Abstract
We evaluated the safety and clinical outcome of autologous nonmyeloablative hematopoietic stem cell transplantation (HSCT) in patients with severe Crohn disease (CD) defined as a Crohn Disease Activity Index (CDAI) greater than 250, and/or Crohn Severity Index greater than 16 despite anti–tumor necrosis factor therapy. Stem cells were mobilized from the peripheral blood using cyclophosphamide (2.0 g/m2) and G-CSF (10 μg/kg/day), enriched ex vivo by CD34+ selection, and reinfused after immune suppressive conditioning with cyclophosphamide (200 mg/kg) and either equine antithymocyte globulin (ATG, 90 mg/kg) or rabbit ATG (6 mg/kg). Eighteen of 24 patients are 5 or more years after transplantation. All patients went into remission with a CDAI less than 150. The percentage of clinical relapse-free survival defined as the percent free of restarting CD medical therapy after transplantation is 91% at 1 year, 63% at 2 years, 57% at 3 years, 39% at 4 years, and 19% at 5 years. The percentage of patients in remission (CDAI < 150), steroid-free, or medication-free at any posttransplantation evaluation interval more than 5 years after transplantation has remained at or greater than 70%, 80%, and 60%, respectively. This trial was registered at www.clinicaltrials.gov as NCT0027853.
Introduction
Crohn disease (CD) is a chronic illness, immunologically mediated, of unknown etiology but probably induced by an exposure to intestinal bacteria or their component antigens leading to an excessive T helper type 1–mediated chronic inflammation of the gastrointestinal (GI) tract in patients with genetic susceptibility.1,2 Regardless of the therapy used, some patients remain seriously ill with active disease after all therapeutic options have been exhausted.3-12 Although not well characterized, a distinct excessive mortality from CD exists in this group of patients.13-20 In addition, patients with severe, refractory disease suffer from an inability to eat, frequent nausea, vomiting, diarrhea, malnutrition, growth retardation in children, fistulas, abdominal pain, extra-intestinal symptoms, psychologic distress from an ileostomy or colostomy bag, iatrogenic addiction to narcotics, toxicities of standard therapies, and multiple surgeries that may lead to short-gut syndrome, chronic total parenteral nutrition, and liver failure. Because of our experience using nonmyeloablative hematopoietic stem cell transplantation (HSCT) in autoimmune diseases such as systemic lupus erythematosus,21 multiple sclerosis,22 and type 1 diabetes mellitus,23 we began in 2001 on an autologous nonmyeloablative HSCT study for severe, anti–tumor necrosis factor (TNF) refractory CD.
Although autologous HSCT using a myeloablative regimen causes irreversible bone marrow failure, thus requiring mandatory hematopoietic stem cell (HSC) reinfusion, nonmyeloablative regimens, as used in this study, halt inflammation without irreversibly altering the ability of bone marrow to recover.24 Following a nonmyeloablative regimen, autologous stem cells are infused as a supportive blood product to hasten recovery and shorten duration of neutropenia and cytopenia.
The rationale for autologous HSCT is that the conditioning regimen induces an immediate immune cease-fire, whereas the stem cells regenerate self-tolerant lymphocytes in the posttransplantation noninflammatory terroir (environment).24 In support of this rationale, we have demonstrated in patients with multiple sclerosis, an “immune reset” accompanied by a surge in recent thymic emigrants and generation of a new and diverse T cell repertoire after transplantation.25 We have also reported in patients with systemic lupus erythematosus that HSCT results in posttransplant tolerance to lupus-specific histone epitopes as well as a surge in CD8+CD103+FoxP3+ and CD4+CD25+FoxP3+ regulatory T cells (Treg) cells.26
Methods
Study design
This phase 1/2 study was designed to investigate safety and efficacy of nonmyeloablative autologous HSCT using high-dose cyclophosphamide, antithymocyte globulin (ATG), and autologous CD34+ cell–enriched HSC in chronic active CD patients refractory to conventional therapies including anti-TNF inhibitor.
