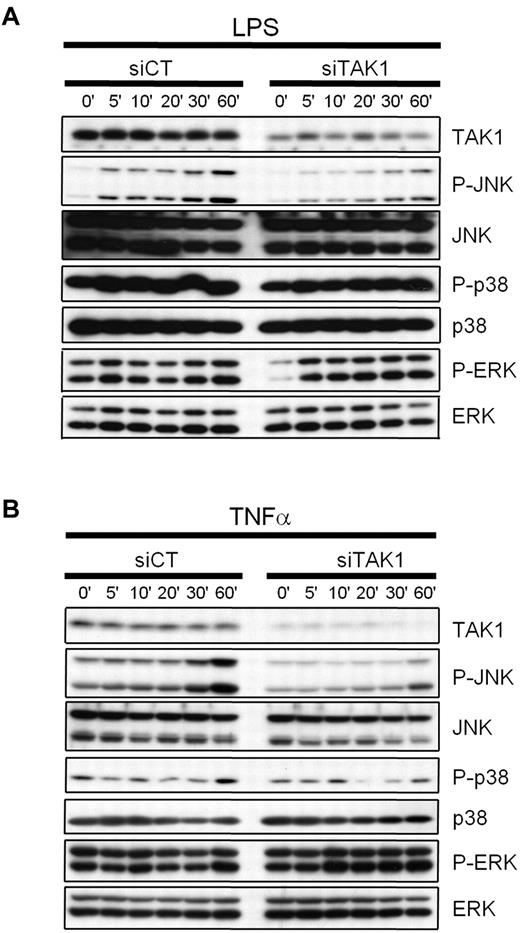
To determine the efficacy of targeting TAK1 by RNAi to interfere with TLR- or cytokine-induced signal transduction in cells from the MPS, the mouse monocytic cell line J774.1 was transiently transfected with siRNAs against TAK1 (siTAK1), or with a nontargeting control siRNA (siCT), and treated with 1 μg/mL of LPS (Figure 1A) or 20 ng/mL of TNF-α (Figure 1B). Compared with siCT transfection, RNAi-mediated knockdown of TAK1 in monocytes led to a reduction of TNF- and LPS-induced JNK activation, whereas no effect on p38 and ERK activation was observed.
Effect of TAK1 gene silencing on LPS- and TNF-induced MAPK signaling in monocytes. Mouse monocytic cells (J774.1) were transiently transfected with siRNAs against TAK1 (siTAK1) or with a nontargeting control siRNA (siCT) as described in the Methods. Twenty-four hours after lipofection, the cells were treated with 1 μg/mL of LPS (A) or 20 ng/mL of TNF-α (B) for the indicated time periods. Whole-cell extracts were prepared, and the inhibition of TAK1 and the activation of JNK, p38, and ERK1/2 were determined by Western blot analyses. Knockdown of TAK1 leads to a reduction of TNF-α- and LPS-induced JNK activation, whereas no effect was evidenced on p38 and ERK activation, compared with siCT-treated cells.
Effect of TAK1 gene silencing on LPS- and TNF-induced MAPK signaling in monocytes. Mouse monocytic cells (J774.1) were transiently transfected with siRNAs against TAK1 (siTAK1) or with a nontargeting control siRNA (siCT) as described in the Methods. Twenty-four hours after lipofection, the cells were treated with 1 μg/mL of LPS (A) or 20 ng/mL of TNF-α (B) for the indicated time periods. Whole-cell extracts were prepared, and the inhibition of TAK1 and the activation of JNK, p38, and ERK1/2 were determined by Western blot analyses. Knockdown of TAK1 leads to a reduction of TNF-α- and LPS-induced JNK activation, whereas no effect was evidenced on p38 and ERK activation, compared with siCT-treated cells.
Systemically delivered siRNA lipoplexes are taken up by circulating and tissue-resident myeloid cells
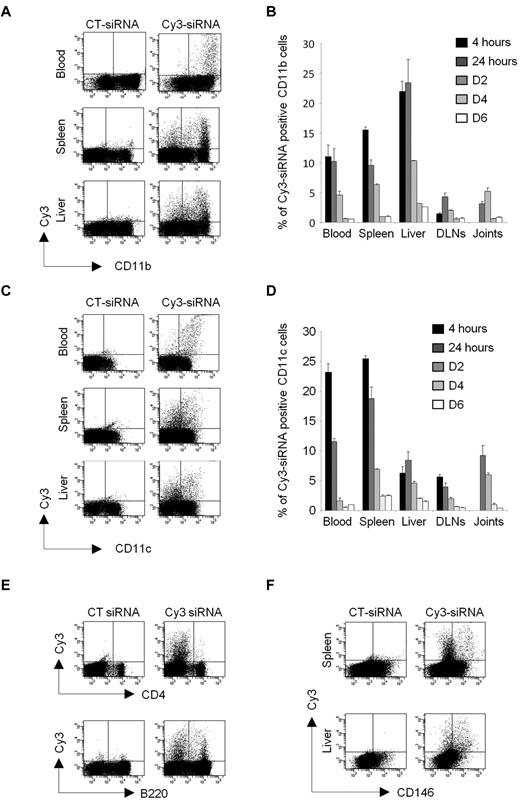
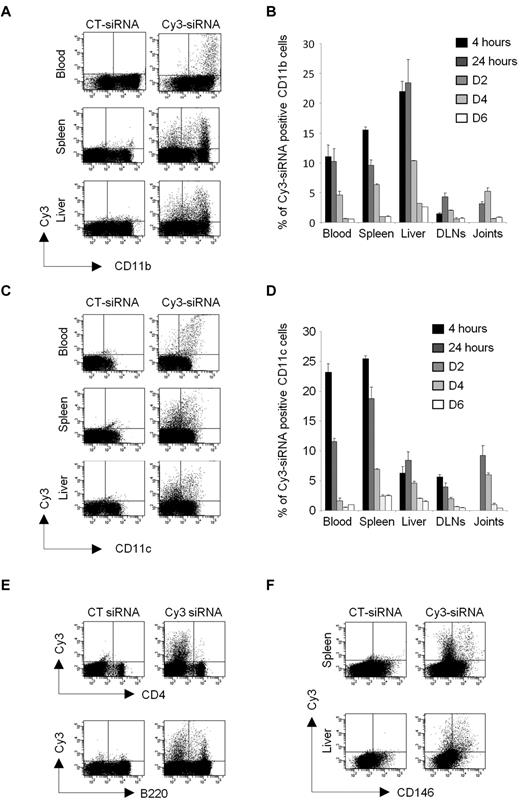
To investigate in vivo the role of TAK1-dependent cascades in cells from the MPS, in the context of inflammatory disorder, we first investigated the biodistribution and kinetics of a siRNA lipoplex formulation designed for in vivo RNAi. For this purpose, mice with experimental autoimmune arthritis were injected intravenously with a single dose of Cy3-labeled siRNA (10 μg) formulated with the cationic liposome, RPR209120/DOPE, and a cargo DNA. Blood, spleen, liver, inflamed joints, and draining lymph nodes (DLNs) were collected at 5 time points (from 4 hours to 6 days) for in vivo examination by flow cytometry of the cellular siRNA uptake (Figure 2). The liposome-encapsulated siRNAs were detected in all of the tissues investigated. During the first 24 hours after injection, approximately 10%-15% of CD11b+ cells (Figure 2A-B) and approximately 10%-25% of CD11c+ cells (Figure 2C-D) from the blood and spleen were positive for the fluorescent siRNA. Not surprisingly, approximately 20%-25% of the CD11b+ cells and approximately 5%-10% of CD11c+ cells from the liver had engulfed the Cy3-siRNA lipoplexes. Importantly, a lower but notable proportion of CD11b- and CD11c-positive cells isolated from the joints and DLNs of arthritic DBA/1 mice had also entrapped the siRNAs. Then, the level of fluorescence declined gradually from day 2 after injection. The siRNA complex was also found to be incorporated, to a lesser extent, by the lymphoid lineage with approximately 1% and 5% of T and B cells from the spleen, respectively, displaying fluorescence at early time points (Figure 2E). None of the T and B lymphocytes in the joints showed positive staining for the siRNA (data not