Abstract
Accumulation of leukemic cells in patients with chronic lymphocytic leukemia (CLL) is due to prolonged cell survival rather than increased proliferation. Survival of CLL cells depends on microenvironmental factors. Even though long-lived in vivo, CLL cells rapidly die by spontaneous apoptosis in vitro unless cocultured with stromal cells or their conditioned medium. In the present study, we show that survival of CLL cells is maintained in high cell density cultures, where the main prosurvival activity is delivered by monocytes. Cytokine array and enzyme-linked immunosorbent assay studies revealed increased expression of soluble CD14 by monocytes in the presence of CLL cells. The addition of recombinant soluble CD14 to primary CLL cells resulted in significantly increased cell survival rates, which were associated with higher activity nuclear factor κB. Quantification of serum levels of soluble CD14 revealed abnormally high levels of this protein in CLL patients, indicating a potential role of soluble CD14 in vivo. In summary, the presented data show that monocytes help in the survival of CLL cells by secreting soluble CD14, which induces nuclear factor κB activation in these cells, and that CLL cells actively shape their microenvironment by inducing CD14 secretion in accessory monocytes.
Introduction
In comparison to normal B lymphocytes, chronic lymphocytic leukemia (CLL) cells show prolonged survival in vivo and therefore accumulate in peripheral blood, bone marrow and lymphoid organs of the patients.1 There is currently no cure for CLL, and even though standard chemotherapeutic regimens decrease the amount of leukemic cells initially, the majority of patients relapse and thereafter show increased drug resistance.2 Both, intrinsic defects affecting the regulation of programmed cell death (apoptosis) as well as an altered, survival-stimulating microenvironment are discussed as major pathogenic factors for CLL.1,3 The importance of external survival factors is exemplified by the fact that CLL cells rapidly undergo spontaneous apoptosis under culture conditions that support the growth of human B-cell lines.4 On the other hand, CLL cells can be rescued from apoptosis ex vivo, if cocultured with bone marrow–derived stromal cells, fibroblasts, dendritic cells, or nurse-like cells.5-9 These cells provide a variety of survival stimuli for CLL cells, mediated via soluble factors, extracellular matrix components, and signals derived from cell-cell contact.10 The importance of soluble survival factors was demonstrated by in vitro studies using autologous blood serum or conditioned medium of stromal cells, leading to increased survival of CLL cells in culture.9,11 By using recombinant cytokines and blocking antibodies targeting selected cytokines, survival-inducing capacity for CLL cells were identified for interferon-α (INF-α), interleukin-1 (IL-1), IL-4, IL-6, IL-8, CD40L, stromal cell–derived factor-1α (SDF1α), B cell–activating factor of the tumor necrosis factor family (BAFF), and a proliferation-inducing ligand (APRIL).5,12-16 It was shown that some of these factors are not only provided by accessory cells, but also are produced by CLL cells themselves, allowing a paracrine and autocrine activation of a variety of signaling pathways in CLL cells.15 Of importance are phosphatidylinositol 3-kinase/AKT, nuclear factor (NF) κB, mitogen-activated protein kinase/extracellular signal-regulated kinase, WNT, and Notch signaling, which are known to be involved in CLL cell survival in vitro.17-22
Recently, the selective expression and function of Toll-like receptors (TLRs) in CLL cells have been described and TLR-signaling was shown to protect CLL cells from spontaneous apoptosis in vitro via activation of the transcription factor NFκB.23 TLRs play a key role in innate immunity in that they recognize structurally conserved molecules derived from microbes. CD14, which is mainly expressed by myeloid cells, acts as a coreceptor for TLR-4 for the detection of bacterial lipopolysaccharide (LPS).24 In addition to the membrane-bound form, CD14 exists also as a soluble protein, which appears either after shedding of membrane-bound CD14 or is directly secreted from intracellular vesicles. Soluble CD14 is secreted by the liver and by monocytes and is sufficient in low concentrations to confer LPS-responsiveness to cells that otherwise do not express CD14.25
In the present study, we analyzed the impact of monocytes and soluble factors produced by these cells on primary CLL cells and could show that CLL cell survival ex vivo is maintained in cocultures with monocytes. We further identified soluble CD14 as a novel survival factor for CLL cells, induced in monocytes by the presence of CLL cells and present in increased levels in the serum of CLL patients.
Methods
Primary cells and cell lines
Peripheral blood (PB) and serum samples were obtained from CLL patients (Table 1) and healthy donors after informed consent in accordance with the Declaration of Helsinki. All studies involving patient samples were approved by the ethics committee of the University of Ulm. All CLL cases used in this study matched the standard diagnostic criteria for CLL. They were selected for their high content of CD5+CD19+ cells in PB. The majority of patients were previously untreated. Only 7 patients had received treatment, which was in all cases more than 1 year ago. The data obtained with these samples were carefully evaluated and not divergent in comparison to samples from previously untreated patients, which were used in the same experimental setup. The human bone marrow (BM)–derived stromal cell line HS-5 was purchased from ATCC.
Cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient. Thereafter, CLL cell preparations consisted of at least 85% CD5+CD19+ cells as measured by flow cytometry using fluorescein isothiocyanate–conjugated mAb with specificity for CD5 (clone L17F12), and APC-conjugated mAb specific for CD19 (clone HIB19; both Abs: BD Biosciences). To enrich CLL cells or normal B cells, magnetic bead activated cell sorting (MACS) using CD19-specific MicroBeads was performed following the instructions of the manufacturer (Miltenyi Biotec). This lead to purities of higher than 98% CD19+ cells. To determine the effect of cell sorting, we compared survival rates of CLL cells either isolated by positive selection or enriched by depletion of non-B cells resulting in untouched CLL cell preparations (B Cell Isolation Kit II; Miltenyi Biotec), and observed no significant differences.
Peripheral blood monocytes were enriched from blood samples of healthy donors by MACS using CD14-specific MicroBeads. Purities of monocyte preparations were analyzed by flow cytometry using fluorescein isothiocyanate-conjugated mAb with specificity for CD14 (clone M5E2) and were higher than 80%. All cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, 4mM L-Glutamine, 4.5 g/L glucose, 100 U/mL penicillin, and 100 μg/mL streptomycin (complete medium), and were cultured at 37°C in a 10% CO2 humified incubator. CLL/HS-5 cocultures were established as described.9
Cell viability
Apoptotic cell death was detected by flow cytometry using annexin V–phycoerythrin (PE) and 7-amino-actinomycin (7-AAD) staining. Cells were harvested and resuspended in annexin V–binding buffer (10mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]/NaOH, pH 7.4, 140mM NaCl, 2.5mM CaCl2) containing 10% annexin V–PE and 10% 7-AAD staining solutions (BD Biosciences). After an incubation time of 15 minutes at 4°C, stained cells were analyzed by flow cytometry, gating on lymphocytes. Double-negative cells were counted as viable cells. The results were confirmed based on changes in forward light scattering properties of dead cells, which showed decreased cell size. All flow cytometric analyses were carried out using a FACSCalibur or FACSCanto flow cytometer equipped with CellQuest Pro 5.2 or FACSDiva V6.1.2 software (BD Biosciences). In addition to the presented annexin V and 7-AAD measurements, we have analyzed absolute counts of viable cells by using a Vi-Cell Automated Cell Viability Analyzer (Beckman Coulter). The obtained cell counting data clearly underlined our statements.
Cytokine array
Supernatants of 1 × 107 primary CLL cells, cocultured for 3 days with 1 × 106 HS-5 cells in 4 mL of serum-free medium, and of 1 × 106 HS-5 cells only cultured as control samples, were analyzed with RayBiotech human cytokine antibody array G series 2000 following the instructions of the manufacturer (RayBiotech). Briefly, after blocking the array slides, 100 μL of the collected culture supernatants were incubated on the array for 2 hours, washed and incubated with biotin-conjugated primary antibodies, and subsequently with streptavidin-conjugated secondary antibodies. Finally, after washing and drying, the arrays were promptly scanned with an Axon GenePix 4000A Microarray Scanner. Data analysis was performed using GenePix Pro 6.0 software. Mean values of spotted duplicates were calculated and normalized against mean values of internal positive controls. To indicate relative expression levels of cytokines, mean fold change values of cytokine-specific data in comparison to positive controls of 3 independently performed experiments were calculated and used to rank the expression of cytokines.
qRT-PCR
Total RNA was isolated from frozen cell pellets by Trizol extraction (Invitrogen) and purified on RNeasy Mini spin columns (QIAGEN). The quality of RNA preparations was examined with an Agilent Bioanalyzer using an RNA 6000 Nano Chip (Agilent). cDNA templates were generated from the corresponding mRNA using SuperScript II and anchored oligo-d(T)20 primer (Invitrogen). For amplification and quantification, SYBR Green ROX Mix (Abgene) was used. To prevent amplification of genomic DNA, all amplicons were designed to span exon-exon boundaries and were tested using genomic DNA as negative control. Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was performed in an ABI Prism 7900RT Sequence Detection System (Applied Biosystems) with the following settings: initial denaturation at 95°C for 15 minutes, amplification and quantification at 95°C, 15 seconds; 60°C, 10 seconds; 72°C, 60 seconds for 40 cycles with single fluorescence measurement. Products were analyzed by melting-curve analysis (60°C to 95°C with 0.1 K/s continuous fluorescence measurements). Calculation of efficiency and relative quantification versus nonregulated housekeeping genes were performed as described previously.26 The qRT-PCR primer sequences used are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
ELISA
Indicated numbers of sorted CLL cells and monocytes were seeded in 250 μL of complete medium in 24-well plates, and cell-culture supernatants were collected after 2 days of culture at 37°C and 10% CO2. Quantification of soluble CD14 in cell-culture supernatants or diluted PB serum samples was performed with human quantikine enzyme-linked immunosorbent assay (ELISA) kit as described by the manufacturer (R&D Systems). Briefly, ELISA plates were incubated with samples for 3 hours, washed, and subsequently incubated with horseradish peroxidise–conjugated polyclonal antibody. After addition of hydrogen peroxide/chromogen mixture, colorimetric changes were measured at 450 nm.