Patient selection
The patients and/or their parents read and signed an informed consent in accordance with the Declaration of Helsinki under Institutional Review Board and the United States Food and Drug Administration (FDA) approved study IDE 7846. Candidates for HSCT had to have clinical and histologic evidence of CD, be between age 14 and 60, and failed conventional therapy including the chimeric anti-TNF monoclonal antibody infliximab. With the advent of FDA approval of adalimumab (humanized anti-TNF antibody) in 2007, we included failure to respond to adalimumab in those allergic to infliximab. Each subject had a persistent Crohn Disease Activity Index (CDAI) of 250 to 400 or a Crohn Severity Index (CSI) greater than 16 (Table 1). All of the 12 original study subjects had severity indices greater than 16 in addition to CDAI greater than 250.28 Because of limitations of the CDAI, the FDA approved the enlisting of subsequent subjects with CSI at 17 or greater even though some did not meet the CDAI score of 250. Subjects with significant heart disease, liver disease unrelated to CD, hematologic disease, active infection, toxic megacolon, active bowel obstruction, or intestinal perforation were excluded. Fistulizing and stricturing CD were not excluded.
Peripheral blood stem cell harvest
Peripheral blood stem cells were mobilized with cyclophosphamide 2 g/m2 followed by granulocyte colony-stimulating factor (G-CSF) 10 mcg/kg/day beginning 5 days after cyclophosphamide. On day 10 after cyclophosphamide, the white blood cells rebounded, and leukaphereses were continued daily until an enriched target CD34+ cell count (2.0 × 106/kg) was achieved. T-cell depletion was performed by enrichment of CD34+ cells using Isolex 300i magnetic immunoselection (Baxter). The HSC graft was cryopreserved until the date of transplantation (reinfusion).
Conditioning regimen
The conditioning regimen consisted of cyclophosphamide 50 mg/kg/day for the 4 days before HSC infusion; in the first 10 subjects, 30 mg/kg/day equine ATG for 3 days or, in the last 14 subjects, 6 mg/kg rabbit ATG, divided over 5 days before HSC reinfusion. Mesna was administered along with the cyclophosphamide to prevent hemorrhagic cystitis, and 1.0 g/day methylprednisolone was administered before each dose of ATG to prevent infusion reactions. G-CSF, 10 mcg/kg/day, was started 5 days after reinfusion and continued until the absolute neutrophil count reached 500/μL.
Supportive care
Patients were treated on a high-efficiency particulate air–filtered medical floor. A low microbial diet, 500 mg oral ciprofloxacin twice daily, 400 mg fluconazole once daily, 500 mg metronidazole 3 times daily, 500 mg acyclovir 3 times daily, and aerosolized pentamidine, 300 mg upon admission, were administered. When the absolute neutrophil count dropped to less than 500/μL, the ciprofloxacin was discontinued and intravenous piperacillin/tazobactam, 3.375 g every 4 hours, was started. Metronidazole and piperacillin/tazobactam were stopped upon neutrophil recovery. Acyclovir at 400 mg twice daily and fluconazole 400 mg once daily were continued for 12 and 6 months after HSCT, respectively. Trimethoprim/sulfamethoxazole double strength (160/800 mg) 3 times weekly was started upon hematopoietic engraftment and continued for 6 months after HSCT. Hemoglobin levels and platelet counts were maintained above 8 g/dL and 20 000/μL, respectively, with leukoreduced, irradiated and cytomegalovirus-safe blood transfusions. All immunosuppressive and disease modifying agents were discontinued upon the stem cell mobilization except systemic corticosteroids that were tapered as out patients according to local physicians.
Assessment of outcomes
Study parameters including clinical, laboratory, and CDAI and CSI28 (Table 1) were performed before transplantation, at 6 and 12 months after HSCT, and then yearly for 5 years. For patients unable to return for follow up, phone contact was initiated with documentation of interval medications, surgeries, hospitalizations, symptoms, and local laboratory values. CDAI and CSI scores were obtained in patients who returned for physician evaluation. The primary outcomes were safety and clinical remission, defined as either a CDAI score less than 150 and a CSI less than 12 or being asymptomatic and requiring no immunosuppressive medications. Secondary outcomes included: (1) medication-free remission defined as free of any CD-related medication for at least 60 days before evaluation; (2) steroid-free remission defined as being off all steroids of any type (absorbable or nonabsorbable) for at least 60 days before evaluation; and (3) clinical relapse-free survival defined as time free of CD medical therapy (excluding posttransplantation corticosteroid taper) before restarting CD medication for symptomatic disease after transplantation.