shown). As systemically administered siRNAs might also be taken up by endothelial cells (ECs),28 known to have a high capacity for antigen uptake and processing, the siRNA fluorescence was also investigated in CD146-positive cells from the spleen and liver (Figure 2F). Two days after injection, up to 3% of the ECs from the spleen and 10% of the liver sinusoidal endothelial cells (LSECs) had taken up the siRNAs, the levels decreasing rapidly thereafter. Together, these data show that liposome-encapsulated siRNAs accumulate, in many organs, upon intravenous injection and highlight a higher uptake of the lipoplexes by cells of the MPS, such as monocytes/macrophages and dendritic cells (DCs).
In vivo biodistribution and kinetics of siRNA lipoplexes after single systemic administration. Arthritic DBA/1 mice were injected intravenously with 10 μg Cy3-siRNA formulated with the cationic liposome, RPR209120/DOPE, and a DNA carrier, as described in “siRNA lipoplexes treatment.” As controls, mice were injected with a nonfluorescent control siRNA (siCT). Tissue biodistribution and mononuclear cell uptake of the Cy3-siRNAs was evaluated by flow cytometry from 4 hours to day 6 after injection using anti-CD11b (A-B), -CD11c (C-D), -B220, -CD4 (E), or anti-CD146 mAbs (F). Representative dot plots showing Cy3-positive cells for the indicated phenotypic markers and organs are shown from CT siRNA- (left panels) or Cy3-siRNA- (right panels) lipoplex-injected mice at day 2 after administration. (B-D) Histograms show percentages of Cy3-siRNA uptake within the gated CD11b (B) and CD11c (D) positive cells from the indicated time points. Data are representative of 2 independent experiments, with 3 mice enrolled in each group.
In vivo biodistribution and kinetics of siRNA lipoplexes after single systemic administration. Arthritic DBA/1 mice were injected intravenously with 10 μg Cy3-siRNA formulated with the cationic liposome, RPR209120/DOPE, and a DNA carrier, as described in “siRNA lipoplexes treatment.” As controls, mice were injected with a nonfluorescent control siRNA (siCT). Tissue biodistribution and mononuclear cell uptake of the Cy3-siRNAs was evaluated by flow cytometry from 4 hours to day 6 after injection using anti-CD11b (A-B), -CD11c (C-D), -B220, -CD4 (E), or anti-CD146 mAbs (F). Representative dot plots showing Cy3-positive cells for the indicated phenotypic markers and organs are shown from CT siRNA- (left panels) or Cy3-siRNA- (right panels) lipoplex-injected mice at day 2 after administration. (B-D) Histograms show percentages of Cy3-siRNA uptake within the gated CD11b (B) and CD11c (D) positive cells from the indicated time points. Data are representative of 2 independent experiments, with 3 mice enrolled in each group.
In vivo inhibition of TAK1 expression and activity in cells of the MPS
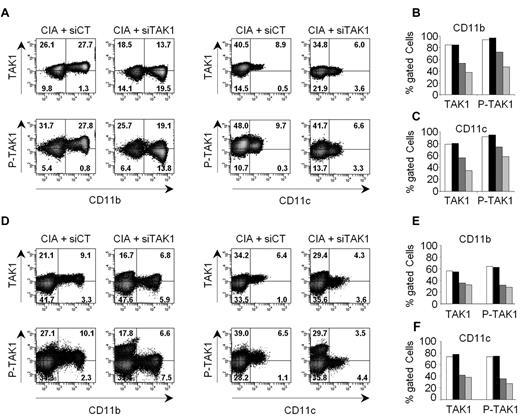
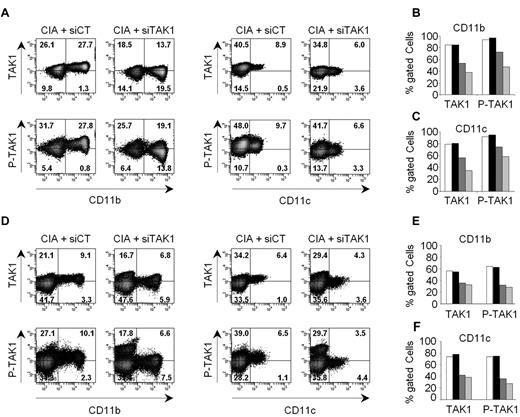
We next determined whether TAK1 expression in myeloid cells could be specifically silenced in vivo using siRNA lipoplexes. Arthritic mice were injected intravenously with either anti-TAK1 siRNA (siTAK1)– or nontargeting control siRNA (siCT)–containing lipoplexes and killed at different time points (from days 2 to 6) for evaluation of TAK1 expression and phosphorylation status by a flow cytometry–based intracellular assay (Figure 3). siTAK1 delivered by the cationic liposome formulation potently suppressed TAK1 protein expression and activity (indicated by p-TAK1 levels) in myeloid cells from blood (Figure 3A-C) and spleen (Figure 3D-F) at day 4 after injection. No silencing was observed in cells from mice injected with a nontargeting control siRNA-containing lipoplex, confirming the specificity of the silencing. Importantly, up to 80% inhibition of TAK1 protein levels and 60% inhibition of p-TAK1 was still evident 6 days after a single administration of siTAK1 lipoplexes.
