Abstract
Human cytomegalovirus (HCMV) is linked to the acceleration of vascular diseases such as atherosclerosis and transplant vasculopathy. One of the hallmarks of these diseases is angiogenesis (AG) and neovessel formation. Endothelial cells (ECs) are an integral part of AG and are sites of HCMV persistence. AG requires multiple synchronous processes that include EC proliferation, migration, and vessel stabilization. Virus-free supernatant (secretome) from HCMV-infected ECs induces AG. To identify factor(s) involved in this process, we performed a human cytokine array. Several cytokines were significantly induced in the HCMV secretomes including interleukin-6 (IL-6), granulocyte macrophage colony-stimulating factor, and IL-8/CXCL8. Using in vitro AG assays, neutralization of IL-6 significantly reduced neovessel formation. Addition of the HCMV secretome to preformed vessels extended neovessel survival, but this effect was blocked by neutralization of IL-6. In these cells, IL-6 prevented apoptosis by blocking caspase-3 and -7 activation through the induction of survivin. Neutralization of IL-6 receptor on ECs abolished the ability of HCMV secretome to increase survivin expression and activated effector caspases. Moreover, survivin shRNA expression induced rapid regression of tubule capillary networks in ECs stimulated with HCMV secretome and activated effector caspases. These observations may explain how CMV accelerates vascular disease despite limited infection in tissues.
Introduction
Human cytomegalovirus (HCMV) is an opportunistic pathogen that causes significant morbidity and mortality in immunocompromised populations, including transplant recipients. There is also increasing evidence to associate CMV infection with vascular complications and transplant loss.1,2 Specific examples of vascular disorders in which CMV may play a role include coronary artery disease, restenosis after angioplasty procedures, and transplant vascular sclerosis (TVS) in chronic graft rejection.2-6 Additional proof to HCMV effect on graft TVS comes from the finding of a higher incidence of viral DNA detected in the explanted vascular intima of patients with cardiac allograft TVS than in explants without vasculopathy.7 In an animal model, rat CMV infection is associated with the acceleration of TVS, leading to graft failure.8-14 As reported for humans, ganciclovir therapy can eliminate virus-induced TVS and also significantly prolongs graft survival in these animal model systems.15,16 The mechanisms through which CMV affects the pathogenesis of TVS and accelerates chronic graft rejection are for largely unknown. TVS is characterized histologically by diffuse concentric intimal proliferation that ultimately occludes the vessel.17 A sparse number of macrophages, T cells, natural killer cells, and B cells are seen in early lesions, while late lesions are associated with thickening of the allograft arterial wall containing smooth muscle cells (SMCs) interspersed with macrophages.18 TVS development involves chronic perivascular inflammation, endothelial cell (EC) dysfunction, SMC migration from the media to the intima, and proliferation that results in deposition of extracellular matrix (ECM). These events result in vessel narrowing, occlusion, and graft failure.1,17,19 A growing body of evidence supports a role for angiogenesis (AG) and tissue repair processes in the development of vascular disease, including TVS.20,21
AG is a physiological process involving growth of new blood vessels from pre-existing vessels. This process involves coordinated EC proliferation, invasion, migration, and tube formation.22 AG is a normal and vital process in growth and development, as well as in wound healing (WH). During these events, the endothelium remains inactive due to a balance of positive and negative regulatory factors. When vessel growth is required, the regulatory balance tips toward proangiogenic factors. Restoration of steady state is achieved by increasing AG inhibitors and vessel stabilization factors. Breakdown of the tightly regulated angiogenic balance leads to abnormal AG as, for example, in cardiovascular diseases.22 While animal models have provided solid in vivo evidence for the link between CMV and the acceleration of vascular disease processes, in vitro models allow exploration of the underlying molecular and cellular mechanisms associated with this link. We and others have demonstrated that HCMV infection alters the types and quantities of bioactive proteins released from infected cells, which we designate as HCMV secretome.23 Many of these factors have important roles in vascular diseases, and we hypothesize that a major role of CMV infection in the acceleration of TVS occurs through the increased production of AG and WH factors in the allograft. Mass spectrometry analysis of secretomes from mock- and HCMV-infected fibroblasts identified more than 1200 proteins of which more than 1000 were specific to or highly enriched in the HCMV secretome. Pathway analysis indicated that many (approximately 100) AG/WH proteins were present in the HCMV secretome, such as proteins involved in transforming growth factor-β (TGF-β) and other growth factor signaling pathways, cytokines, and chemokines, factors involved in ECM remodeling including a number of matrix metalloproteinases (MMP-1, -2, -3, -10, -12, and -19), cathepsins (B, D, F, K, L, and S), and tissue inhibitors of matrix metalloproteinases (TIMP-1, -2, and -3). A human cytokine antibody array was used to validate the mass spectrometry studies. Among the most highly abundant AG/WH-associated cellular factors identified in the HCMV secretome are cytokines/chemokines (interleukin-6 [IL-6], osteoprotegerin, macrophage inflammatory protein-1β [MIP-1α]/ C-C motif chemokine ligand 3 [CCL3], regulated upon activation normal T-cell expressed, and presumably secreted [RANTES]/CCL5, monocyte chemoattractant protein-3 [MCP-3]/CCL7, MIP-3α/CCL20, growth regulated oncogene-alpha [GROα]/C-X-C motif chemokine ligand 1 [CXCL1], epithelial neutrophil attractant-78 [ENA]-78/CXCL5, and CXCL16), receptors (tumor necrosis factor receptor I [TNF-RI] and TNF-RII, and intercellular adhesion molecule 1 [ICAM-1]), growth factors (transforming growth factor [TGF]-β1, hepatocyte growth factor [HGF], and granulocyte macrophage colony-stimulating factor [GM-CSF]), ECM modifiers (MMP-1, TIMP-1, TIMP-2, and TIMP-4), and the angiogenic RNase angiogenin.23,24 Interestingly, many of the genes identified as up-regulated by microarray analysis in the rat allograft hearts were also found in the HCMV secretome, suggesting that the factors involved in HCMV-induced AG and wound healing are similar to those expressed in the RCMV-infected allografts.14
IL-6 is a potent, pleiotropic, inflammatory cytokine that mediates a plethora of physiological functions, including the differentiation of lymphocytes, cell proliferation, cell survival, and inhibition of apoptotic signals.25-28 IL-6 levels are elevated in chronic inflammatory conditions, such as rheumatoid arthritis, and indeed antibodies against IL-6 and the soluble form of its receptor have been used therapeutically for this condition.29,30 The inflammatory effects of IL-6 have also been implicated as a factor in the failure of organ and tissue graft; moreover, elevated levels of the cytokine have been reported to accompany HCMV replication in transplanted lungs and bone marrow during episodes of inflammation or rejection.31-34
In this study, we identified several cytokines significantly induced in the HCMV secretome of ECs including IL-6, GM-CSF, and IL-8/CXCL8. Our results indicate that HCMV-induced IL-6 is the major mediator of neovessel formation and survival through the up-regulation of the antiapoptotic factor survivin.