CD4+CD25+ T cells
In the first 13 subjects CD4+CD25bright Treg cells were assessed in terms of percentage of T cells and absolute number, using flow cytometry before and at 6, 12, 24, and 36 months after the HSCT. Peripheral blood mononuclear cells were separated using standard Ficoll gradient centrifugation. The relative and absolute number of CD4+CD25bright cells was calculated using cell cytometry. Fluorochrome-conjugated monoclonal antibody against CD4, CD25, and CD3 and appropriate isotopic controls were purchased from BD Pharmingen. Flow cytometry was performed on an Epics XL (Beckman Coulter) running with CellQuest software. The Treg cell counts of each subject were compared before and after transplantation and with normal controls.
Genetic testing
The first 21 patients underwent genetic testing by molecular methods for genotyping nucleotide binding oligomerization domain 2 (NOD2) variants and Toll-like receptor 5 (TLR5) polymorphisms.29-35 All samples were analyzed in one laboratory (Dr de Villiers, University of Kentucky Medical Center). For NOD2, the allelic variants G908R, L1007P, and R702W were assayed by polymerase chain reaction (PCR), amplification of the entire sequence of exon 8 and exon 11, and the relevant segment of the large exon 4 of the NOD2/CARD15 gene, followed by restriction fragment length polymorphism analysis. Genomic DNA was extracted from patient whole blood using the Aqua Pure Genomic DNA kit from Bio-Rad. Three separate PCR amplifications for each sample were carried out with the following primer pairs: G908R for 5′-CCCAGCTCCTCCCTCTTC-3′/G908Rrev 5′-AAGTCTGTAATGTAAAGCCAC-3′; L1007 for 5′-GGCAGAAGCCCTCCTGCAGGGCC-3′/L1007fsrev 5′-CCTCAAAATTCTGCCATTCC-3′; and R702W for 5′-AGATCACAGCAGCCTTCCTG-3′/R702Wrev 5′-CACGCTCTTGGCCTCACC-3′. Oligonucleotide primers were custom synthesized by Integrated DNA Technologies. PCR was carried out for 30 cycles with a temperature of 55°C. Restriction fragment length polymorphism analysis was carried out by restriction enzyme digestion (HhaI for G908R, ApaI for L1007P, and MspI for R702W) and subsequent agarose gel electrophoresis. HhaI and ApaI were obtained from Invitrogen, and MspI was obtained from New England Biolabs. Genotypes were determined by band patterns resulting from digestion. For G908R, the absence of a variant yields a 380-bp band. A heterozygote yields a 380-bp band as well as 2 bands at 242 and 138 bp. A homozygote yields only the 242-bp and 138-bp bands. For L1007fs, a wild type yields a single band at 151 bp, whereas the heterozygote yields the 151-bp band as well as 2 bands, at 131 and 20 bp. A homozygote yields only the 131-bp and 20-bp bands. Finally, for the R702W assay, the wild type yields bands at 20, 35, 54, and 76 bp. The heterozygote yields the aforementioned bands as well as a band at 130 bp, and the homozygote yields only the bands at 130, 35, and 20 bp.
TLR5 polymorphism genotyping was performed by PCR amplification of TLR5 from genomic DNA followed by DdeI restriction enzyme digestion. Genomic DNA was extracted from patient whole blood using a Bio-Rad Aqua Pure Genomic DNA kit. For genotyping by DdeI restriction digestion, a 276-bp PCR product was first generated with primers 5′-GGTAGCCTACATTGATTTGC-3′ (forward) and 5′-GAG AAT CTG GAG ATG AGG TAC CCG-3′ (reverse), digested with DdeI (New England Biolabs) overnight at 37°C and separated on a 1.8% agarose gel. A cytosine-thymidine transition at bp 1174 changes the arginine at amino acid 392 to a stop codon. This change results in a prematurely truncated TLR5 in the extracellular domain and causes the loss of the transmembrane domain and the entire signaling cytoplasmic tail. DdeI restriction enzyme digestion selectively cuts the 276-bp PCR product encompassing the 1174T variant.