In vivo silencing of TAK1 expression and activity in myeloid cells. Arthritic DBA/1 mice received a single intravenous injection (0.5 mg/kg) of either anti-TAK1 siRNAs or nontargeting control siRNAs, formulated as lipoplexes. At days 2, 4, and 6 after siRNA lipoplex injection, TAK1 protein expression and phosphorylation were determined in CD11b- and CD11c-positive cells by flow cytometry after staining and permeabilization of mononuclear cells from blood (A-C) and spleen (D-F). (A,D) Representative dot plots are shown for siCT- (left panels) and siTAK1- (right panels) injected mice, 6 days after vehicle administration. Gating was performed on mononuclear cells after exclusion of doublets and dead cells. Histograms show mean percentages of TAK1 and P-TAK1 expression within the gated phenotypic markers indicated, in either blood (B-C) and spleen (E-F), at days 2 (white and black), 4 (dark gray), and 6 (light gray) after injection with either siCT- (white bars) or siTAK1- (black and gray bars) lipoplexes. Mean of cumulative data from 2 independent experiments, with 2 mice enrolled in each group are presented.
In vivo silencing of TAK1 expression and activity in myeloid cells. Arthritic DBA/1 mice received a single intravenous injection (0.5 mg/kg) of either anti-TAK1 siRNAs or nontargeting control siRNAs, formulated as lipoplexes. At days 2, 4, and 6 after siRNA lipoplex injection, TAK1 protein expression and phosphorylation were determined in CD11b- and CD11c-positive cells by flow cytometry after staining and permeabilization of mononuclear cells from blood (A-C) and spleen (D-F). (A,D) Representative dot plots are shown for siCT- (left panels) and siTAK1- (right panels) injected mice, 6 days after vehicle administration. Gating was performed on mononuclear cells after exclusion of doublets and dead cells. Histograms show mean percentages of TAK1 and P-TAK1 expression within the gated phenotypic markers indicated, in either blood (B-C) and spleen (E-F), at days 2 (white and black), 4 (dark gray), and 6 (light gray) after injection with either siCT- (white bars) or siTAK1- (black and gray bars) lipoplexes. Mean of cumulative data from 2 independent experiments, with 2 mice enrolled in each group are presented.
Systemic TAK1 siRNA lipoplex delivery inhibits ongoing inflammation
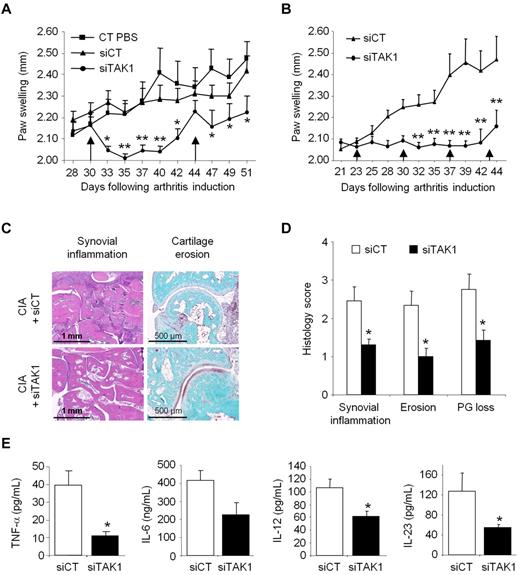
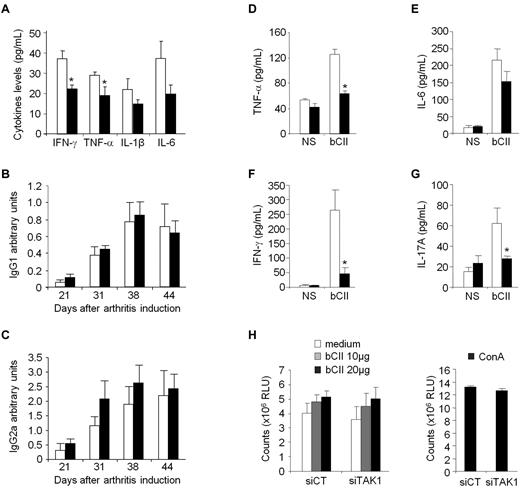
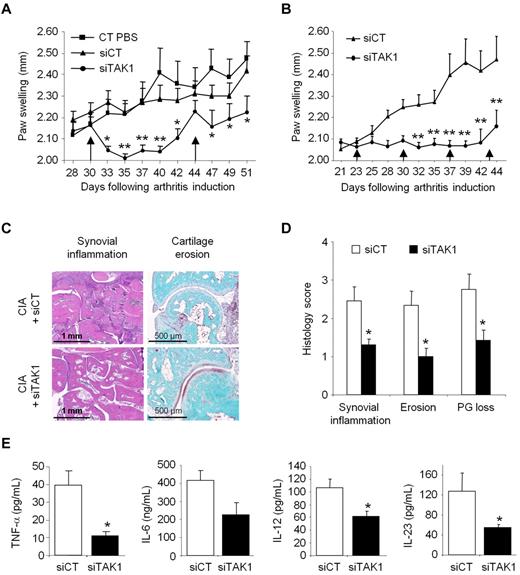
Combining our delivery system with functional anti-TAK1 siRNAs that allows silencing the expression of this master MAP3K, notably in cells from the MPS, we then evaluated the immunomodulatory potential of such an approach in an ongoing systemic inflammation. Collagen II–immunized DBA/1 mice were injected intravenously from disease onset (day 30) with either phosphate-buffered saline (PBS), siCT-, or siTAK1-containing liposomes (0.5 mg/kg). A single injection of siTAK1 lipoplexes significantly reduced clinical signs of arthritis, compared with both control groups, as assessed by paw swellings (Figure 4A). The maximum effect was observed 5 days after injection and persisted for 10 days (P < .01). On day 44, when the siTAK1-treated animals relapsed, each group received a second injection. Although partial, mice treated with the siTAK1 lipoplexes showed a significant reduction in paw swellings, compared with both control groups. Considering the duration of the TAK1 silencing as well as its beneficial effect on CIA, weekly injection of the siRNA lipoplexes were used in the following in vivo experiments. As shown in Figure 4B, arthritic mice weekly injected with siTAK1 lipoplexes from disease onset (day 23) were significantly protected from disease progression, compared with siCT lipoplex-treated animals (P < .01). In agreement with the clinical features, histologic analyses of paw sections showed a significantly decreased synovial inflammation, cartilage proteoglycan loss, and erosion scores in the siTAK1-treated mice, compared with the siCT-injected animals (Figures 4C-D). In the control group at euthanasia, synovial membranes showed areas of hyperplasia, proteoglycan loss, and cartilage surface erosion as well as bone damage. Coincident with these data, local production of inflammatory mediators, such as TNF-α, IL-6, IL-12, and IL-23, were decreased in the knee joint tissues of siTAK1-injected mice (Figure 4E). The striking effect observed in siTAK1-treated mice was associated with decreased systemic inflammation, as evidenced by lower serum levels of IFN-γ, TNF-α, IL-1β, and IL-6 proinflammatory cytokines (Figure 5A).
