Patients ≥ 70 years of age with acute myeloid leukemia (AML) have a poor prognosis. Recent studies suggested that intensive AML-type therapy is tolerated and may benefit most. We analyzed 446 patients ≥ 70 years of age with AML (≥ 20% blasts) treated with cytarabine-based intensive chemotherapy between 1990 and 2008 to identify risk groups for high induction (8-week) mortality. Excluding patients with favorable karyotypes, the overall complete response rate was 45%, 4-week mortality was 26%, and 8-week mortality was 36%. The median survival was 4.6 months, and the 1-year survival rate was 28%. Survival was similar among patients treated before 2000 and since 2000. A multivariate analysis of prognostic factors for 8-week mortality identified the following to be independently adverse: age ≥ 80 years, complex karyotypes, (≥ 3 abnormalities), poor performance (2-4 Eastern Cooperative Oncology Group), and elevated creatinine > 1.3 mg/dL. Patients with none (28%), 1 (40%), 2 (23%), or ≥ 3 factors (9%) had estimated 8-week mortality rates of 16%, 31%, 55%, and 71% respectively. The 8-week mortality model also predicted for differences in complete response and survival rates. In summary, the prognosis of most patients (72%) ≥ 70 years of age with AML is poor with intensive chemotherapy (8-week mortality ≥ 30%; median survival < 6 months).
MedscapeCME Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians. Medscape, LLC designates this educational activity for a maximum of 0.5 AMA PRA Category 1 credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://cme.medscape.com/journal/blood; and (4) view/print certificate. For CME questions, see page 4731.
Disclosures
Elias Jabbour received honoraria from BMS and Novartis Oncology (consultancy and advisory board). Jorge Cortes received research support from Ambit and Chroma Therapeutics. The remaining authors; the Editor Cynthia E. Dunbar; and the CME questions author Charles P. Vega, University of California, Irvine, CA, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Identify medications commonly used in frontline intensive chemotherapy of AML
Analyze factors associated with complete response to chemotherapy among older adults with AML
Evaluate which older adult patients with AML might be at high risk for early mortality with intensive chemotherapy
Introduction
In patients with acute myeloid leukemia (AML), intensive chemotherapy combinations with different dose schedules of cytarabine and anthracycline result in complete response (CR) rates of 40%-80% and in cure rates of 5%-60%, depending on the patient and disease characteristics, including age, performance status, cytogenetic abnormalities in the leukemic cells, molecular abnormalities, and organ functions.1,,,–5 Patients with higher risk myelodysplastic syndrome (MDS), particularly those with ≥ 20% blasts, are often treated like AML. The World Health Organization classification categorizes patients with ≥ 20% blasts as having AML.6
Standard frontline therapy of AML is accepted to be regimens that include cytarabine 100-200 mg/m2 daily × 5-7 days and daunorubicin 45-90 mg/m2 daily × 3 days.7,8 This is referred to as the 3 + 7 regimen. Recent studies have suggested that idarubicin (12 mg/m2 intravenously daily × 3) or high-dose daunorubicin (90 mg/m2) may be superior to standard daunorubicin 45-60 mg/m2 daily × 3, at least in some subsets of patients.7,,,,,,,,–16 The use of high-dose cytarabine during induction12,13 and the addition of adenosine nucleoside analogues to induction therapy12,14 may also improve outcome.
