Abstract
T helper type 17 (Th17) cells have been characterized based on production of interleukin-17 (IL-17) and association with autoimmune diseases. We studied the role of Th17 cells in aplastic anemia (AA) by isolating Th17 cells from patients blood (n = 41) and bone marrow (BM) mononuclear cells (n = 7). The frequency and total number of CD3+CD4+IL-17–producing T cells were increased in AA patients at presentation compared with healthy controls (P = .0007 and .02, respectively) and correlated with disease activity. There was an inverse relationship between the numbers of Th17 cells and CD4+CD25highFoxP3+ regulatory T cells (Tregs) in the blood of AA patients. Concomitant with the classical Th1 response, we detected the presence of CD4+ and CD8+ IL-17-producing T cells in a mouse model of lymph node infusion–induced BM failure. Although anti–IL-17 treatment did not abrogate BM failure, early treatment with the anti–IL-17 antibody reduced the severity of BM failure with significantly higher platelet (P < .01) and total BM cell (P < .05) counts at day 10. Recipients that received anti-IL-17 treatment had significantly fewer Th1 cells (P < .01) and more Treg cells (P < .05) at day 10 after lymph node infusion. Th17 immune responses contribute to AA pathophysiology, especially at the early stage during disease progression.
Introduction
Th17 cells have been characterized recently in mice as a novel subset of CD4+ T cells that produce interleukin-17A (IL-17A), IL-17-F, and IL-22,1,2 and serve as immune effectors in various settings, including inflammation, infection, and autoimmunity.3,4 Th17 cells produce a large amount of IL-17A, a cytokine that coordinates tissue inflammation by inducing the expression of proinflammatory cytokines (such as IL-6 and tumor necrosis factor [TNF]), chemokines (such as KC, MCP-1, and MIP-2), and matrix metalloproteases that mediate tissue infiltration and tissue destruction.5 In mice, the differentiation program of Th17 cells from naive CD4+ T cells requires the activation of the transcription factor, orphan nuclear receptor RORγt,6 and the presence of IL-6 and transforming growth factor-β (TGF-β).7,8 In humans, Th17 differentiation is under the control of IL-1β, IL-6, and IL-23.9,10 Several studies have reported the association of IL-17 with inflammatory disorders, such as rheumatoid arthritis, asthma, multiple sclerosis, and lupus,11 as well as hematologic disorders, such as myelodysplastic syndrome12,13 and acute myeloid leukemia.14
Aplastic anemia (AA), a disease characterized by peripheral blood pancytopenia and bone marrow (BM) hypoplasia,15 is an immune-mediated disorder in most cases with active destruction of hematopoietic cells by effector T lymphocytes.16 Recovery of autologous hematopoiesis in patients who failed to engraft after conditioning and stem cell transplantation,17 and responsiveness of patients to immunosuppressive therapies,18 provided powerful evidence for the pivotal role of the immune system in the disease pathophysiology. Immoderate production of interferon-γ (IFN-γ), TNF-α, and IL-2 from patients' T cells suggests that the hematopoietic cells are destroyed through a Th1 response,19-21 as illustrated by the up-regulation of the transcription factor T-bet in patient T cells.22 The description of nonrandom skewing of the Vβ chain families of the T-cell receptor in patient peripheral blood (PB) revealed that expanded oligoclonal or monoclonal specific Vβ subfamilies selectively induced apoptosis of hematopoietic progenitor cells.23 Regulatory T cells (Tregs), which control and suppress autoreactive T cells, are decreased at disease presentation in almost all patients.24
We have developed murine models for immune-mediated BM failure by the infusion of allogeneic lymph node (LN) cells into sublethally irradiated recipients for which treatment with limited number of Treg cells,25 or anti–IFN-γ and anti–TNF-α antibodies26 effectively mitigated BM destruction. Using T-bet-deficient LN cells as effectors, we recently found that lack of Th1 immune response resulting from T-bet deficiency significantly abrogated the immune responses, but recipient mice still experienced mild BM destruction.27 Because Th17-mediated immune responses have been reported in autoimmune disorders, we hypothesized that Th17 cells could contribute to the development of BM failure in mice, as in some AA patients.28 However, a recent report showed a very limited role of Th17 cells in AA patients,29 whereas other studies revealed reciprocal developmental pathways for the generation of pathogenic effector Th17 and Treg cells.8,30 Here we examined the role of Th17 immune responses in AA by assessing Th17 and Treg cells in the presence in AA patients before and after immunosuppressive therapy and by testing the preventive/therapeutic effects of anti–IL-17 antibody in abrogating BM destruction in our mouse model. Results from this study suggest that Th17 immune response plays an important role in immune-mediated BM failure.
Methods
Patient information
Heparinized PB and/or BM samples were collected from 41 patients (age range, 18-82 years) with acquired AA after informed consent in accordance with approved protocols by the Institutional Review Board of the National Heart, Lung, and Blood Institute and the Declaration of Helsinki. The diagnosis of AA was based on the criteria of the International Agranulocytosis and AA Study. Blood samples from 10 healthy volunteers (age range, 18-60 years) were used as controls. All patients and controls had normal BM cytogenetics. For young adults (younger than 40 years), chromosomes were assayed after in vitro exposure of lymphocytes to diepoxybutane and to mitomycin C to exclude Fanconi anemia. All patients were screened for telomerase mutation and tested for paroxysmal nocturnal hemoglobinuria by flow cytometry (positive in case of absence of glycosylphosphatidylinositol-anchored proteins on > 1% of neutrophils or red cells). Patients were divided into 3 groups: (1) de novo: PB (n = 21) and BM (n = 7) samples were obtained from patients at time of diagnosis before any treatment; (2) complete remission (CR): samples were obtained from 13 patients in long-term CR with normal complete blood counts without further treatment; and (3) poor responders (PR): samples were taken from 16 patients who had responded poorly to treatment. Clinical information is summarized in Table 1.