Systemic TAK1-siRNA lipoplexes delivery alleviates inflammation in experimental autoimmune arthritis. DBA/1 mice were immunized with bovine type II collagen and boosted on day 21. Mice were intravenously administered (arrows) with 0.5 mg/kg of TAK1 siRNA (siTAK1). Control groups were injected with either PBS or a nontargeting control siRNA sequence (siCT). All siRNA duplexes were formulated with the cationic liposome, RPR209120/DOPE, and a DNA carrier, as described in “siRNA lipoplexes treatment.” (A) Mice with ongoing inflammation were administered with siTAK1 lipoplexes on days 30 and 44, and paw swellings were measured overtime. (B) From arthritis onset (day 23), mice were injected weekly with siCT or siTAK1 lipoplexes at the indicated time points. (C) HE (synovial inflammation; scale bar, 1 mm)–, safranin O/Fast green (cartilage erosion and proteoglycan loss; scale bar, 500 μm)–stained representative ankle-joint sections from experiment shown in B. (D) Histologic scores of synovial inflammation, cartilage erosion, and cartilage proteoglycan (PG) loss from the experiment shown in B. (E) Cytokine levels of TNF-α, IL-6, IL-12, and IL-23 in 24-hour conditioned media from the knee joints of control and TAK1 siRNA-treated mice were measured by ELISA at euthanasia. Results in panel A are the mean ± SEM of 10 mice per group, and results in panels B-E are the mean ± SEM of 7-8 mice per group. * P < .05, ** P < .01.
Systemic TAK1-siRNA lipoplexes delivery alleviates inflammation in experimental autoimmune arthritis. DBA/1 mice were immunized with bovine type II collagen and boosted on day 21. Mice were intravenously administered (arrows) with 0.5 mg/kg of TAK1 siRNA (siTAK1). Control groups were injected with either PBS or a nontargeting control siRNA sequence (siCT). All siRNA duplexes were formulated with the cationic liposome, RPR209120/DOPE, and a DNA carrier, as described in “siRNA lipoplexes treatment.” (A) Mice with ongoing inflammation were administered with siTAK1 lipoplexes on days 30 and 44, and paw swellings were measured overtime. (B) From arthritis onset (day 23), mice were injected weekly with siCT or siTAK1 lipoplexes at the indicated time points. (C) HE (synovial inflammation; scale bar, 1 mm)–, safranin O/Fast green (cartilage erosion and proteoglycan loss; scale bar, 500 μm)–stained representative ankle-joint sections from experiment shown in B. (D) Histologic scores of synovial inflammation, cartilage erosion, and cartilage proteoglycan (PG) loss from the experiment shown in B. (E) Cytokine levels of TNF-α, IL-6, IL-12, and IL-23 in 24-hour conditioned media from the knee joints of control and TAK1 siRNA-treated mice were measured by ELISA at euthanasia. Results in panel A are the mean ± SEM of 10 mice per group, and results in panels B-E are the mean ± SEM of 7-8 mice per group. * P < .05, ** P < .01.
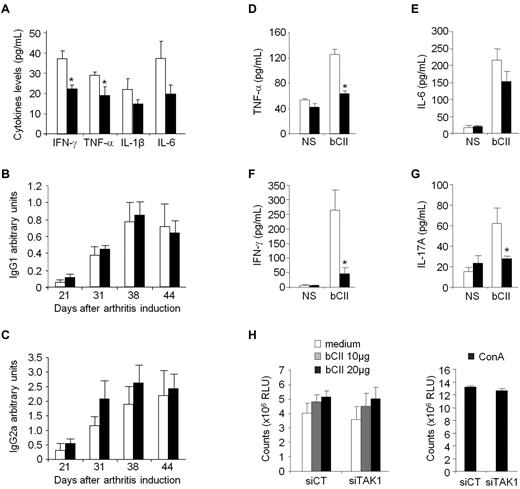
Effect of in vivo TAK1 gene silencing on systemic CIA hallmarks. DBA/1 mice were immunized with bovine type II collagen (CII) on day 0 and boosted on day 21. From arthritis onset, mice were weekly injected intravenously with 0.5 mg/kg of either siTAK1- (black bars) or siCT- (white bars) lipoplexes. Serum samples from the experiment shown in Figure 4B were collected overtime. (A) Serum levels of IFN-γ, TNF-α, IL-1β, and IL-6 were analyzed on day 44 by ELISA. (B-C) Anti-CII IgG1 (B) or IgG2a (C) isotype antibody levels were measured by ELISA in control- and TAK1- siRNA-treated mice. Results are expressed as arbitrary units, as described in “Anti–type II collagen assays.” (D-G) At euthanasia, splenocytes from the different groups were collected, and isolated cells were either not stimulated (NS) or stimulated with bCII antigen (20 μg/mL). Levels of TNF-α (D), IL-6 (E), IFN-γ (F), and IL-17A (G) were determined by ELISA after 24 hours of culture. (H) Splenic T cells were stimulated with increasing concentrations of bCII. As a positive control, splenocytes were stimulated with concanavalin A (5 μg/mL). Antigen-specific T-cell proliferation was quantified by the CellTiter-Glo luminescent cell viability assay 3 days later. Results are the mean ± SEM of 7-8 mice per group and are representative of 3 separate experiments. * P < .05, ** P < .01.
Effect of in vivo TAK1 gene silencing on systemic CIA hallmarks. DBA/1 mice were immunized with bovine type II collagen (CII) on day 0 and boosted on day 21. From arthritis onset, mice were weekly injected intravenously with 0.5 mg/kg of either siTAK1- (black bars) or siCT- (white bars) lipoplexes. Serum samples from the experiment shown in Figure 4B were collected overtime. (A) Serum levels of IFN-γ, TNF-α, IL-1β, and IL-6 were analyzed on day 44 by ELISA. (B-C) Anti-CII IgG1 (B) or IgG2a (C) isotype antibody levels were measured by ELISA in control- and TAK1- siRNA-treated mice. Results are expressed as arbitrary units, as described in “Anti–type II collagen assays.” (D-G) At euthanasia, splenocytes from the different groups were collected, and isolated cells were either not stimulated (NS) or stimulated with bCII antigen (20 μg/mL). Levels of TNF-α (D), IL-6 (E), IFN-γ (F), and IL-17A (G) were determined by ELISA after 24 hours of culture. (H) Splenic T cells were stimulated with increasing concentrations of bCII. As a positive control, splenocytes were stimulated with concanavalin A (5 μg/mL). Antigen-specific T-cell proliferation was quantified by the CellTiter-Glo luminescent cell viability assay 3 days later. Results are the mean ± SEM of 7-8 mice per group and are representative of 3 separate experiments. * P < .05, ** P < .01.