Statistical methods
Nonparametric one sample Wilcoxon Rank sum test was used to compare previous HSCT values with the follow-up values at 6, 12, 24, 36, 48, and 60 months after HSCT for CDAI and CSI. A comparison between pre- and post-HSCT values was tested using the null hypothesis.
Results
Patient demographics and pre-HSCT disease manifestations
A total of 25 patients were enrolled, but 1 patient was removed from analysis because of clerical error–related infusion of an unmanipulated graft (Table 2). The 24 patients in the study were white (12 female, 12 male; ages 15–52 years; mean, 27 years). All patients had severe refractory disease having tried and failed extensive prior medical therapies (Table 2), whereas 71% (17/24) had prior CD-related surgeries. Eighteen patients had required medications for pain control; 8 patients had a history of total parenteral nutrition or tube feeding; 18 patients had a history of fistulas and or strictures, 8 patients had CD-related fevers. Mean pretransplantation disease duration, CDAI, and CSI were 10 years, 235, and 23, respectively. Sixteen patients had significant pre-HSCT complications from standard immunosuppressive therapies, including hepatotoxicity, sepsis, pancreatitis, anaphylaxis, and/or pancytopenia (Table 2).
Engraftment
The mean number of infused CD34+ and CD3+ cells were 6.35 × 106 cells/kg and 1.23 × 104 cells/kg, respectively (Table 3). Neutrophil (> 1000 cells/μL) and platelet (> 20 000 cells/μL and transfusion independent) engraftment occurred at a mean of 9 days, range 7 to 11 days and 8 to 16 days, respectively. The mean number for platelet and red blood cell transfusions were 4 and 5, respectively. Hospital discharge occurred on day 11 (range 8-18 days).
Toxicity
Besides fever that was common and neutropenic- or CD-related, 6 patients had bacteremia with positive blood cultures and/or central line infection (Table 4). Patients with CD-related fever before HSCT generally continued to have peritransplantation fever that resolved after neutrophil engraftment. In the first year after transplantation, infectious complications (with number of patients affected) included central line sepsis (1), sepsis not related to a central line (1), abdominal abscess (1), urinary tract infection (3), urosepsis (1), atypical mycobacterium pneumonia (1), varicella zoster virus dermatomal reactivation (1), and resection of a pulmonary aspergilloma, 2 years after HSCT (1; Table 4).
Interval response to harvesting stem cells
The interval between mobilization and HSCT varied from 3 to 7 weeks. Formal studies were not repeated during this interval and symptoms persisted. Although there was generally noted mild improvement in symptoms, these observations were not quantified.
Survival