Methods
Cells and virus preparation
Human umbilical vein ECs (HUVECs) were purchased from Lonza (cc-2519) and cultured in endothelial growth medium (EGM-2; Clonetics) containing 2% fetal bovine serum, human recombinant vascular endothelial growth factor, basic fibroblast growth factor, human epidermal growth factor, insulin growth factor, hydrocortisone, ascorbic acid, heparin, gentamicin, and amphotericin B (1 μg/mL each; high-serum medium). The cells were seeded into 100-mm culture dishes coated with 0.2% gelatin. Experiments were carried out with cells at passages 2 to 6. Low-passage normal human dermal fibroblasts (NHDFs; ATCC) were grown as monolayers in Dulbecco modified Eagle medium (cellgro; Mediatech) supplemented with 4.5 g/L glucose, l-glutamine, and sodium pyruvate, 10% heat-inactivated fetal calf serum (GIBCO-BRL), and antibiotics (penicillin [100 U/mL], streptomycin [100 mg/mL]). HCMV strain VR1814 (a gift from Dr Gerna, Servizio di Virologia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy) was produced by infected NHDFs. Infected cell supernatants were recovered at maximum cytopathic effect and cleared of cellular debris by centrifugation (10 000g for 15 minutes). Virus was pelleted through 10% sorbitol (Sigma-Aldrich) in phosphate-buffered saline at 22 000 rpm (Beckman model SW28 unit) for 60 minutes. Pelleted virus was resuspended in serum-free Dulbecco modified Eagle medium, and titers were determined with NHDFs.35
Preparation of the serum- and virus-free HCMV secretomes
Secretomes (cell and virus-free supernatants) were generated from HCMV- and mock-infected cells as follows: 100-mm dishes of 80% confluent HUVECs were washed 3 times with serum-free M199 medium and incubated for 12 hours in serum-free EC medium (SF-EBM2; Lonza). Subsequently, HUVECs were infected with HCMV at a multiplicity of infection of 3 in SF-EBM2 or mock infected for 3 hours at 37°C. Cells were washed 3× with SF-EBM2 and incubated for an additional 96 hours. Supernatants were centrifuged (10 000g for 15 minutes), followed by 2 high-speed centrifugations at 25 000 rpm (Beckman model SW28 unit) for 60 minutes each to remove virus particles. The clarified supernatants from HCMV- and mock-infected cells were tested for infectious virus by plaque assay and immunostaining for immediate-early protein (IE)72. Supernatants devoid of infectious virus and IE72 were used in secretome experiments.
AG assay
Secretome AG activity was evaluated using a modification of the standard Matrigel in vitro tubule formation assay.36 HUVEC monolayers were nutrient starved for 3 hours in SF-EBM2 before they were resuspended and plated (2 × 104 cells/well in 33 μL SF-EBM2) on 50 μL preformed growth factor–reduced BD Matrigel plugs (BD Biosciences) in a 96-well tray. Subsequently, 100 μL secretome were added to triplicate wells. Control wells received either 100 μL SF-EBM2 (negative control) or complete EGM2 (positive control). After 24 hours incubation, wells were analyzed for the formation of tubule structures by examination with an Axiovert 40 CFL microscope (10× and 20× objective; Carl Zeiss Microscopy), and the center of each well was digitally photographed with a Canon PowerShot A630. To quantitatively compare levels of tubule formation, digital images were analyzed for their degree of branching by counting branch points and for the extent of their network formation by counting enclosed polygonal spaces delimited by tubules and branch points (lumens). Data were compared for significance using Student t test. P values of ≤ .05 were considered significant.
Cytokines and anticytokine antibodies
All of the recombinant cytokines and anticytokine antibodies used in these studies are commercially available and are listed below with the company from which they were purchased. Recombinant human anti–IL-6, anti–IL-6 receptor, anti–GM-CSF, and anti–IL-8/CXCL8 (R&D Systems) are mouse monoclonal immunoglobulin G antibodies. For neutralizing experiments, the secretome with anti–IL-6 or anti–GM-CSF or anti–IL-8/CXCL8 antibodies (2 μg/mL; R&D Systems) was first incubated for 1 hour at 37°C, and then used to stimulate HUVECs. For IL-6 receptor blocking experiments, HUVECs were pretreated with anti–IL-6 receptor (1 μg/mL; R&D Systems) at 37°C for 1 hour to block IL-6 receptor, and then the secretome was added for 48 hours.
IL-6, GM-CSF, and IL-8/CXCL8 immunoassay
A double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) for the quantitative determination of human IL-6, GM-CSF, and IL-8/CXCL8 in tissue culture media was performed on the stored supernatants, according to the manufacturer's recommended procedure (R&D Systems) with a detection limit of 0.7 pg/mL for IL-6, 3 pg/mL for GM-CSF, and 3.5 pg/mL for IL-8/CXCL8. The plates were read with VersaMax microplate reader (Molecular Devices).
Human cytokine protein array
RayBio human cytokine array G Series 2000 antibody arrays (RayBiotech) were used to assay secretomes generated from mock and HCMV VR1814-infected HUVECs. A total of 174 factors were evaluated (for a complete list of factors, refer to www.raybiotech.com/G_Series.asp). Triplicate biological replicates of HCMV and mock secretomes were concentrated 5-fold using Amicon Ultra-4 (Millipore), and 50 μL of sample were incubated on the array. Arrays were processed according to the manufacturer's instructions, scanned using a Bioscience GeneScan Lite laser scanner, and analyzed using Imagene Version 3.5.1 digital processing software (Biodiscovery). Data were subtracted for local background and normalized to internal positive and negative controls. Cutoff values for detection were set at an average intensity of 500 over background. Mock- and HCMV-infected samples were compared for significance using Student t test. P values ≤ .05 were considered significant.
Immunoblotting
HUVECs were grown to subconfluence, nutrient starved for 12 hours, and incubated with secretome from HUVEC-infected cells or mock-infected cells. Whole-cell protein extracts were prepared by resuspending the cells in lysis buffer containing 50mM Tris-Cl, pH 6.8, 2% sodium dodecyl sulfate (SDS), and 1× protease inhibitor cocktail (Sigma-Aldrich P8340). After incubation at 4°C for 30 minutes, soluble proteins were collected by centrifugation at 15 000 × g for 10 minutes. Supernatants were analyzed for protein concentration with a Bio-Rad Dc protein assay kit (Bio-Rad Laboratories) and stored at −80°C. Proteins were separated by 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE; 50 μg protein per lane), and then transferred to Immobilon-P membranes (Millipore). The filters were blocked in 5% nonfat dry milk in 10mM Tris-CL, pH 7.5, 100mM NaCl, and 0.1% Tween 20, and immunostained with rabbit polyclonal against cleaved caspase-3-Asp175 (9664, 1:500; Cell Signaling), rabbit polyclonal against cleaved caspase-7-Asp198 (9491, 1:500; Cell Signaling), rabbit polyclonal against survivin (AF886, 1:1000; R&D Systems), rabbit polyclonal against STAT-3 (4904; Cell Signaling), and a mouse monoclonal α-tubulin (sc-58667, 1:1000; Santa Cruz Biotechnology) as an internal control. Immunocomplexes were detected with donkey anti–mouse (Jackson ImmunoResearch) or mouse anti–rabbit (Sigma-Aldrich) conjugated to horseradish peroxidase and visualized by enhanced chemiluminescence (ECL plus; Amersham/GE). Films were digitalized and quantified densitometrically using the image analysis system and software ImageJ 1.43 (National Institutes of Health).
Caspase assays
Caspase-3/7 activity in HUVECs was measured using a Caspase-Glo assay kit (Promega). Briefly, the proluminescent substrate containing the DEVD (the sequence is in a single-letter amino acid code) is cleaved by caspase-3/7. After caspase cleavage, a substrate for luciferase (aminoluciferin) is released. This results in the luciferase reaction and the production of luminescent signal. Cytosolic extracts from HUVECs incubated with the secretome for 24, 48, 72, and 96 hours or treated with exogenous IL-6 (10 ng/mL) for 48 hours were prepared in hypotonic extraction buffer (25mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.5, 5mM MgCl2, 1mM EGTA [ethyleneglycoltetraacetic acid], 1mM Pefablock, and 1 μg/mL each pepstatin, leupeptin, and aprotinin) and subsequently centrifuged (15 minutes at 13 000 rpm at 4°C). The protein concentration of the supernatant was adjusted to 1 mg/mL with extraction buffer and stored at −80°C. An equal volume of reagents and 10 μg/mL cytosolic protein were added to a white-walled 96-well plate and incubated at room temperature for 1 hour. The luminescence of each sample was measured with GloMax-96 Microplate Luminometer (Turner BioSystems).