Analysis of NFκB activity
Detection of NFκB activity was performed by using a sensitive oligo-based chemiluminescent ELISA as described previously.27 Briefly, protein extracts of primary CLL cells were hybridized to 96-well plates coated with either wild-type (wt) DNA oligonucleotides containing a single copy of the NFκB consensus binding sequence, or oligonucleotides with a mutated NFκB consensus binding sequence. Bound NFκB protein was thereafter detected with antibodies specific for p50 or p65 subunits (anti-p50 no. sc-7178, anti-p65 no. sc-372; Santa Cruz Biotechnology) and chemiluminescent detection (GE Healthcare). NFκB activity was calculated as the difference between signals from wt oligos and mutated oligos. The IκB kinase-2 inhibitor, 2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide (TPCA-1; Sigma-Aldrich) was added at 1μM to the CLL cells.
Results
CLL cell survival in vitro is increased in high cell density cultures of unsorted CLL cell samples
We and others have previously shown that CLL cells in vitro die within one week by spontaneous apoptosis, unless cocultured with stromal cells or their conditioned medium. To more extensively study survival rates of CLL cells without stromal cell support, we seeded increasing numbers of mononuclear cells isolated from peripheral blood samples of CLL patients, which consisted of 85% to 95% CD5+CD19+ cells, and measured apoptotic cell death at various time points over one week by annexin V–PE/7-AAD staining. As expected, CLL cell survival dropped to zero within that time, when 2.5 × 105 PBMCs were seeded in 0.5 mL of complete medium per well in 24-well plates. However, by increasing the amount of seeded cells per well to up to 1.6 × 107, a correlative increase in cell survival could be observed, reaching survival rates of approximately 70% after 1 week of culture (Figure 1A-B). Cell survival rates in high cell density cultures were comparable with those observed when CLL cells were cultured in conditioned medium of HS-5 stromal cells, and slightly weaker than survival rates in HS-5 stromal cell cocultures (data not shown).
Survival of CLL cells is maintained in high cell density cultures of unsorted PBMCs. (A) The indicated numbers of CLL cells were cultured in 0.5 mL of complete medium per well in 24-well plates, and cell viability was determined after 4 days of culture by flow cytometry after annexin V–PE/7-AAD staining. (B) CLL cells were seeded as described in (A) and cell viability was determined by annexin V–PE/7-AAD staining after 3, 4, 5, and 6 days of culture. (C) PBMCs isolated from CLL samples were enriched for CD19-positive cells by MACS or treated equally without addition of CD19-specific beads. Unsorted and CD19-sorted CLL cells (CD19+) were then cultured under the following 3 conditions: 3 × 105 CLL cells per well in 24-well plates were cultured in 1 mL of complete medium (Control), or in coculture with 3 × 105 preseeded HS-5 cells (Coculture). For high cell density cultures, 3 × 106 CLL cells per well were seeded in 1 mL of complete medium (Cell dense). Cell viability was determined by annexin V–PE/7-AAD staining after 2, 5, and 7 days of culture. Error bars in panels B-C indicate standard deviations of duplicates of one representative example of 3 independently performed experiments.
Survival of CLL cells is maintained in high cell density cultures of unsorted PBMCs. (A) The indicated numbers of CLL cells were cultured in 0.5 mL of complete medium per well in 24-well plates, and cell viability was determined after 4 days of culture by flow cytometry after annexin V–PE/7-AAD staining. (B) CLL cells were seeded as described in (A) and cell viability was determined by annexin V–PE/7-AAD staining after 3, 4, 5, and 6 days of culture. (C) PBMCs isolated from CLL samples were enriched for CD19-positive cells by MACS or treated equally without addition of CD19-specific beads. Unsorted and CD19-sorted CLL cells (CD19+) were then cultured under the following 3 conditions: 3 × 105 CLL cells per well in 24-well plates were cultured in 1 mL of complete medium (Control), or in coculture with 3 × 105 preseeded HS-5 cells (Coculture). For high cell density cultures, 3 × 106 CLL cells per well were seeded in 1 mL of complete medium (Cell dense). Cell viability was determined by annexin V–PE/7-AAD staining after 2, 5, and 7 days of culture. Error bars in panels B-C indicate standard deviations of duplicates of one representative example of 3 independently performed experiments.