Most AML trials exclude older patients (age 55-60 or older) with AML.15,16 Still the 3 + 7 regimens are applied by inference to older patients. Despite achievement of reasonable CR rates (CR rates. 35%-55%), intensive chemotherapy is associated with a high incidence of 4-week mortality and with 3- to 5-year survival rates of < 10%.5,8,15,,,,–20
Recent research trends have emphasized investigational low-intensity and targeted therapies in older patients with AML, hoping to reduce the early mortality and to improve the benefit/risk ratio for long-term survival.21,,,,,,,,–30 Investigational therapies included low-dose cytarabine, arsenic trioxide, gemtuzumab ozogamycin, clofarabine, hypomethylating agents, and others.21,,,,,,,,–30
Several studies had previously addressed the outcome of older patients with AML. Some suggested that such patients may be offered supportive care only, low-intensity therapy, or investigational (including phase 1-2) strategies, particularly if patients were older than 70-80 years, rather than older than 55-70 years. Others found a beneficial effect for intensive chemotherapy in candidate patients.29 Many oncologists feel uncomfortable offering intensive chemotherapy to older patients because of the high risks of induction mortality and morbidities, and many elderly patients with AML may refuse intensive regimens in favor of alternative approaches. Several studies have analyzed the significance of pretreatment host and leukemia-related characteristics to identify subsets of older patients with AML who may benefit more from intensive chemotherapy.20,30,,,–34 We had previously reported our experience in older patients (age ≥ 65 years) with AML and higher risk MDS, delineating their poor prognosis with intensive chemotherapy. We proposed that most older patients were “unfit for intensive chemotherapy,” based on an 8-week mortality exceeding 20%.20 Our previous analysis had limitations because of the inclusion of (1) patients 65-69 years old (better tolerance to intensive chemotherapy, (2) patients with higher-risk MDS (blasts 10%-19%), (3) patients who received noncytarabine regimens, and (4) patients treated in earlier times (1980-1989) when supportive care measures might have been suboptimal. In this analysis, we focus on patients ≥ 70 years with AML (≥ 20% blasts) who received cytarabine-based intensive chemotherapy since 1990 to evaluate whether there are any subsets in this age group who might benefit from this high-risk (intensive chemotherapy) strategy. We also excluded the laminar airflow room (LAFR) as an initial variable in the prognostic models (because LAFR is not available in most treatment centers) and added it only after the risk model was developed (to show its added independent prognostic effect but to allow for a risk model applicable to general practice).
The primary aim was to identify patients at high risk of 8-week mortality with intensive chemotherapy. A mortality of ≥ 30% with intensive chemotherapy could be used as a cautionary signal for physicians and patients to decide on whether the risk/benefit ratio is worthwhile or whether they may prefer investigational lower- intensity therapies.
Methods
Study group
Adults with a diagnosis of AML who were ≥ 70 years treated on frontline cytarabine-based intensive chemotherapy regimens from 1990 until 2008 were evaluated. For this analysis, AML was defined by the presence of ≥ 20% myeloblasts in the marrow or peripheral blood.6 Patients with acute promyelocytic leukemia were excluded from this analysis. All studies with patients received approval from the institutional review board of the M. D. Anderson Cancer Center.
Therapy
Induction therapy varied by treatment period (Table 1). Variations in these regimens included different anthracyclines (daunorubicin, idarubicin, liposomal daunorubicin), topoisomerase I inhibitors (topotecan), other nucleoside analogues (fludarabine, clofarabine), with or without cytokines and differentiation agents.35,–37 Patients receiving noncytarabine regimens were excluded from the analysis.
Response criteria and statistical methods
Response criteria were as previously established.6 A CR required normalization of bone marrow structure with ≤ 5% blasts, and of peripheral counts with granulocytes of ≥ 109/L and platelets of ≥ 100 × 109/L.
Induction mortality was defined by the 8-week mortality, because this was the time when the weekly mortality rate was reduced from the high initial rates to the lower rates noted during maintenance. It is also a measure of the combined effect of treatment-associated mortality and of mortality because of ineffective therapy that allows for persistence of disease and cytopenias. This definition avoids subjectivity in attributing mortality to therapy versus leukemia. In addition, we reported a 4-week mortality, which is used in many published series, for the purpose of comparisons.
Differences among variables were compared by the χ2 tests. Survival was calculated by the Kaplan-Meier method. Survival among different categories were compared by the log-rank test.38 Variables showing significant differences (P < .1 by univariate analyses) were included in the multivariate analysis. Multivariate analyses of prognostic factors used the logistic regression methods for CR and mortality and the Cox proportional hazard method for survival.39,–41 A P value < .05 was considered significant.
Karyotypic abnormalities were categorized as follows: (1) t(8;21) or inversion 16 (alone or with other changes; later analyzed separately); (2) normal or with loss of chromosome Y; (3) chromosome 5 and/or 7 abnormalities, ± 1 other abnormality; (4) other noncomplex abnormalities (1 or 2 cytogenetic abnormalities); and (5) complex abnormalities (≥ 3 abnormalities).
In the univariate and multivariate analyses, age was first tested as a continuous variable. After identifying it as an important factor for 8-week mortality and survival, we investigated age in incremental age groups of 5 years, to develop a user-friendly risk model. The most discriminating age cutoff was 80 years; this was used as the cutoff in the final multivariate analysis and in the development of the risk model.
Because the analysis spanned over a long period, and because patients were selected to various regimens by different criteria, interactions between different treatments and patient or other characteristics, other than age, were evaluated in all the multivariate analyses.