Apoptotic cell death assay
To analyze DNA fragmentation as a sign of apoptosis, HUVECs were grown on coverslips, nutrient-starved for 12 hours, and then incubated with secretome from HUVEC-infected or mock-infected cells. Nuclear DNA fragmentation was detected using a terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay (DeadEnd Fluometric TUNEL System; Promega) and evaluated using an Axiovert 40 CFL microscope (Carl Zeiss Microscopy).
Inhibition of survivin protein expression
HUVECs were infected twice with a replication-incompetent recombinant retrovirus expressing either a survivin-directed short hairpin RNA (shRNA) cassette (5′-GATGGCCGAGGCTGGCTTCATCCACTGCC-3′) or a nonspecific 29-mer scrambled shRNA cassette (5′-GCACTACCAGAGCTAACTCAGATAGTACT-3′). Retrovirus was grown on as ϕNX-A cells described in the HuSH shRNA Plasmid Panels (29-mer) Application Guide (TG315477; OriGene Technology). To investigate the effects of survivin-specific shRNA on the HCMV secretome-dependent AG, transiently retrovirus infected HUVECs were nutrient-starved for 3 hours in SF-EBM2 before they were plated on growth factor-reduced Matrigel plugs in the presence of secretome from HUVEC-infected or mock-infected cells. At the same time, total cell extracts were prepared from transiently retrovirus-infected HUVECs treated with HCMV secretome or mock secretome for 24 hours and analyzed by immunoblotting with rabbit anti–STAT-3, anti-survivin, anti–cleaved caspase-3 and -7 antibodies.
Statistical analysis
Values are expressed as the mean ± SD of at least 3 independent experiments. Data were analyzed for significance using 1-way analysis of variance with Bonferroni posttest correction for multiple comparisons. Data were considered significant at P ≤ .05. All data were analyzed with GraphPad Prism4 software.
Results
The HUVEC-derived HCMV secretome induces AG
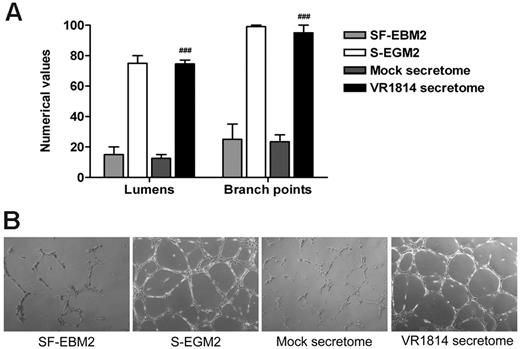
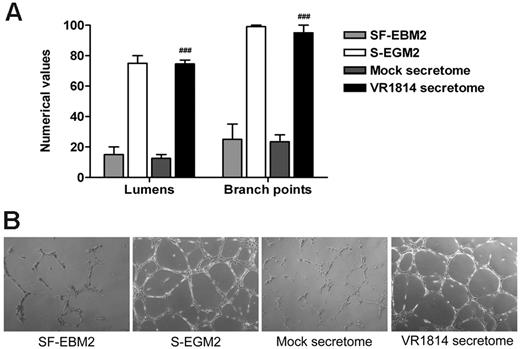
Previous experiments using the secretome from HCMV-infected fibroblasts have shown that the virus induces factors that promote AG. To verify that this effect was not a cell type-specific or viral strain-specific phenomenon, we nutrient-starved low passage primary HUVECs in EBM2 before harvest and plating in 96-well trays containing polymerized plugs of growth factor-reduced Matrigel in the presence of mock and HUVEC-derived HCMV VR1814 secretome. Controls included HUVECs grown in SF-EBM2 (negative control) or complete S-EGM2 (positive control). The extent of AG was quantified by determining the number of lumens delimited by intact capillary-like tubule structures, as well as the number of interconnecting branch points. HUVECs cultured for 24 hours in complete S-EGM2 or in the presence of the HCMV VR1814 secretome supported the formation of an extensive polygonal capillary network, while ECs cultured in SF-EBM2 or mock secretome were unable to form a consistent network of interconnecting tubules (Figure 1A-B).
Supernatants from HCMV-infected ECs induce AG. (A) Quantitative measures of AG consisted of the number of lumens and branch points. The results are expressed as mean ± SD, ##P < .01 versus mock. (B) Representative examples of each culture condition are shown as a low-power image (magnification, ×10).
Supernatants from HCMV-infected ECs induce AG. (A) Quantitative measures of AG consisted of the number of lumens and branch points. The results are expressed as mean ± SD, ##P < .01 versus mock. (B) Representative examples of each culture condition are shown as a low-power image (magnification, ×10).
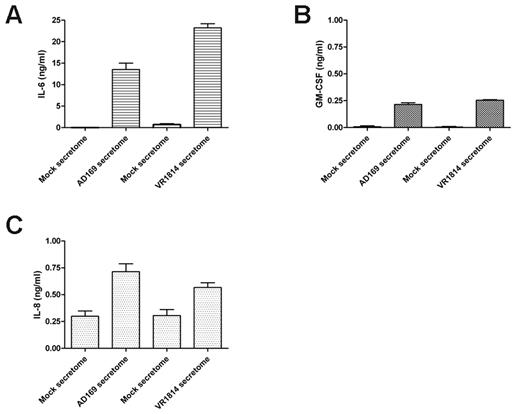
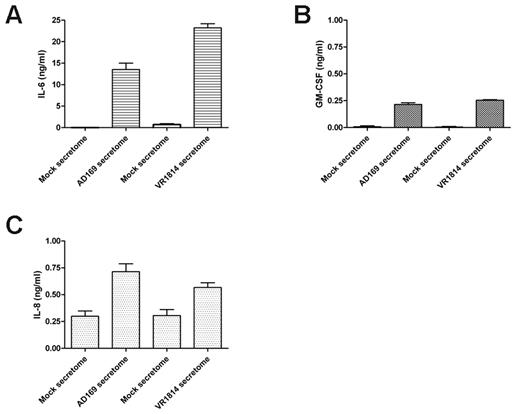
To determine whether the cytokines in the HCMV VR1814-infected HUVEC secretome are similar those in the AD169-infected fibroblast secretome,23 we analyzed the HCMV VR1814-infected HUVEC secretome using a RayBiotech human cytokines/growth factors array. In 3 replicated experiments, we detected 174 proteins in both the HCMV and the mock secretomes. A comparison between the viral and mock secretomes indicated 29 significantly induced and 6 down-regulated factors (Table 1). The most highly abundant AG-associated cytokines/chemokines present in the secretome of both fibroblasts and endothelial-infected cells included the IL-6, GM-CSF, interleukin-8 (IL-8/CXCL8), MIP-1β/CCL4, MCP-3/CCL7, MIP-3α/CCL20, and interferon-inducible T cell α-chemoattractant (I-TAC/CXCL11; Table 2). ELISA analysis confirmed that the proangiogenic proteins IL-6, GM-CSF, and IL-8/CXCL8 were present in the secretome from infected cells between 10 to 20 ng/mL for IL-6 (Figure 2A), 250 pg/mL for GM-CSF (Figure 2B), and 500 to 750 pg/mL for IL-8/CXCL8 (Figure 2C). These results indicate that the HCMV-EC secretome induces AG and contains the proangiogenic factor IL-6, GM-CSF, and IL-8/CXCL8 that might be involved in this process.