To evaluate the relevance of nonmalignant leukocytes for the observed prosurvival effect in high cell density cultures, we enriched CD19+ cells from CLL samples to purities of at least 98% and analyzed survival of these cells in high and low cell density cultures, as well as in stromal cell cocultures. For high cell density cultures, cell numbers were chosen to achieve complete coverage of the bottom of the plates with cells, as assessed by light microscopy. This was obtained when 3 × 106 cells/well were seeded in 24-well plates. Our results give clear evidence that survival of sorted CLL cells in the absence of HS-5 stromal cells is in general lower compared with unsorted CLL cells, which were equally treated without addition of CD19-specific magnetic beads. But the data also show that pure CLL cell cultures likewise benefit from high cell density by slightly enhanced cell survival rates compared with low cell density control cultures (Figure 1C). In comparison to that, no significant differences in survival rates of sorted and unsorted CLL cells in HS-5 stromal cell cocultures were observed.
Even though the presented culture conditions enabled long-term survival of CLL cells in vitro, proliferating CLL cells could not be detected in any of the culture systems tested (data not shown). As observed with CLL cells freshly isolated from PB samples, also cultured CLL cells remained arrested in G0/G1 phase of the cell cycle.
Monocytes prevent apoptosis of primary CLL cells in vitro
To assess the survival-inducing impact of monocytes in high cell density cultures of unsorted CLL samples, we compared apoptosis rates of highly enriched CLL cells (purities of > 98%) cultured in the presence or absence of increasing numbers of monocytes, which were isolated from peripheral blood of healthy donors by CD14-specific MACS. The results clearly show that CLL cell survival in vitro positively correlates with the amount of monocytes added to the cultures, reaching 90% CLL cell viability after 5 days of culture in the presence of 20% monocytes (Figure 2).
The presence of monocytes increases survival of CLL cells in vitro. CD19-sorted CLL cells (5 × 105) were cultured in the absence or presence of the indicated numbers of monocytes in 1 mL of complete medium per well in 24-well plates. Survival rates of CLL cells were determined after 5 days of culture by flow cytometry after annexin V–PE/7-AAD staining by gating on lymphocytes. Mean values of 2 replicates of one representative CLL sample of 3 independently performed experiments are depicted. Error bars indicate the technical reproducibility and low variability of the assay.
The presence of monocytes increases survival of CLL cells in vitro. CD19-sorted CLL cells (5 × 105) were cultured in the absence or presence of the indicated numbers of monocytes in 1 mL of complete medium per well in 24-well plates. Survival rates of CLL cells were determined after 5 days of culture by flow cytometry after annexin V–PE/7-AAD staining by gating on lymphocytes. Mean values of 2 replicates of one representative CLL sample of 3 independently performed experiments are depicted. Error bars indicate the technical reproducibility and low variability of the assay.
CLL cells induce CD14 secretion in monocytes in vitro
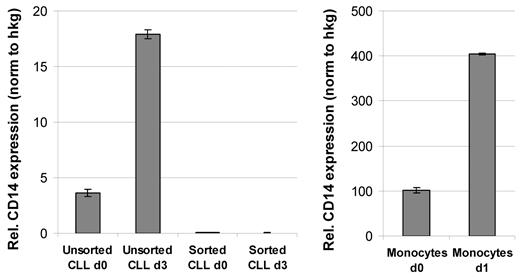
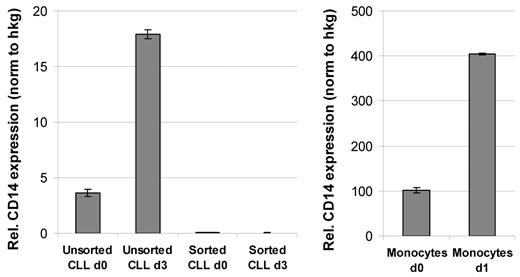
Microarray-based expression profiling of unsorted PBMCs isolated from CLL patients revealed an up-regulation of a variety of inflammatory cytokines and chemokines in high cell density cultures (A.S., Grischa Toedt, Thorsten Zenz, S.S., P.L., and M.S.; manuscript submitted April 2010; Gene Expression Omnibus [GEO; National Center for Biotechnology Information] accession number GSE18192). High protein levels of these cytokines could be further detected in supernatants of CLL cultures by cytokine antibody arrays. The top 8 proteins detected in CLL/HS-5 stroma cocultures, but not in corresponding HS-5 control cultures, were CCL4, CCL3, CXCL7, CD170, CCL20, CD14, CCL24, and CCL8. Among these proteins were factors known to be mainly expressed and secreted by monocytes (for example soluble CD14). To confirm an up-regulation of CD14 expression in CLL high density cultures and to identify the cellular source of CD14, we performed qRT-PCR analyses with unsorted or highly enriched CLL cells before and after culture. The results shown in Figure 3A confirm an up-regulation of CD14 in the unsorted CLL sample, but not in CD19-sorted cells, arguing for an induction of CD14 in non-B cells. Because it is very likely that monocytes are the source of CD14 in the unsorted CLL cultures, we performed qRT-PCR analysis with magnetic bead-sorted monocytes before and after 1 day of culture and detected a similar up-regulation of CD14 (Figure 3B). However, the expression levels measured in relation to housekeeping genes were approximately 20-fold higher compared with the unsorted CLL sample. These data suggest that PB-derived monocytes are the source of CD14 in unsorted CLL cultures.