Mice and cell preparation
Inbred C57BL/6J (B6) and hybrid (B6 × BALB/cBy)F1 (CByB6F1) mice were obtained from The Jackson Laboratory and were bred and maintained in the National Institutes of Health animal facility under standard care and nutrition. Mice were used at 2 to 6 months of age and were sex-matched between donors and recipients in each experiment. All animal studies were approved by the National Heart, Lung, and Blood Institute's Animal Care and Use Committee. Blood was obtained through retro-orbital sinus bleeding. Inguinal, brachial, and axillary LNs were obtained from B6 mice, homogenized using a cell grinder, washed in Iscove modified Dulbecco medium, filtered through 90-μm nylon mesh to obtain single-cell suspension, and counted using a ViCell counter (Coulter Cooperation). BM cells were extracted from bilateral femurs and tibiae.
Induction of BM failure
LN cells from B6 donors were infused into CByB6F1 mice at 5 × 106 cells per recipient to induce BM failure as previously described.25,26 All recipient mice received a sublethal dose of 5 Gy total body irradiation (TBI) from a Shepherd Mark 1 137cesium γ irradiator (J. L. Shepherd) 6 hours before cell infusion. In each experiment, mice that received 5 Gy TBI-only without LN cell infusion and/or mice received no treatment were used as controls. Animals were bled at 7, 10, 15, and 19 days after LN cell infusion for complete blood counts (CBCs) using a Hemavet 950 analyzer (Drew Scientific) and were then killed to extract BM cells from tibiae and femurs. Sternebrae were collected for various analyses as specified in each experiment.
Immunohistochemistry and confocal microscopy
BM was obtained from a biopsy core, extracted from the posterior iliac crest of AA patients. BM, encased in bone, was lightly fixed in paraformaldehyde, and then permeabilized in 0.05% Triton X-100 (Sigma-Aldrich) and stained as previously described.31 BM whole-mount specimens were incubated with AB blood type serum to block nonspecific binding. The samples were incubated with the anti-IL-17 primary antibodies (eBioscience) and then with fluorescein isothiocyanate-labeled secondary antibodies. Additional stains used 4,6-diamidino-2-phenylindole (Invitrogen) for nuclei. Samples were washed 3 times with phosphate-buffered saline between each staining and were subjected to confocal microscopy within a few hours. Images were acquired by confocal laser scanning microscopy with the Zeiss LSM 710 systems (Carl Zeiss MicroImaging).
IL-17 depletion in vivo
To neutralize IL-17, 100 μg of antimouse IL-17 monoclonal antibody (clone 50104, R&D Systems) was resuspended in phosphate-buffered saline and then injected intraperitoneally into each mouse every 2 days for a total of 4 injections starting from day −3 (early treatment: days −3, −1, 1, and 3) or day 7 (late treatment: days 7, 9, 11, and 13) of LN cell infusion. Mice that received TBI-only and TBI plus B6 LN cell infusion without anti–IL-17 antibody injection were used as controls. Recipients were killed at day 14 after LN cell infusion for CBC and BM cell composition analysis. Blood plasma samples were collected for cytokine analysis as detailed in “Flow cytometry and intracellular cytokine staining.”
Flow cytometry and intracellular cytokine staining
Human PB mononuclear cells (PBMCs) and BM mononuclear cells (BMMCs) were separated by density gradient centrifugation with lymphocytes separation medium (Organon). Cells were washed, resuspended in fixation/permeabilization solution (BD Biosciences), and stained for intracellular antigens after the manufacturer's instructions. For the detection of Th1/Tc1 and Th17 cells, PBMCs were incubated for 6 hours with 50 ng/mL phorbol myristate acetate (PMA) and 750 ng/mL ionomycin in the presence of monensin (eBioscience) in tissue culture incubator at 37°C. For mouse samples, blood and BM cells were first incubated with Gey solution for 10 minutes on ice to lyse red blood cells and were then washed and stained with various antibody mixtures. The same procedures were followed for intracellular staining.
Monoclonal antibodies for human CD3 (clone SK7), CD4 (clone RPA-T4), CD8 (clone SK1), and IFN-γ (clone B27) were from BD Biosciences, whereas monoclonal antibodies for human CD25 (clone BC96), FOXP3 (clone PCH101), and IL-17 (clone eBio64DEC17) were from eBioscience. Similarly, monoclonal antibodies for mouse CD3 (clone 145-2C11), CD4 (clone GK 1.5), CD8 (clone 53-6.72), and IFN-γ (clone XMG1.2) were from BD Biosciences, whereas rat anti–mouse IL-17A (clone eBio17B7), CD25 (clone PC61.5), and FoxP3 (clone FJK-16s) antibodies were from eBioscience. All human and mouse antibodies were conjugated to either fluorescein isothiocyanate, phycoerythrin, phycoerythrin–cyanin 5, allophycocyanin, allophycocyanin-Cy7, or Amcyan. Stained cells were analyzed by a BD LSR II flow cytometer (BD Biosciences), and data were analyzed by a FlowJo software Version 7.6 (TreeStar).
Pathology
Sternebrae were fixed in 10% neutral buffered formalin and embodied, sectioned, and stained with hematoxylin and eosin (Histoserve). Slides were viewed using an Olympus IX50 microscope (Optical Elements), and photographic images of BM morphology were captured at 4× and 10× magnification using a SPOT INSIGHT camera with the SPOT Version 4.0.8 software.
Enzyme-linked immunosorbent assay
Blood was collected by orbital sinus bleeding into tubes containing 10 μL 0.5M ethylenediaminetetraacetic acid and centrifuged at 1000g for 10 minutes. Plasma was removed and stored at −20°C. Plasma cytokine concentrations were measured using the Multi-Analyte ELISArray Kits (SABiosciences) as previously described.32
Statistical methods
Summary statistics, such as percentages, medians, means, SDs, and 95% confidence intervals, were used to describe the patients' baseline characteristics and mice samples. Pairwise comparisons between groups were conducted using Student t tests and further verified by the Wilcoxon tests based on ranks. The t test P values were reported for all the pairwise comparisons and compared with the 5% statistical significance level. When multiple groups are present, the analysis of variance models and the analysis of variance F tests were used to evaluate the overall differences among these groups. Multiple linear regression models were used when continuous independent variables or covariates were present. Numerical results were computed using the S-PLUS software package (TIBCO). Significance level was set at 5% for all the statistical tests.