Treatment-related mortality was zero. Overall mortality was 5% because 1 patient, more than 3 years after transplantation, died an accidental death. Postmortem examination revealed no evidence of CD. Although study follow up was limited to 5 years, 18 patients are alive more than 5 years since transplantation. Except for the 1 accidental death, all are alive without interval malignancies.
Disease response/remission
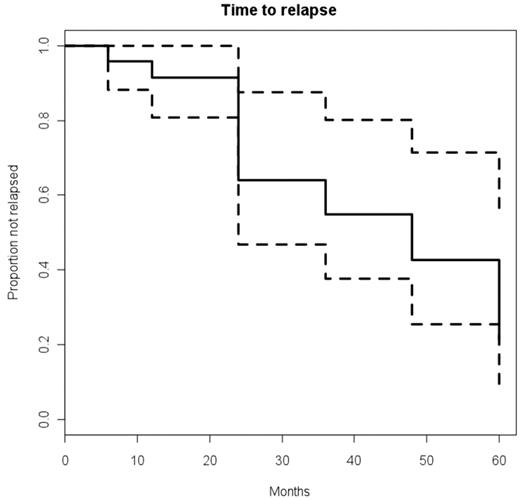
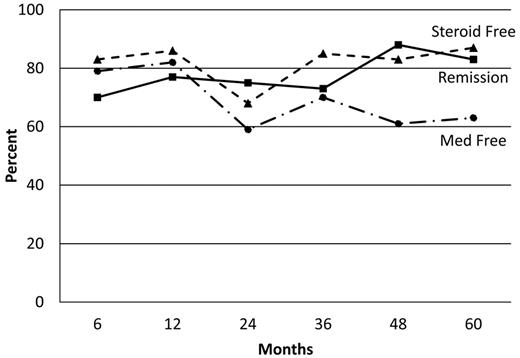
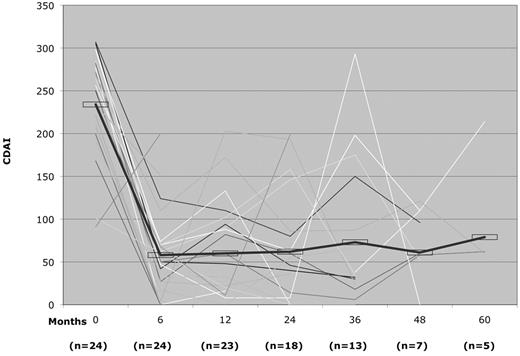
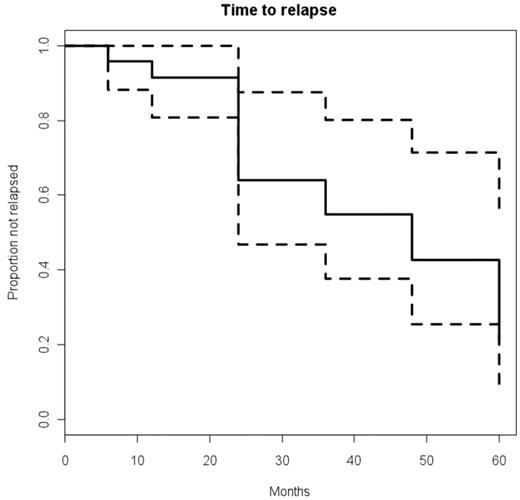
The CDAI had a rapid and significant post-HSCT improvement: P < 0.5 × 10−4 at 6 and 12 months, P < .005 at 2 and 3 years, P < .004 at 4 years, and P < .06 at 5 years (Figure 1). Similarly, the CSI had a rapid and significant improvement post-HSCT: P < 0.5 × 10−4 at 6 and 12 months, P < .004 at 2 years, P < .002 at 3 years, P < .02 at 4 years, and P < .06 at 5 years (Figure 2). The probability of clinical relapse-free survival (defined as the percentage not restarting CD medication) after HSCT is 91% at 1 year, 63% at 2 years, 57% at 3 years, 39% at 4 years, and 19% at 5 years (Figure 3). Nine patients have not relapsed to date, and of 15 patients who restarted therapy, 8 patients subsequently became medication-free for a second time (Table 5). Thus, the percentage of patients who remained in remission independent of therapy was between 70% to 80% at each annual evaluation for 5 years after transplantation (Figure 4). The medication-free survival was always above 60% and steroid-free survival approximately 80% for each of 5 years (Figure 4).
CDAI pre- and post-HSCT disease response. The thick line indicates mean CDAI for all patients.
CDAI pre- and post-HSCT disease response. The thick line indicates mean CDAI for all patients.
CSI pre- and post-HSCT disease response. The thick line indicates mean Craig CSI for all patients.
CSI pre- and post-HSCT disease response. The thick line indicates mean Craig CSI for all patients.
Probability of clinical relapse-free survival after HSCT. Probability is defined as time to restarting CD medication. Dashed lines represent the upper and lower limits of 95% confidence interval.
Probability of clinical relapse-free survival after HSCT. Probability is defined as time to restarting CD medication. Dashed lines represent the upper and lower limits of 95% confidence interval.
Percentage of remission, medication-free, and steroid-free survival after HSCT. The solid line represents the percentage in remission defined as CDAI less than 150 and CSI less than 12. The dashed line represents the percentage of steroid-free survivors. A dashed-dotted line represents the percentage of free immune suppressive and immune modulating medications.
Percentage of remission, medication-free, and steroid-free survival after HSCT. The solid line represents the percentage in remission defined as CDAI less than 150 and CSI less than 12. The dashed line represents the percentage of steroid-free survivors. A dashed-dotted line represents the percentage of free immune suppressive and immune modulating medications.