As high levels of circulating Abs directed against bCII accompany the development of CIA, the serum levels of bCII-specific IgG1 and IgG2a isotype Abs were measured during the course of disease. Levels of bCII-specific IgGs Abs were not modified after administration of either control or TAK1 siRNA-lipoplexes (Figure 5B-C). Although we cannot rule out that TAK1 does not affect B-cell responses in vivo, as TAK1 was silenced after arthritis induction, when anti-CII Ab responses are expected to already be established, these data suggest that the systemic RNAi-mediated TAK1 intervention did not dampen the ongoing B-cell response. Finally, to test whether impaired T-cell functions in siTAK1-treated mice lead to CIA inhibition, we first measured the levels of proinflammatory cytokines produced by spleen cells from CIA mice stimulated in vitro with bCII (Figure 5D-G). Mice treated weekly with siTAK1 lipoplexes showed significantly decreased TNF-α, IFN-γ, and IL-17A secretion (P < .05), compared with siCT-injected animals. However, when testing the effect of siRNA lipoplexes on CII-specific proliferative T-cell response, a comparable proliferation rate of splenocytes, from both siCT- and siTAK1-treated mice, was measured in response to increasing doses of bCII (Figure 5H), indicating that TAK1 siRNA administration during CIA development alters T-helper cytokine production without affecting their clonal expansion in response to bCII challenge.
In vivo siTAK1 lipoplex administration is associated with decreased Th1 and Th17 cells
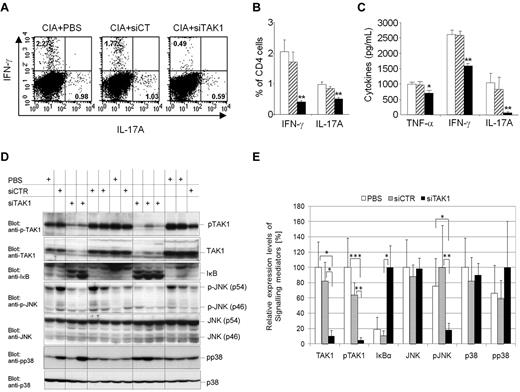
It has been previously reported that subsets of effector T cells that produce IFN-γ (Th1) or IL-17A (Th17) play an important role in mediating inflammation and development of mouse arthritis models. Moreover, the development of Th1 and Th17 cells is regulated by antigen presenting cell–derived cytokines.29,30 To examine whether decreased proinflammatory cytokine milieu, measured locally and systemically after injection of the siTAK1 lipoplexes, has an influence on the development of effector T helper (Th) cell subsets in CIA, we analyzed the frequency of pathogenic Th1 and Th17 cells in arthritic mice after treatment, focusing on the spleen, where the TAK1 gene silencing was optimal (Figure 6A-C). In the siTAK1-treated group, we found significantly reduced frequencies of 2- and 5-fold, respectively, of IL-17A– and IFN-γ–secreting CD4+ T cells in the spleen (Figure 6A-B), compared with controls (P < .01). These results were confirmed by ELISA analysis of TNF-α, IFN-γ, and IL-17A secretion levels from identical cultured spleen (Figure 6C) supernatants. Anti-inflammatory IL-4 and IL-5 cytokine secretions were only detectable at background levels in all of the experiments performed (data not shown).
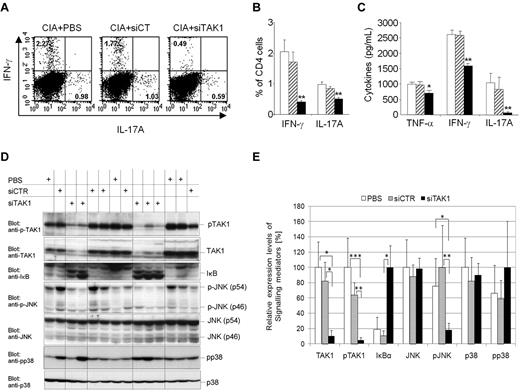
In vivo TAK1 gene silencing in arthritic mice interferes with downstream JNK and NF-κB signaling cascades and pro-inflammatory cytokines. DBA/1 mice were immunized with bovine type II collagen (CII) on day 0 and boosted on day 21. Mice were injected intravenously from arthritis onset (days 25 and 28) with 0.5 mg/kg of TAK-1 siRNA lipoplexes (siTAK1). Control groups were injected with either PBS or the nontargeting siRNA lipoplexes (siCT). Twenty-four hours after the last injection, spleen cells were isolated from siTAK1-, siCT-, or PBS-treated mice for subsequent experiments. (A-B) Cultured splenic cells were activated overnight with anti-CD3/CD28 Abs, and cells were stained with anti-CD4 fluorescein isothiocyanate together with anti–IL-17A phycoerythrin and anti–IFN-γ APC mAbs. Gating was performed on the basis of CD4+ cells. (A) Representative dot plots for the detection of IFN-γ- and IL-17A-expressing CD4+ T cells in the spleen by intracellular cytokine staining assay and FACS analysis. (B) Histograms indicate mean percentages of IL-17A (Th17)– and IFN-γ (Th1)–expressing CD4+ T cells in the spleen. (C) ELISA analyses of TNF-α, IFN-γ, and IL-17A secretion in cultured splenic cells activated overnight with anti-CD3/CD28 Abs. (D-E) Protein extracts were prepared from freshly isolated splenocytes. (D) Western blot analyses of TAK1 inhibition and downstream signaling cascades. (E) Relative expression levels of signaling mediators in arthritic mice were quantified as described in the Methods. The highest expression level of the indicated signaling mediator in each group (PBS, siCT, and siTAK1) was arbitrarily considered as 100%. Results in panels A-E are the mean ± SEM of 5-6 mice per group. * P < .05; ** P < .01; *** P < .001 versus controls.