Results
Th17 cells in the BM of AA patients
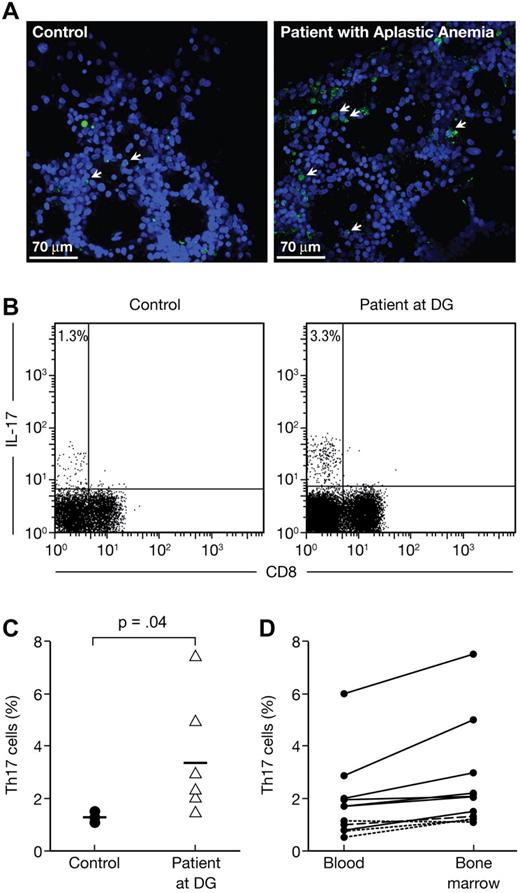
To define the role of Th17 cells in the development of AA, we first stained BM samples from 2 AA patients (one moderate and one severe AA) and analyzed the images using a confocal microscope. Patient samples showed a notable decrease in BM cellularity with an increased presence of IL-17+ cells compared with samples from controls (Figure 1A). We then isolated and stimulated BMMCs with PMA and ionomycin in the presence of monensin from patients (n = 7) and controls (n = 4). Because of reduced CD4 expression after stimulation in human samples, we focused on CD3+CD8− IL-17+ T cells as Th17 cells33 and identified a population of CD3+CD8− IL-17+ T cells (Th17 cells) in patient samples (Figure 1B), which was significantly (P = .04) larger than in healthy controls (3.3% ± 2.1% vs 1.3% ± 0.2%, Figure 1C). We also assessed the presence of Th17 cells in the PBMCs and found that the increased expression of intracellular IL-17 in BMMCs and PBMCs was highly correlated (r = 0.939, P < .01) among patients but not among controls (Figure 1D).
Increased frequencies of Th17 cells in the BM of aplastic patients (AA) at diagnosis. BM from 2 patients (1 moderate and 1 severe AA) and 1 healthy control were stained with 4,6-diamidino-2-phenylindole and anti-IL-17 and then analyzed using confocal microscopy. BMMCs from healthy controls (n = 4) and AA patients at diagnosis (n = 7) were stained with anti-CD3 and anti-CD8 antibodies followed by intracellular staining with anti-IL-17 antibody. Cells were analyzed by flow cytometry after stimulation for 6 hours with PMA and ionomycin in the presence of monensin. (A) Representative confocal analysis of the BM from healthy control (top panel) and AA patient (bottom panel). (B) Representative flow cytometric analysis of IL-17 expressions in CD8− T-cell subsets (Th17 cells) in healthy control (left panel) as well as in AA at diagnosis (right panel). The mean value of each group is indicated. (C) Frequencies of Th17 cells in healthy controls (●, n = 4) and in AA at diagnosis (▵, n = 7). The mean value of each group is represented (solid line). (D) Correlation between the frequencies of PBMC and BMMC Th17 cells in AA at diagnosis (n = 7). Normal donors (4 healthy controls) are represented by a dashed line.
Increased frequencies of Th17 cells in the BM of aplastic patients (AA) at diagnosis. BM from 2 patients (1 moderate and 1 severe AA) and 1 healthy control were stained with 4,6-diamidino-2-phenylindole and anti-IL-17 and then analyzed using confocal microscopy. BMMCs from healthy controls (n = 4) and AA patients at diagnosis (n = 7) were stained with anti-CD3 and anti-CD8 antibodies followed by intracellular staining with anti-IL-17 antibody. Cells were analyzed by flow cytometry after stimulation for 6 hours with PMA and ionomycin in the presence of monensin. (A) Representative confocal analysis of the BM from healthy control (top panel) and AA patient (bottom panel). (B) Representative flow cytometric analysis of IL-17 expressions in CD8− T-cell subsets (Th17 cells) in healthy control (left panel) as well as in AA at diagnosis (right panel). The mean value of each group is indicated. (C) Frequencies of Th17 cells in healthy controls (●, n = 4) and in AA at diagnosis (▵, n = 7). The mean value of each group is represented (solid line). (D) Correlation between the frequencies of PBMC and BMMC Th17 cells in AA at diagnosis (n = 7). Normal donors (4 healthy controls) are represented by a dashed line.