HCMV induces secretion of the angiogenic factors IL-6, GM-CSF, and IL-8. (A) ELISA quantification of IL-6 levels in 3 different supernatants of fibroblasts infected with HCMV AD169 and HUVECs infected with HCMV VR1814. (B) ELISA quantification of GM-CSF levels in 3 different supernatants of fibroblasts infected with HCMV AD169 and HUVECs infected with HCMV VR1814. (C) ELISA quantification of IL-8/CXCL8 levels in 3 different supernatants of fibroblasts infected with HCMV AD169 and HUVECs infected with HCMV VR1814.
HCMV induces secretion of the angiogenic factors IL-6, GM-CSF, and IL-8. (A) ELISA quantification of IL-6 levels in 3 different supernatants of fibroblasts infected with HCMV AD169 and HUVECs infected with HCMV VR1814. (B) ELISA quantification of GM-CSF levels in 3 different supernatants of fibroblasts infected with HCMV AD169 and HUVECs infected with HCMV VR1814. (C) ELISA quantification of IL-8/CXCL8 levels in 3 different supernatants of fibroblasts infected with HCMV AD169 and HUVECs infected with HCMV VR1814.
Depletion of IL-6 from HCMV secretome decreases tubule formation and stabilization
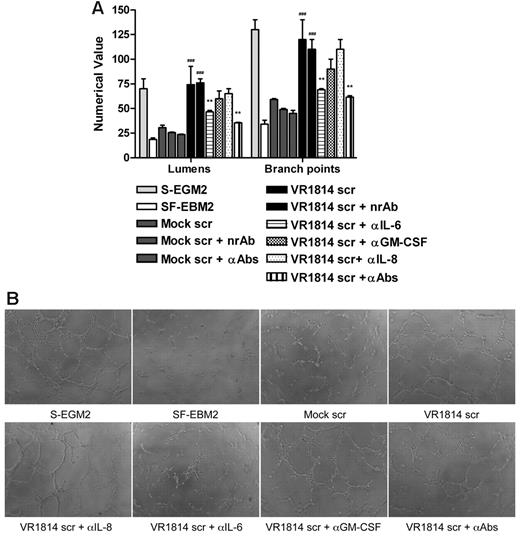
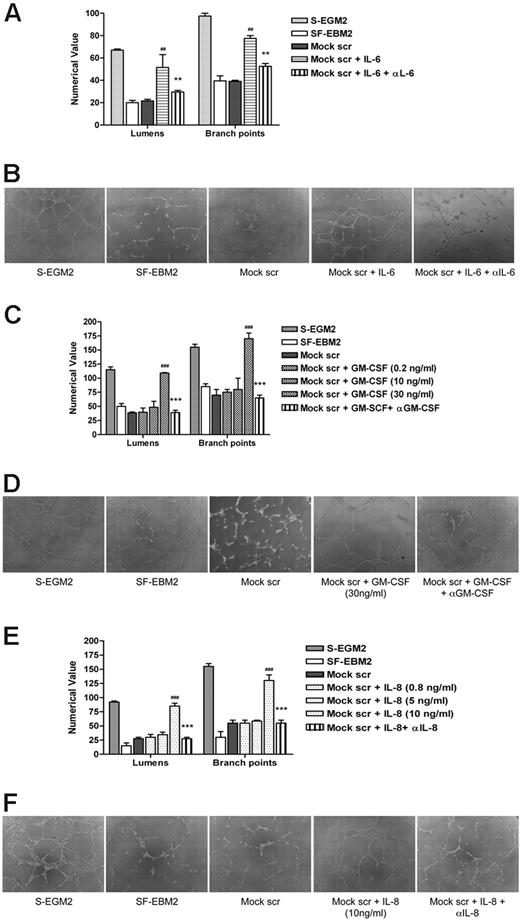
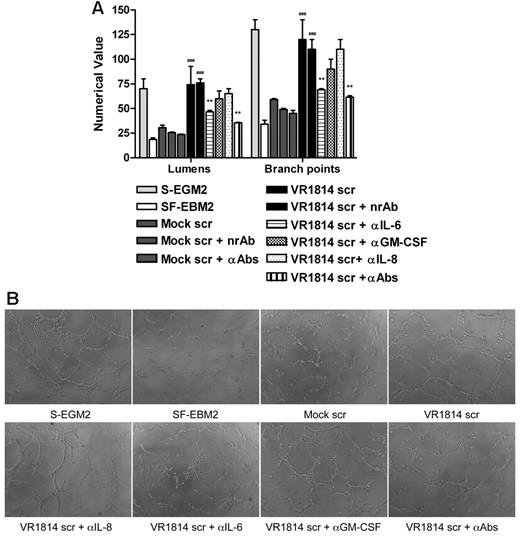
To investigate the role of IL-6, GM-CSF, and IL-8/CXCL8 in AG, secretomes from mock-infected and infected-HUVECs were preincubated with anti–IL-6, anti–GM-CSF, and anti–IL-8/CXCL8 antibodies alone or in combination and then tested for their ability to induce tubule formation on Matrigel. Controls included HUVECs grown in SF-EBM2 (negative control), in complete S-EGM2 (positive control), or in secretome-treated with a nonrelated antibody IL-1R. Neutralization of IL-6 activity resulted in 50% reduction of AG either with secretome from infected-HUVECs (Figure 3A-B) or from infected-NHDF (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), while GM-CSF and IL-8/CXCL8 neutralization did not significantly affect the angiogenic response (P > .05). Moreover, the angiogenic effect observed 24 hours after secretome stimulation persisted for up to 1 week after treatment (Figure 3C). The tubules formed in the presence of VR1814 secretome remained intact, while those induced by mock secretome or VR1814 secretome treated with IL-6 neutralizing antibodies had degenerated severely. The result obtained from the neutralization of IL-6, GM-CSF, and IL-8/CXCL8 in the same secretome is comparable with the neutralization effect of IL-6 alone, suggesting that the concentrations of GM-CSF and IL-8 in the secretome are not sufficient to support AG in vitro. To verify this hypothesis, a Matrigel assay was run after stimulation of HUVECs with exogenous human recombinant IL-6, GM-CSF, or IL-8/CXCL8. Mock secretome was tested alone or in the presence of recombinant IL-6, GM-CSF, or IL-8/CXCL8. The presence of IL-6 at the same concentration in the HCMV secretome can rescue the angiogenic activity of the mock-secretome and, as expected, the neutralization of IL-6 inhibits tubule formation (Figure 4A-B). The same experiment run with the exogenous GM-CSF and IL-8/CXCL8 clearly shown that the optimal dose for these 2 factors for stimulating tube formation is higher than the concentration present in the secretome (Figure 4C-F). To demonstrate that the GM-CSF and IL-8/CXCL8 antibodies can neutralize these cytokines, we used the neutralizing antibodies with concentrations of IL-8/CXCL8 and GM-CSF that can induce AG and show that the antibodies neutralize the effect (Figure 4C-F). Taken together, these results indicate a crucial role for IL-6 not only in the HCMV secretome-dependent induction of AG, but also in the stabilization of neovessels.
Neutralization of IL-6 decreases AG and vessel stabilization at 1 week. (A) Quantification of Matrigel tube formation assay after 24 hours in the presence of mock secretome (Mock scr), mock secretome with a nonrelated antibody (Mock scr + nrAb), mock secretome with anti–IL-6, anti–GM-CSF, and anti–IL-8/CXCL8 (Mock scr + αAbs), HCMV VR1814 secretome (VR1814 scr), HCMV VR1814 secretome with a nonrelated antibody (VR1814 scr + nrAb), HCMV VR1814 secretome with anti–IL-6 antibody (VR1814 scr + αIL-6), HCMV VR1814 secretome with anti–GM-CSF antibody (VR1814 scr + αGM-CSF), HCMV VR1814 secretome with anti–IL-8/CXCL8 antibody (VR1814 scr + αIL-8), and HCMV VR1814 secretome with anti–IL-6, anti–GM-CSF, and anti–IL-8/CXCL8 antibodies (VR1814 scr + αAbs). The results are expressed as mean ± SD, ###P < .001 versus mock, **P < .01 versus HCMV VR1814 secretome. (B) Representative examples of each culture conditions are shown as a low-power image (magnification, ×10). (C) Tubule survival is shown after 1 week on Matrigel in the presence of mock secretome, HCMV VR1814 secretome, HCMV VR1814 secretome with anti–IL-6 antibody (VR1814 scr + αIL-6) at low-power image (magnification, ×10), and at high-power image (magnification, ×20).
