Abstract
Abstract 1461
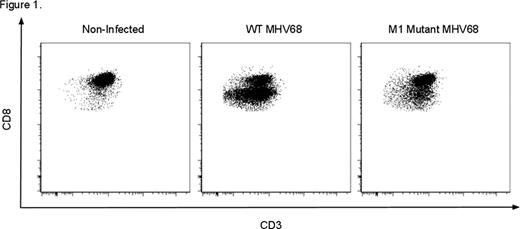
Latent herpesvirus infections are ubiquitous and are important causes of transplant complications mediated by both direct viral pathology and anti-viral-related immune dysregulation. An emerging paradigm, heterologous immunity, suggests that alloreactivity arises in a subset of anti-viral memory T cells during infection, putting patients at increased risk for rejection. Murine γ-herpesvirus 68 (MHV68) provides a rodent model of human herpesvirus infections, including EBV, allowing the study of herpesvirus infections in transplantation. We have previously shown that latent infection with wild type (WT) MHV68 induces resistance to engraftment in a non-myeloablative, costimulation-blockade- (CoB) based bone marrow transplant model. Resistance to donor-engraftment was not observed in mice infected with a mutant virus lacking the viral M1 gene. (Stapler, et al. J Immunol 2008). Based on these findings, we sought to determine the mechanism(s) by which MHV68 induces heterologous alloimmunity and transplant rejection. B6 mice were infected i.p. with WT MHV68 or M1 mutant MHV68 and viral latency was defined as 6–8 weeks post infection (p.i.) after which some mice received allogeneic (BALB/c) BMT or skin grafts (SG). Transplanted mice received 0.5 mg each of anti-CD154 and CTLA4-Ig on days 0, 2, 4, and 6 relative to transplantation. BMT mice additionally received 0.6 mg busulfan on the day before transplant. Analysis of graft survival and multicolor flow cytometric analysis of immune phenotype was then performed. In addition to inducing rejection of donor bone marrow, as we previously observed, our new results indicate that latent infection with WT MHV68 decreases the length of allogeneic SG survival. While SG in non-infected, CoB-treated recipients have a mean survival time (MST) of 26 days (n = 5), MHV68 decreases this to 13 days (n=10, p=.0004*), similar to that in non-CoB treated recipients (11 days, n=5). This suggests a broad impact of latent MHV68 infection on transplant rejection. Flow cytometric analysis has revealed a unique cell population that may increase the risk of transplant rejection in MHV68-infected mice. MHV68 infection is associated with the expansion of a distinct CD8 T cell population with decreased per-cell CD8 expression and a unique phenotype, CD8Int. In non-infected mice, CD8Int are uncommon (11% of CD8s), but are highly induced by WT MHV68 (76%) and less so by an M1 mutant strain (35%, p<.0001*, Fig 1). CD8Int appear by 7 days p.i., and have been observed through >165 days p.i. In infected mice, T cells specific for two MHV68 epitopes (p56 and p79) are exclusively CD8Int by tetramer staining, identifying the CD8Int compartment as containing anti-viral T cells. Given the correlation of high-level induction of CD8Int and costimulation blockade-resistant transplant rejection, we sought to determine the mechanisms by which these cells might heterologously induce graft rejection. After infection with WT MHV68, CD8Int are significantly more activated vs CD8Nml (CD8 T cells with unchanged CD8 expression) as assessed by increased expression of CD44 (mean fluorescence intensity [MFI] 2746 vs 1640, p=.0025*) and KLRG1 (2553 vs 1003, p=.004*). Despite high KLRG1 expression, a significant fraction of CD8Int also express the memory marker CD127 suggesting that CD8Int consists of both effector and effector memory T cells. CD8Int exhibit decreased expression of the costimulatory molecules ICOS (MFI Int vs Nml, 171 vs 329, p<.0001*), 4-1BB (26 vs 84, p<0.0001*), and CD28 (416 vs 556, p=.078), consistent with a role for these cells in CoB-resistant rejection. Importantly, CD8Int demonstrate increased expression of the adhesion molecules LFA-1 (MFI Int vs Nml, 12965 vs 5630, p=.0001*) and VLA-4 (1511 vs 805, p=.002*). The specific upregulation of these molecules on activated T cells has been correlated with increased rejection risk in other models of transplant rejection. This observation thus raises the possibility that the CD8Int subpopulation may use heightened expression of adhesion molecules to subvert costimulation-blockade, and suggests that adjuvant therapy with clinically available agents blocking either the LFA-1 or VLA-4 pathways may be a therapeutic strategy to overcome herpesvirus-induced heterologous alloimmunity and rejection. We are currently testing these hypotheses directly in our murine models of MHV68-mediated rejection of both skin and bone marrow allografts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.