Validation of the model was conducted in older patients with AML treated from 1980 to 1989, using similar criteria: age ≥ 70 years; treatment with frontline cytarabine-based intensive regimens; blasts ≥ 20%; exclusion of acute promyelocytic leukemia and core binding factor (CBF) leukemia.
Results
A total of 446 patients were analyzed. Their median age was 74 years (range, 70-88 years); 13% were ≥ 80 years (Table 2). Two hundred forty-two patients (54%) had unfavorable karyotypes: chromosome 5 or 7 abnormalities ± 1 other abnormality in 28 patients (6%), chromosome 5 or 7 complex (total ≥ 3 abnormalities) in 97 patients (22%), other noncomplex in 79 patients (18%), and other complex in 38 patients (9%). Prior malignancy was noted in 139 patients (31%) and prior chemotherapy for the primary malignancy in 55 patients (12%; 40% of prior malignancies). The chemotherapy regimens are listed in Table 1. The characteristics of the study group are detailed in Table 2.
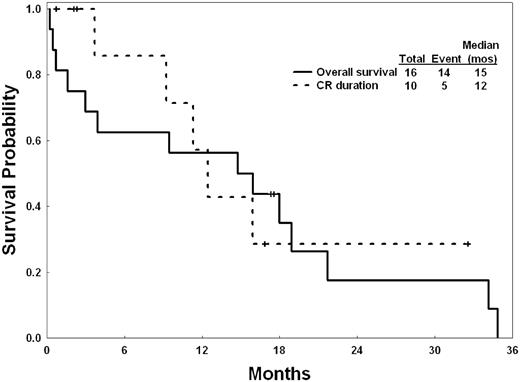
Among 16 patients with CBF leukemia, 13 had inversion 16, and 3 had t(8;21). Overall, 10 achieved CR (63%; Table 3). The median CR duration was 12 months. The median survival was 15 months; the 2-year survival rate was 18% (Figure 1).
Survival and CR duration of the 16 elderly patients with CBF leukemias.
Survival and CR duration of the 16 elderly patients with CBF leukemias.
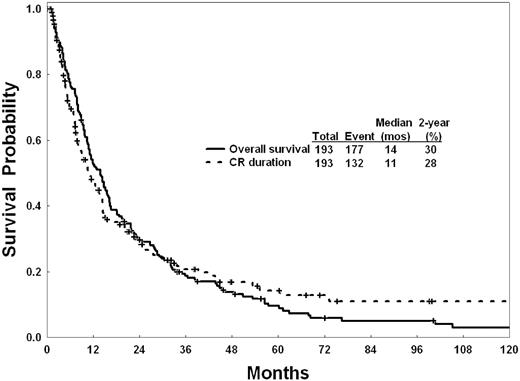
Outcome of the remaining 430 patients was then analyzed. Response to intensive chemotherapy is shown in Table 3. Overall, 193 patients achieved CR (45%), and 154 (36%) died during remission induction (8-week mortality). Among the 193 patients achieving CR, 183 (95%) achieved CR after 1 course, 9 after 2 courses, and 1 after 3 courses. The survival of patients was not different among patients achieving CR after 1 course or > 1 course, considering the small numbers of patients in the latter category. The median CR duration was 10.8 months, and the median survival in patients achieving CR was 13.8 months (Figure 2). The median survival of the total study group (n = 430) was 4.6 months (95% confidence interval, 3.9-6.0). The 1-, 2-, and 3-year survival rates were 28%, 16%, and 10%, respectively (Figure 3). The CR rates were 49% in patients treated before 2000 and 38% in patients treated since 2000. The median survivals were not different in the 2 decades (Figure 3).
Survival of 430 elderly patients with AML, excluding CBF leukemias, by year of therapy.
Survival of 430 elderly patients with AML, excluding CBF leukemias, by year of therapy.
Prognostic factors associated with response, 8-week mortality, and survival
Table 4 details response, 8-week mortality, and survival by pretreatment characteristics.