Neutralization of IL-6 decreases AG and vessel stabilization at 1 week. (A) Quantification of Matrigel tube formation assay after 24 hours in the presence of mock secretome (Mock scr), mock secretome with a nonrelated antibody (Mock scr + nrAb), mock secretome with anti–IL-6, anti–GM-CSF, and anti–IL-8/CXCL8 (Mock scr + αAbs), HCMV VR1814 secretome (VR1814 scr), HCMV VR1814 secretome with a nonrelated antibody (VR1814 scr + nrAb), HCMV VR1814 secretome with anti–IL-6 antibody (VR1814 scr + αIL-6), HCMV VR1814 secretome with anti–GM-CSF antibody (VR1814 scr + αGM-CSF), HCMV VR1814 secretome with anti–IL-8/CXCL8 antibody (VR1814 scr + αIL-8), and HCMV VR1814 secretome with anti–IL-6, anti–GM-CSF, and anti–IL-8/CXCL8 antibodies (VR1814 scr + αAbs). The results are expressed as mean ± SD, ###P < .001 versus mock, **P < .01 versus HCMV VR1814 secretome. (B) Representative examples of each culture conditions are shown as a low-power image (magnification, ×10). (C) Tubule survival is shown after 1 week on Matrigel in the presence of mock secretome, HCMV VR1814 secretome, HCMV VR1814 secretome with anti–IL-6 antibody (VR1814 scr + αIL-6) at low-power image (magnification, ×10), and at high-power image (magnification, ×20).
Exogenous IL-6, GM-CSF, and IL-8 promote HUVEC tube formation. (A) Quantification of Matrigel tube formation assay after 24 hours in the presence of human recombinant IL-6 (10 ng/mL). The results are expressed as mean ± SD, ##P < .01 versus mock, **P < .01 versus mock + IL-6. (B) Representative examples of each culture condition are shown as a low-power image (magnification, ×10). (C) Quantification of Matrigel tube formation assay after 24 hours in the presence of increasing concentrations of GM-CSF. The results are expressed as mean ± SD, ###P < .001 versus mock, ***P < .001 versus mock + GM-CSF (30 ng/mL). (D) Representative examples of each culture condition are shown as a low-power image (magnification, ×10). (E) Quantification of Matrigel tube formation assay after 24 hours in the presence of increasing concentrations of IL-8/CXCL8. The results are expressed as mean ± SD, ##P < .01 versus mock, **P < .01 versus mock + IL-8/CXCL8. (F) Representative examples of each culture condition are shown as a low-power image (magnification, ×10).
Exogenous IL-6, GM-CSF, and IL-8 promote HUVEC tube formation. (A) Quantification of Matrigel tube formation assay after 24 hours in the presence of human recombinant IL-6 (10 ng/mL). The results are expressed as mean ± SD, ##P < .01 versus mock, **P < .01 versus mock + IL-6. (B) Representative examples of each culture condition are shown as a low-power image (magnification, ×10). (C) Quantification of Matrigel tube formation assay after 24 hours in the presence of increasing concentrations of GM-CSF. The results are expressed as mean ± SD, ###P < .001 versus mock, ***P < .001 versus mock + GM-CSF (30 ng/mL). (D) Representative examples of each culture condition are shown as a low-power image (magnification, ×10). (E) Quantification of Matrigel tube formation assay after 24 hours in the presence of increasing concentrations of IL-8/CXCL8. The results are expressed as mean ± SD, ##P < .01 versus mock, **P < .01 versus mock + IL-8/CXCL8. (F) Representative examples of each culture condition are shown as a low-power image (magnification, ×10).
Secretion of IL-6 from HCMV-infected cells prevents apoptosis by blocking the activation of caspases-3 and -7 through induction of survivin
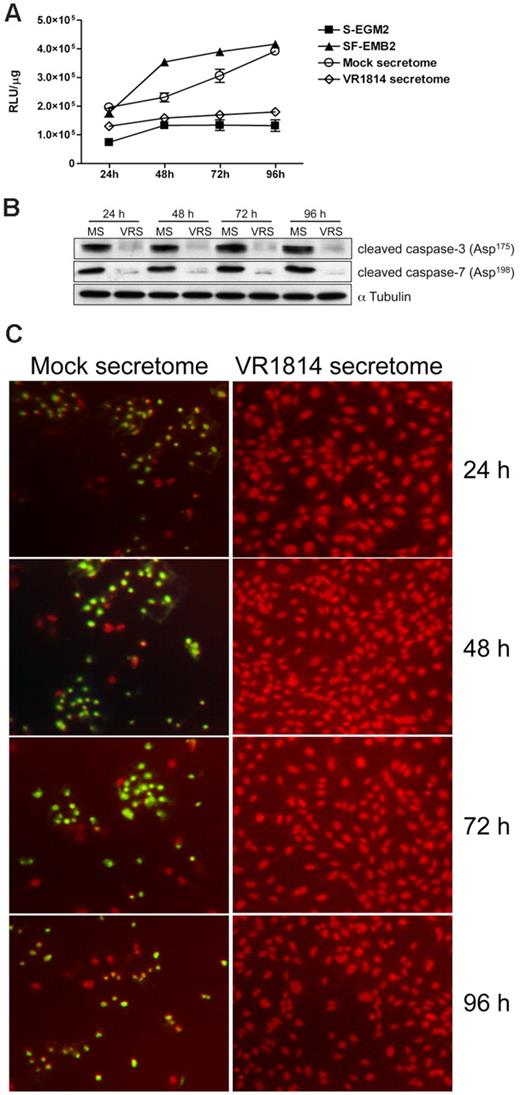
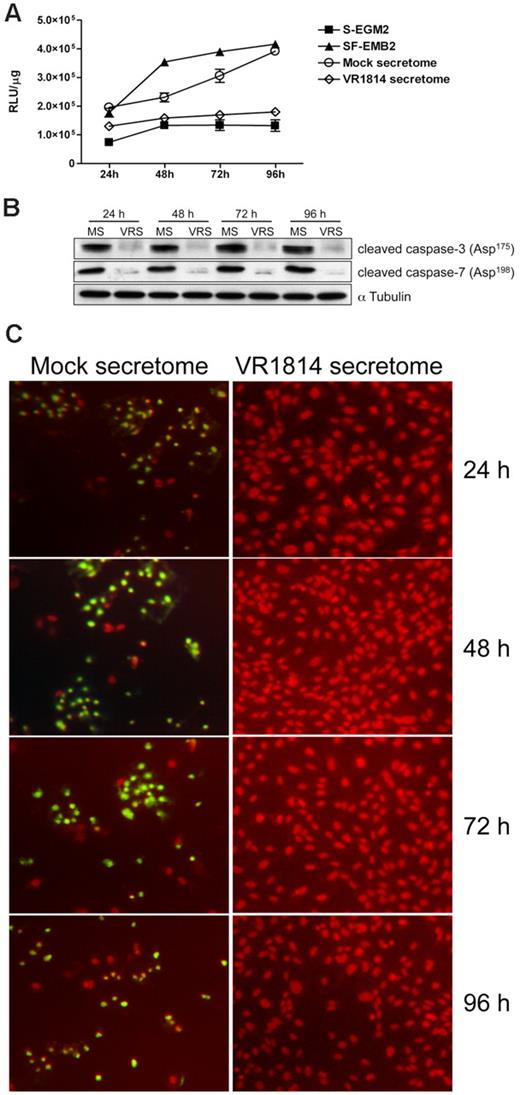
To determine the mechanism through which the HCMV secretome induces vessel stabilization, we analyzed the effect of virus-free supernatants on the apoptosis pathway. Because caspase-3 and -7 are the key factors in apoptosis, we examined their activation in ECs cultured in the HCMV-infected EC secretomes. Controls included HUVECs grown in SF-EBM2 (positive control) or complete S-EGM2 (negative control). Protein extracts were prepared after 24, 48, 72, and 96 hours after treatment and tested for caspase-3 and -7 activity using a luciferase assay. As shown in Figure 5A,C, secretomes from infected HUVECs or NHDF cells (supplemental Figure 2A-B) prevented caspase-3 and -7 activation at each time point analyzed, with a major effect at 48, 72, and 96 hours (4-fold reduction). The mock-treated ECs showed levels of caspase-3 and -7 activity comparable with the positive control. Immunoblot analysis confirmed a 93% to 97% increase of cleaved caspase-3 and -7 in mock secretome-treated cells compared with HCMV secretome stimulation (Figure 5B,D). To further confirm the antiapoptotic effect of the HCMV secretome we performed a TUNEL assay in HUVECs stimulated with HCMV secretome over time. As shown in Figure 6C, fragmented DNA is predominant in cells treated with mock secretome. To determine whether the antiapoptotic effect was due to the presence of IL-6, IL-8/CXCL8, or GM-CSF, we analyzed the activation of effector caspases and DNA fragmentation in ECs treated for 48 hours with the HCMV secretomes neutralized with anti–IL-6, anti–GM-CSF, anti–IL-8/CXL8 antibodies, or IL-1R (control). As shown in Figure 6A,C, neutralization of IL-6 activity in HCMV-infected ECs restores the apoptotic effect observed with the mock secretome or the serum-free media. Substantial differences in caspase-3 and -7 activation were not observed in secretome-stimulated ECs upon treatment with anti–GM-CSF, anti–IL-8, or with a nonrelated antibody. The introduction of exogenous IL-6 in the mock secretome led to a 3-fold reduction in caspase-3 and -7 activation (Figure 6B) and brought the expression of cleaved caspases back to the level observed in the ECs treated with complete media. Comparable results were obtained with the TUNEL assay (Figure 6C).