qRT-PCR analysis demonstrated an up-regulation of CD14 expression in cultured monocytes. qRT-PCR analysis was performed with RNA isolated from either unsorted or CD19-sorted CLL samples at the time of isolation (d0) and 3 days after HS-5 coculture (d3) with a primer set specific for CD14. Respective qRT-PCR analyses were performed with CD14-sorted monocytes at the time of isolation (d0) and after 1 day of culture in HS-5 conditioned medium (d1). qRT-PCR results were normalized to 3 housekeeping genes (PGK1, DCTN2, and HPRT for CLL cells; SDHA, PBGD, and HPRT for monocytes), and are shown as relative expression normalized to the housekeeping genes (hkg).
qRT-PCR analysis demonstrated an up-regulation of CD14 expression in cultured monocytes. qRT-PCR analysis was performed with RNA isolated from either unsorted or CD19-sorted CLL samples at the time of isolation (d0) and 3 days after HS-5 coculture (d3) with a primer set specific for CD14. Respective qRT-PCR analyses were performed with CD14-sorted monocytes at the time of isolation (d0) and after 1 day of culture in HS-5 conditioned medium (d1). qRT-PCR results were normalized to 3 housekeeping genes (PGK1, DCTN2, and HPRT for CLL cells; SDHA, PBGD, and HPRT for monocytes), and are shown as relative expression normalized to the housekeeping genes (hkg).
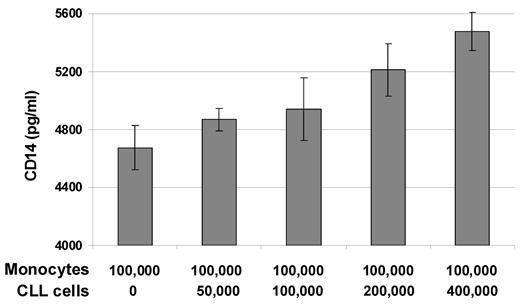
Further evidence for monocyte-derived CD14 secretion was obtained by ELISA, where soluble CD14 was detected only in cultures with monocytes. Interestingly, we observed an increase in CD14 levels in correlation with the number of CLL cells present in the cultures (Figure 4), indicating that CLL cells induce the secretion of soluble CD14 in monocytes. The purity of the CLL cell samples used in these experiments was at least 98% CD5+CD19+ cells, and only minor amounts of CD14 could be detected in CLL cell cultures without addition of monocytes (supplemental Figure 1), which excludes the possibility that remaining monocytes within the CLL cell preparations are responsible for the increased CD14 protein levels. To further assess whether increased levels of CD14 secretion is due to proliferation of monocytes in culture, we used Click-iT EdU Flow Cytometry Assay Kit (Invitrogen). After 48 hours of incubation of CLL/monocyte cocultures with EdU thymidine analog, no cells that have entered the cell cycle could be detected (data not shown).
CD14 secretion by monocytes is increased in the presence of CLL cells. CD14-sorted monocytes (1 × 105) were cultured in the absence or presence of the indicated numbers of CD19-sorted CLL cells in 1 mL of complete medium per well in 24-well plates for 2 days. Cell-culture supernatants were analyzed by ELISA for the presence of soluble CD14. Mean values and standard deviations of duplicates of one representative example of 3 independently performed experiments are shown.
CD14 secretion by monocytes is increased in the presence of CLL cells. CD14-sorted monocytes (1 × 105) were cultured in the absence or presence of the indicated numbers of CD19-sorted CLL cells in 1 mL of complete medium per well in 24-well plates for 2 days. Cell-culture supernatants were analyzed by ELISA for the presence of soluble CD14. Mean values and standard deviations of duplicates of one representative example of 3 independently performed experiments are shown.
To assess whether induction of CD14 secretion in monocytes is specific for CLL cells, we performed corresponding experiments with normal B cells isolated from PB samples of healthy donors by MACS. Thereby, we observed that the survival-inducing effect of monocytes and the induction of CD14 secretion are comparable using CLL cells or normal B cells (data not shown), indicating that the observed cross-talk of monocytes and B cells is of general nature.
Soluble CD14 increases survival of CLL cells in vitro
To test for a potential involvement of soluble CD14 in CLL cell survival, we added recombinant human CD14 to CLL cultures and quantified apoptotic cells by annexin V–PE and 7-AAD staining. As shown in Figure 5, in the presence of 10 μg/mL soluble CD14 survival of CLL cells was significantly increased by 9.6% ± 5.1%, ranging from 2.4% to 17.9% (n = 14) compared with control cells in complete medium (P = .00001). As a positive control, we used SDF1α, which was already described as a survival factor for CLL cells.5 By analyzing the same CLL samples in the presence of 1 μg/mL recombinant human SDF1α, we observed increased cell survival of 17.2% ± 7.2%, ranging from 6.5% to 31.3% (n = 10). Both these survival-inducing effects of CD14 or SDF1α were generally lower compared with CLL cell survival induced in stromal cell cocultures or in conditioned medium of stromal cells, arguing for a complex mechanism ensuring long-term survival of CLL cells.