Association of Th17 cells with disease activity
In AA, IFN-γ is a marker of the Th1/cytotoxic T cells (Tc1) immune response that has been directly implicated in hematopoietic cell destruction.16 We assessed Th1/Tc1 and Th17 responses in our cohort of AA patients (21 at diagnosis, 13 in CR, and 16 in PR) along with 10 healthy controls. The frequency and total number of IFN-γ+ cells showed a strong association with disease activity in our cohort of AA patients, similar to those reported previously.21,22 Among PR patients, we observed a significant increase in the frequency, but not in absolute numbers, of IFN-γ+ cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
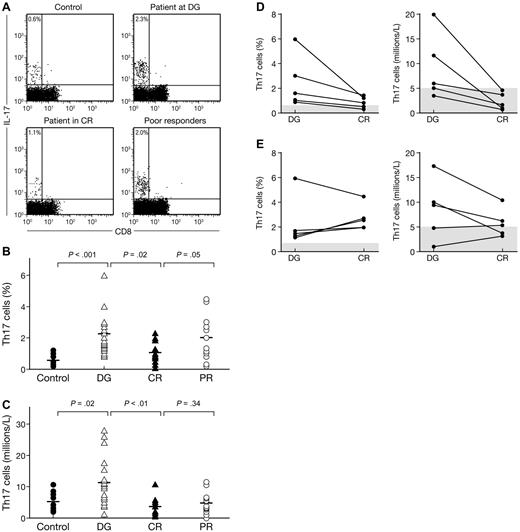
We further assessed Th17 contribution to AA. The percentage of CD3+CD8−IL-17+ T cells (Th17 cells) was measured by flow cytometry after stimulation with PMA and ionomycin, and was detectable in all groups of patients (Figure 2A). The percentage of Th17 cells was significantly higher (P < .001) in the 21 newly diagnosed AA patients (2.3% ± 1.5%) than in the 10 healthy controls (0.6% ± 0.3%; Figure 2B), resulting in a significantly higher (P < .01) Th17 T-cell concentration in patients (11.2 ± 7.6 × 106/L) than in controls (5.1 ± 2.9 × 106/L, Figure 2C). Patients with moderate AA had higher proportion and higher circulating number of Th17 T cells (3% ± 1.7% and 15.9 ± 9.4 × 106/L) than did patients with severe AA (2.2% ± 1.4% and 10 ± 7.1 × 106/L), but these differences were not statistically significant. We did not find any difference in the frequency and number of Th17 cells according to the paroxysmal nocturnal hemoglobinuria status. Among the 16 patients who received treatment, 11 responded at 6 months (69%; 6 CR and 5 partial remissions). The initial pretreatment proportion and absolute number of Th17 cells were, respectively, 2.5% ± 1.6% and 11.2 ± 7.2 × 106/L in responders compared with 1.7% ± 0.2% and 8.2 ± 6.5 × 106/L in nonresponders (not statistically significant). We then compared the proportion and absolute number of Th17 T cells in patients at diagnosis with patients in CR (Figure 2B-C). Patients in CR had lower proportion and fewer absolute number of Th17 cells than patients at diagnosis (0.6% ± 0.3% and 3.7 ± 2.9 × 106/L vs 1.1% ± 0.7% and 5.1 ± 2.9 × 106/L; P = .02 and P < .01, respectively). Proportions and absolute numbers in PR patients are indicated (Figure 2B-C).
Frequencies and numbers of Th17 cells in aplastic patients (AA) correlate with disease activity. PBMCs from healthy controls (n = 10) and AA patients (AA) at diagnosis (n = 18), in CR (n = 12) or in PRs (n = 12) were stained with anti-CD3 and anti-CD8 antibodies followed by intracellular IL-17 antibody and examined by flow cytometry after stimulation for 6 hours with PMA and ionomycin. (A) Representative flow cytometric analysis of IL-17 expression in CD8− T-cell subsets (Th17 cells) in healthy control (left panel) as well as in AA at diagnosis (left middle panel), in CR (right middle panel), or in PR (right panel). The mean value of each group is indicated. (B) Frequencies of Th17 cells in healthy controls (●) and in AA at diagnosis (▵), in CR (▴) and in PR (○). The mean value of each group is represented (solid line). (C) Absolute numbers of Th17 cells in healthy controls (●) and in AA at diagnosis (▵), in CR (▴) and in PR (○). The mean value of each group is represented (solid line). (D) Frequencies (left panel) and absolute numbers (right panel) of Th17 cells in patients who obtained a CR. Normal values are indicated by gray bars. (E) Frequencies (left panel) and absolute numbers (right panel) of Th17 cells in patients who were PRs. Normal values are indicated by gray bars.
Frequencies and numbers of Th17 cells in aplastic patients (AA) correlate with disease activity. PBMCs from healthy controls (n = 10) and AA patients (AA) at diagnosis (n = 18), in CR (n = 12) or in PRs (n = 12) were stained with anti-CD3 and anti-CD8 antibodies followed by intracellular IL-17 antibody and examined by flow cytometry after stimulation for 6 hours with PMA and ionomycin. (A) Representative flow cytometric analysis of IL-17 expression in CD8− T-cell subsets (Th17 cells) in healthy control (left panel) as well as in AA at diagnosis (left middle panel), in CR (right middle panel), or in PR (right panel). The mean value of each group is indicated. (B) Frequencies of Th17 cells in healthy controls (●) and in AA at diagnosis (▵), in CR (▴) and in PR (○). The mean value of each group is represented (solid line). (C) Absolute numbers of Th17 cells in healthy controls (●) and in AA at diagnosis (▵), in CR (▴) and in PR (○). The mean value of each group is represented (solid line). (D) Frequencies (left panel) and absolute numbers (right panel) of Th17 cells in patients who obtained a CR. Normal values are indicated by gray bars. (E) Frequencies (left panel) and absolute numbers (right panel) of Th17 cells in patients who were PRs. Normal values are indicated by gray bars.
We measured Th17 cell presence in 10 patients (5 in CR and 5 as PR) before and after treatment. All 5 patients in CR had marked decreases in the proportion and absolute number of Th17 cells after treatment (Figure 2D). In the 5 PR patients, 4 of them showed an increased proportion of Th17 T cells (Figure 2E left panel). However, 3 of the 5 PR patients had reduced absolute number of Th17 T cells after treatment (Figure 2E right panel).
Inverse relationship between Th17 and Treg cells in patients with AA
Treg cells play a fundamental role in the maintenance of immune tolerance to prevent autoimmune diseases. A reciprocal developmental pathway for the generation of pathogenic Th17 cells and protective Treg has been reported.8 Treg cells were defined in the current study by the cell surface phenotype CD4+CD25high and the intracellular marker FOXP3.32 We found that Treg cell percentage was decreased in AA patients at diagnosis, as we have reported previously.24 We analyzed the correlation between the proportion of natural Treg cells and Th17 cells comparing patients at diagnosis, in CR and in PR (Figure 3). Using a linear regression model, we found that healthy controls had higher proportion of Treg cells and lower proportion of Th17 cells than did patients at diagnosis, also showing different distribution patterns (P < .001). Using the same model, we found also different distribution patterns regarding patients at diagnosis and CR patients (P < .01). Moreover, this difference diminished but was still significant comparing CR patients with PRs (P = .02). These data suggest an inverse relationship between Th17 and Treg cells during disease activity in patients with AA.
Expansion of the Th17 cell population correlates with the depletion of natural regulatory T cells. PBMCs from healthy controls (n = 10) and AA patients at diagnosis (n = 21), in CR (n = 12), or in PRs (n = 15) were stained with anti-CD3, anti-CD4, anti-CD25 antibodies followed by intracellular FOXP3 antibody and examined by flow cytometry. Correlation between CD4+CD25highFOXP3+ T cells (natural regulatory T cells) and CD8− IL-17+ T cells (Th17 cells) was studied according to the stage of the disease. Frequencies of natural regulatory T cells and Th17 cells are indicated in healthy controls (●) and in AA at diagnosis (▵), in complete remission (▴), and in PR AA (○).