HCMV secretomes decrease caspase-3 and -7 activity. HUVECs were starved overnight and then stimulated with mock- or HCMV-secretomes during a time course analysis. (A) Cytosolic extracts from HUVECs incubated with controls, mock- and HCMV VR1814-secretome for 24, 48, 72, and 96 hours were prepared in hypotonic extraction buffer. An equal volume of a proluminescent substrate (DEVD), and cytosolic proteins were added to a white-walled 96-well plate and incubated at room temperature for 1 hour. The luminescence of each sample run in triplicate was measured in a plate-reading luminometer. The graphic shows the mean ± SD of 3 independent experiments. (B) Cell lysates from HUVECs incubated with mock-secretome (MS) and HCMV VR1814 (VRS) were subjected to SDS-PAGE followed by Western blotting to evaluate the expression of activated/cleaved caspase-3 and -7. αTubulin served as an internal control. (C) HUVECs were stimulated as above, and nuclear DNA fragmentation as a sign of apoptosis was determined over the time using a TUNEL assay. Fragmented DNA is indicated by green fluorescence. Nuclei were stained with propidium iodide (red fluorescence).
HCMV secretomes decrease caspase-3 and -7 activity. HUVECs were starved overnight and then stimulated with mock- or HCMV-secretomes during a time course analysis. (A) Cytosolic extracts from HUVECs incubated with controls, mock- and HCMV VR1814-secretome for 24, 48, 72, and 96 hours were prepared in hypotonic extraction buffer. An equal volume of a proluminescent substrate (DEVD), and cytosolic proteins were added to a white-walled 96-well plate and incubated at room temperature for 1 hour. The luminescence of each sample run in triplicate was measured in a plate-reading luminometer. The graphic shows the mean ± SD of 3 independent experiments. (B) Cell lysates from HUVECs incubated with mock-secretome (MS) and HCMV VR1814 (VRS) were subjected to SDS-PAGE followed by Western blotting to evaluate the expression of activated/cleaved caspase-3 and -7. αTubulin served as an internal control. (C) HUVECs were stimulated as above, and nuclear DNA fragmentation as a sign of apoptosis was determined over the time using a TUNEL assay. Fragmented DNA is indicated by green fluorescence. Nuclei were stained with propidium iodide (red fluorescence).
Neutralization of IL-6 activity in the HCMV secretomes induces apoptosis. HUVECs were starved overnight and then stimulated with mock-secretome, HCMV-secretome, and neutralized HCMV-secretomes for 48 hours. (A) Cytosolic extracts from HUVECs incubated with controls, mock-secretome, HCMV VR1814-secretome, and HCMV VR1814-secretome neutralized with anti–IL-6, anti–GM-CSF, anti–IL-8/CXCL8, or nonrelated antibody were prepared in hypotonic extraction buffer. An equal volume of a proluminescent substrate (DEVD) and cytosolic proteins were added to a white-walled 96-well plate and incubated at room temperature for 1 hour. The luminescence of each sample run in triplicate was measured in a plate-reading luminometer. The results are expressed as mean ± SD of 3 independent experiments, ***P < .001 versus mock. (B) HUVECs were starved overnight and then stimulated with mock-secretome, mock-secretome with IL-6 (10 ng/mL), and mock-secretome with IL-6 (10 ng/mL) + anti–IL-6 Ab (2 μg/mL) for 48 hours. Cytosolic extracts were prepared as above and the luminescence of each sample run in triplicate was measured in a plate-reading luminometer. The results are expressed as mean ± SD of 3 independent experiments, **P < .01 versus mock. (C) TUNEL assay performed in the same experimental conditions as above. Fragmented DNA is indicated by green fluorescence. Nuclei were stained with propidium iodide (red fluorescence).
Neutralization of IL-6 activity in the HCMV secretomes induces apoptosis. HUVECs were starved overnight and then stimulated with mock-secretome, HCMV-secretome, and neutralized HCMV-secretomes for 48 hours. (A) Cytosolic extracts from HUVECs incubated with controls, mock-secretome, HCMV VR1814-secretome, and HCMV VR1814-secretome neutralized with anti–IL-6, anti–GM-CSF, anti–IL-8/CXCL8, or nonrelated antibody were prepared in hypotonic extraction buffer. An equal volume of a proluminescent substrate (DEVD) and cytosolic proteins were added to a white-walled 96-well plate and incubated at room temperature for 1 hour. The luminescence of each sample run in triplicate was measured in a plate-reading luminometer. The results are expressed as mean ± SD of 3 independent experiments, ***P < .001 versus mock. (B) HUVECs were starved overnight and then stimulated with mock-secretome, mock-secretome with IL-6 (10 ng/mL), and mock-secretome with IL-6 (10 ng/mL) + anti–IL-6 Ab (2 μg/mL) for 48 hours. Cytosolic extracts were prepared as above and the luminescence of each sample run in triplicate was measured in a plate-reading luminometer. The results are expressed as mean ± SD of 3 independent experiments, **P < .01 versus mock. (C) TUNEL assay performed in the same experimental conditions as above. Fragmented DNA is indicated by green fluorescence. Nuclei were stained with propidium iodide (red fluorescence).