Soluble CD14 induces survival of CLL cells in vitro. Unsorted CLL cells (2 × 105) were cultured in 0.5 mL of complete medium per well in 24-well plates. Recombinant human CD14 at 10 μg/mL (n = 13) or SDF1α at 1 μg/mL (n = 9) were added to the cultures and survival of CLL cells was analyzed after 2 days by flow cytometry after annexin V–PE/7-AAD staining. Relative survival rates (values of medium control cultures were set as 1) are shown, and calculated P values are indicated.
Soluble CD14 induces survival of CLL cells in vitro. Unsorted CLL cells (2 × 105) were cultured in 0.5 mL of complete medium per well in 24-well plates. Recombinant human CD14 at 10 μg/mL (n = 13) or SDF1α at 1 μg/mL (n = 9) were added to the cultures and survival of CLL cells was analyzed after 2 days by flow cytometry after annexin V–PE/7-AAD staining. Relative survival rates (values of medium control cultures were set as 1) are shown, and calculated P values are indicated.
To evaluate whether the pro-survival activity of soluble CD14 is specific for CLL cells, we also analyzed B cells isolated from PB samples of healthy donors. Survival of these cells increased in the presence of soluble CD14 similarly to CLL cells (data not shown). We conclude that CD14-mediated survival pathways are shared between normal B cells and CLL cells.
Soluble CD14 induces NFκB activity in CLL cells
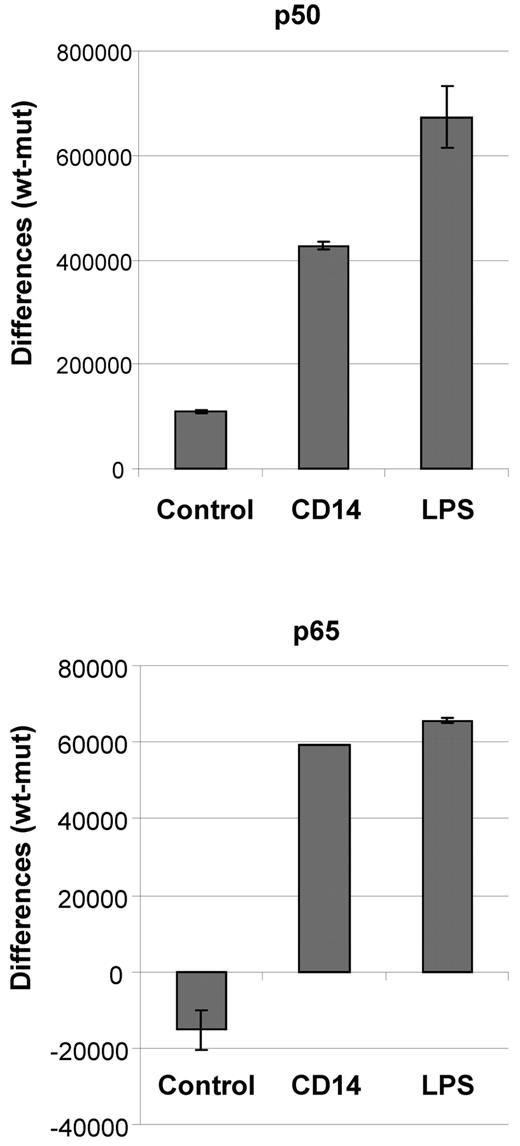
Survival of CLL cells is associated with constitutive activity of NFκB. To test whether treatment of CLL cells with soluble CD14 results in an increase in NFκB activity, we used an oligo-based chemiluminescent ELISA detecting binding of the NFκB subunits p50 and p65 to immobilized oligonucleotides harboring consensus binding sequences of NFκB. CLL cells cultured for 20 hours in the presence or absence of 10 μg/mL soluble CD14 or 10 μg/mL LPS were analyzed. The results clearly demonstrate that stimulation of CLL cells with soluble CD14 or LPS increases the activity of both NFκB subunits of the canonical pathway (Figure 6). Addition of the IκB kinase-2 inhibitor TPCA-1 in these experiments lead to a reduction of p50 activity by 30% to 40% (data not shown). We therefore conclude that soluble CD14 induces survival of CLL cells via activation of NFκB signaling pathway.