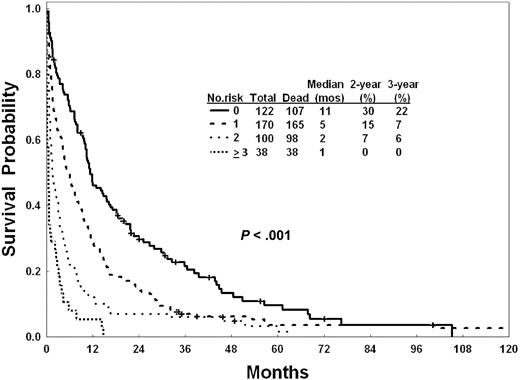
Because the primary objective of this analysis was to identify risk events for high mortality with intensive chemotherapy, we conducted first a multivariate analysis of prognostic factors associated with 8-week mortality. This identified the following to have independent adverse significance: older age ≥ 80 years, poor performance status (Eastern Cooperative Oncology Group 2-4), complex karyotype (≥ 3 abnormalities regardless of the presence or absence of chromosome 5 or 7 abnormalities), and creatinine level > 1.3 mg/dL (Table 5). Patients could be predicted to have 8-week low mortality rate (estimated 8-week mortality < 20%; 28% of patients; no adverse factors), intermediate mortality rate (estimated 8-week mortality 31%; 40% of patients; 1 adverse factor), and higher mortality rate (estimated 8-week mortality > 50%; 32% of patients; ≥ 2 adverse factors). Because our previous study had shown consistent adverse factors predictive of mortality, CR, and survival,20 we applied the 8-week mortality risk model for prediction of CR and survival. As shown in Table 5, the risk model for mortality was predictive for differences in CR rates and for survival (Figure 4). Separate multivariate analyses conducted for CR and survival identified the same 3 factors (poor performance, complex karyotype, creatinine level > 1.3 mg/dL) as independently adverse for CR (others identified were antecedent hematologic disorder ≥ 12 months, prior chemotherapy for other cancers, and high lactate dehydrogenase level), and for survival (others identified were older age [as for mortality], prior chemotherapy for other malignancy, elevated lactate dehydrogenase level, and lower albumin levels; supplemental Tables 1-2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Survival of 430 elderly patients with AML by number of independent risk factors for 8-week mortality.
Survival of 430 elderly patients with AML by number of independent risk factors for 8-week mortality.
The inclusion of the LAFR after accounting for the other independent prognostic factors identified LAFR to remain an independent favorable factor associated with a reduction of 8-week induction mortality (hazard ratio, 0.35; P < .001), improvement of the CR rate (odds ratio, 3.09; P < .001), and improvement of survival (hazard ratio, 0.6; P < .001).
Validation of the 8-week mortality risk model
A total of 72 patients ≥ 70 years with AML (≥ 20% blasts), excluding acute promyelocytic leukemia, were treated with frontline induction cytarabine-based intensive chemotherapy from 1980 through 1989. Their median age was 74 years (range, 70-89 years), similar to the 1990-2008 study group. Other disease characteristics were also similar (data not shown). Overall, 33 patients (46%) achieved CR. The median survival was 3.3 months, and the 2-year survival rate was 3%, significantly worse than the survival of patients treated since 1990, thus justifying the restriction of this study to the more modern era.
However, for the purpose of this model validation, the application of the proposed risk model to this independent study group provided validation of the risk model in an independent study group (Table 6; P = .03).
Discussion
In a review of the Surveillance, Epidemiology, and End Results data in elderly AML,19,42 among 3439 patients > 65 years of age analyzed (1991-1996), the median survival was only 2.4 months and the 2-year survival rate was 6%. Only 34% of patients received chemotherapy during their course. Although the cost of therapy was 3 times higher with chemotherapy, the median survival of such patients was longer.
Recent studies have suggested that intensive chemotherapy may be well tolerated in most elderly patients with AML and have implied that it might improve their prognosis.8,43 The Food and Drug Administration and many AML experts consider intensive chemotherapy (3 + 7 regimens) as an acceptable standard of care against which novel investigational strategies would be compared. An editorial by C. Schiffer44 also suggested that older patients with AML tolerated intensive chemotherapy well, with an estimated mortality of < 10%, and that many may benefit from intensive chemotherapy.
Our single institutional experience with the use of intensive chemotherapy in patients ≥ 70 years with AML showed that, despite the achievement of reasonable CR rates of 40%-50%, the overall survival of these patients remained poor, with a median survival of 4.6 months. This outcome was unchanged over the past 2 decades despite variations in intensive chemotherapy and improvements in supportive care measures. The 8-week mortality of 36% in our study group was prohibitively high.