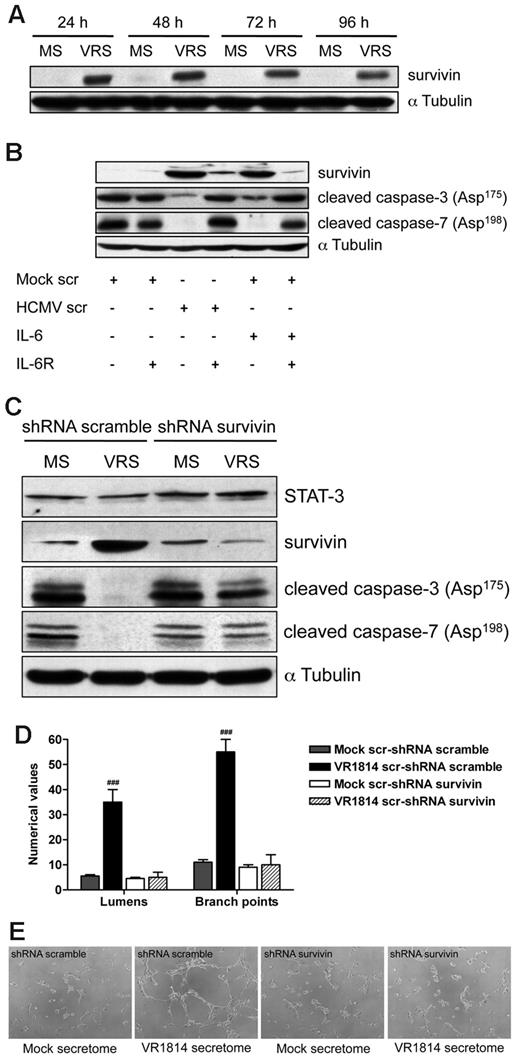
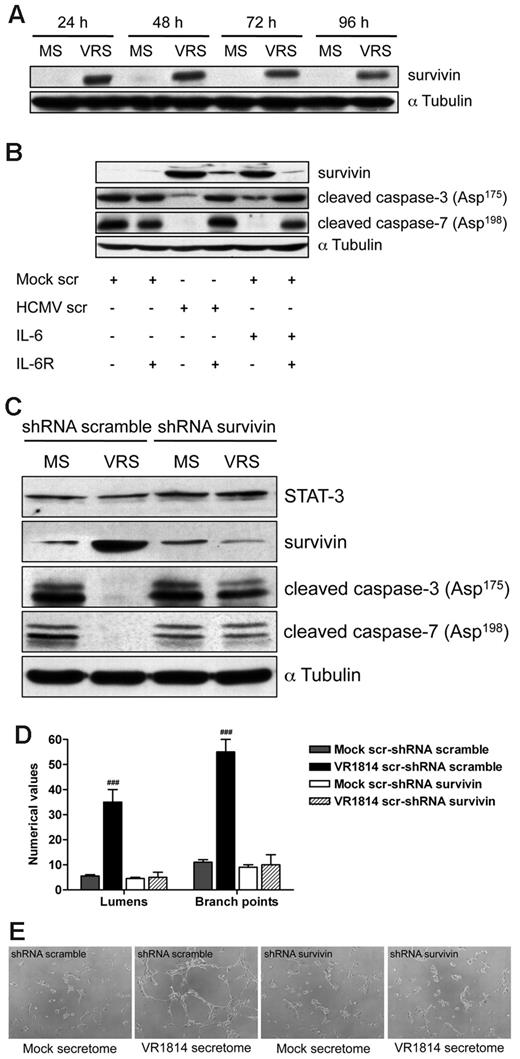
Activated IL-6 receptor has been reported to result in STAT-3 phosphorylation, which subsequently regulates the expression of nuclear genes involved in apoptosis, including survivin.37,38 It has been shown that inhibition of STAT3 signaling induces apoptosis and diminishes survivin expression in primary effusion lymphoma.39 Therefore to determine whether survivin is involved in secretome-mediated apoptosis, we examined levels of this protein in ECs treated with the HCMV secretome. As shown in Figure 7A, HCMV-secretome significantly increased survivin expression at each time point analyzed. Neutralization of IL-6 receptor on ECs abolished the ability of HCMV secretome to increase survivin and caused the activation of caspase-3 and -7 (Figure 7B). These results suggest that the HCMV secretome promotes survival of ECs through the induction of survivin via IL-6 receptor–mediated signaling.
HCMV secretome stimulates survivin expression through IL-6 receptor blocking apoptosis and promoting AG. (A) Cell lysates from HUVECs incubated with mock-secretome (MS) and HCMV VR1814 (VRS) for 24, 48, 72, and 96 hours were subjected to SDS-PAGE followed by Western blotting to evaluate the expression of survivin. αTubulin served as an internal control. (B) HUVECs were treated with anti–IL-6R antibody and stimulated with the secretome for 48 hours. Expression of survivin, cleaved caspase-3, and caspase-7 was determined by Western blotting. αTubulin immunodetected with mAb served as an internal control. (C) HUVECs were infected with recombinant retroviruses expressing either a survivin-directed shRNA or a nonspecific control (scramble) shRNA followed by stimulation with the HCMV secretome. Expression of STAT-3, survivin, cleaved caspase-3, and caspase-7 was determined by Western blotting. αTubulin served as an internal control. (D) Quantification of Matrigel tube formation assay after 24 hours of HUVECs in the same experimental condition as above. The results are expressed as mean ± SD, ###P < .01 versus mock. (B) Representative examples of each culture condition are shown as a low-power image (magnification, ×10).
HCMV secretome stimulates survivin expression through IL-6 receptor blocking apoptosis and promoting AG. (A) Cell lysates from HUVECs incubated with mock-secretome (MS) and HCMV VR1814 (VRS) for 24, 48, 72, and 96 hours were subjected to SDS-PAGE followed by Western blotting to evaluate the expression of survivin. αTubulin served as an internal control. (B) HUVECs were treated with anti–IL-6R antibody and stimulated with the secretome for 48 hours. Expression of survivin, cleaved caspase-3, and caspase-7 was determined by Western blotting. αTubulin immunodetected with mAb served as an internal control. (C) HUVECs were infected with recombinant retroviruses expressing either a survivin-directed shRNA or a nonspecific control (scramble) shRNA followed by stimulation with the HCMV secretome. Expression of STAT-3, survivin, cleaved caspase-3, and caspase-7 was determined by Western blotting. αTubulin served as an internal control. (D) Quantification of Matrigel tube formation assay after 24 hours of HUVECs in the same experimental condition as above. The results are expressed as mean ± SD, ###P < .01 versus mock. (B) Representative examples of each culture condition are shown as a low-power image (magnification, ×10).
shRNA targeting survivin suppresses HCMV secretome-dependent AG and induces apoptosis in ECs
We next wanted to determine whether the targeting of survivin affected the antiapoptotic activity of the HCMV secretome as well as neovessel formation/stabilization. HUVECs were infected with recombinant retroviruses expressing either a survivin-directed shRNA or a nonspecific control (scramble) shRNA followed by stimulation with the HCMV secretome. As shown in Figure 7C, the expression of survivin shRNA after mock or HCMV secretome stimulation resulted in 85%-90% decrease of protein level and in the induction of caspase-3 and -7 cleavage. However, exposure to the HCMV secretome still induced survivin and blocked effector caspase activation in the control shRNA-transduced HUVECs (Figure 7C). Comparable levels of STAT-3 were observed in the survivin and scramble shRNA transduced cells demonstrating the integrity of the IL-6 signaling pathway. Under these conditions, we analyzed the role of survivin in HCMV secretome-dependent AG. As we previously observed, stimulation with HCMV secretome in the control shRNA-transduced HUVECs resulted in the persistence of viable capillary-like structures (Figure 7D-E). In contrast, viable capillaries were not formed in the presence of survivin-directed shRNA and incubated with either HCMV or mock secretome (Figure 7D-E). These results indicate a functional role for survivin in the control of HCMV secretome-mediated apoptosis and ultimately in the induction of AG.