Soluble CD14 induces NFκB activity in CLL cells. CLL cells (1 × 107) were cultured for 20 hours in 1 mL of complete medium containing either 10 μg/mL soluble CD14 or 10 μg/mL LPS, or in plain medium. Cells were harvested, lysed and 5 μg total protein was used per well in an oligo-based chemiluminescent ELISA detecting the binding of p50 and p65 to oligos containing the NFκB consensus binding sequence. Values represent differences between signal and background (wt oligo − mut oligo) and are given as means and SDs of duplicates of one representative example of 3 independently performed experiments.
Soluble CD14 induces NFκB activity in CLL cells. CLL cells (1 × 107) were cultured for 20 hours in 1 mL of complete medium containing either 10 μg/mL soluble CD14 or 10 μg/mL LPS, or in plain medium. Cells were harvested, lysed and 5 μg total protein was used per well in an oligo-based chemiluminescent ELISA detecting the binding of p50 and p65 to oligos containing the NFκB consensus binding sequence. Values represent differences between signal and background (wt oligo − mut oligo) and are given as means and SDs of duplicates of one representative example of 3 independently performed experiments.
Serum levels of soluble CD14 are increased in CLL patients
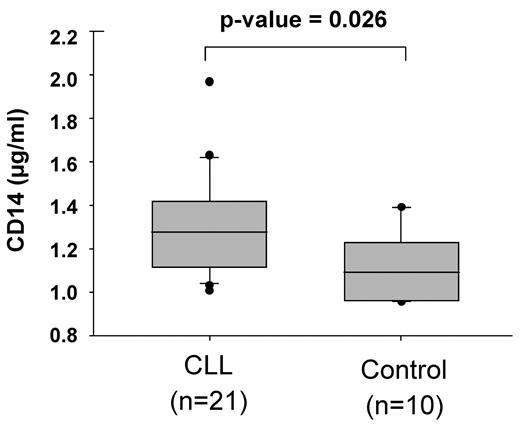
To evaluate a potential role of soluble CD14 in CLL in vivo, we analyzed CD14 serum levels of 21 CLL patients and 10 healthy donors by ELISA. Our data show that CD14 levels are significantly increased in CLL patients compared with healthy individuals (1.31 ± 0.22 μg/mL vs 1.12 ± 0.14 μg/mL; Figure 7).
CD14 serum levels are increased in CLL patients in comparison to healthy individuals. Blood serum levels of soluble CD14 in 21 CLL samples (see Table 1) and 10 healthy controls were analyzed by ELISA.
CD14 serum levels are increased in CLL patients in comparison to healthy individuals. Blood serum levels of soluble CD14 in 21 CLL samples (see Table 1) and 10 healthy controls were analyzed by ELISA.
Discussion
The dependence of CLL cells on external survival-inducing stimuli is exemplified by the rapid apoptosis of these otherwise long-lived cells, when removed from their natural microenvironment. Bone marrow–derived stromal cells (eg, the cell line HS-5) were shown to efficiently provide stimuli for long-term survival of CLL cells and are therefore used in coculture model systems to mimic the in vivo microenvironment for in vitro studies of CLL cells. The importance of soluble factors in CLL cell survival was further demonstrated by studies showing increased survival of CLL cells cultured in HS-5–conditioned medium.9 The data presented in this manuscript show that culturing unsorted PB-derived CLL cells at cell densities, which allow intercellular contact, prevents spontaneous apoptosis of these cells. This could be due to survival signals transmitted via interactions of cell surface receptors on CLL cells and other nonmalignant leukocytes present in these cultures, or due to higher concentration levels of soluble survival factors secreted into the culture medium compared with cultures with lower cell densities. The prosurvival activity in high cell density cultures was dramatically reduced when CD19-sorted CLL cells of the same patients were used. These data demonstrate the importance of nonmalignant leukocytes in survival of CLL cells in vitro. They further show that CLL cells themselves, if cultured densely, maintain survival in an autocrine fashion. Therefore, we conclude that CLL cell survival is regulated by both autocrine and paracrine soluble factors and/or intercellular interactions, which can be either stromal cell–derived or provided by nonmalignant leukocytes. This is in accordance with results shown by Bomstein et al, demonstrating that apoptotic levels of isolated CLL cells in culture can be decreased by cultivating them at high cell density (1 × 106 cells/mL).28
Extensive DNA microarray and cytokine antibody array data using survival-inducing CLL cultures identified a variety of inflammatory cytokines and signaling pathways, which showed up-regulated expression and increased secretion or activation. Because many of those cytokines are known to be specific for monocytes and macrophages, we hypothesized that blood-derived monocytes might be the source of the observed survival-inducing activity. In vitro studies using sorted CLL cells with and without the addition of monocytes clearly showed that this is indeed the case. Experiments with normal B cells isolated from PB samples of healthy donors further indicate that monocytes harbor survival-inducing activity for B cells in general. This is in concordance with previously published data showing that survival and proliferation of normal B cells and diffuse large B-cell lymphoma cells are induced in the presence of monocytes.29 Further publications describe increased survival rates of CLL cells when cultured in the presence of monocytes or monocyte-derived nurse-like cells.5,30 In recent years, there is increasing evidence, that the tumor microenvironment plays an important role in the development and progression of many different malignancies. As part of the microenvironment, tumor-associated macrophages were identified as major players in cancer-related inflammation.31 High levels of tumor-associated macrophages are often correlated with bad prognosis in a variety of tumor entities.32 Our findings, showing increased survival of CLL cells in the presence of monocytes in vitro, indicate their importance as tumor-associated accessory cells in CLL, and therapeutical approaches targeting monocytes or macrophages should therefore be considered.