Surgeries
Twelve patients required one or more surgical procedures after HSCT. Some surgeries were, however, not related to active CD: 1 underwent cholecystectomy for cholilithiasis; 1 had a pretransplant colovesical fistula that persisted after transplantation and required a diverting colostomy because of recurrent urinary tract infections; and 2 had progressive ileal strictures that required surgical resection without pathologic evidence of inflammation; and 1 had closure of a pretransplantation loop ileostomy. For patients with active pretransplantation fistulas: 3 had severe anorectocutaneous fistulas with gradual, but incomplete, improvement; 1 had a severe anovulvar fistula that gradually, completely resolved over 3 years; and 1 required a total proctocolectomy 1 year later for severe rectosigmoid fistulizing and stricturing disease. For patients with active pretransplantation stricturing disease: 1 had a severe colonic stricture that completely resolved; 1 had severe anorectal stricturing that completely resolved, permitting ileostomy take-down with restoration of bowel continuity; and 1 had recurrent stricturing disease accompanying inflammatory recurrence.
Treg cell studies
The first 13 patients (Table 6) had peripheral blood analysis for Treg cells. Before HSCT, the mean percentage of CD4+CD25bright cells in peripheral blood of CD patients (2.62% ± 2.01%, n = 13) was reduced (P < .01) compared with normal volunteers (3.86% ± 1.67%, n = 8). Post-HSCT, compared with pre-HSCT, a robust increase in the percentage of CD4+CD25bright cells was observed: pre-HSCT 2.6 %± 2.01%, n = 13); 6 months post-HSCT 2.91% ± 1.74% (P < .05, n = 9); 1 year post-HSCT (3.55% ± 1.63%, n = 13, P < .05); 2 years post-HSCT (3.36% ± 1.63%, n = 10, P < .05); 3 years post-HSCT (3.99% ± 0.78%, n = 4, P = not significant).
Genetic testing
None of the subjects tested positively for the NOD2 genetic variants G908R or L1007P (Table 5). Only one subject tested positively for the heterozygous NOD2 genetic variant R702W (Table 5). He had only small bowel disease and required a small bowel resection 2 years after HSCT for small bowel obstruction, and has thereafter remained in clinical remission. Three patients showed heterozygosity for the genetic TLR5 polymorphism. Each of the 3 patients has had long-term remissions (Table 5).
Discussion
We now report the long-term follow up of nonmyeloablative HSCT for severe and refractory CD, an immune-mediated but probably not autoimmune disorder of the GI tract. The current medical therapy for severe CD is addition to standard immune suppressive drugs of an anti-TNF inhibitor (infliximab, adalimumab, or certolizumab). In randomized trials, anti-TNF therapy administered to anti-TNF naive patients who generally did not have fistulizing or stricturing disease resulted in medication-dependent clinical remissions (CDAI < 150) at 6 months of 46% to 59% and at 12 months of 35% to 41%.36-39 Remission duration with longer treatment (2 years) has only occasionally been reported.40
By convention, the definition of remission in anti-TNF trials is a CDAI less than 150 while the patient is receiving ongoing maintenance anti-TNF and may also be receiving other CD medications.36-41 Conversely, the accepted criteria for remission of cancer, that is, treatment free with no evidence of disease, is not applicable to CD. Therefore, to be consistent with and allow comparison to other trials for CD, our primary end point is remission defined by CDAI. However, HSCT, unlike other therapies for CD, results in a prolonged posttransplantation treatment-free interval without clinical symptoms that ranged from 6 months to greater than 5 years. To convey this concept, we, therefore, also defined “clinical relapse-free survival” after HSCT as the time to recurrence of symptomatic disease requiring medical therapy. For other nontransplantation CD-related therapy, the time to restarting medical therapy (ie, clinical relapse-free survival) is a non sequitur because patients were never removed from maintenance medical therapy. On the other hand, steroid-free remission in anti-TNF trials is achievable and has generally been reported to be between 18% and 36% at 1 year with CDAI defined remission of approximately 40%.36,38 In comparison, we report a consistent steroid-free survival of approximately 80%, CDAI remission of between 70% to 80%, and medication-free remission of greater than 60% for 5 years.