In vivo TAK1 gene silencing in arthritic mice interferes with downstream JNK and NF-κB signaling cascades and pro-inflammatory cytokines. DBA/1 mice were immunized with bovine type II collagen (CII) on day 0 and boosted on day 21. Mice were injected intravenously from arthritis onset (days 25 and 28) with 0.5 mg/kg of TAK-1 siRNA lipoplexes (siTAK1). Control groups were injected with either PBS or the nontargeting siRNA lipoplexes (siCT). Twenty-four hours after the last injection, spleen cells were isolated from siTAK1-, siCT-, or PBS-treated mice for subsequent experiments. (A-B) Cultured splenic cells were activated overnight with anti-CD3/CD28 Abs, and cells were stained with anti-CD4 fluorescein isothiocyanate together with anti–IL-17A phycoerythrin and anti–IFN-γ APC mAbs. Gating was performed on the basis of CD4+ cells. (A) Representative dot plots for the detection of IFN-γ- and IL-17A-expressing CD4+ T cells in the spleen by intracellular cytokine staining assay and FACS analysis. (B) Histograms indicate mean percentages of IL-17A (Th17)– and IFN-γ (Th1)–expressing CD4+ T cells in the spleen. (C) ELISA analyses of TNF-α, IFN-γ, and IL-17A secretion in cultured splenic cells activated overnight with anti-CD3/CD28 Abs. (D-E) Protein extracts were prepared from freshly isolated splenocytes. (D) Western blot analyses of TAK1 inhibition and downstream signaling cascades. (E) Relative expression levels of signaling mediators in arthritic mice were quantified as described in the Methods. The highest expression level of the indicated signaling mediator in each group (PBS, siCT, and siTAK1) was arbitrarily considered as 100%. Results in panels A-E are the mean ± SEM of 5-6 mice per group. * P < .05; ** P < .01; *** P < .001 versus controls.
Interestingly, the percentage of CD4+CD25+FoxP3+ regulatory T cells (Tregs) remained unchanged among several tissues investigated from siTAK1-injected mice, compared with controls (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Indeed, no substantial variation of Tregs frequencies in the blood, spleen, liver, and DLNs of mice subjected to siRNA-dependent systemic TAK1 knockdown was evidenced by flow cytometry, compared with control groups. Taken together, these findings indicate that systemic RNAi-mediated TAK1 gene silencing leads to decreased abundance of Th1 and Th17 pathogenic cells, without increasing the frequency of Treg cells, nor inducing a bias toward a Th2 phenotype.
In vivo knockdown of TAK1 interferes with NF-κB and JNK signaling pathways
To address the involvement of TAK1 in the activation of downstream signaling cascades, splenocytes isolated from mice injected with either PBS, siCT, or siTAK1 lipoplexes were analyzed by Western blot for native and phosphorylated forms of TAK1 and for the downstream inflammatory NF-κB, JNK, and p38 signaling molecules (Figure 6D-E). The siRNA-dependent TAK1 silencing efficiently and significantly interfered with TAK1 expression (P < .05) and activation (P < .01), compared with siCT-treated mice, as assessed by 70% and 80% decreases of the native and phosphorylated forms of TAK1, respectively. In addition, TAK1 gene silencing dramatically affected the inflammatory NF-κB-signaling cascade, as evidenced by the expression levels of the protease-sensitive inhibitor IκBα. Upon phosphorylation mediated by upstream kinases, this inhibitor of the NFκB-pathway is rapidly turned over, initiating NF-κB–mediated gene activation. Here, systemic TAK1 knock down led to the accumulation of substantial amounts of IκBα (80% increase), reflecting the effective shut-off of inflammatory NF-κB–dependent signals. In contrast, in the presence of activated TAK1, resulting from the inflammatory arthritic environment, substantial levels of IκBα were barely monitored, indicative of an unrestricted activity of the NF-κB pathway in the spleen of arthritic mice (Figures 6D-E). Similarly, JNK pathway activation seemed to be severely attenuated by TAK1-dependent gene silencing as evidenced by the reduced phosphorylation of p54 and p46 JNK kinases, whereas total protein levels were unchanged in comparison with the PBS- or the nontargeting control siRNA-treated mice. Consistent with previous studies as well as our own in vitro data performed on the mouse monocytic cell line J774 challenged with TNF-α or LPS (Figure 1), p38 phosphorylation, however, was apparently not affected by TAK1 deficiency. In 3 of 5 mice subjected to siRNA-dependent TAK1-silencing, p38 signaling was active, indicating that, in splenocytes, p38 activation is mediated by a TAK1-independent pathway and that, therefore, p38 signaling in this organ seems not to be involved in the manifestation of the arthritic phenotype. Although TAK1 was reported to be critical for survival of hematopoietic cells,19 our multilineage analysis did not show significantly reduced numbers of leukocyte populations in the spleen, as assessed by flow cytometric analyses of phenotypic markers such as B220, CD4, CD8, GR-1, CD11b, and F4/80 between the siCT and siTAK1 lipoplex-injected groups (supplemental Figure 2A).
In vitro TAK1 gene silencing in myeloid cells attenuates generation of Th1 cells
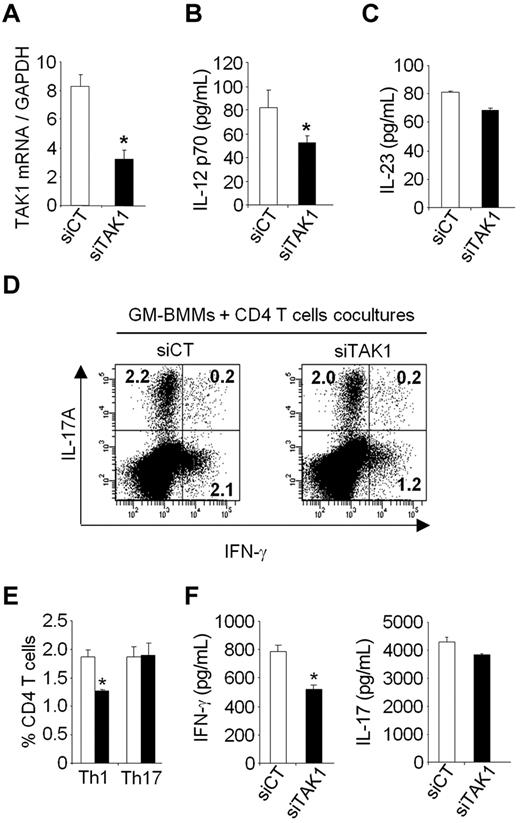
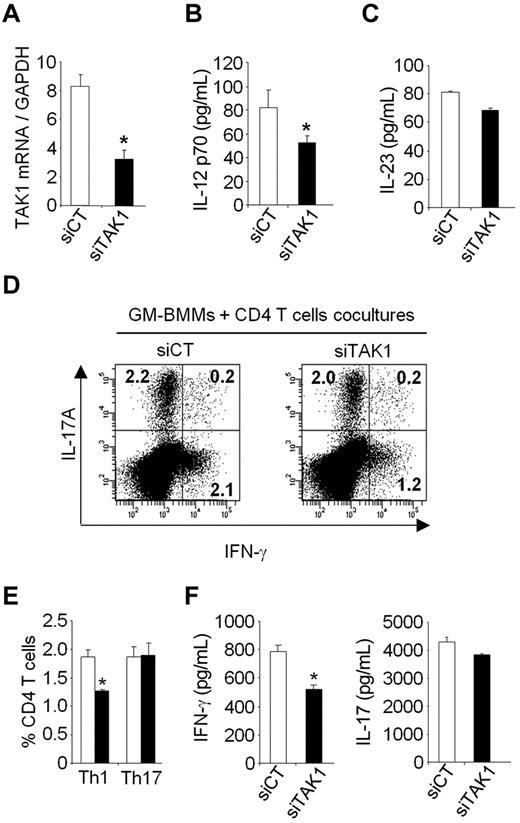
To investigate whether the in vivo effect of TAK1 silencing directly contributes to the decreased Th1 and Th17 cells, we examined in vitro the ability of TAK1 knockdown within myeloid cells to alter Th1 and Th17 cells production. To test this hypothesis, bone marrow–derived mouse macrophages grown in GM-CSF (GM-BMMs) were lipofected with siCT or siTAK1 and stimulated with TNF-α for 24 hours, then cocultured with CD4+ T cells purified from naïve mice in the presence of anti-CD3 antibody for 5 days (Figure 7). Compared with siCT-treated cells, 60% inhibition of TAK1 mRNA was associated with a significant reduction of IL-12p70 secretion in the supernatants of GM-BMMs transfected with TAK1 siRNA. The frequencies of IFN-γ-producing CD4+ T cells, as well as the IFN-γ secretion in supernatants, were decreased when CD4+ T cells were cocultured with siTAK1-lipofected GM-BMMs, compared with siCT-lipofected GM-BMMs. The proportion of IL-17A-secreting CD4+ T cells, as well as the secretion levels of IL-17A, were however unchanged between coculture conditions. These in vitro data suggest that the marked in vivo reduction of Th1-cell frequencies observed after siTAK1 systemic injections might be mediated, at least in part, by a direct effect of TAK1 silencing within myeloid cells. Together, our results suggest that inhibition of TAK1 gene expression and downstream signaling events in myeloid cells are important for negatively regulating Th1 responses.
