Abstract
Abstract 1462
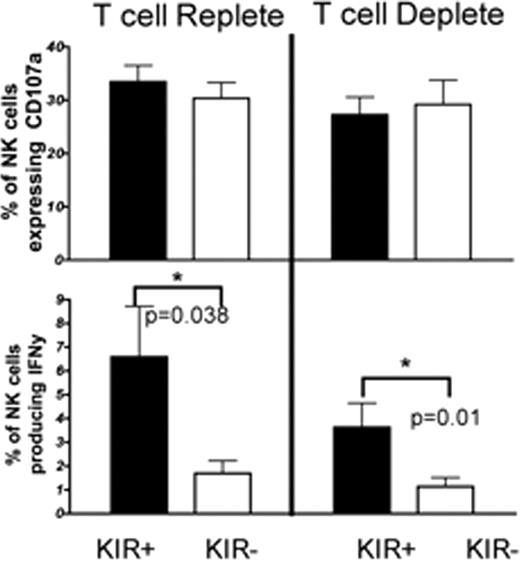
NK cells are the first lymphocyte subset to reconstitute following hematopoietic stem cell transplantation (HSCT) and they play a pivotal role in mediation of the graft versus leukemia (GvL) effect in myeloid leukemia. We hypothesized that for NK cells to mediate GvL, they must be fully functional via a process termed “licensing” or “education”. Although it has been presumed that NK cell functions (cytotoxicity and cytokine production) develop in parallel through interactions with their class I recognizing inhibitory receptors, new data suggests that this may not be the case. To address this issues we developed a 9-color flow cytometric-based assay to simultaneously measure both CD107a expression and IFNy production by CD56+ NK cells in the context of expression of inhibitory receptors for self-class I human leukocyte antigen (HLA). We tested a cohort of 30 patients who received either unmanipulated (T cell replete) or potently T cell depleted (CD34+ selected) grafts from adult unrelated donors. Thawed peripheral blood mononuclear cells (PBMC) were rested overnight in cytokine free media and then incubated with K562 cells to trigger cytotoxicity and cytokine production. PBMC were stained with CD107a (a surrogate for cytotoxicity), IFNy, CD56, CD3, CD45, CD158a, CD158b, CD158e and CD159a simultaneously. The same normal volunteer and the actual transplant donor were used as positive controls in each assay. Cytotoxicity or IFNy production was calculated as a percentage of the normal positive control. Cytotoxicity was intact but modestly suppressed (∼35%) at 3 months after both T cell deplete and T cell replete HSCT with further recovery of killing at 6 months. By contrast, at 3 months after T cell replete HSCT there was potent and sustained suppression of IFNy production by CD56+ cells (57%±11% suppression, p=0.009). The cohort of patients receiving T cell deplete (CD34-selected) grafts also exhibited significant suppression of IFNy at 3 months after HSCT (73%±9.6%, p=0.018), suggesting that the use of post-transplant immune suppression medications did not explain the effect. Suppression of IFNy production when exposed to targets continued through 6 months post-transplant in both cohorts and was partially restored with low concentrations of IL-15. Cells stimulated overnight with IL-12 and IL-18 produced IFNy at 3 and 6 months. Thus the cells were not globally hyporesponsive, suggesting the defect was based on physiologic interactions with the target. NK cells become educated following engagement of inhibitory receptors (eg. Killer-immunoglobulin-like receptors [KIR]) with self class I HLA. Therefore we compared NK cells that expressed at least one KIR with KIR negative NK cells. At 3 months post transplant, KIR expression had no effect on cytotoxicity. In contrast, KIR positive cells produced significantly higher amounts of IFNy than KIR negative cells at 3 (Figure 1) and 6 (data not shown) months post-transplant. Therefore following HSCT, expression of KIR discriminates a population of NK cells that produce IFNy, but does not correlate with cytotoxicity. While NK cell cytotoxicity is only partially suppressed following HSCT, IFNy production is significantly reduced. Consistent with this we found that while all IFNy producing cells degranulate, only a small fraction of CD107a+ cells also make IFNy. This effect is not a result of post-transplant immune suppression or graft versus host disease, as patients receiving CD34+ selected grafts had neither. Perhaps NKG2A, highly expressed on almost all NK cells early after transplant, selectively mediates education for cytotoxcity. In conclusion, our data shows distinct defects in NK cell education for either cytotoxicity or cytokine production. This highlights the importance of analyzing both cytotoxicity and cytokine production when assessing NK cell function post HSCT. Because of their critical anti-tumor and infection protection roles, methods to enhance broad in vivo NK cell function, such as the use of post-transplant IL-15 administration, are warranted.
KIR expression on NK cells at 3 months after HSCT correlates with IFNg production but not cytotoxicity.
KIR expression on NK cells at 3 months after HSCT correlates with IFNg production but not cytotoxicity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal