Abstract
CXCL4 and CXCL4L1 are 2 closely related CXC chemokines that exhibit potent antiangiogenic activity. Because interactions with glycosaminoglycans play a crucial role in chemokines activity, we determined the binding parameters of CXCL4 and CXCL4L1 for heparin, heparan sulfate, and chondroitin sulfate B. We further demonstrated that the Leu67/His67 substitution is critical for the decrease in glycan binding of CXCL4L1 but also for the increase of its angiostatic activities. Using a set of mutants, we show that glycan affinity and angiostatic properties are not completely related. These data are reinforced using a monoclonal antibody that specifically recognizes structural modifications in CXCL4L1 due to the presence of His67 and that blocks its biologic activity. In vivo, half-life and diffusibility of CXCL4L1 compared with CXCL4 is strongly increased. As opposed to CXCL4L1, CXCL4 is preferentially retained at its site of expression. These findings establish that, despite small differences in the primary structure, CXCL4L1 is highly distinct from CXCL4. These observations are not only of great significance for the antiangiogenic activity of CXCL4L1 and for its potential use in clinical development but also for other biologic processes such as inflammation, thrombosis or tissue repair.
Introduction
The formation of new blood vessels (angiogenesis) is essential for embryonic development, postnatal growth, and wound healing. Angiogenesis also significantly contributes to pathologic conditions. Insufficient angiogenesis leads to tissue ischemia, whereas excessive vascular growth promotes cancer, chronic inflammatory disorders, or ocular neovascular disease.1 Many positive and negative angiogenesis regulators have been identified including growth factors (such as vascular endothelial growth factors and fibroblast growth factors), guidance molecules (such as netrins), thrombospondins, or chemokines.1
Chemokines are broad-range regulators that play important roles in development, inflammation, HIV pathophysiology, and cancer.2 Chemokines are divided into 4 subfamilies, based on structural properties and primary amino acid sequence, as CXC, CC, C, or CX3C.2 Cell responses to chemokines involve interactions with glycosaminoglycans (GAGs), integrins, and receptors (GPCRs).3 Concentration gradients of chemokines may be formed due to their interaction with GAGs. In vivo, these gradients are needed for cell migration and leukocyte arrest at inflammatory sites.3 GAGs have a carbohydrate structure and are found on the cell surface and in the matrix. Heparan sulfate, chondroïtin sulfate, and keratan sulfate, the most common GAGs identified on cell surfaces, vary by their lengths, repeating disaccharide units, and sulfation pattern.4 Heparin is more often a circulating GAG.5 The difference in GAG structure has consequences for their interaction with chemokines. This may lead to differences in localization, local or systemic concentration, and availability in vivo.
CXC chemokines represent a large family of homologous peptides exhibiting positive or negative activities on the control of angiogenesis.6 Angiostatic CXC chemokines could play an important role in tumor development and dissemination. For example, overexpression of CXCL4 or CXCL10 has been shown to block tumor progression and to induce regression of metastasis.7,8 We have extensively contributed in the study of CXCL4 (Platelet Factor 4 [PF4]).9–13 We have demonstrated antiangiogenic, anti-invasive and antitumor properties of a C-terminal fragment of CXCL4.13 Furthermore, we have partially elucidated its interaction with angiogenic growth factors and integrins.12,14 In addition, it has also been shown that CXCL4 interacts with an alternatively spliced variant of the CXCR3 receptor.15
CXCL4L1 (pf4v1 or pf4alt) has arisen by recent duplication of the CXCL4 gene and is only present in humans, chimpanzees, and monkeys. Both CXCL4 and CXCL4L1 genes are localized on chromosome 4, albeit in inversed orientation. Mature CXCL4L1 is highly homologous to CXCL4 and only differs in 3 amino acids. These 2 chemokines share several properties such as antiangiogenic activity and antitumor effects in vivo when administered as protein.16 Despite the apparent similarity of both chemokines, crucial differences may exist such as in binding to GAGs, export, diffusibility, and interaction with receptors or oligomerization. It has been already reported that CXCL4L1 is very different to CXCL4 with regard to the mechanism of export from cells since CXCL4, but not CXCL4L1, is released from cells through dense core granules (DCCs) by a protein kinase C (PKC)–dependent mechanism.17
In this study, we aimed to elucidate more precisely the functional differences between CXCL4L1 and CXCL4 especially with relation to their interaction with GAGs. We clearly demonstrated that CXCL4L1 and CXCL4 are very different in terms of in vitro glycan binding, in vivo diffusion, and biologic activity. These differences are conferred by single amino acid substitutions at the C-terminus of the molecule. These observations are not only of great significance for the antiangiogenic activity of CXCL4L1 and its potential use in clinical development, but also for other biologic processes such as inflammation, thrombosis, or tissue repair.
Methods
Cell lines, culture, and transfection
Bovine aortic endothelial cells (BAECs) were grown in Dulbecco modified Eagle medium (DMEM) 1 g/L glucose (Invitrogen) containing antibiotics (gentamicin), 1% L-glutamine, and 10% new born calf serum (NbCS). Human embryonic kidney (HEK)293T cells (GenHunter, Q401) were grown in DMEM containing 4.5 g/L glucose (Gibco), supplemented with 10% fetal calf serum (FCS), 1% glutamine, and antibiotics (penicillin/streptomycin). BAEC and HEK293T cells were cultured at 37°C in a humidified 5% CO2 atmosphere. Human umbilical vein endothelial cells (HUVECs; Lonza) were maintained in endothelial basal medium-2 (Lonza) supplemented with endothelial growth medium-2 SingleQuots (Lonza), which contains 2% FCS and were incubated at 37°C in 5% CO2. For transient transfection experiments, cells were seeded onto 6-well dishes (5 × 105 cells/well) 24 hours before transfection. HEK293T cells were transfected with 50 ng of vectors using the JetPEI transfection reagent (Polyplus Transfection) according to the manufacturer's instructions.
Cell proliferation assays
BAECs and HUVECs were seeded in 96-well plates at 5 × 103 and 1 × 104 cells/well, respectively and allowed to adhere overnight. Complete medium was replaced by serum-free medium and cells were treated in triplicate with 10 ng/mL recombinant basic fibroblast growth factor (FGF2) in the presence or absence of different concentrations of recombinants chemokines during 48 hours. As control, we carried out the tests in absence of FGF2 or recombinants proteins. Cell proliferation was measured at 490 nm using the CellTiter 96 AQueous One Solution cell proliferation assay (Promega) following the manufacturer's instructions.
Migration assays
HUVEC migration assays were carried out using a transwell assay (membrane filter with 8-μm pore size; BD Biosciences). Cells (1 × 105) in serum-free medium were seeded per insert and allowed to migrate for 6 hours at 37°C with serum-free medium 0.5% FBS in the lower chamber as a chemoattractant. Migrated cells were fixed, stained with Coomassie blue and counted. BAEC migration was tested by the scratch assay as described previously.18 Surface recovery after migration was determined using National Institutes of Health ImageJ Version 1.44 software.
HUVEC tube formation assays
Twenty-four–well culture plates (Nunc) were coated with 250 μL of Matrigel (BD Biosciences), and incubated at 37°C for 30 minutes. HUVECs (40 000 cells per well) were suspended in a culture medium (150 μL) containing 0.5% FCS, FGF2 (20 ng/mL) with or without the different conditioned media (350 μL) harvested from HEK 293 cells that were transfected with the different plasmids encoding the various chemokines or mutants. Before the assay, the concentration of the chemokines or mutant proteins was determined using the commercial CXCL4-enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems). Equal amounts of protein concentrations (1 μg/mL) were used for the assay. Untransfected conditioned medium was used as control. Finally, the cells were added to the Matrigel-coated wells (40 000 cells per well). HUVECs were incubated for 10 hours at 37°C, and 4 digitized pictures were made per well to determine the number of branching points.
Secretion and membrane retention assays
Twenty-four hours after transient transfection, media (soluble fraction) were collected, HEK 293T were washed and then treated or not with Heparinase II (1 U/mL, 1 hour, 37°C), or 2M NaCl in phosphate-buffered saline (1 minute, 37°C). Treatment solutions were collected and analyzed (membrane-associated fraction). In addition, cell were harvested and lysed with phosphate-buffered saline containing 1% nonyl phenoxypolyethoxylethanol (NP40) and the extracts were analyzed (intracellular fraction). In another set of experiments, cells were treated with 20 to 50mM NaClO3 24 hours after transient transfection for 24 additional hours. The different fractions were then collected as described above and analyzed.
CXCL4/CXCL4L1-ELISA assays
The concentrations of CXCL4, CXCL4L1, and mutant proteins were determined according to the manufacturer's indications with the commercial CXCL4-ELISA kit (R&D Systems), which does not distinguish between CXCL4 and CXCL4L1. We also developed a specific CXCL4L1 (and derived mutants) test using part of the commercial CXCL4-ELISA kits where the first mouse monoclonal CXCL4 antibody was substituted by a mouse monoclonal CXCL4L1 antibody (Mab-L1). Assays were performed in triplicate and results analyzed using the Softmax Pro4.0 software (Molecular Devices).
Animal studies
RAG-γ/c and BALB/c mice were housed and treated in the animal facility of Bordeaux University (Animalerie Mutualisée Bordeaux I). All animal procedures were done according to institutional guidelines and were approved by the Inserm institutional animal care committee. Then, 500 μL of blood were collected into each tube containing ethylenediaminetetraacetic acid (EDTA), mixed, and immediately placed in an ice bath for 30 minutes. Plasma was obtained by spinning the tubes in a refrigerated centrifuge for 30 minutes. Plasma samples were stored at −70°C until analysis with the commercially available CXCL4-ELISA kit that recognizes human but not mouse chemokines CXCL4 (R&D Systems).
CXCL4 and CXCL4L1 labeling with IRdye800W and mice imaging
Eight-week-old RAG-γ/c mice (male, n = 6 per group) were injected intravenously with 2nM rCXCL4 and rCXCL4L1 proteins labeled with IRDye800CW (Protein Labeling Kit–HighMW#928-38040; LI-COR Biosciences). Infrared (IR) fluorescence imaging of live animals was done at each time point using Odyssey Infrared Imaging System (LI-COR Biosciences) equipped with the MousePOD. Furthermore, blood was collected intracardially and organs were removed and scanned with the Odyssey Imaging System at baseline and at 1/2, 1, 2, 4, 12, 24, and 48 hours after administration of labeled chemokines.
Electrotransfer of plasmids encoding CXCL4 or CXCL4L1 in the tibialis anterior of mice
Fifteen micrograms of control pSCT DNA plasmid or pSCT DNA plasmids encoding CXCL4 (pSCT-CXCL4) or CXCL4L1 (pSCT-CXCL4L1) were injected into the tibialis anterior of 6-week-old BALB/c mice (female, n = 6 per group). Electroporation was performed as described using an ECM 830 electroporator (BTX Division of Genetronics Inc).19 For determination of CXCL4 or CXCL4L1 expression in the muscle, the electroporated tibialis anterior was excised and protein extracts, obtained after homogenization in a tissue extraction reagent (Invitrogen), were analyzed by Western blotting and densitometry.
SPR
Real-time binding experiments were performed with a BIAcore 3000 biosensor instrument (BIAcore AB) or Proteon XPR36 (Bio-Rad) biosensor instrument. Heparin (Hep), heparan sulfate (HS), or chondroitin sulfate B (CSB) were biotinylated (with the EZ-Link Biotin-LC-Hydrazide kit; Pierce) and were immobilized (140, 120, and 120 resonance units [RU], respectively) on a streptavidin-coated sensorchip (chip SA, BIAcore AB; NLC Sensorchip, Bio-Rad). Mab-L4 (mAb7952; R&D Systems) and Mab-L1 antibodies were immobilized (10 000 RU) on a CM5 sensorchip (BIAcore AB). Sensorgrams are representative of specific interactions (differential response) and results are expressed as RU as a function of time in seconds. A kinetic analysis to determine association, dissociation, and affinity constants (ka, kd, and KD, respectively) was carried out by injecting different protein concentrations over immobilized GAGs (16-2000nM; 30 μL/min; 600 second-association phase; 300-second dissociation phase) or over immobilized antibodies (31.25-500 nM; 30 μL/min; 150-second association phase; 300-second dissociation phase). The dissociation rate of the complexes of recombinants proteins with GAGs or with antibodies were not influenced by the contact time (4-8 minutes, data not shown). Binding parameters were obtained by fitting the overlaid sensorgrams with the 1:1 Langmuir binding model of the BIAevaluation 3.1 software.
Statistic analysis
Data are presented as mean ± SD. Statistical analyses were performed using the Student t test. *P < .05; **P < .01; ***P < .001. Half inhibitory concentration (IC50) values were determinate with equation log (inhibitor) versus response variable slope (4 parameters), and half-lives were determined with equation 2 phases exponential decay (Graphpad Prism Software Version 1.0).
Reagents, construction of expression plasmids, protein expression, purification, Western blot analysis, CXCL4L1- specific monoclonal antibody, and cell death assays
These items are described in detail in supplemental Methods and supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
In vitro functional characterization of recombinant CXCL4 and CXCL4L1
Mature CXCL4 and CXCL4L1 only differs by 3 amino acids (Figure 1A) located in the C-terminal α-helix (Figure 1A, gray shade). CXCL4 (rCXCL4) and -CXCL4L1 (rCXCL4L1) fused to glutathione S-transferase (GST) were expressed in Escherichia coli, purified by glutathion sepharose affinity chromatography and verified by sodium dodecyl sulfate polyacrylamide gel electrophoresis, Coomassie staining, and Western blot (supplemental Figure 1A). The biologic activity of rCXCL4 and rCXCL4L1 was evaluated through their ability to inhibit migration and/or the proliferation of endothelial cells in vitro.16 Using the scratch wound assay we first confirmed that rCXCL4L1 is at least a 250-fold more potent inhibitor of cell migration than rCXCL4 for bovine aortic endothelial cells (BAECs; Figure 1B-C). The chemotactic activity of FGF2, tested on HUVECs by Boyden chamber assays, was drastically inhibited after 16 hours exposure to 20 ng/mL (2.5nM) of rCXCL4L1, whereas rCXCL4 only did so for much higher concentrations ( ≥ 1000 ng/mL, 125nM; Figure 1D). Moreover, we evaluated the effect of rCXCL4 and rCXCL4L1 on FGF2-dependent proliferation of HUVECs and BAECs. As previously described, rCXCL4 inhibited proliferation of HUVECs20 and BAECs with an IC50 of 2.3 μg/mL. We determined an IC50 value of 0.053 μg/mL for rCXCL4L1 indicating that the antiproliferative effect of CXCL4L1 is 43× greater than that of CXCL4 (Figure 1C). In addition, no difference in activity was observed between rCXCL4 (CXCL4 fused to GST), CXCL4 purified after removal of GST (CXCL4c) or commercially available CXCL4 (supplemental Figure 1B). Similarly, GST fusion had no effect on CXCL4L1 activity (supplemental Figure 1B). In addition, rCXCL4L1 or rCXCL4 had no effect on nonendothelial cells such as HEK 293 (supplemental Figure 1C) and did not induce toxicity nor apoptosis of endothelial cells (supplemental Figure 1D-E). Taken together, this indicates that CXCL4L1 and, to a lesser extend CXCL4, act both on endothelial cell proliferation and migration without induction of cell death.
Functional characterization of recombinants rCXCL4 and rCXCL4L1 in vitro. (A) Alignment of CXCL4, CXCL4L1 and CXCL4-241 amino acid sequences. The fully conserved (.) and substituted residues were indicated. α-helix (gray shade) of CXCL4 is represented. The alignment was constructed using ClustalW. (B) In vitro endothelial cell migration assay using the scratch assay. BAECs were stimulated with FGF2 (10 ng/mL) in the presence or absence of rCXCL4L1 (0.02 and 0.05 μg/mL) or rCXCL4 (1 and 5 μg/mL). (C) Quantification of the scratch assay results (n = 6). (D) In vitro endothelial cell migration assay using Boyden chambers. In comparison, the effect of CXCL4L1 or CXCL4 on endothelial cell migration was also tested on HUVECs (n = 6). (E-F) In vitro endothelial cell proliferation using MTT assay. BAECs and HUVECs were stimulated with FGF2 (10 ng/mL) in presence or absence of various concentrations of rCXCL4 or rCXCL4L1 and cell proliferation was determined.
Functional characterization of recombinants rCXCL4 and rCXCL4L1 in vitro. (A) Alignment of CXCL4, CXCL4L1 and CXCL4-241 amino acid sequences. The fully conserved (.) and substituted residues were indicated. α-helix (gray shade) of CXCL4 is represented. The alignment was constructed using ClustalW. (B) In vitro endothelial cell migration assay using the scratch assay. BAECs were stimulated with FGF2 (10 ng/mL) in the presence or absence of rCXCL4L1 (0.02 and 0.05 μg/mL) or rCXCL4 (1 and 5 μg/mL). (C) Quantification of the scratch assay results (n = 6). (D) In vitro endothelial cell migration assay using Boyden chambers. In comparison, the effect of CXCL4L1 or CXCL4 on endothelial cell migration was also tested on HUVECs (n = 6). (E-F) In vitro endothelial cell proliferation using MTT assay. BAECs and HUVECs were stimulated with FGF2 (10 ng/mL) in presence or absence of various concentrations of rCXCL4 or rCXCL4L1 and cell proliferation was determined.
rCXCL4 and rCXCL4L1 GAG affinities
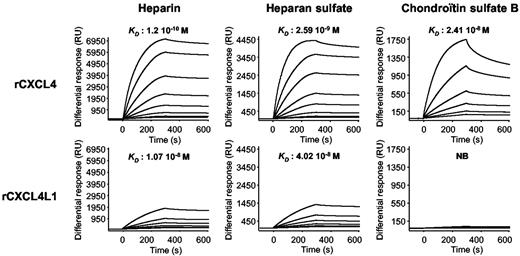
GAG binding is of great importance for the biologic activity of a large number of chemokines and growth factors. It has previously been shown CXCL4 can interact in vitro and in vivo with sulfated GAGs21–24 and that the C-terminal region (in which lies the differences between CXCL4 and CXCL4L1) is required for these interactions.20 Using surface plasmon resonance (SPR) experiments, we determined the affinity constants of the 2 chemokines for GAGs. To this aim, Hep, HS, and CSB were biotinylated and immobilized onto a streptavidin-coated sensor chip. As shown in Figure 2, rCXCL4 strongly bound to the immobilized GAGs with affinity constants of 1.2 × 10−10M for Hep, 2.59 × 10−9M for HS and 2.41 × 10−9M for CSB. On the other hand, rCXCL4L1 bound to Hep and HS with moderate affinity of 1.07 × 10−8M and 4.02 × 10−8M, respectively. More surprisingly, no significant binding of rCXCL4L1 to CSB was observed (KD > 10−2). These results clearly indicate that a difference of 3 amino acids between rCXCL4 and rCXCL4L1 has dramatic consequences for their interactions with GAGs. We next set out to examine the biologic consequences of these differences in cellulo and in vivo.
Overlaid sensograms for the binding of rCXCL4 and rCXCL4L1 to purified GAGs. Sensorgrams and representative KD from the injection of different concentrations (15.6-2000nM) of rCXCL4 and rCXCL4L1 over immobilized GAGs are depicted. NB indicates no binding.
Overlaid sensograms for the binding of rCXCL4 and rCXCL4L1 to purified GAGs. Sensorgrams and representative KD from the injection of different concentrations (15.6-2000nM) of rCXCL4 and rCXCL4L1 over immobilized GAGs are depicted. NB indicates no binding.
In cellulo diffusion of CXCL4 and CXCL4L1
First, HEK293 cells were treated with recombinant chemokines. We also included CXCL4-241 (initially named PF4-241) as a control. In the latter, the 4 lysines located at C-terminus of CXCL4 are mutated (Figure 1A) leading to a complete loss of heparin binding.20 After 4 hours incubation, cell membrane fractions were analyzed by Western blotting using an anti-CXCL4 antibody that recognizes equally well the 3 proteins.16,25 CXCL4 and CXCL4L1 were differentially bound to the cell membranes whereas CXCL4-241 was never found membrane-associated (Figure 3A). These results are in good agreement with the binding experiments (Figure 2).
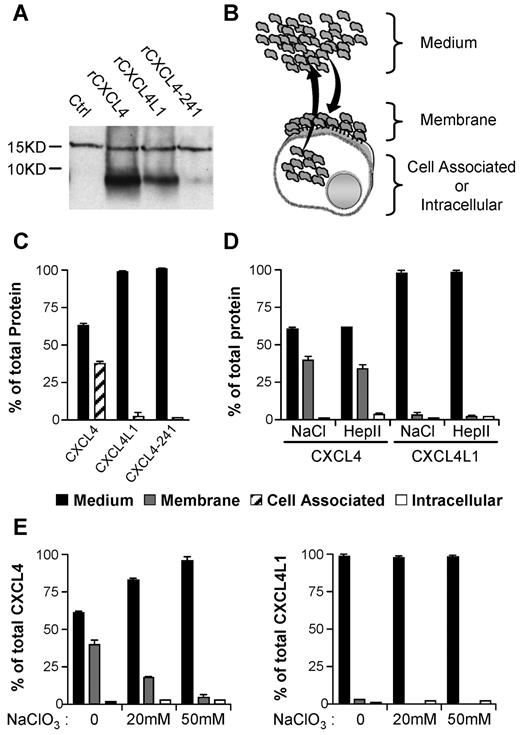
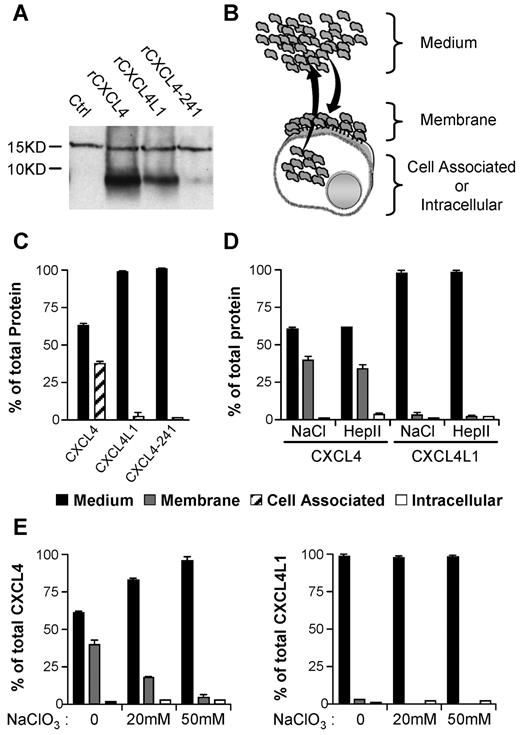
In vitro association properties of CXCL4 and CXCL4L1 to GAGs sulfated membranes. (A) Western blot with Mab-L4 antibody, which neither discriminates between CXCL4, CXCL4L1, or CXCL4-241, of membrane fractions of cells treated with 1 μg/mL recombinant proteins. (B) Schematic representation of membrane retention or diffusion of secreted protein after transfection. (C) CXCL4-ELISA assay on the different fractions of transfected HEK293T cells. CXCL4, CXCL4L1, and CXCL4-241 released in the medium (1) or remaining cell-associated (2), were quantified. (D) Distribution of CXCL4 and CXCL4L1 in medium and the membrane bound or intracellular fraction. Membrane-bound chemokines were collected with high salt (NaCl) or heparinase II (Hep II) treatment. (E) Membrane binding of CXCL4 and CXCL4L1 in cells treated or not with sodium chlorate (NaClO3). Results are represented as percent of total proteins expressed.
In vitro association properties of CXCL4 and CXCL4L1 to GAGs sulfated membranes. (A) Western blot with Mab-L4 antibody, which neither discriminates between CXCL4, CXCL4L1, or CXCL4-241, of membrane fractions of cells treated with 1 μg/mL recombinant proteins. (B) Schematic representation of membrane retention or diffusion of secreted protein after transfection. (C) CXCL4-ELISA assay on the different fractions of transfected HEK293T cells. CXCL4, CXCL4L1, and CXCL4-241 released in the medium (1) or remaining cell-associated (2), were quantified. (D) Distribution of CXCL4 and CXCL4L1 in medium and the membrane bound or intracellular fraction. Membrane-bound chemokines were collected with high salt (NaCl) or heparinase II (Hep II) treatment. (E) Membrane binding of CXCL4 and CXCL4L1 in cells treated or not with sodium chlorate (NaClO3). Results are represented as percent of total proteins expressed.
We next determined whether the differences in GAGs affinities have consequences for the release when chemokines are expressed by the cells. To this goal, HEK293 were transiently transfected and a comparable level of CXCL4, CXCL4L1, or CXCL4-241 was obtained (supplemental Figure 2C). The protein content of different fractions (cell-associated, intracellular and membrane-bound fractions, and the culture medium) was determined (Figure 3B-C). The majority of CXCL4L1 was found in the medium, whereas a significant part of CXCL4 remained associated to the cells (Figure 3C). As expected, CXCL4-241 was only detected in the medium (Figure 3C). To identify whether cell-associated chemokines were bound to the cell surface or remained intracellular, various treatments that dissociate molecules from GAGs were used. High salt treatment completely removed CXCL4 and CXCL4L1 indicating that chemokines were associated with the cell surface in a noncovalent manner (Figure 3C). Heparanase II treatment indicated that HS-GAGs are the major cell surface binding sites for both, CXCL4 and CXCL4L1 (Figure 3D). After 48 hours pretreatment of HEK293 cells by sodium chlorate (inhibition of GAGs sulfation), a drastic reduction in protein content in the membrane fraction was observed for CXCL4. In addition, CXCL4L1 which was initially already low, was further decreased by sodium chlorate pretreatment (Figure 3E). This indicates that sulfate groups are required for binding. Furthermore, our results clearly indicate that CXCL4 was much better recovered from the membrane than CXCL4L1 (39% for CXCL4 versus 2% for CXCL4L1 in the membrane fraction). Significantly more CXCL4L1 than CXCL4 was found in the medium (98% for CXCL4L1 versus 60% for CXCL4). Thus, CXCL4L1 is less tightly associated to the cell surface than CXCL4 and diffuses much more efficiently after secretion.
It has been recently published that the secretion of CXCL4 is much lesser efficient than that of CXCL4L1 due to their respective signal peptides that are much more divergent than the mature protein.17 Because the differences in the signal sequence could greatly influence our analysis, we design expression vectors in which the signal peptides were exchanged or replaced by the vascular endothelial growth factor signal sequence (supplemental Figure 2A). Whatever the signal peptide used, secretion efficiency was very high, and no effect on the distribution of CXCL4 or CXCL4L1 within the different fractions (membrane, intracellular, medium) was observed (supplemental Figure 2B). In the absence of signal peptide, there was no release of CXCL4, even if transfected cells were washed with a high-salt solution to remove membrane-bound chemokines (supplemental Figure 2). Thus, although the signal peptides exhibit 38% amino acid divergence, both chemokines are released with similar efficiency.
Consequently, the differences in the distribution of both chemokines in the different cell compartments are due to the 3 amino acids divergence of the C-terminus.
In vivo behavior of CXCL4 and CXCL4L1
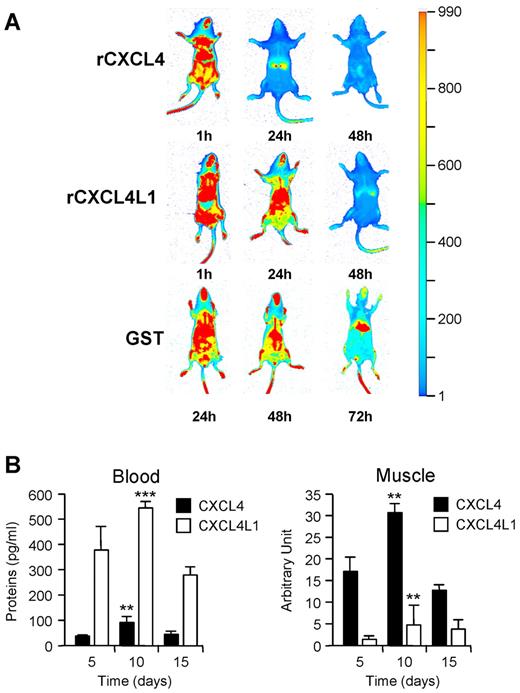
Because the in cellulo diffusibility of CXCL4L1 was greatly enhanced compared with CXCL4, we examined the bioavailability and the clearance of these 2 molecules in vivo. To this aim, rCXCL4 and rCXCL4L1 were labeled with IR dye (supplemental Figure 3A). Biologic activities were verified before injection (supplemental Figure 3B). A single intravenous injection of 2 × 10-9 moles (17 μg) of labeled chemokine was given to mice. After 24 hours, fluorescence imaging revealed a much more rapid clearance for rCXCL4 than rCXCL4L1 (Figure 4A) and a greater accumulation of rCXCL4 in the liver (Figure 4A and supplemental Figure 3C). Serum levels of rCXCL4 and rCXCL4L1 were determined at different times after injection into mice using a specific ELISA test that does not recognize mouse CXCL4. In agreement with previous studies,22,26 our results indicated that rCXCL4 was very rapidly cleared from the circulation in a biphasic pattern with half-lives of 2.1 minute and 30-40 minutes. rCXCL4L1 showed also a biphasic pattern of disappearance from the circulation with half-life of 5.33 minutes and 58 minutes (supplemental Figure 3D).
In vivo diffusion of CXCL4 and CXCL4L1. (A) Injection of rCXCL4, rCXCL4L1, or GST labeled with IRDye800CW in RAG-γ/c mice (n = 6). Biodistribution was monitored with IR signal at 1, 24, and 48 hours after injection. GST was still detected at 72 hours, whereas CXCL4 and CXCL4L1 were completely cleared from the mice. (B) In vivo diffusion of human CXCL4 and CXCL4L1 expressed in mice using electrotransfer in the tibialis anterior muscle (n = 6). Plasma levels were determined using a human CXCL4-ELISA (left panel) specific for human chemokines. No signal was detected in controls (mouse tissue). Expression of human CXCL4 and CXCL4L1 in the tibialis anterior muscle was also detected by Western blot using Mab-L4 (right panel). The graphs represent the densitometric analysis. No signal was observed for control mice electrotransfered with the empty vector.
In vivo diffusion of CXCL4 and CXCL4L1. (A) Injection of rCXCL4, rCXCL4L1, or GST labeled with IRDye800CW in RAG-γ/c mice (n = 6). Biodistribution was monitored with IR signal at 1, 24, and 48 hours after injection. GST was still detected at 72 hours, whereas CXCL4 and CXCL4L1 were completely cleared from the mice. (B) In vivo diffusion of human CXCL4 and CXCL4L1 expressed in mice using electrotransfer in the tibialis anterior muscle (n = 6). Plasma levels were determined using a human CXCL4-ELISA (left panel) specific for human chemokines. No signal was detected in controls (mouse tissue). Expression of human CXCL4 and CXCL4L1 in the tibialis anterior muscle was also detected by Western blot using Mab-L4 (right panel). The graphs represent the densitometric analysis. No signal was observed for control mice electrotransfered with the empty vector.
We next investigated the in vivo bioavailability of CXCL4 and CXCL4L1 when expressed continuously by a mouse tissue. To this aim, plasmids expressing human CXCL4 or CXCL4L1 cDNAs were electrotransfered into the tibialis anterior muscle of Balb/c mice. At specified time points after electrotransfer, chemokine levels were measured both in the plasma and muscle using ELISA test that only recognizes human CXCL4L1 or CXCL4. As shown in Figure 4B, the amount of circulating CXCL4L1 was much greater than that of CXCL4 (left panel). This was inversed in the muscle, where much more CXCL4 than CXCL4L1 was recovered (right panel). These data clearly show that CXCL4L1 is highly diffusible, in contrast to CXCL4, which remains sequestered in the muscle, the site where it is produced.
Leucine versus Histidine at position 67 is critical for GAGs affinities
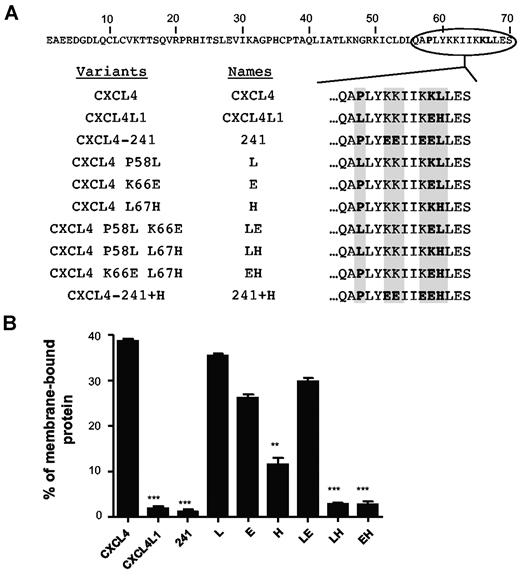
Because mature CXCL4L1 only differs from CXCL4 by 3 amino acids, we determined which substitution is responsible for the variations in GAG affinity. We produced a series of CXCL4 mutants (Figure 5A and supplemental Figure 1A). It is of note that the variant CXCL4 L67H could not be produced, probably because of a defect in the stability of the recombinant protein in Escherichia coli. Using SPR analysis, all the purified proteins were tested and their affinity constants for immobilized GAGs were determined (Table 1). Each individual substitution had more or less an effect on GAGs binding. Remarkably, all molecules with His67 (including CXCL4L1) had very reduced affinities for Hep and HS and did not bind CSB. On the other hand, proteins with Leu67 (including CXCL4) showed high affinities to Hep (KD values at the picomolar to nanomolar range) and HS. Within this last class of proteins, the Pro58 substitution (L and LE proteins) abolished the binding to CSB. This indicates that, together with Leu67, Pro58 is required for CSB binding of CXCL4. The Lys/Glu66 substitution had only a moderate effect.
Histidine 67 is critical for the membrane binding of CXCL4L1. (A) Schematic representation of the amino acide sequences of CXCL4, CXCL4L1, or mutants. (B) In cellulo binding properties of CXCL4, CXCL4L1 and mutants to cell membranes. HEK293T cells were transfected with plasmids expressing CXCL4, CXCL4L1, or mutants. The amount of proteins associated with the membrane was determined using the human CXCL4-ELISA, which detects CXCL4, CXCL4L1, and all variants.
Histidine 67 is critical for the membrane binding of CXCL4L1. (A) Schematic representation of the amino acide sequences of CXCL4, CXCL4L1, or mutants. (B) In cellulo binding properties of CXCL4, CXCL4L1 and mutants to cell membranes. HEK293T cells were transfected with plasmids expressing CXCL4, CXCL4L1, or mutants. The amount of proteins associated with the membrane was determined using the human CXCL4-ELISA, which detects CXCL4, CXCL4L1, and all variants.
To validate these observations in the living cell, we performed in cellulo diffusion assays. As shown in Figure 5, the different chemokines, when transfected in HEK 293 cells, were efficiently produced and secreted (supplemental Figure 2C-D). The results clearly show that the presence of His67 inhibited chemokine retention at the cell membrane. These results are in agreement with affinities for GAGs observed by SPR (Table 1) confirming that GAGs are essential for cell surface retention. Interestingly, the Lys/Glu66 substitution (Table 1) showed a greater effect on membrane retention than on GAG affinity (Figure 5B). This indicates that other membrane constituents (eg, lipids) may also contribute to surface retention.
Histidine 67 is critical for inhibition of cell proliferation and in vitro angiogenesis
We then assessed the ability of rCXCL4 mutants to inhibit the proliferation of BAECs. IC50 and Imax presented in Table 2 (complete data are presented in supplemental Figure 4) clearly show that 2 groups could be distinguished: proteins with Leu67 and proteins with His67. Proteins with Leu67 (including CXCL4) showed an IC50 ranged between 0.8 and 2.3 μg/mL whereas those with the His67 (including CXCL4L1) have an IC50 ranged between 0.04 and 0.15 μg/mL. In accordance with previous results, we showed that CXCL4-241 present a biologic activity similar to CXCL4 indicating that the inhibitory effect is not dependent on GAG affinity. More importantly, when Leu67 is replaced by His67, at least a 5.5-fold increase in inhibitory activity was observed (Table 2). This indicates that the 2 different effects of His67 substitution (ie, modification of GAG binding and biologic activity) are not necessary connected.
Mutants with single mutation were tested in a tube-formation assay. Once again, replacement of Leu67 by His67 induced an increased inhibitory effect (Figure 6) indicating that in vitro angiogenesis was also sensitive to the single amino acid substitution.
Histidine 67 is critical for inhibiting the FGF2-induced capillary tube formation of HUVECs by CXCL4L1. (A) Representative images of HUVECs on Matrigel stimuled with FGF2 and in the presence or not of the indicated chemokine. (B) Quantitative analysis of the experiments shown in panel A obtained by counting the number of branching points from 4 fields.
Histidine 67 is critical for inhibiting the FGF2-induced capillary tube formation of HUVECs by CXCL4L1. (A) Representative images of HUVECs on Matrigel stimuled with FGF2 and in the presence or not of the indicated chemokine. (B) Quantitative analysis of the experiments shown in panel A obtained by counting the number of branching points from 4 fields.
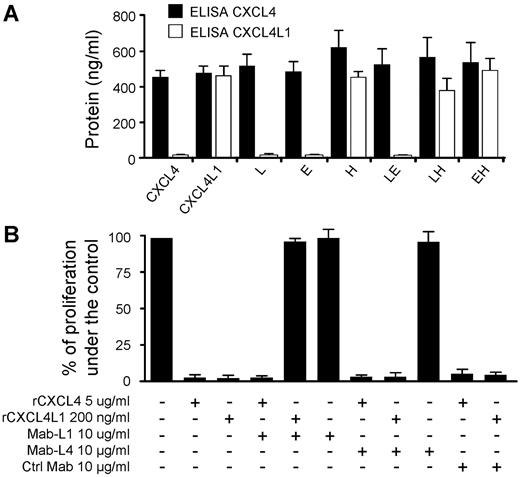
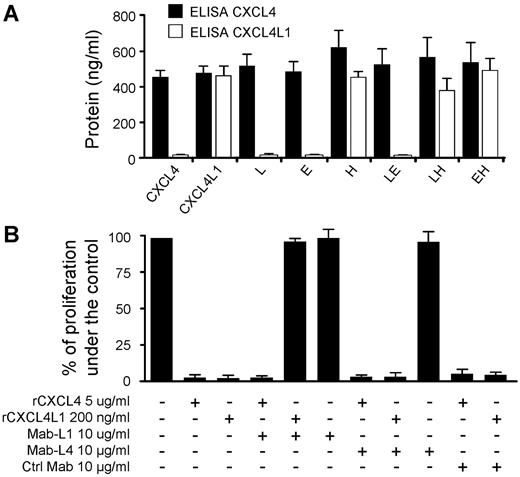
To better characterize the CXCL4L1 activity, we developed mouse monoclonal antibodies (Mab-L1) directed against a peptide that corresponds to the 20 amino acids of the C-terminal part of CXCL4L1. Mab-L1 tightly bound rCXCL4L1 but not rCXCL4 as determined by BIAcore analysis and slot-blot assay (supplemental Figure 5A-B). Mab-L1 was used to perform ELISA to detect the different proteins expressed by transfected cells (supplemental Figure 5C). Our results clearly show that Mab-L1 only recognized CXC4L1 or mutants that contain His67 (Figure 7A). This indicates that His67 was crucial for the epitope recognized by Mab-L1. His67 may contribute to the epitope by itself, or induce a conformational change within the C-terminal region.
Specific anti-CXCL4L1 monoclonal antibody blocks CXCL4L1's biologic activity. (A) The specific anti-CXCL4L1 monoclonal antibody (Mab-L1) detects specifically proteins possessing His67. Conditioned media of HEK293T expressing CXCL4, CXCL4L1, or mutants were tested with CXCL4-ELISA (Mab-L4) or with a specific CXCL4L1-ELISA (Mab-L1). (B) Effect of Mab-L1 (10 μg/mL) on the proliferation of BAEC treated or not with rCXCL4 (5 μg/mL) or rCXCL4L1 (0.2 μg/mL). Mab-L4 and unrelated Immunoglobulin G were used as controls.
Specific anti-CXCL4L1 monoclonal antibody blocks CXCL4L1's biologic activity. (A) The specific anti-CXCL4L1 monoclonal antibody (Mab-L1) detects specifically proteins possessing His67. Conditioned media of HEK293T expressing CXCL4, CXCL4L1, or mutants were tested with CXCL4-ELISA (Mab-L4) or with a specific CXCL4L1-ELISA (Mab-L1). (B) Effect of Mab-L1 (10 μg/mL) on the proliferation of BAEC treated or not with rCXCL4 (5 μg/mL) or rCXCL4L1 (0.2 μg/mL). Mab-L4 and unrelated Immunoglobulin G were used as controls.
Mab-L1 was then used in proliferation assays on BAECs and HUVECs. As shown in Figure 6 B, Mab-L1 completely blocked the inhibitory effect of CXCL4L1 but had no effect on CXCL4 activity. In opposite, Mab-L4, that recognized both chemokines, had no influence on their inhibitory activity (Figure 7B). Taken together, these results indicate that the motif involved in the inhibitory effect of CXCL4L1 lies in its carboxyl terminal part. This is in accordance with previous results, which demonstrate that peptides derived from the C-terminal part of CXCL4 have angiostatic properties.10,11
Discussion
CXCL4 is a CXC chemokine that has pleiotropic effects and plays a role in blood coagulation,27 angiogenesis,6,8 modulation of the immune system28 and tumor growth.8 CXCL4 has also been proposed to play critical role in other human diseases including heparin-induced thrombocytopenia (HIT) and progression of multiple myeloma.29,30 During evolution, recent gene duplication gave rise to a second CXCL4 form, named PF4alt/PF4V1 or CXCL4L1.31 CXCL4L1 is only found in some primates. Except for the parts of the genes that encode the mature polypeptides (95% identity), the genetic conservation between the 2 genes is poor (promoters, introns, 5′ untranslated region, and 3′ untranslated region). This indicates that the regulation of expression has drastically diverged between the CXCL4 and CXCL4L1 genes. As for the signal sequence, the situation is particular, because, despite significant genetic divergence (difference in 13 of 33 amino acids), the efficiency of secretion is similar as demonstrated in this work. Among the 3 diverging amino acids, leucine 67 (for CXCL4) is replaced by histidine (for CXCL4L1) in rhesus monkey, chimpanzee, and orangutan or by arginine in marmoset. Proline 58 is replaced by leucine only in chimpanzee and orangutan whereas glutamate 66 is only found in human. This underlines the fact that the leucine 67 mutation is the first event that evolutionary distinguishes CXCL4L1 from CXCL4. This is in line with our results showing that this event is also the most significant in terms of biologic activity and glycan binding. This highlights the fact that a single amino acid substitution has significant consequences on the biologic properties of regulatory molecules.
We have clearly shown that these 2 chemokines mainly differ by their cell surface retention and that the 3 amino acid substitutions within the carboxyl terminus are responsible for this effect. It is well known that chemokines exert their biologic activity through high-affinity interactions with cell-surface receptors, but their interactions with proteoglycans remain crucial because they may facilitate high local concentrations required for cell activation.3 We have determined striking differences in the affinity constants for GAGs between CXCL4, CXCL4L1 and the different mutants and fusion proteins. First, GAG affinity was directly correlated with membrane retention as shown by transfection experiments. Second, affinities for heparin and heparan sulfate were significantly reduced for CXCL4L1 and, most interestingly, binding to chondroïtin sulfate B was abolished. Because chemokines/GAGs interactions are highly dependent on positively charged amino acids, it was surprising that the Lys/Glu substitution had only a limited impact. On the other hand, the Leu/His substitution had dramatic consequences for all GAG affinities. In between was the Pro/Leu substitution that mainly affected CSB affinity.
The interaction of CXCL4 with chondroïtin sulfate B is of great biologic significance and includes leukocyte activation,32 binding of monocytes to the endothelium,33 adhesion of progenitors34 and interaction with the LDL receptor.35 We show herein that the substitutions of 2 amino acid is involved in the inability of CXCL4L1 to bind CSB. This could explain the loss in its inhibitory activity of monocytes or neutrophils,36 a property that seems to be restricted to CXCL4.
CXCL4L1 has a much more potent angiostatic activity than CXCL4. We initially sought that this increase is related to the decreased affinity in proteoglycan binding, making CXCL4L1 more available for interaction with a potential receptor. However, when the Leu/His67 mutation was introduced into CXCL4-241 (PF4-241) which does not bind proteoglycans and which has similar activity than CXCL4,20 a significant increase in biologic activity was also observed. This strongly suggests that the Leu/His mutation has 2 potentially unrelated effects ie decrease of GAG binding and increase in biologic activity. The role of His67 in the biologic activity of CXCL4L1 is reinforced by inhibition experiments using a monoclonal antibody. This antibody, Mab-L1, recognized specifically His67 of CXCL4L1 or a conformational change of the C-terminus induced by substitution of the amino acid. Nevertheless, Mab-L1 was able to completely block CXCL4L1 activity whereas an antibody directed against the amino-terminal half of the molecule had no effect. These findings mostly argue for a structural re-organization of the carboxyl terminal domain of CXCL4L1, which is responsible for the specific effects of the molecule. It is also not to exclude that difference in biologic activity may be related to different oligomeric state of CXCXL4L1 in comparison to CXCL4. Structure biology experiments are underway to address this issue.
Finally, we show that CXCL4L1 and CXCL4 are nonredundant chemokines because they differ in many aspects from each other. In particular, binding to GAGs and diffusibility are strikingly different. The high local concentration of CXCL4 at the surface of producing cells, could explain a juxtacrine effect that involves vicinity with target endothelial cells. Conversely, we have shown in vivo that CXCL4L1 has a high diffusibility, an enhanced half-life and, in vitro, a low IC50 value for endothelial cell inhibition. These parameters clearly indicate that CXCL4L1 is a paracrine regulator acting over longer distances than CXCL4 does. Consequently, when CXCL4 and CXCL4L1 are produced by the same cells such as megakaryocytes or platelets, their juxtacrine and paracrine modes of actions are complementary rather than redundant. Nevertheless, little is known about the spatial and temporal expression of these 2 chemokines and it will be of great importance to better characterize their respective regulation of gene expression in normal and pathologic tissues.
Existing data point out to a role of CXCL4 chemokines as a biomarker for disease. It has been proposed that circulating platelet-derived CXCL4 could represent a suitable biomarker for solid tumors,37 cardiovascular disease or trauma.38 However, the lack of specific antibodies could be responsible for misinterpretations because the reagents available do not discriminate between CXCL4 and CXCL4L1. It has not escaped our notice that the better diffusibility and stability makes CXCL4L1 a potential better biomarker than CXCL4. Antibodies that discriminate between the 2 chemokines may thus be of importance not only for research but also as diagnostic tools for monitoring patients and the response to therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank A. Rayssac for technical assistance with the electrotransfer experiments in mice.
This work was supported by grants from the ACI Cancéropoles 2007 (Institut National du Cancer) to H.P. and A.B., Agence National de la Recherche (ANR, ANR Blanc Angio_ANR_NSC) to A.B. and W.-G.W., The Institut National du Cancer (INCA, ChemoRencan project) to A.B. and H.P., Association de la Recherche sur le Cancer (ARC) to A.B., and Ligue contre le Cancer to A.B.
Authorship
Contribution: A.D., A.B., and H.P. designed research; A.D., C.Q., C.Z., and E.L. performed research; A.D. and F.L. performed SPR experiments and analysis; A.D., C.Q., F.L., W.-G.W., A.B., and H.P. analyzed data; and A.D., W.-G.W., A.B., and H.P. drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Bikfalvi, Insem U920, University Bordeaux I, Avenue des Facultes, 33405 Talence, France; e-mail: a.bikfalvi@angio.u-bordeaux1.fr.
References
Author notes
A.B. and H.P. are co-senior authors.