TAK1 gene silencing results in the diminution of Th1 cells in vitro. Bone marrow–derived macrophages grown in GM-CSF (GM-BMMs) were generated as described in the Methods. GM-BMMs were first transiently transfected with siRNAs against TAK1 (siTAK1) or with a nontargeting control siRNA (siCT). After lipofection, the cells were stimulated with TNF (20 ng/mL) for 24 hours and then cultured with CD4+ T cells in the presence of anti-CD3 (1 μg/mL) for 5 days. (A) After lipofection, GM-BMMs stimulated with TNF were harvested and the expression of TAK1 was determined by qPCR. (B-C) IL-12 and IL-23 secretion in GM-BMM supernatants were measured by ELISA. (D) Flow cytometric analysis of Th1 and Th17 cells in CD4+ T cells cocultured with GM-BMM. Cells were stained for surface CD4 and intracellular IL-17A and IFN-γ. Representative dot plots were gated on CD4+ T cells. (E) Histograms indicate mean percentages of IL-17A and IFN-γ–producing CD4+ T cells. (F) IFN-γ and IL-17A production in the coculture of CD4+ T cells and GM-BMMs were measured by ELISA. Results are representative of 2 independent experiments performed in triplicate. *P < .05.
TAK1 gene silencing results in the diminution of Th1 cells in vitro. Bone marrow–derived macrophages grown in GM-CSF (GM-BMMs) were generated as described in the Methods. GM-BMMs were first transiently transfected with siRNAs against TAK1 (siTAK1) or with a nontargeting control siRNA (siCT). After lipofection, the cells were stimulated with TNF (20 ng/mL) for 24 hours and then cultured with CD4+ T cells in the presence of anti-CD3 (1 μg/mL) for 5 days. (A) After lipofection, GM-BMMs stimulated with TNF were harvested and the expression of TAK1 was determined by qPCR. (B-C) IL-12 and IL-23 secretion in GM-BMM supernatants were measured by ELISA. (D) Flow cytometric analysis of Th1 and Th17 cells in CD4+ T cells cocultured with GM-BMM. Cells were stained for surface CD4 and intracellular IL-17A and IFN-γ. Representative dot plots were gated on CD4+ T cells. (E) Histograms indicate mean percentages of IL-17A and IFN-γ–producing CD4+ T cells. (F) IFN-γ and IL-17A production in the coculture of CD4+ T cells and GM-BMMs were measured by ELISA. Results are representative of 2 independent experiments performed in triplicate. *P < .05.
Discussion
Targeting innate immunity signaling cascades in inflammation has become a major challenge for the drug design of inflammatory and autoimmune disorders. Among the key signaling components that are involved in acute and chronic inflammation, protein kinases represent attractive drug targets to modulate inflammatory responses. Several small-molecule kinase inhibitors have been designed and evaluated in inflammatory models and have even reached phase II clinical trials.31 Among possible targets, inhibition of p38 MAPK demonstrated good efficiencies in animal models32-34 and underwent clinical trials for the treatment of rheumatoid arthritis (RA) and psoriasis, but none of the compounds progressed to phase III because of toxicity concerns. Such side effects are thought to be due to the inhibition of p38 MAPK feedback control loops, leading to hyperactivation of TAK1 and JNK kinases.35 Other upstream protein kinases may be more advantageous for reducing both the synthesis of proinflammatory cytokines and their downstream pathways. Because of the special feature of TAK1 as an integrator of multiple proinflammatory signaling pathways,36 the range of efficiency on inflammation for TAK1 inhibition could be enhanced, in comparison to the inhibition of a single MAPK or NF-κB.
Due to the decisive role of TAK1 in innate, adaptive immune responses and in inflammation, TAK1 might provide a potent target for anti-inflammatory strategies in immune-mediated inflammatory disorders. However, no in vivo functional data exist to support such a hypothesis. We thus decided to determine the in vivo impact of TAK1 gene silencing on both joint inflammation and integrity using RNAi-engineered lipoplexes in a preclinical model of autoimmune arthritis. Indeed, in the context of degenerative joint disorders, such as RA, TAK1-mediated signal transduction has been reported to play an important role in the regulation of catabolic events and proinflammatory processes.37 Interestingly, MEK kinase (MEKK) 1 and 2 and TAK1 are the most abundant MAP3Ks in inflamed rheumatoid joints, as well as in IL-1–induced fibroblast-like RA synoviocytes (RA-FLSs) in vitro.38 Moreover, TLR2 and TLR4 that trigger TAK1 signaling have been shown to contribute to the progression of experimental arthritis.39 To address in vivo the effect of TAK1 silencing in CIA as a prototype model of RA, we took advantage of a simple, efficient RNAi-based lipoplex formulation that we previously developed.24 Although RNAi has emerged as a potent technology for silencing gene expression, the use of RNAi-based therapeutics has to overcome major obstacles, mainly effective delivery to target cells. We have previously demonstrated that the cationic liposome, RPR209120/DOPE, in combination with a carrier DNA, and siRNAs, provides high nucleic acid stability and effective lipofection rates at low amounts of formulated siRNAs.40 The present work provides direct evidence that, after intravenous administration, the siRNA lipoplexes are mainly taken up by cells of the MPS, namely circulating and tissue-resident CD11b+ and CD11c+ cells, but also incorporated, to a lesser extent, by T and B lymphocytes, as well as ECs. Importantly, a significant amount of RNAi-engineered MPS cells were also detected within the inflamed joints.