An important question is whether there are subsets of patients ≥ 70 years in whom the benefit from intensive chemotherapy would outweigh the risks. These would be, for example, patients who may have an estimated 8-week mortality of < 20%, a CR rate of > 50%, and a 3-year survival rate of > 10%. This appears to hold true for the uncommon subset of favorable karyotypic leukemias (CBF; 3.5% of total study group). Among the remaining patients the multivariate analysis identified consistent adverse prognostic factors for 8-week mortality: age ≥ 80 years, complex karyotype (≥ 3 abnormalities), poor performance (Eastern Cooperative Oncology Group 2-4), and creatinine level > 1.3 mg/dL. Patients with ≥ 1 adverse factor (72%) had 8-week mortality rates of 31%-71% and median survivals of 0.5-5.3 months (Table 5). It is reasonable that such patients, who constituted 72% of elderly patients with AML, would be considered for alternative investigational therapies. However, 28% of elderly patients, who have none of these adverse factors, have a reasonable outcome with expected CR rates > 50%, 8-week mortality rates < 20%, and a median survival of 11.3 months. Such patients should be encouraged to undergo intensive chemotherapy in leukemia centers with expertise in intensive supportive care. In such patients, supportive care only or investigational strategies that do not include cytarabine-based programs may not be justified. However, low-intensity combination regimens, including low-dose cytarabine, should still be compared with standard intensive chemotherapy.
To allow for the proposed risk model to be universally applicable, we did not include LAFR as a factor in the risk model. However, LAFR remained an independent favorable risk factor for reducing the risk of 8-week mortality after accounting for the other independent adverse factor (hazard ratio, 0.35; P < .001). This suggests that the use of intensive supportive measures (reverse isolation, gloves, face masks, no plants, restriction of visits, prophylactic antibiotics) may reduce the induction mortality and improve outcome.
Recently, several investigational agents attempted to fill this area of medical necessity, namely the treatment of elderly AML “not fit for intensive chemotherapy.” These have included clofarabine, decitabine, azacitidine, sapacitabine, tipifarnib and others.21,,,,,,–28,45,46 Inability to receive intensive chemotherapy has been subjective, based on the physician's opinion, on patient's refusal of intensive chemotherapy, or because of poor performance status or comorbid conditions (diabetes mellitus, hypertension, heart conditions, chronic lung disease). By defining “intolerance to standard chemotherapy” as a condition that results in an 8-week mortality in excess of a certain percentage, for example, > 30%, such patients would then be objectively defined as poor risk for intensive chemotherapy and offered new approaches. The 8-week mortality, rather than “induction death” or “treatment-associated death,” also removes the bias associated with these measures, because the 8-week mortality is a combined measure of the toxicity as well as lack of efficacy of a particular approach.
The ongoing debate about treatment of older patients with AML is not whether they can receive intensive chemotherapy. It is clear from our institutional experience that they can; the real question is whether they should.44,47 The answer is not clear cut and depends on what the treating oncologist and the patient consider a reasonable risk for a reasonable benefit with intensive chemotherapy. Most would probably agree that an 8-week mortality of < 10% to 20% to achieve a CR rate of > 40% and a 3-year survival of > 10% would be an acceptable benefit/risk ratio with intensive chemotherapy. This is the case, in our study, for patients with CBF leukemia and those with none of the 4 independent adverse factors. Most would also probably agree that an 8-week mortality rate of > 30% or a 3-year survival rate of < 10% or both with intensive chemotherapy is prohibitive. Arguments may be offered for or against intensive chemotherapy for patients whose risks and benefits are between these 2 extremes (eg, patients with an estimated 8-week modality of 10%-30% and an estimated 3-year survival rate > 10%).
In summary, our analysis suggested that, although intensive chemotherapy can be delivered to older patients (age ≥ 70 years) with AML, it may not be beneficial to most, and it could be harmful to some. Excluding older patients with CBF leukemias and those without any of the 4 identified adverse risk factors for 8-week mortality (age < 80 years and performance 0-1 and noncomplex karyotype and creatinine level ≤ 1.3 mg/dL; 28% of patients), all others may be considered for investigational low-intensity strategies or can be offered trials comparing intensive versus low-intensity therapies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by a research grant from Genzyme, Celgene, Eisai.
Authorship
Contribution: H.K. defined the research, performed the analysis, and wrote the paper; F.R., S.O., J.C., S.F., G.G.-M., E.J., W.W., T.K., M.K., and E.J.F. treated patients, reviewed data, and analyzed, reviewed, and approved final manuscript; S.P. and J.S. performed the analysis and reviewed data.
Conflict-of-interest disclosure: E.J. received honoraria from BMS and Novartis Oncology (consultancy and advisory board). J.C. received research support from Ambit and Chroma Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Hagop Kantarjian, Department of Leukemia, M.D. Anderson Cancer Center, 1515 Holcombe Blvd Box 428, Houston, TX 77030; e-mail: hkantarj@mdanderson.org.