Abstract
Abstract 237
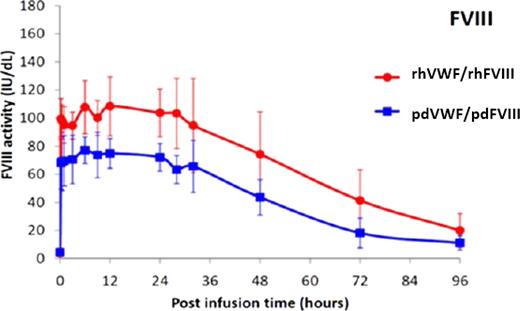
Preliminary PK data from 8 subjects post-infusion of either rhVWF/rhFVIII or pdVWF/pdFVIII. Endogenous FVIII activity reached a plateau after 6 hours and remained stable for at least 30 hours. FVIII was still elevated well above baseline at 96 hours
Preliminary PK data from 8 subjects post-infusion of either rhVWF/rhFVIII or pdVWF/pdFVIII. Endogenous FVIII activity reached a plateau after 6 hours and remained stable for at least 30 hours. FVIII was still elevated well above baseline at 96 hours
Suiter:Baxter BioScience: Employment. Laffan:Baxter BioScience: Consultancy. Mannucci:Baxter BioScience: Consultancy. Kempton:Baxter BioScience: Consultancy. Romond:Baxter BioScience: Consultancy. Shapiro: Baxter BioSci- ence: Consultancy. Birschmann:Baxter BioScience: Consultancy. Gill:Baxter BioScience: Consultancy. Ragni:Baxter BioScience: Consultancy. Turecek:Baxter BioScience: Employment. Ewenstein:Baxter Bioscience: Employment. Baxter BioScience:Baxter BioScience: Employment.
Author notes
Asterisk with author names denotes non-ASH members.