Expansion of the Th17 cell population correlates with the depletion of natural regulatory T cells. PBMCs from healthy controls (n = 10) and AA patients at diagnosis (n = 21), in CR (n = 12), or in PRs (n = 15) were stained with anti-CD3, anti-CD4, anti-CD25 antibodies followed by intracellular FOXP3 antibody and examined by flow cytometry. Correlation between CD4+CD25highFOXP3+ T cells (natural regulatory T cells) and CD8− IL-17+ T cells (Th17 cells) was studied according to the stage of the disease. Frequencies of natural regulatory T cells and Th17 cells are indicated in healthy controls (●) and in AA at diagnosis (▵), in complete remission (▴), and in PR AA (○).
Dynamics of Th1 and Th17 immune responses in a mouse model of immune-mediated BM failure
To further examine the role of Th17 immune responses in immune-mediated BM failure, we analyzed intracellular IL-17A expression in BM CD4+ and CD8+ T cells as a representative marker for the Th17 immune response in a mouse model of LN cell infusion-induced BM failure.25,26,34 At 10 days after LN injection, the proportion of IL-17A positive CD4+ cells in B6 LN-cell–infused animals (15.0% ± 1.1%) was significantly higher (P < .01) than that in TBI-only controls (6.5% ± 1.3%). Almost no IL-17A positive CD4+ cells were detectable in untreated animals (Figure 4A upper panels). Similarly, the proportion of IL-17A+ CD8+ T cells was also significantly higher (16% ± 2.3%, P < .01) in B6 LN-cell–infused mice than in TBI-only controls (6.0% ± 1.3%). Again, this population was barely visible in untreated animals (Figure 4A lower panels). This difference along with the overall T-cell expansion resulted in a 32-fold increase in total IL-17A+ CD4+ T cells and a 28-fold increase in total IL-17A+ CD8+ T cells in the BM of B6 LN-cell-infused animals than in TBI-only controls (Figure 4B). We also noted that the majority of IL-17A+ T cells were Th17 specific, and only minor fractions of CD4+ and CD8+ T cells were IL-17A+IFN-γ+ double positive (Figure 4C).
Induction of BM failure with T-cell expansion in a mouse model. CByB6F1 mice (n = 5) were irradiated (5 Gy TBI) and injected with 5 × 106 B6 LN cells and then bled and killed 10 days later along with untreated control mice (irradiated non–LN-injected, TBI-only, n = 5; non-irradiated non–LN-injected, untreated, n = 5). CBCs were performed using a Hemavet analyzer. BM cells were stained with anti-CD4 and anti-CD8 antibodies and examined by flow cytometry. Experiments are represented as mean ± SD. P less than .05 is considered statistically significant. (A) Representative flow cytometric analysis of IL-17 expressions in CD4+ (top panel) and CD8+ (bottom panel) T-cell subsets (Th17 cells) in B6LN, TBI-only, and untreated mice. The mean value of each group is indicated. (B) Absolute numbers of CD4+ (top panel) and CD8+ (bottom panel) T-cell subsets (Th17 cells) in B6 LN, TBI-only, and untreated mice. Experiments are represented as mean ± SD. (C) Representative flow cytometric analysis of IL-17 and IFN-γ coexpression in CD4+ (top panel) and CD8+ (bottom panel) T-cell subsets (Th17 cells) in B6 LN mice. The mean value of each group is indicated.
Induction of BM failure with T-cell expansion in a mouse model. CByB6F1 mice (n = 5) were irradiated (5 Gy TBI) and injected with 5 × 106 B6 LN cells and then bled and killed 10 days later along with untreated control mice (irradiated non–LN-injected, TBI-only, n = 5; non-irradiated non–LN-injected, untreated, n = 5). CBCs were performed using a Hemavet analyzer. BM cells were stained with anti-CD4 and anti-CD8 antibodies and examined by flow cytometry. Experiments are represented as mean ± SD. P less than .05 is considered statistically significant. (A) Representative flow cytometric analysis of IL-17 expressions in CD4+ (top panel) and CD8+ (bottom panel) T-cell subsets (Th17 cells) in B6LN, TBI-only, and untreated mice. The mean value of each group is indicated. (B) Absolute numbers of CD4+ (top panel) and CD8+ (bottom panel) T-cell subsets (Th17 cells) in B6 LN, TBI-only, and untreated mice. Experiments are represented as mean ± SD. (C) Representative flow cytometric analysis of IL-17 and IFN-γ coexpression in CD4+ (top panel) and CD8+ (bottom panel) T-cell subsets (Th17 cells) in B6 LN mice. The mean value of each group is indicated.
During the induction of BM failure in our mouse model, LN donor T-cell expansion and activation are associated with a Th1 response, with increased IFN-γ and TNF-α production.26,34 We decided to analyze the kinetics of both Th1 and Th17 immune responses in the BM on days 7, 10, 15, and 19 after LN donor infusion by after the intracellular expression of both IL-17 and IFN-γ in CD4+ and CD8+ T-cell subsets. As described previously, we observed marked expansion of both CD4+ and CD8+ T cells in LN-cell–infused animals at day 15 (Figure 5A). BM failure was already apparent at day 7 after LN injection and was complete in all animals at day 19. The percentage of CD4+IFN-γ+ (Th1) cells as well as CD8+IFN-γ+ cells increased over time. As shown in Figure 5B, at day 15, 35% of CD4+ and CD8+ subsets were positive for IFN-γ compared with less than 10% in both subsets in TBI-only controls. At the same time, the frequencies of CD4+ IL-17+ (Th17) and CD8+ IL-17+ cells increased but to a less extent. At day 15, CD4+ and CD8+ cells positive for IL-17 were at approximately 20% in LN-cell–infused animals compared with 7% in TBI-only control animals (Figure 5B). In absolute number, CD4+ and CD8+ IFN-γ+ and IL-17+ cells increased with time after LN injections until day 15 and then decreased sharply when the BM failure was complete (Figure 5C). Moreover, the absolute number of IL-17–producing cells was consistently between 2- and 5-fold lower at each time point analyzed after LN injection. Taken together, these results indicate that the frequency and absolute number of IL-17–producing cells increase with time in our mouse model, but the level of Th17 cell increase is much lower than the level of increase in Th1 cells.
Increase frequencies and numbers of Th17 cells during the induction of BM failure in the mouse. CByB6F1 mice were irradiated (5 Gy TBI) and injected with 5 × 106 B6 LN cells. Three B6 LN mice as well as 3 controls (irradiated non–LN-injected, TBI-only) were killed at days 7, 10, 15, and 19 after LN injection. At each time point, BMMCs were stained with anti-CD4, anti-CD8 followed by intracellular IL-17, and IFN-γ antibody and examined by flow cytometry. Experiments are represented as mean ± SD. (A) Absolute numbers of CD4+ (top panel) and CD8+ (bottom panel) cells in the BM of B6 LN and control mice (TBI-only) at the indicated days after LN injection. (B) Representative flow cytometric analysis at day 15 of IFN-γ (top panels) and IL-17 (bottom panels) expression in CD4+ (left panels) and CD8+ (right panel) subsets, in TBI-only and LN B6 mice. The mean value of each group is indicated. (C) Absolute numbers of IL-17+ and IFN-γ+ cells in CD4+ (top panel) and CD8+ (bottom panel) compartment at the indicated days. Experiments are represented as mean plus or minus SD.
Increase frequencies and numbers of Th17 cells during the induction of BM failure in the mouse. CByB6F1 mice were irradiated (5 Gy TBI) and injected with 5 × 106 B6 LN cells. Three B6 LN mice as well as 3 controls (irradiated non–LN-injected, TBI-only) were killed at days 7, 10, 15, and 19 after LN injection. At each time point, BMMCs were stained with anti-CD4, anti-CD8 followed by intracellular IL-17, and IFN-γ antibody and examined by flow cytometry. Experiments are represented as mean ± SD. (A) Absolute numbers of CD4+ (top panel) and CD8+ (bottom panel) cells in the BM of B6 LN and control mice (TBI-only) at the indicated days after LN injection. (B) Representative flow cytometric analysis at day 15 of IFN-γ (top panels) and IL-17 (bottom panels) expression in CD4+ (left panels) and CD8+ (right panel) subsets, in TBI-only and LN B6 mice. The mean value of each group is indicated. (C) Absolute numbers of IL-17+ and IFN-γ+ cells in CD4+ (top panel) and CD8+ (bottom panel) compartment at the indicated days. Experiments are represented as mean plus or minus SD.
Reduced severity of BM failure after inhibition of Th17 cells
Modulation of Th17 immune responses has been effective in several studies using neutralizing anti–IL-17 antibodies. Indeed, a single injection of anti–IL-17 antibody prevented inflammation and bone erosion and reduced Th17 cell number in experimental rheumatoid arthritis,35 and multiple doses of anti–IL-17 dramatically reduced inflammatory lesions and neurologic symptoms in experimental autoimmune encephalomyelitis.36 To directly assess the role of Th17 cells in our model of BM failure, we depleted Th17 cells using 2 different schedules of anti–IL-17 injections (Figure 6A). Early treatment with anti–IL-17 reduced the severity of BM failure at day 10, as illustrated by the cell counts in BM as well as in blood. Total BM cells and neutrophils, red blood cells, and platelets were all higher in the group receiving early injection of anti–IL-17, reaching statistical significance for platelets (P = .01) and total BM cell number (P = .02; Figure 6B). Histologic examination of BM from BM failure mice that received early anti–IL-17 treatment detected increased BM cellularity and reduced areas of hemorrhage compared with those from BM failure mice without anti–IL-17 treatment (Figure 6C). Delayed anti–IL-17 antibody treatment to day 7 after LN cell infusion had much less effect, as histologic analysis (correlated with blood count) revealed severe loss of BM cellularity in these animals (Figure 6B-C). We also did not find any difference at day 16 between mice that received early anti–IL-17 treatment and mice that injected with LN without anti–IL-17 treatment (data not shown), as both groups had BM hypocellularity, consistent with the notion that early anti–IL-17 treatment is capable of delaying but not abrogating the onset of the disease. Thus, Th17 immune responses appear to play a role with Th1 immune response in the development of BM failure.
Early inhibition of Th17 cells with neutralizing anti–IL-17 antibody delays the induction of BM failure in mice. Anti–IL-17 was administered at 100 μg intraperitoneally on alternating days (4 total injections) to the BM failure induction mouse model. Early anti–IL-17 neutralization consisted of 100 μg intraperitoneal injections on alternating days from 3 days before the LN injection to 3 days after (n = 5). Controls consisted of LN-injected mice (n = 3) and mice only irradiated (n = 2). Late anti–IL-17 neutralization consisted of 100 μg intraperitoneal injections on alternating days from 7 to 13 days after LN injections (n = 5). Controls consisted of LN-injected mice (n = 3) and mice only irradiated (n = 2). CBCs were performed using a Hemavet analyzer. Sternebrae were sectioned, hematoxylin and eosin–stained, and photographed. Experiments are represented as mean ± SD. P <.05 is considered statistically significant. (A) Schema of the anti–IL-17 neutralization experiment. (B) CBCs and total BM cells in TBI-only, B6 LN, and in B6 LN with anti–IL-17 antibody mice after early or late neutralization. (C) Representative hematoxylin and eosin sternebrae section (original magnification ×4) in TBI-only, B6 LN, and B6 LN with anti–IL-17 antibody after early (top panels) or late (bottom panels) neutralization.
Early inhibition of Th17 cells with neutralizing anti–IL-17 antibody delays the induction of BM failure in mice. Anti–IL-17 was administered at 100 μg intraperitoneally on alternating days (4 total injections) to the BM failure induction mouse model. Early anti–IL-17 neutralization consisted of 100 μg intraperitoneal injections on alternating days from 3 days before the LN injection to 3 days after (n = 5). Controls consisted of LN-injected mice (n = 3) and mice only irradiated (n = 2). Late anti–IL-17 neutralization consisted of 100 μg intraperitoneal injections on alternating days from 7 to 13 days after LN injections (n = 5). Controls consisted of LN-injected mice (n = 3) and mice only irradiated (n = 2). CBCs were performed using a Hemavet analyzer. Sternebrae were sectioned, hematoxylin and eosin–stained, and photographed. Experiments are represented as mean ± SD. P <.05 is considered statistically significant. (A) Schema of the anti–IL-17 neutralization experiment. (B) CBCs and total BM cells in TBI-only, B6 LN, and in B6 LN with anti–IL-17 antibody mice after early or late neutralization. (C) Representative hematoxylin and eosin sternebrae section (original magnification ×4) in TBI-only, B6 LN, and B6 LN with anti–IL-17 antibody after early (top panels) or late (bottom panels) neutralization.