Discussion
In this study, we report for the first time a proangiogenic effect of the secretome from HCMV infected-ECs, supporting a critical role of these cells not only during the initial response to vessel injury but also in enhancing the chronic inflammatory response. Interestingly, the HCMV secretome contains high levels of IL-6 that promotes long-term EC survival. Neutralization of IL-6 leads to vessel destabilization and activation of effector caspases-3 and -7. The antiapoptotic mechanism involves the up-regulation of survivin through the IL-6 receptor-mediated signaling and, more importantly, suppression of survivin induces apoptosis and rapid regression of tubule capillary networks in ECs stimulated with HCMV secretome. These observations may explain how CMV accelerates vascular diseases such as chronic rejection in solid organ transplant patients as well as atherosclerosis despite limited infection in tissues.
The vascular endothelium by virtue of its strategic location between the plasma and the underlying tissue and its constitutive properties is endowed with a large array of functions that are vital for homeostasis. Under normal physiological conditions, ECs monitor the transport of plasma molecules, using bidirectional receptor-mediated and receptor-independent transcytosis and endocytosis, to regulate vascular tone and to synthesize and secrete a large variety of factors. Under pathological conditions alterations in endothelial function precede, for example, the development of atherosclerotic plaques and contribute decisively to their perpetuation and to the clinical manifestations of vascular diseases.40 Vascular disease involves multiple components including the primary injury event, the local response to injury through platelet aggregation, and the production of cytokines and growth factors, followed rapidly by inflammation. HCMV infection of the vasculature may contribute to any one or all of these components, thereby accelerating the disease process. Here, we show that HCMV-infected ECs secrete a number of cytokines (IL-6, GCP-2/CXCL6, MPIF-1/CCL23, GM-CSF, osteoprotegerin, TNFα) and chemokines (RANTES/CCL5, MCP-2/CCL8, MIP-3α/CCL20, IP-10/CXCL11, MIP-1α/CCL3, and MIP-1β/CCL4, MCP-3/CCL7, I-TAC/CXCL11, GRO-α/CXCL1, IL-8/CXCL8) that can indirectly contribute to switch the growth/differentiation process from a natural healing to a pathological process. Importantly, CMV encodes chemokine homologs that also recruit and stimulate a multitude of host cellular infiltrates that mediate the inflammatory response.41,42 In vivo studies have demonstrated that acute CMV infection is associated with a subendothelial inflammation of allograft vascular structures both in human and in rat.43 Nonactivated T lymphocytes and monocytes predominate the inflammatory lesion in the subendothelium, suggesting that the virus-linked vascular wall inflammation may play a role in the immune injury toward allograft vascular structures, particularly to endothelium, and thus contribute to and accelerate TVS.
Vascular disorders are linked to an increase of AG and inhibition of cell death.44,45 Many of the factors present in the HCMV secretome derived from infected-HUVECs regulate these processes, a finding corroborated by our demonstration that the HCMV secretome not only promotes EC tubule formation but also stabilizes the tubule network. Although GM-CSF and IL-8/CXCL8 in HCMV EC secretome have the potential to regulate AG and apoptosis and they might play a role in vivo, our results indicate that the concentration of IL-6 in our model is sufficient to mediate these processes. IL-6 is a potent proangiogenic cytokine, that participates in AG during tumor progression, wound healing, and brain vascular system development.46 Moreover, IL-6 produced by ECs inhibits apoptosis of B-chronic lymphocytic leukemia cells.28 Our results clearly show that neutralization of IL-6 in the HCMV secretome impairs tubule formation and vessel stabilization. The survival of the ECs in the Matrigel is mediated by an antiapoptotic activity of IL-6; indeed depletion of this factor from the HCMV secretome increases the activation of caspase-3 and -7.
The increase of IL-6 in the secretome after HCMV infection of ECs parallels the observation of increased IL-6 in CMV-infected tissues. In one study, IL-6 was significantly induced in lung transplants during CMV pneumonia and to a lesser extent during allograft rejection in these patients.32 Studies by our group have shown that IL-6 mRNA is significantly up-regulated in RCMV-infected heart tissues both at 21 and 28 days after transplantation in a rat allograft model during the development of TVS.8,13 Thus, the findings presented in this paper together with the observations for the RCMV allograft model provide a possible mechanism of CMV infection-accelerated TVS through secretion of IL-6 from infected cells, which involves induction of a pathological tissue-remodeling process that leads to EC proliferation and survival.
We also observed an increase of the antiapoptotic factor survivin in the ECs treated with the HCMV secretome (Figure 7A). Survivin is a member of the inhibitor of apoptosis (IAP) gene family,47 also known as Birc5, and is found in the cytoplasm and in the nucleus, yet biological functions of the protein differ within each cellular compartment. In the cytoplasm, survivin serves as an efficient inhibitor of apoptosis controlling both caspase-dependent (external) and mitochondria (internal) apoptosis cascades. Intranuclear survivin plays a pivotal role in cell division being as a chromosomal passenger protein.48 Recent studies have suggested a potential beneficial role of survivin to prevent EC apoptosis in myocardial ischemia, pressure overload cardiac remodeling, and heart failure.49,50 Moreover, results from Simosa et al51 strongly suggest that survivin is an important modulator of the generalized injury response in the vessel wall and a potential unique marker of dedifferentiated cells that are involved in the formation of the neointima. Interestingly, persistent activation of STAT-3 signaling induces survivin gene expression and confers resistance to apoptosis in tumor cells, and activated IL-6 receptor has been reported to phosphorylate STAT-3.37-39 Here we shown that HCMV secretome increases survivin expression in ECs (Figure 7A) and the block of IL-6 receptor on the cells abrogates the antiapoptotic activity of the HCMV secretome (Figure 7B). Finally, suppression of survivin impairs not only the activation of effector caspase-3 and -7 (Figure C), but also the formation and the persistence of viable capillary-like structures on Matrigel (Figure 7D-E).
One question that arises from these studies is how would HCMV induction of cellular cytokines that block apoptosis be beneficial for the virus? HCMV is known to encode at least 2 antiapoptic proteins, vICA and vMIA, with the later gene being indispensible for viral replication.52 In addition, HCMV has been reported to induce the antiapoptotic cellular proteins AKT, Bcl-2, and Np73.53 Clearly, these observations indicate that blocking cellular apoptosis is an essential characteristic of the virus. Therefore, one explanation for the HCMV induction of IL-6–mediated antiapoptosis described in this report is that this pathway may represent a redundant mechanism for the virus to maintain cellular survival of persistently HCMV-infected ECs. However, a byproduct of this process is the stimulation of AG that can lead to pathological consequences such as atherosclerosis or TVS.
In conclusion, results from these experiments show for the first time the activation of the normally dormant antiapoptotic survivin in a viral-related in vitro model and provide new insights into the pathophysiology of HCMV-accelerated vascular diseases. Understanding of the IL-6-survivin pathway may lead to the development of more effective therapies targeting the survivin or caspase pathway against a thus far incurable and fatal disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jay Nelson for the helpful discussions and for critical reading of the manuscript.
This work was supported by National Institutes of Health grant HL 088603 and grant HL 083194 (to D.N.S.).
National Institutes of Health
Authorship
Contribution: S.B. performed research and analyzed data; D.N.S. analyzed data and wrote the manuscript; V.D. analyzed data and wrote the manuscript; L.W., C.N.K., and P.P.S. performed research; and P.C. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patrizia Caposio, Vaccine and Gene Therapy Institute, Oregon Health & Science University, 505 NW 185th Ave, Beaverton, OR 97006; e-mail: caposiop@ohsu.edu.