Recently, a short-term randomized trial of azathrioprine versus infliximab versus combined infliximab and azathioprine de-monstrated at 26 weeks a steroid-free clinical remission (CDAI < 150) of 30%, 44%, and 57%, respectively.42 However, steroid-free remission in that trial was defined as receiving equal to or less than 6 mg of nonsystemically absorbable budesonide (equivalent to 30 mg prednisone)43 a day for at least 3 weeks.42 In comparison, in our study, we define steroid-free as no steroids of any type or dose for at least 60 days and report a consistent steroid-free survival of approximately 80% for 5 years. Also in contrast to anti-TNF trials, transplant candidates had already failed anti-TNF therapy and had severe and often fistulizing and/or stricturing disease.
Transplantation using a CD34+ selected graft and nonmyeloablative regimen of cyclophosphamide and ATG achieves impressive results in otherwise refractory CD coma is to date the most effective therapy for achieving and maintaining remission and is the only documented therapy to achieve extended treatment-free clinical remissions. However, with the current conditioning regimen, it is not a cure, in that eventually standard medical therapies and or surgery are intermittently needed to maintain remission for most patients.
The procedure was safe, without mortality, even in patients heavily pretreated with anti-TNF therapy and with ongoing fistulas. The prevalence of bacteremia during transplantation is higher than our experience in patients with lupus,21 type 1 diabetes mellitus,23 and multiple sclerosis,22 and is probably secondary to a combination of prior immune suppression including anti-TNF treatments and ongoing inflammation and fistulas with disease-related breakdown in mucosal barriers. Thus, although CD itself may be the etiology of fever, during periods of neutropenia, positive blood cultures are common and aggressive preemptive antibiotic therapy is essential.
That defined mutations or polymorphisms are not always involved in CD is underscored in our study in which only 1 subject had a heterozygotic NOD2 variant, and 3 subjects were heterozygous for TLR5 polymorphism. The limited sample size, however, precludes definitive analysis of NOD2 or TLR5 polymorphism and response to autologous HSCT. Our patients had a robust rise in their Treg cells from pre- to posttransplantaion, lending support for a role for these cells in the salutary effect that we have observed. However, because of sample size, we cannot definitively draw a correlation between the pre- or post-HSCT Treg cells and disease response and or relapse.
To assess risk versus benefit in any new therapy for CD, it is necessary to compare the anticipated mortality for the new therapy to the intrinsic mortality from severe CD and its therapy. Although data are varied, most studies show increased mortality from CD above expected mortality rates for given populations.13-20 For example, a recent European study showed a mortality rate nearly twice as high as expected over 10 years.20 The subjects who have been selected for our study all had very severe disease and have failed all usual therapy, and presumably have a higher intrinsic mortality rate than the general CD population.
We found the CSI score (Table 1) to be a more reliable index in this group of refractory and heavily pretreated patients who often have had multiple prior surgeries, as it allows for improved assessment of disease activity for small bowel disease, which unlike colonic disease, is not associated with significant diarrhea; allows for better scoring of subjects with ileostomy; is more inclusive in terms of more measurements of disease activity; and is less subjective in terms of diarrhea, well being, abdominal pain, and presence or absence of an abdominal mass.
Although relapses have occurred in our patients after using a cyclophosphamide ATG nonmyeloablative regimen, we have achieved treatment-free remissions for as long as 5 years, and remission (CDAI < 150, CSI < 12) rates of between 70% to 80% for 5 years. Because approximately 40% of patients with CD develop intolerable side effects or lose response to anti-TNF,44 further investigation of stem cell therapy including the role of CD34 graft selection and type of conditioning regimen or other methods to maintain remission without surgery for anti-TNF refractory CD appears warranted.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was supported by a grant from the Broad Research Program of The Eli and Edythe Broad Foundation.
Authorship
Contribution: R.K.B. was principal investigator to protocol; R.M.C. was principal investigator to gastroenterology study; F.M. helped with data collection; K.Q. contributed to outpatient care; P.G. contributed to outpatient care; J.B. contributed to inpatient care; A.T. contributed to inpatient care; A.H. contributed to gastroenterology surgery; L.V. helped with Treg cell assays; W.J.S.d.V. helped with NOD2 and TLR5 genetic assays; B.J. helped with stats; and Y.O. contributed to inpatient and outpatient care.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard K. Burt, MD, Division of Immunotherapy, Feinberg School of Medicine, Northwestern University, 750 N Lake Shore Dr, Ste 647, Chicago, IL 60611; e-mail: rburt@northwestern.edu.