Treg cell up-regulation and Th1 down-regulation after anti–IL-17 treatment
To further understand the mechanism underlying the effect of early anti–IL-17 treatment in our BM failure model, we analyzed the contribution of different T-cell populations and activation markers during induction of disease. We did not find any difference in the expansion of CD4+ and CD8+ T cells with or without anti–IL-17 treatment (data not shown). As expected, we observed a significant, 5-fold decrease of Th17 cells (P = .01) and 2-fold decrease (P = .01) in CD8+ IL-17+ T cells in mice that received early anti–IL-17 treatment (Figure 7A). Early anti–IL-17 antibody treatment also caused a significant (P = .01) decrease in IFN-γ+CD4+ T cells (Figure 7A). At day 10 after LN injection, there was a significant increase (P = .04) in the percentage of donor-derived CD4+ FoxP3+ T cells in recipients that received the anti–IL-17 treatment (Figure 7B). We also analyzed cytokine levels in the plasma on day 10 after LN injections and found decreased levels of IL-6 (P = .15) and IFN-γ (P = .29) in the plasma of recipients that received anti–IL-17 neutralization (Figure 7C).
Mice treated with early anti–IL-17 antibody have an increased percentage of Tregs in the BM and decreased IFN-γ levels in the plasma at day 10. CByB6F1 mice were irradiated (5 Gy TBI) and injected with 5 × 106 B6 LN cells. Early anti-IL-17 neutralization consisted of 100-μg intraperitoneal injections on alternating days from 3 days before the LN injection to 3 days after (n = 5). Controls consisted of LN-injected mice (n = 3) and mice only irradiated (n = 2). CD4+ and CD8+ BM cells were assessed at day 10 for IL-17 and IFN-γ expression. CD4+FOXP3+ cells were also measured. Cytokine levels in the plasma were measured by enzyme-linked immunosorbent assay. White bars represent the group of mice who did not receive anti–IL-17 neutralization; gray bars, mice that did receive anti–IL-17 neutralization; and black bars, TBI-only controls. (A) Absolute numbers of IL-17+ and IFN-γ+ cells in CD4+ (left panel) and CD8+ (right panel) subsets at day 10, according to the anti–IL-17 neutralization. Experiments are represented as mean ± SD. (B) Frequencies of CD4+ FOXP3+ cells. Experiments are represented as mean ± SD. (C) Cytokine levels in the plasma measured by enzyme-linked immunosorbent assay at day 10. Experiments are represented as mean ± SD.
Mice treated with early anti–IL-17 antibody have an increased percentage of Tregs in the BM and decreased IFN-γ levels in the plasma at day 10. CByB6F1 mice were irradiated (5 Gy TBI) and injected with 5 × 106 B6 LN cells. Early anti-IL-17 neutralization consisted of 100-μg intraperitoneal injections on alternating days from 3 days before the LN injection to 3 days after (n = 5). Controls consisted of LN-injected mice (n = 3) and mice only irradiated (n = 2). CD4+ and CD8+ BM cells were assessed at day 10 for IL-17 and IFN-γ expression. CD4+FOXP3+ cells were also measured. Cytokine levels in the plasma were measured by enzyme-linked immunosorbent assay. White bars represent the group of mice who did not receive anti–IL-17 neutralization; gray bars, mice that did receive anti–IL-17 neutralization; and black bars, TBI-only controls. (A) Absolute numbers of IL-17+ and IFN-γ+ cells in CD4+ (left panel) and CD8+ (right panel) subsets at day 10, according to the anti–IL-17 neutralization. Experiments are represented as mean ± SD. (B) Frequencies of CD4+ FOXP3+ cells. Experiments are represented as mean ± SD. (C) Cytokine levels in the plasma measured by enzyme-linked immunosorbent assay at day 10. Experiments are represented as mean ± SD.
Discussion
In the current study, we first confirm the role of Th1/Tc1 immune response in AA development and then characterized the involvement of Th17 cells in the pathophysiology of AA. We found an increased number and frequency of Th17 cells in BMMCs and PBMCs in patients with AA at diagnosis along with a reduction in Treg cells. In patients who responded to treatment, we found that number and frequency of Th17 cells were both comparable with healthy controls, concomitantly with Treg recovery. In a mouse model of BM failure, we found expansion of IL-17–producing T cells during the induction of the disease. Early treatment with anti–IL-17 antibody reduced the severity of BM failure at day 10 but not at day 16, illustrating that Th17 immune responses may contribute to the recruitment of Th1 cells and may be important for the proinflammatory cytokine milieu in the BM during the early stages of BM failure.
Until now, the potential role of IL-17 in the pathophysiology of AA is still discussed. A previous study in humans showed elevated expression of IL-17A mRNA in BMMCs and PBMCs in 28 patients with severe AA before any specific therapy,28 which is concordant with our findings. However, others found no difference in the frequencies and numbers of Th17 cells between patients with AA and healthy controls.29 We speculate that the presence of moderate AA patients in our cohort, displaying a higher number of Th17 cells than did patients with severe AA, may have contributed to the different findings. Whereas the debate is still open regarding the assessment of IL-17-producing cells in patients with severe AA, on the other hand, IL-17 related cytokines seem not to be elevated in the plasma of most patients with this disease. Indeed, IL-17 was not detectable in all but one study published to date in the plasma of patients with severe AA.28,29,37 IL-17 might play a role directly at the site of BM destruction, as it is the case for other inflammatory cytokines. These cytokines were not detected either in the plasma of patients with AA in a very recent comprehensive cytokine analysis.36
The local cytokine environment is a determining factor in the development of Th17 cells. Polymorphism in the IL-6 gene, associated with an increase immune response, was suggested in AA38 ; IL-6 is a cytokine implicated with IL-1β in the generation of human Th17 cells.9,10 IL-17 coordinates tissue inflammation by inducing the expression of proinflammatory cytokines.5 IL-17 was found to induce in vitro the secretion of IL-6, IL-8, and TNF-α by the macrophage in patients with AA, in a higher extent than what was observed with macrophages from normal controls.28 Moreover, the IL-17 cytokine family members themselves are inhibitors of human hematopoietic progenitor proliferation39 and can thus have a direct effect on hematopoiesis in patients with severe AA. A local Th1/Th17 response in severe AA where Th17 cells could participate in T cell–mediated destruction of the BM.