A single administration of target-specific siRNA lipoplexes triggered a clear knock down of TAK1 protein expression, as well as its phosphorylated active form, within myeloid cells of investigated tissues, assayed 1 week after injection. When delivered weekly over 3 weeks, TAK1 siRNA lipoplexes resulted in a similar level of silencing of TAK1 expression and activity in the spleen. As expected, TAK1 gene silencing was associated with a marked decrease in downstream inflammation-induced JNK and NF-κB activation. In accordance with previously reported data for primary RA synovial fibroblasts,37 we also show that TAK1 down-regulation does not affect p38 activation in vivo, nor in vitro, as evidenced by a mouse monocytic cell line transfected with siTAK1 after TNF-α or LPS challenges. Although we cannot exclude that the direct silencing of TAK1 within other cell types, including T and B lymphocytes, as well as ECs (Figure 2 and supplemental Figure 3), might be responsible for the pronounced inhibition of TAK1 down-stream signaling pathways, an indirect effect through TAK1-silenced, MPS-derived cytokines is more likely to contribute to the decreased TAK1 activation observed in the whole spleen. Importantly, the siTAK1 regimen was able to efficiently interfere with ongoing systemic and joint inflammation, as evidenced by decreased clinical arthritis features and proinflammatory cytokine production. Indeed, the systemic TAK1 blockade led to significantly impaired production of TNF-α, IFN-γ, and IL17A after a secondary stimulation of splenocytes with collagen or anti-CD3/CD28 mAbs, without interfering with their proliferative capacities. As these cytokines reflect the secretion pattern of the pathogenic Th1 and Th17 cells in CIA, we then determined whether the anti-TAK1 siRNA treatment of arthritic mice would impact these T effector populations. Indeed, our results show a decreased frequency of both IFN-γ- and IL17A-producing CD4+ T cells in siTAK1-injected mice, suggesting a novel aspect of TAK1 in immune-mediated inflammatory disorders. Interestingly, TAK1 gene silencing had no effect on Treg frequencies or on the production of Th2-type cytokines. Our biodistribution data, together with the clinical and biologic analyses of TAK1 silencing in the CIA model, emphasize the particular interest of developing strategies optimized to target in vivo cells from the MPS,41 as MPS-derived cytokines may contribute to forming the cytokine milieu for the generation and persistence of potentially arthritogenic autoimmune T cells.42,43 Although the mechanisms underlying the effects of TAK1 RNAi in vivo on the reduced pathogenic Th1- and Th17-cell frequencies have not been studied in detail, various cytokines produced by macrophages have been shown to contribute to disease pathogenesis and to promote T-effector cell differentiation and/or expansion. In vitro, we found that siTAK1-lipofected myeloid cells were able to decrease IFN-γ production when cocultured with CD4+ T cells, while Th17 cells were not altered. These findings underscore the role of the myeloid lineage in modulating the inflammatory environment and shaping Th1 cell responses through a TAK1-dependent cascade.
Finally, although RNAi-based therapies have already been entered into clinical trials for diseases, such as macular degeneration, hepatitis, AIDS, diabetes, and cancers,44 siRNA therapeutics have to deal with safety concerns. It is now well established that unmodified siRNAs can trigger off-target effects by activating the innate immune response, and therefore there is real potential for siRNA to elicit nonspecific therapeutic effects in a wide range of disease models. Although judicious chemical modifications have been engineered to stabilize the siRNA and minimize their immunostimulatory capacities, we have previously shown that no type I IFN response could be observed upon intravenous injection of 0.5 mg/kg of unmodified siRNA duplexes formulated with our delivery system.24,40,45 Different types of cells or tissues have been reported to be sensitive for TAK1 deletion and undergone apoptotic cell death.19 Here, we show that targeted organs of TAK1-siRNA lipoplex–injected mice exhibited similar numbers of mononuclear cells, indicating that TAK1 RNAi-mediated knockdown does not induce cell death.
In conclusion, the present study demonstrates that systemic administration of the RPR209120/DOPE cationic liposome-encapsulated TAK1 siRNA particles potently protects mice from arthritis progression, decreasing both systemic and local inflammatory features (ie, proinflammatory key cytokines and joint destruction. Our data suggest that the protective effect of TAK1 gene silencing in ongoing experimental arthritis is mainly mediated through the inhibition of TAK1 signaling pathways–induced inflammatory reactions, but also involves the inhibition of Th1 and Th17 cells, thereby impairing the loss of joint integrity in treated animals. Although RNAi-mediated gene therapy using nonviral delivery systems in RA might not be ready for clinical application, our findings confirm that our cationic liposome formulation represents a potent experimental tool for specific screening of potential novel drugs in preclinical models of autoimmune disorders. Moreover, targeting upstream kinases such as MAP3K that integrate many signaling events might efficiently steer the course of an immune response, representing a promising anti-inflammatory target for a large number of chronic inflammatory disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Philippe Alzieu for animal care, Chantal Ripoll for technical help from histology platform, all members of Inserm U844 for help during CIA experiments, and we are also grateful to Dr Danièle Noël, Pascale Louis-Plence, and Patrick Caroll for their constructive comments on the manuscript.
This work was supported by grants from Inserm, the European community (Autocure No. LSHB-CT-2006-018661), the University of Montpellier I, the French Society of Rheumatology, the Medical Research Foundation (FRM), and the Deutsche Forschungsgemeinschaft (SFB 599).
This publication reflects only the authors' views, and the European Community is not liable for any use that may be made of the information herein.
Authorship
Contribution: G.C. designed and performed experiments, analyzed data, and wrote the manuscript; A.H. designed and performed experiments, analyzed data, and participated in writing the manuscript; V.S., J.P. J.Z., G.R., N.Z., and J.J.K. contributed to experimental work and analyzed data; V.E. and D.S. generated essential reagents (the liposome formulation) and participated in the writing of the manuscript; S.W. and C.J. contributed to experimental work and participated in the writing of the manuscript; and F.A. and G.G. designed and supervised the overall research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gabriel Courties, Inserm U844, 80 rue Augustin Fliche, 34295 Montpellier, France; e-mail: gabriel.courties@inserm.fr.
References
Author notes
G.C. and V.S., contributed equally to this work as first authors.
F.A. and G.G. contributed equally to this work.