CD14 is a cell-surface receptor present on monocytes and macrophages, and to a lesser extent on neutrophils and dendritic cells. By binding bacterial LPS, CD14 helps in the activation of TLR-4 signaling, which subsequently results in the activity of NFκB, AP1, and IRF3 transcription factors. Although LPS is considered its main ligand, CD14 also recognizes other pathogen associated molecules. A soluble form of CD14 is present in body fluids, like blood, saliva or breast milk, where it is involved in innate immunity by conferring its signaling activity to cells that do not express CD14. By adding recombinant soluble CD14 to CLL cells in culture, we observed increased CLL cell viability. Consistent with this we found that stimulation of CLL cells with soluble CD14 results in an augmented activity of the NFκB components p50 and p65, which are known to be constitutively active in primary CLL cells and are associated with their survival.17 Our data suggest that soluble CD14 mediates TLR-signaling in CLL cells, which leads to NFκB activity and cell survival. Since TLR4 is not expressed on CLL cells, the question remains which receptor is involved in the submission of CD14-mediated signals in these cells.
Our data show that monocytes and the secreted form of CD14 have survival-inducing activity for mature B cells in general and that the induction of CD14 secretion in monocytes is similar in the presence of CLL cells or normal B cells. A detailed comparative analysis of monocyte interactions with normal versus malignant B cells is planned to detect potential differences that might help to elucidate a pathomechanistic role of monocytes and soluble CD14 in CLL. Our in vivo data, showing significantly higher CD14 serum levels in CLL patients compared with healthy individuals, implicate a pathogenic effect for this factor. This finding is in accordance with data published by Callea et al, who measured serum levels of a variety of soluble factors in a cohort of 47 CLL patients, and found soluble CD14 to be significantly increased.33 Considering our data showing a positive correlation of CD14 secretion in monocytes with the number of B cells present in culture, the increased CD14 serum levels in CLL patients can be explained by the high CLL cell counts in the blood. Accumulation of these cells is known to be nourished by a proliferating pool of malignant cells localized in the BM or lymph nodes. Our data suggest that, after entering the blood stream, CLL cells stimulate monocytes to secrete CD14, and most likely further survival factors, which are then responsible to maintain long-term survival of CLL cells in the blood. In addition, there are several studies describing the presence of follicular dendritic cells, a potential source of soluble CD14, in the lymph nodes and BM of CLL patients.34,35 But this issue is still under debate. However, there is evidence that CD14-expressing cells are found in close proximity to CLL cells in lymph nodes (Nupur Bhattacharya, Susanne Diener, Irina S. Idler, Thomas F. Barth, Judith Rauen, Hauke Busch, Annett Habermann, Thorsten Zenz, Peter Möller, H.D., S.S., and Daniel Mertens, manuscript submitted, 2010). Experiments that address the potential in vivo role of monocytes and soluble CD14 in CLL development and progression may elucidate, whether substances that inhibit secretion of CD14 or its interaction with CLL cells reduce survival rates of CLL cells in vitro and/or in vivo and could therefore lead to new therapeutic avenues for CLL patients.
In summary, our data demonstrate that autologous and allogeneic blood–derived monocytes protect CLL cells from apoptosis in vitro. This finding is of interest, as it provides a novel culturing method for primary CLL cells in the absence of stromal cells. It further provides a new mechanism by which hematopoietic accessory cells can regulate survival of CLL cells even outside the bone marrow microenvironment. In addition, we identified soluble CD14 as a novel, monocyte-derived survival factor for primary CLL cells. The relevance of this protein in CLL pathogenesis and its potential usage as a novel therapeutical target needs to be evaluated by future studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Nupur Bhattacharya and Antonio Sarno for technical advice concerning the NFκB ELISA, as well as Daniel Mertens and Thorsten Zenz for helpful discussions and suggestions.
This research was supported by the German José Carreras Foundation, grant no. DJCLS R 08/22v.
Authorship
Contribution: M.S. provided budget support, designed and performed research, analyzed data, and wrote the paper; A.S. designed and performed research and analyzed data; S.O. performed research; H.D. and S.S. provided clinical data and participated in planning the work; and P.L. established the research plan, provided logistic and budget support, and approved the data and the final version of the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Lichter, German Cancer Research Center, Department for Molecular Genetics, Im Neuenheimer Feld 280, 69120 Heidelberg, Germany; e-mail: peter.lichter@dkfz.de.