Immune and autoimmune responses are regulated by a fine balance between effector and regulatory T cells. In AA, 30% of patients showed autoreactivity and 50% showed alloreactivity in lymphocyte toxicity assays using autologous or human leukocyte antigen-identical target cells.40 Treg cells are decreased in most patients with AA,24 and infusion of Treg cells in a minor antigen H60-mediated AA mouse model aborted H60-specific T-cell expansion and prevented BM destruction.25 We found an inverse correlation between Treg and Th17 cells in AA patients at diagnosis. Our results are in accordance with the dichotomy already reported in the generation of pathogenic Th17 cells responsible for autoimmunity and Tregs that inhibit autoimmune tissue injury.8 Our findings support the notion that the immune system is polarized through a Th1/Th17 response during the induction of BM failure with a Treg deficiency leading to the increased autoreactive T-cell activity and the development of clinical AA.
Our study also raises some questions concerning PR patients. We used stringent criteria to classify patients into different groups in which patients in CR all had normal CBC at the time of sampling, whereas PR patients had been highly treated before sampling. Whereas the proportions of Th1/Tc1 or Th17 cells were significantly different between PR and CR patients and normal persons, the absolute numbers of these cell subsets were not different among different groups. Moreover, 5 patients showed a roughly normal balanced Th17/Tregs distribution while being PRs (Figure 3). It is possible that the autoimmune insult was adequately suppressed with therapy in these patients but that the destruction of primitive hematopoietic elements was such that precluded resumption of more efficient hematopoiesis.
In our mouse model for LN infusion–induced BM failure,25,34 we knew from a previous study that T-bet deletion did not completely abolish the ability of LN cells to induce BM damage.27 Th17 cells became a good candidate to explain this feature because either Th17 or Th1 effector response can drive autoimmunity.41 Besides the classic Th1 response, we detected both CD4+IL-17+ and CD8+IL-17+ T cells during the induction of the disease. This latter subset has been identified in mice and in humans.42,43 We did not find CD8+IL-17+ T cells in our cohort of severe aplastic anemia patients, which does not suggest a role of this subset in the pathophysiology of the disease in humans. In our mouse model, we were able to deplete effectively both IL-17–producing T-cell subsets with anti–IL-17 antibody and showed that early depletion delayed BM failure onset and reduced severity. Nevertheless, later IL-17 cells depletion did not modify the course of BM failure. These results indicate that Th17 cell populations are involved in the initiation of the disease. Although IL-17 could be produced by either CD4+ or CD8+ T cells, we were unable to separately deplete either of the 2 cell subsets. Although it has been shown that CD8+IL-17+ T cells do not express granzyme B and could not mediate cell lysis in vitro,44 those cells could be critically important effectors in the pathogenesis of autoimmune disease43 or in CD8+ T cell-based adoptive immunotherapy.45 Altogether, it is possible that Th17 cells might be generated more rapidly than Th1 cells during inflammation and that Th17 cells might participate in the initial acute inflammation, whereas Th1 cells might function to prolong and perpetuate tissue destruction.4
We then explored the impact of Th17 depletion on the immune system in our mouse model of BM failure. Mice treated with anti–IL-17 have a decreased frequency and absolute number of donor-derived Th1 cells in the BM at day 10, whereas no change was observed at day 16 (data not shown). We also found a nonsignificant decrease in IFN-γ level at day 10 in the plasma of the mouse who received LN injection and anti–IL-17. Those data are very similar to the recipients of IL-17−/− CD4+ T in a graft-versus-host disease model46 and correlate with other studies regarding IL-17 and recruitment of Th1 cells.47,48 Collectively, these data suggest that IL-17 may directly or indirectly modulate Th1 cells. Moreover, our findings that inhibition of Th17 cells with anti–IL-17 antibody leads to enrichment in BM FoxP3+ cells is not only consistent with Th17/Treg paradigm49 but indicate that treatment with anti IL-17 antibody resulted in enrichment in Treg cell populations, which probably control the pathogenic effects of alloreactive Th1 cells in the BM. Inhibition of Th17 cells thus allows for donor Treg population to persist and counteract the development of alloimmunity in this model, underlying the major role of the balance between effector and Treg in the pathophysiology of the disease.
In conclusion, we show here that Th17 immune response plays a role in the early phase of AA physiopathology. We also found a reciprocal relationship between Treg and Th17 cells in AA, illustrating the underlying autoimmune process in this disease. IL-17 thus emerges as a potential new target for therapeutic intervention. Monoclonal antibodies against IL-17 have been developed for clinical application. Phase 2 trials are under way for psoriasis, rheumatoid arthritis, Crohn disease, and psoriatic arthritis.50 Anti–IL-17 treatment could thus be evaluated in patients with AA to shift the balance between Treg and Th17 cells, to restore the function of Treg cells in severe AA.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health. R.P.d.L. received a bursary award from the AA and Myelodysplastic Syndrome International Foundation and a grant from France HPN.
National Institutes of Health
Authorship
Contribution: R.P.d.L. designed the study, executed the research plan, collected and analyzed the data, and wrote the paper; V.V. performed experiments, analyzed data, and wrote the paper; T.T. performed the immunochemistry and confocal analysis; C.W. helped with the statistical analysis; A.J.E., A.K.S., M.J.D., P.S., K.K., and O.N. performed experiments and collected data; J.C. designed the study, analyzed the data, and wrote the paper; and N.S.Y. designed the study and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Regis Peffault de Latour, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bldg 10, Clinical Research Center, Rm 3E-5232, 10 Center Dr, Bethesda, MD 20892-1202; e-mail: regis.peffaultdelatour@sls.aphp.fr.